Abstract
Gangliosides in the brain of the knockout mouse deficient in the activity of β1,4 N-acetylgalactosaminyl transferase (β1,4 GalNAc-T)(GM2 synthase) consisted of nearly exclusively of GM3- and GD3-gangliosides as expected from the known substrate specificity of the enzyme and in confirmation of the initial reports from two laboratories that generated the mutant mouse experimentally. The total molar amount of gangliosides was approximately 30% higher in the mutant mouse brain than that in the wild-type brain. However, contrary to the initial reports, one-fourth of total GD3-ganglioside was O-acetylated. It reacted positively with an anti-O-acetylated GD3 monoclonal antibody and disappeared with a corresponding increase in GD3-ganglioside after mild alkaline treatment. The absence of O-acetylated GD3 in the initial reports can be explained by the saponification step included in their analytical procedures. Although quantitatively much less and identification tentative, we also detected GT3 and O-acetylated GT3. Anti-GD3 and anti-O-acetylated GD3 monoclonal antibodies gave positive reactions in the brain of mutant mouse as expected from the analytical results. Either antibody barely stained wild-type brain except for immunoreactivity of GD3 in the cerebellar Purkinje cells. The distributions of GD3 and O-acetylated GD3 in the brain of mutant mouse were similar but differential localization was noted in the cerebellar Purkinje cells and cerebral cortex.
Keywords: Sphingolipid, ganglioside, O-acetylated ganglioside, immunohistochemistry, GM2-synthase, knockout mouse
Introduction
The β1,4 N-acetylgalactosaminyl transferase (β1,4 GalNAc-T; EC2.4.1.92) (GM2 synthase) that synthesizes both GM2- and GD2-ganglio-sides from GM3 and GD3, respectively, was first cloned in 19921) and then also in 1995.2) Essentially no higher gangliosides beyond GM3 and GD3 can be generated in the absence of this enzyme. Subsequent to cloning of the gene, GM2-synthase-deficient mice (GalNAcT−/−) were generated independently in two laboratories.3),4) In view of the postulated multiple physiological functions of gangliosides, dire consequences had been predicted. However, when the knockout mouse was generated by the gene-targeting technology, the phenotype was unexpectedly mild with apparently normal anatomical and cellular development of the brain, although later studies indicated numerous subtle behavioral, anatomical and physiological abnormalities.3),5) Outside of the nervous system, the testis is drastically abnormal in these mice.6)
The initial biochemical characterization indicated, as expected, that the major brain ganglioside appeared to be GD3.3),4) The present work was prompted by the initial observation, in a study comparing these mice to mice doubly deficient in GM2-synthase and NPC1, that an additional large sialic acid-containing band was present between GM1- and GM2-gangliosides and that it was in fact O-acetylated GD3.7) This was indicated without description of how the identification was made and without quantitative information. That a large proportion of GD3 was O-acetylated appears to have been missed by both laboratories that generated the GalNAcT−/− mouse, because their analytical procedures included a step of saponification procedure, which hydrolyzed the O-acetyl group from sialic acid. In view of the recent interest in the possible biological functions of O-acetylated GD3-ganglioside in normal as well as in pathological conditions, we followed up our finding with more quantitative analyses and immunological identification. We also carried out immunohistochemical studies of GD3 and O-acetylated GD3 in the brain of young adult wild-type and GalNAcT−/− mice.
Materials and methods
Animals
The GalNAcT−/− mice originally generated and kindly provided to us by R. L. Proia at the NIH were used for this study. A colony was maintained in the facility for the experimental animals at the University of North Carolina at Chapel Hill in strict accordance with the Federal and local regulations governing use and care of experimental animals. All experimental procedures were approved by the institutional review committee. Analyses and quantitation of brain gangliosides were done in Lyon on the tissue shipped frozen.
Lipid studies
General lipid extraction: Two mouse brains, one each of wild-type and GalNAcT−/− mice at 64 days, were analyzed for brain ganglioside composition. The initial extraction was based on the method of Svennerholm and Fredman8) as described in detail by Svennerholm et al.9) The tissue was homogenized with 0.6 ml of water in an all-glass Potter-Elvehjem homogenizer and extracted by addition of 2 ml of methanol and 1 ml of chloroform. After 30 min., the extract was centrifuged at 1,000 g for 10 min. and the supernate transferred to a glass tube. The pellet was resuspended in 0.7 ml water and re-extracted with additional 3 ml of chloroform-methanol, 1:2 (v/v). After centrifugation, the combined supernates were adjusted to 8 ml with C-M 1:2 (v/v).
