Abstract
Patient: Female, 36
Final Diagnosis: Small cell sarcoma
Symptoms: Mass to right upper thigh
Medication: —
Clinical Procedure: Surgical resection
Specialty: Oncology
Objective:
Rare disease
Background:
A subset of undifferentiated small round cell sarcomas (USRCSs) is currently being recognized as emerging entities with unique gene fusions: CIC-DUX4 (the area of focus in this article), BCOR-CCNB3, or CIC-FOXO4 gene fusions. CIC-DUX4 and CIC-FOXO4 fusions have been reported in soft tissue tumors, while BCOR-CCNB3 fusion with an X chromosomal inversion was described in both bone and soft tissue tumors. CIC-DUX4 fusion can either harbor t(4;19)(q35;q13.1) or t(10;19)(q26.3;q13), while t(4;19)(q35;q13.1) is reported more commonly.
Case Report:
The aim of this study is to share a new case report of a 36-year-old woman who had a rapidly growing mass in her right upper thigh, which was found to be an undifferentiated small round cell sarcoma with t(4;19) (q35;q13.1) CIC-DUX4 fusion was confirmed by cytogenetic testing. Combined modality treatment with surgery, radiation, and chemotherapy was used and achieved a good response. A review of the literature of the reported cases with CIC-DUX4 fusions including both t(4;19) and t(10;19) translocations revealed a total of 44 cases reported. Out of these 44 cases, 33 showed t(4;19)(q35;q13.1) translocation compared to 11 cases with t(10;19)(q26.3;q13).
Conclusions:
Undifferentiated small round cell sarcomas are aggressive tumors. Their treatment includes surgery, chemo-therapy, and radiation. Resistance to chemotherapy is common. Lung and brain are common sites of metastasis, with associated poor prognosis. Generally, median survival is less than 2 years. Newer techniques have been developed recently which helped identify a subset of previously unclassifiable sarcomas, with promising prognostic value.
MeSH Keywords: Genes, Homeobox; Sarcoma, Small Cell; Translocation, Genetic
Background
Undifferentiated small round cell sarcomas (USRCS), also called small blue round cell tumors (SBRCTs), comprise a rare subset of highly aggressive EWSR1-negative sarcomas that occur in younger populations. This subset of undifferentiated small round blue cells sarcomas are also referred to as ‘Ewing’s-like tumors’ [1]. When viewed under a microscope, they share similar morphological features with the Ewing sarcoma family of tumors (EST). “They demonstrate small to medium-sized round to oval cells, packed in solid sheets with minimal or absent intervening collagen” [2], and they share with them an aggressive clinical course [2]. Upon conducting next-generation sequencing, unique mutations are being recognized: recurrent BCOR-CCNB3 fusion (in primitive bone and soft tissue sarcoma) [3]; CIC-FOXO4 fusion (in soft tissue sarcoma) [4] Or CICDUX4 fusion as a result of either t(4;19) or t(10;19) translocations (in primitive soft tissue sarcoma) [2]. The clinical features in our case harboring t(4;19) are compatible with those described in the literature.
Case Report
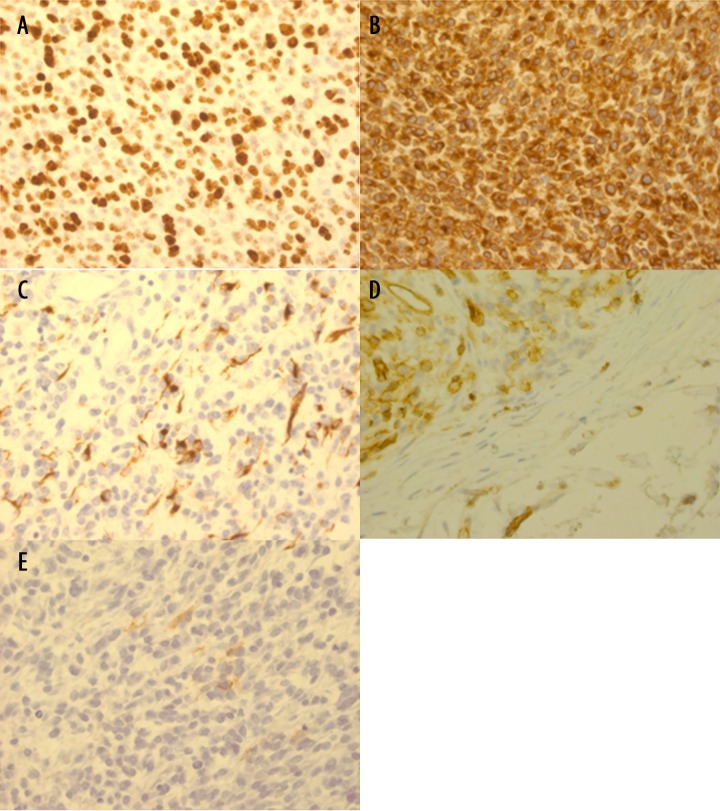
A healthy 36-year-old African-American nonsmoker nonalcoholic woman presented to the hospital 11 months ago with pain and swelling in her right groin. Two weeks prior to that, she noticed a small boil which rapidly increased to an “orangesize”. Physical exam revealed a tender, fluctuant mass on the right upper medial thigh. An incision and drainage was performed and it revealed blood clots. Ultrasound showed a complex 4.8×3.5×6 cm heterogeneous swelling consistent with hematoma. A CT scan of the right femur, (Figure 1A), revealed a complex mass with hematoma suspicious for malignancy. The patient subsequently underwent an elective wide local excision (>10 cm). Pathology (Figure 2A, 2B), revealed a high-grade un-differentiated small round cell sarcoma. Immunohistochemical staining (Figure 3A–3E) showed very focally positive staining for CD 99, and positive staining for CD 31; Pan-Cytokeratin, and Bcl-2 was strongly and diffusely positive. FISH studies for EWSR1 (Ewing sarcoma) and SYT (synovial sarcoma) rearrangements were negative. t(4: 19)(q35;q13.1) CIC-DUX4 gene fusion was confirmed by FISH (Figure 4). Margins were clear, with no lymph node involvement. Within a few weeks, the patient began adjuvant radiation treatment. She received 45 Gy to the tumor bed plus a wide margin followed by a cone down to a total dose of 66.6Gy. Radiation treatment was delivered using intensity modulation (IMRT) to maximize dose to the coverage of the tumor volume, while minimizing dose to the perineal skin and femur/femoral neck. Subsequently chemotherapy was started; where the patient was given 4 cycles of Doxorubicin and ifosfamide along with mesna. During the fourth and the final cycle, the patient was unable to receive ifosfamide secondary to the adverse effects during the third cycle. She reported that her tongue was “swollen and twisted” and that she had some memory problems. Otherwise, the patient was clinically stable. A follow-up MRI femur with contrast performed 11 months later (Figure 1B) and CT abdomen/pelvis showed no convincing evidence of residual neoplasm or metastatic disease.
Figure 1.
(A) CT scan of the femur at diagnosis which shows a 4.3×5.6×6.2 cm of an extra muscular mass in the proximal, medial right thigh, see arrow. (B) MRI of the right femur 11 months later showing no residual lesions or masses.
Figure 2.

(A) 100× magnification Round cell sarcoma at center of photo, left: geographic tumoral necrosis, right: fibrous septa. (B) 400× magnification Round cell sarcoma with moderate nuclear pleomorphism, coarse nuclear chromatin and prominent nucleoli, variably scant cytoplasm, and numerous abnormal mitotic figures.
Figure 3.
Immunohistochemical stain (400×): (A) Ki-67: Strong nuclear staining in >90% of tumor cells. (B) Bcl-2: 100% strong cytoplasmic staining of tumor cells. (C) Pankeratin: Focal, strong cytoplasmic staining of tumor cells. (D) CD31: Patchy, strong cytoplasmic staining of tumor cells. (E) CD99: Focal, weak membranous staining pattern of tumor cells.
Figure 4.