Separation and analysis of gangliosides: Brain lipids (starting from a 6 ml aliquot of the total lipid extract) were fractionated to neutral and acidic fractions using a reverse phase column as described by Kyrklund et al.10) (Varian Bond Elute C-18, 3 ml/500 mg). This procedure allows clean separation of acidic lipids including gangliosides and sulfatides from other lipids without loss of the less polar gangliosides, and simple and rapid desalting without a need for saponification prior to ganglioside separation. The total sialic acid content of the ganglioside fraction was determined by the resorcinol method11),12) using N-acetylneuraminic acid as the standard. Thin-layer chromatography was performed with high performance silica gel-coated thin-layer plates (Merck HPTLC 60). The solvent system was chloroform-methanol-0.2% CaCl2 (55:45:10, v/v/v). Gangliosides were visualized by the resorcinol spray.11) For quantitation of individual gangliosides, samples were applied with a CAMAG Linomat IV apparatus at 10 nmoles total sialic acid per lane, and densitometric evaluation of the plates performed using a CAMAG TLC scanner model II operated with the CATS-3 evaluation software. The results were expressed in nmoles of individual gangliosides by taking into account the number of sialic acids per molecule.
Identification of O-acetyl-GD3 ganglioside: Preliminary identification was done by thin-layer chromatography before and after saponification. Aliquots of the acidic lipid fraction obtained by Bond-Elute C-18 chromatography were subjected to saponification by redissolving in 0.9 ml C-M 1:2 (v/v), adding 0.1 ml NaOH 1 mol/l and incubating for 2 h at 37 °C. The saponified extract was desalted through a 1g Sephadex G-25 fine column, and subjected to the thin-layer chromatography as described above. Identification was further assessed by a TLC-immunostaining method using mouse monoclonal antibodies (both IgG3) 7H2, specific for O-acetyl-GD3 which does not bind GD3, and 4F6, specific for GD3, which does not bind O-acetyl GD3.13) HPTLC separation of the unsaponified ganglioside fraction along with a standard of O-acetyl-GD3 purified from rainbow trout liver14) was conducted as above. After drying, immunostaining was performed on the HPTLC plate as described.15)
Immunohistochemical localization of GD3 and O-acetyl-GD3 gangliosides in the brain of GalNAcT−/− mouse
Tissue preparation: Three GalNAcT−/− male mice at 6–7 months and three age-matched wild-type male mice were used. The background strain of all mice was a mixture of 129 Sv and C57BL/6. The mice were anesthetized with ether and decapitated. The brains were rapidly removed, dissected on ice, placed in the O.C.T. compound (Sakura Finetek U.S.A., Inc.,Torrance,CA) and frozen with isopentane in Histobath (Shandon, Pittsburgh, PA). For sectioning, the samples were cut to 7-μm thickness with a cryostat microtome and thaw-mounted on glass slides. The mounted sections were air-dried for 30 min, followed by fixation in cold acetone for 5 min. at −20 °C, after which acetone was removed by airing for 1 hr.
Immunohistochemistry: Sections were rinsed with PBS and incubated with 1.5% normal donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) in PBS for 1 hr. at room temperature. To prevent the non-specific binding of secondary labeled anti-mouse IgG to mouse tissues, the sections were incubated in the M.O.M. mouse IgG blocking reagent (Vector M.O.M. Kit, Vector Laboratories, Inc., Burlingame, CA) for 1 hr. After washing in PBS, the sections were then incubated overnight at room temperature with undiluted culture supernatant of the hybridomas containing either anti-GD3 (4F6) or anti-O-Ac-GD3 (7H2) mouse monoclonal antibodies (IgG) (concentration: 2–5 μg/ml) as a primary antibody. The production and characterization of the antibodies have been described previously.13) On the next day, the sections were washed in PBS and incubated with secondary fluoresceine isothiocyanate (FITC)-conjugated AffiniPure donkey anti-mouse IgG (H+L) antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) at 1:100 dilution for 1 hr at room temperature and washed in PBS. Immunoreactions were examined by a fluorescence microscopy. As a control for non-specific staining, additional sections were subjected to the immunostaining procedure without primary antibody. No staining was observed in control sections. The experiments were repeated 3 times using three different mice sections.