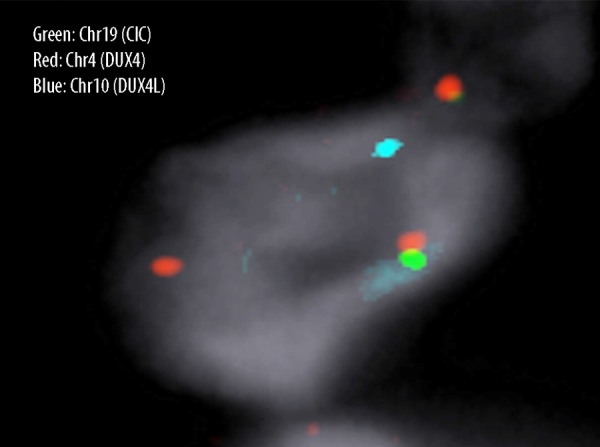
CIC-DUX4 fusion detected by FISH using a dual-color, single-fusion probe: BAC clone RP11-521G19 (green) centromeric to DUX4 on chromosome 4 and RP11-778C1 (red) telomeric to CIC on chromosome 19. CICDUX4 translocation results in 1 fused (yellow), 1 red, and 1 green signal (normal 2R: 2G; fused 1R: 1G: 1Y). RP11-108K14 (centromeric to DUX4L, light blue on chromosome 10) is also shown. There is no evidence of a t10;19 fusion.
Discussion
Small round cell sarcomas (including Ewing sarcoma, small cell osteosarcoma, undifferentiated synovial sarcoma, and rhabdomyosarcoma) have distinctive morphologic features and most of them carry a unique chromosomal translocation. A small subset of undifferentiated small round cell sarcoma remains unclassified, leading to a dilemma in diagnosis and treatment.
Recent studies have identified unique translocations and gene fusions in these unclassifiable highly aggressive undifferentiated small blue round cell sarcomas, which are considered to be of diagnostic value: CIC-DUX4 fusion transcript with either t(4;19) (q35;q13.1) translocation or t(10;19)(q26.3;q13) trans-location, and BCOR-CCNB3 fusion with an X chromosomal inversion, also CIC-FOXO4 fusions, all of which are EWSR1- and SYT-negative on FISH. These new small group, especially those with CIC-DUX4 fusion within the round cell category, have recently been recognized in the new 2013 WHO classification as discrete entities [1].
Recent data suggest that certain genetic events like CICDUX4 fusion are more prevalent than others. Initially, individual cases of undifferentiated small round cell sarcomas with CIC-DUX4 fusion were reported [5–10,17], and as more data have been gathered from 2 larger series, CIC-DUX4 fusion was found in approximately 66% of tumor cases that were EWSR1-negative [2,11,17].
Differential diagnosis of CIC-DUX4 fusion sarcomas includes Ewing-like sarcoma and the Ewing sarcoma family of tumors (ESFT). The morphology and immunophenotype can help to identify tumor type. CIC-FOXO4 gene fusion, however, can be difficult to distinguish from CIC-DUX4 fusion by morphology and immunophenotype. Therefore, testing for gene fusions is needed to make the diagnosis [4].
CIC-DUX4 fusion – mechanism of action
The gene capicua transcriptional repressor (CIC), located on the chromosome 19q13.2, is usually fused to copies of the DUX4 gene (double homeobox 4), which are found on either chromosome 4 (4q35) or chromosome 10 (10q26.3) [2]. CIC-DUX4 fusion, in particular, is known to potentiate the NIH/3T3 fibro-blasts transformation. This potentiation has been suggested by an enhanced CIC transcriptional activity, with resultant deregulation of the CIC downstream targets. Further gene expression analysis shows that CIC-DUX4 fusion recognizes a novel target sequence on the EMR/ETV5 promoter; and it binds directly to it, leading to an up-regulation to its expression [7].
CIC-DUX4 fusion
CIC-DUX4 fusion has been reported in 44 cases of EWSR1-negative undifferentiated small round cell sarcomas, including our patient (Table.1). Age, sex, location, metastasis status, treatment used if reported, life span based on last follow-up, and the source of information were all merged and are detailed in Table 1. Noticeably, t(4;19)(q35;q13.1) translocation has been the most prevalent gene mutation reported (33 cases) compared to t(10,19)(q26.3;q13) translocation (11 cases). The first case ever reported with CIC-DUX4 fusion with t(4;19)(q35;q13.1) was in 1996 by Richkind et al. [5]. Age range for CIC-DUX4 cases was 6–62 years, with a mean age of 27, affecting younger age groups. General, the sex distribution for CIC-DUX4 fusion was slightly in favor of females: (M:F ratio is 1:1.31); however, among cases with t(4;19) translocation M: F ratio was similar (1:1.08). In contrast, t(10;19) is more common among males (M: F ratio of 1.75:1). The most commonly affected body site is the limbs (including groin) 22/44 cases (50%). Unusual primary cerebral localization was reported in 1 case 1/44 (0.02%). Metastasis rate was high in 26/44 reported cases (59%), and the most common place to metastasize was to the lungs 18/44 cases (∼41%), in addition to other body areas like bone, brain, and peritoneum. Treatment modalities vary based on staging, and there is minimal literature available with which to determine a standard therapy for this rare disease. In general, surgery and radiation therapy remain the main treatments for sarcomas. Combinations of doxorubicin and Ifosfamide, or doxorubicin, vincristine, and cyclophosphamide are used (Table 1). Average life span after diagnosis was around 15.4 months and only 6 cases had prolonged survival (22–48 months (Table 1).
Table 1.
Clinico-pathologic features, treatment and survival rates for CIC-DUX-4 fusion cases reported, both with t(4;19) and t(10;19) translocations.
| Case | Age | Sex | Originating site | Mets | CIC-DUX4+: t(4;19)(q35;q13.1) or t(10,19) (q26.3;q13) | Treatment applied: Surgery +/−, Radiation Therapy (RT)+/−, Chemo +/− | Life span after diagnosis (months) | Source |
|---|---|---|---|---|---|---|---|---|
| 1 | 36 | F | Right Thigh/groin | None | t(4;19) | S+, RT+, Chemo+ | 11, no recurrence so far | Current case |
| 2 | 40 | F | Lower part of thoracic wall | Lung/local recurrence | t(4;19) | Chemo+ (pre-surgery), S+ | 10, died of cancer | Panagopoulos et al., 2014 [23] |
| 3 | 33 | F | Right Knee | None | t(4;19) | S+, Local RT+ | 18, no recurrence so far | Smith et al., 2014 [22] |
| 4 | 38 | M | Lower arm | Lung, cubital fossa, lymph node | t(4;19) | S+ (Resection of lung metastasis), RT+ (local palliative) | 17, alive with disease | Smith et al., 2014 [22] |
| 5 | 23 | F | Left upper back | Lung | t(4;19) | S+ (Lung metastasectomy), Chemo+ (Vincristine, Doxorubicin, Cyclophosphamide – Ifosfamide, Etoposide), RT+ (stereotactic radiosurgery to primary and lung metastasis) | 48, no recurrence so far | Smith et al., 2014 [22] |
| 6 | 24 | F | Rt Parieto-Occipital (Brain) | N/A | t(4;19) | S+ three times (two relapses), Chemo+ (Euro-Ewing99 protocol with dose reductions and omitting Doxorubicin), R+ | 4 (until the case was reported) | Bielle et al., 2014 [21] |
| 7 | 36 | F | paraspinal | N/A | t(4;19) | N/A | N/A | Specht et al., 2014 [24] |
| 8 | 19 | F | Stomach | N/A | t(4;19) | N/A | N/A | Specht et al., 2014 [24] |
| 9 | 25 | M | Neck | N/A | t(4;19) | N/A | N/A | Specht et al., 2014 [24] |
| 10 | 36 | F | Shoulder | N/A | t(4;19) | N/A | N/A | Specht et al., 2014 [24] |
| 11 | 25 | F | Paravertebral | N/A | t(4;19) | N/A | N/A | Specht et al., 2014 [24] |
| 12 | 47 | M | Clavicular | N/A | t(4;19) | N/A | N/A | Specht et al., 2014 [24] |
| 13 | 37 | M | Peri-prostatic | N/A | t(4;19) | N/A | N/A | Specht et al., 2014 [24] |
| 14 | 25 | F | Rt Calf | Lung, Brain | t(4;19) | N/A | 10.2, died of cancer | EY Choi et al., 2013 [12] |
| 15 | 32 | F | Rt Buttock/pelvis | Lung | t(4;19) | N/A | 14.1, died of cancer | EY Choi et al., 2013 [12] |
| 16 | 20 | F | Lt Shoulder | Lung | t(4;19) | N/A | 16.8 | EY Choi et al., 2013 [12] |
| 17 | 43 | M | Lt knee | Pelvis | t(4;19) | N/A | 14.2 | EY Choi et al., 2013 [12] |
| 18 | 62 | F | Rt Inguinal Region; & later an additional mass in the lt thigh | Lung | t(4;19) | S+ twice due to local recurrence, Chem+, RT+ | 10–12, died from cancer | B. Kajtar et al., 2013 [14] |
| 19 | 11 | F | Flank | N/A | t(4;19) | N/A | N/A | Graham et al., 2012 [15] |
| 20 | 9 | F | Paraspinal | N/A | t(4;19) | N/A | N/A | Graham et al., 2012 [15] |
| 21 | 11 | F | Inguinal | N/A | t(4;19) | N/A | N/A | Graham et al., 2012 [15] |
| 22 | 19 | M | Foot | N/A | t(4;19) | S+ in all patients but one.Chem+ (vincristine, doxorubicin, cyclophosphamide, Ifosfamide) | N/A | Italiano et al., 2012 [2] |
| 23 | 30 | F | Peritonsil/mandible | Lung | t(4;19) | 13.4 | Italiano et al., 2012 [2] | |
| 24 | 33 | F | Paraspinal | None | t(4;19) | 7.1 | Italiano et al., 2012 [2] | |
| 25 | 35 | F | Groin | Lung | t(4;19) | 28.9 | Italiano et al., 2012 [2] | |
| 26 | 16 | M | Thigh | Lung | t(4;19) | 7 | Italiano et al., 2012 [2] | |
| 27 | 28 | M | Thigh | None | t(4;19) | 6 | Italiano et al., 2012 [2] | |
| 28 | 14 | M | Head/Neck | Lung | t(4;19) | N/A | 9, died of cancer | Yoshimoto et al., 2009 [6] |
| 29 | 6 | M | Lt Hip | Lung | t(4;19) | S+ Gross total resection, Chemo+ (vincristine, doxorubicin, cyclophosphamide), RT+ | No recurrence after 7 wk | Rakheja et al., 2007 [10] |
| 30 | 62 | F | Lt Buttock/Pelvis with vaginal invasion | N/A | t(4;19) | S−, RT+, Chem+ | 10, died of cancer | Kawamura-Saito et al., 2006 [7] |
| 31 | 31 | M | Lt Shoulder | N/A | t(4;19) | Complete surgical resection, Chem+ (cisplatin, adriamycin),RT+ | Healthy without recurrence for 30 post operation | Kawamura-Saito et al., 2006 [7] |
| 32 | 16 | F | Abdominal wall | Lung, Brain, Bone | t(4;19) | N/A | 14, died of cancer | Somers et al,. 2004 [13] |
| 33 | 12 | M | Right ankle | Lung, Groin | t(4;19) | Chem+, RT+ (no response for treatment) | 10, died of cancer | KE Richkind et al., 1996 [5] |
| 34 | 36 | F | Trapezius | Lung | t(10;19) | RT+ (Local), Chemo+ (Vincristine, Doxorubicin, Cyclophosphamide – Ifosfamide, Etoposide), S+ (en bloc re-resection) | 11, died of cancer | Smith et al., 2014 [22] |
| 35 | 37 | M | Rt lower leg | Groin lymph node | t(10;19) | S+ (resection of primary, groin), Chemo+ ((Vincristine, Doxorubicin, Cyclophosphamide – Ifosfamide, Etoposide) | 22, no recurrence so far | Smith et al., 2014 [22] |
| 36 | 50 | F | Peritoneum | N/A | t(10;19) | N/A | N/A | Specht et al., 2014 [24] |
| 37 | 33 | F | Knee | N/A | t(10;19) | N/A | N/A | Specht et al., 2014 [24] |
| 38 | 19 | M | Rt Gluteus | Inguinal lymph nodes | t(10,19) | S+; chemo + (vincristine, adriamycin, and cyclophosphamide alternating with ifosfamide and etoposide), evaluated for RT | N/A | Machado et al., 2013 [16] |
| 39 | 15 | M | Back – superficial | Lung | t(10,19) | S+ in all patients but one. Chem+ (vincristine, doxorubicin, cyclophosphamide, Ifosfamide) | 14.2 | Italiano et al., 2012 [2] |
| 40 | 45 | M | Back – deep | Lung, bone, brain | t(10,19) | 10.4 | Italiano et al., 2012 [2] | |
| 41 | 49 | M | Arm – deep | Lung | t(10,19) | 14.6 | Italiano et al., 2012 [2] | |
| 42 | 26 | M | Chest wall soft tissue – deep | N/A | t(10,19) | 9.6 | Italiano et al., 2012 [2] | |
| 43 | 26 | M | upper arm with skin ulceration- superficial | Peritoneum | t(10,19) | 22.6 | Italiano et al., 2012 [2] | |
| 44 | 29 | F | Arm – deep | N/A | t(10,19) | 46.7 | Italiano et al., 2012 [2] |
N/A – information not available.
Role of radiation
Studies showed that patients with high-grade soft tissue sarcomas who were randomized to amputation versus limb-sparing surgery plus adjuvant radiation had no differences in 5-year disease-free survival and overall survival, establishing limb preservation plus radiation as the standard of care [18].
Patients who underwent limb-sparing surgery were randomized to adjuvant radiation or observation. In patients with high-grade tumors, adjuvant radiation was associated with decreased local failure (0% vs. 19%, SS) [19].
Given the inner thigh location of our patient’s tumor, we decided to use radiation in the post-operative setting to decrease the likelihood of a wound complication, this decision was supported by a randomized trial [20].
Conclusions
High-grade undifferentiated small round cell sarcomas are very aggressive tumors, and quickly develop resistance to traditional chemotherapy regimens. Treatment includes surgery, chemotherapy, and radiation. Despite aggressive treatment, they exhibit high rates of metastasis to lung and brain, often with a poor prognosis, and median survival is less than 2 years. Next-generation sequencing techniques help in identifying subsets of previously unclassifiable sarcoma with distinct mutations and unique histopathology features, and are promising diagnostic and possibly prognostic tools.
Acknowledgments
Thanks to Dr. Dafydd G. Thomas, (The University of Michigan Department of Pathology) for performing the FISH and providing us with CIC-DUX4 fusion picture.
References:
- 1.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. World Health Organization classification of tumours of soft tissue and bone. 4th ed. Lyon: International Agency for Research on Cancer (IARC); 2013. [Google Scholar]
- 2.Italiano A, Sung YS, Zhang L, et al. High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosom. Cancer. 2012;51:207–18. doi: 10.1002/gcc.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierron G, Tirode F, Lucchesi C, et al. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nature Genetics. 2012;44:461–66. doi: 10.1038/ng.1107. [DOI] [PubMed] [Google Scholar]
- 4.Sugita S, Yasuhito A, Akiko T, et al. A Novel CIC-FOXO4 Gene Fusion in Undifferentiated Small Round Cell Sarcoma: A Genetically Distinct Variant of Ewing-like Sarcoma. Am J Surg Pathol. 2014;38(11):1571–76. doi: 10.1097/PAS.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 5.Richkind KE, Romansky SG, Finklestein JZ. t(4;19)(q35;q13.1): a recurrent change in primitive mesenchymal tumors? Cancer Genet Cytogenet. 1996;87:71–74. doi: 10.1016/0165-4608(95)00240-5. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimoto M, Graham C, Chilton-MacNeill S, et al. Detailed cytogenetic and array analysis of pediatric primitive sarcomas reveals a recurrent CIC–DUX4 fusion gene event. Cancer Genet Cytogenet. 2009;195:1–11. doi: 10.1016/j.cancergencyto.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Kawamura-Saito M, Yamazaki Y, Kaneko K, et al. Fusion between CIC and DUX4 up-regulates PEA3 family genes in Ewing-like sarcomas with t (4; 19) (q35; q13) translocation. Hum Mol Gen. 2006;15(13):2125–37. doi: 10.1093/hmg/ddl136. [DOI] [PubMed] [Google Scholar]
- 8.Alaggio R, Bisogno G, Rosato A, et al. Undifferentiated sarcoma: does it exist? A clinicopathologic study of 7 pediatric cases and review of literature. Hum Pathol. 2009;40(11):1600–10. doi: 10.1016/j.humpath.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Sirvent N, Trassard M, Ebran N, et al. Fusion ofEWSR1 with the DUX4 facioscapulohumeral muscular dystrophy region resulting from t (4; 22)(q35; q12) in a case of embryonal rhabdomyosarcoma. Cancer Genet Cytogenet. 2009;195(1):12–18. doi: 10.1016/j.cancergencyto.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Rakheja D, Goldman S, Wilson KS, et al. Translocation (4; 19)(q35; q13. 1)-associated primitive round cell sarcoma: report of a case and review of the literature. Pediatr Dev Pathol. 2007;11(3):239–44. doi: 10.2350/07-06-0296.1. [DOI] [PubMed] [Google Scholar]
- 11.Graham C, Chilton-MacNeill S, Zielenska M, Somers GR. The CIC-DUX4 fusion transcript is present in a subgroup of pediatric primitive round cell sarcomas. Hum Pathol. 2012;43(2):180–89. doi: 10.1016/j.humpath.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Choi EYK, Thomas DG, McHugh JB, et al. Undifferentiated small round cell sarcoma with t (4; 19)(q35; q13. 1) CIC-DUX4 fusion: a novel highly aggressive soft tissue tumor with distinctive histopathology. Am J Surg Pathol. 2013;37(9):1379–86. doi: 10.1097/PAS.0b013e318297a57d. [DOI] [PubMed] [Google Scholar]
- 13.Somers GR, Shago M, Zielenska M, et al. Primary subcutaneous primitive neuroectodermal tumor with aggressive behavior and an unusual karyotype: case report. Pediart Dev Pathol. 2004;7(5):538–45. doi: 10.1007/s10024-004-2024-6. [DOI] [PubMed] [Google Scholar]
- 14.Kajtár B, Tornóczky T, Kálmán E, et al. CD99-positive undifferentiated round cell sarcoma diagnosed on fine needle aspiration cytology, later found to harbour a CIC-DUX4 translocation: a recently described entity. Cytopathology. 2014;25(2):129–32. doi: 10.1111/cyt.12079. [DOI] [PubMed] [Google Scholar]
- 15.Graham C, Chilton-MacNeill S, Zielenska M, Somers GR. The CIC-DUX4 fusion transcript is present in a subgroup of pediatric primitive round cell sarcomas. Hum Pathol. 2012;43(2):180–89. doi: 10.1016/j.humpath.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Machado I, Cruz J, Lavernia J, et al. Superficial EWSR1-negative undifferentiated small round cell sarcoma with CIC/DUX4 gene fusion: a new variant of Ewing-like tumors with locoregional lymph node metastasis. Virchows Archiv. 2013;463(6):837–42. doi: 10.1007/s00428-013-1499-9. [DOI] [PubMed] [Google Scholar]
- 17.Antonescu C. Round cell sarcomas beyond Ewing: emerging entities. Histopathology. 2014;64(1):26–37. doi: 10.1111/his.12281. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg SA, Tepper JOEL, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–15. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 21.Bielle F, Zanello M, Guillemot D, et al. Unusual primary cerebral localization of a CIC-DUX4 translocation tumor of the Ewing sarcoma family. Acta Neuropathol. 2014;128(2):309–11. doi: 10.1007/s00401-014-1312-0. [DOI] [PubMed] [Google Scholar]
- 22.Smith SC, Buehler D, Choi EYK, et al. CIC-DUX sarcomas demonstrate frequent MYC amplification and ETS-family transcription factor expression. Mod Pathol. 2014 doi: 10.1038/modpathol.2014.83. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Panagopoulos I, Gorunova L, Bjerkehagen B, Heim S. The “Grep” Command But Not FusionMap, FusionFinder or ChimeraScan Captures the CIC-DUX4 Fusion Gene from Whole Transcriptome Sequencing Data on a Small Round Cell Tumor with t (4; 19)(q35; q13) PloS One. 2014;9(6):e99439. doi: 10.1371/journal.pone.0099439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Specht K, Sung YS, Zhang L, et al. Distinct transcriptional signature and immunoprofile of CIC-DUX4 fusion–positive round cell tumors compared to EWSR1-rearranged ewing sarcomas: Further evidence toward distinct pathologic entities. Genes Chromosomes Cancer. 2014;53(7):622–63. doi: 10.1002/gcc.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]