Results
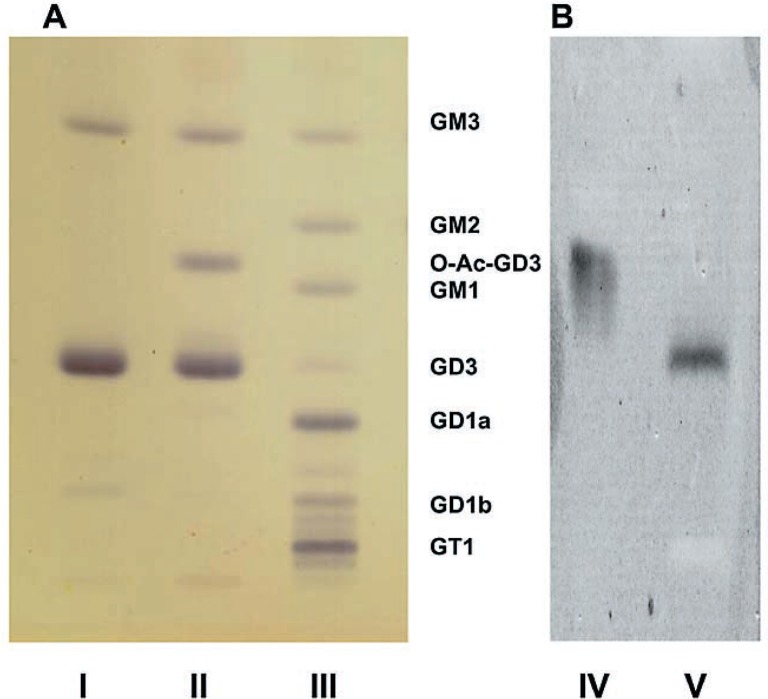
Brain ganglioside analysis (Table I, Fig. 1). On the thin-layer chromatogram, gangliosides in the GalNAcT−/− mouse brain consisted of essentially only three components, GM3, GD3 and another large band with a thin-layer chromatographic mobility between GM2- and GM1-gangliosides (Fig. 1A, lane II). This band disappeared and the band of GD3 became heavier upon mild alkaline treatment (Fig. 1A, lane I). This suggested that the extra band was an alkaline-labile derivative of GD3. This point is further supported by quantitative determination of individual gangliosides (Table I). Upon saponification, this extra band disappeared and the amount of GD3 increased by the same amount. Its identity as O-acetylated GD3 was further confirmed by its reactivity with the monoclonal antibody specific for O-acetylated GD3 (Fig. 1B). The proportion of GD3 to O-acetylated GD3 was approximately 3 to 1, i.e. approximately 25% of total GD3 ganglioside was O-acetylated.
Table I.
Ganglioside concentrations in the brain of 64 day-old control and GalNAcT knockout mice. The results are expressed in nmoles ganglioside/g wet weight tissue. Values for individual gangliosides are expressed up to three significant digits, and the total is their arithmetic sums. The value 0 indicates below the level for reliable quantification. Note: GMx contains one mole of sialic acid per molecule. The corresponding mole numbers of sialic acid are two for GDx, three for GTx, and four for GQ, respectively. Refer to the legend for Fig. 1 regarding GT3 and O-acetylated GT3.
| Control | GalNAcT−/− | GalNAcT−/− saponified | |
|---|---|---|---|
| GM3 | 18 | 679 | 769 |
| GM2 | 29 | 0 | 0 |
| O-Ac-GD3 | 0 | 392 | 0 |
| GM1 | 327 | 0 | 0 |
| GD3 | 69 | 1140 | 1460 |
| GD1a | 579 | 0 | 0 |
| O-Ac-GT3 | 0 | 50 | 0 |
| GT3 | 0 | 20 | 92 |
| GD2 | 44 | 0 | 0 |
| GD1b | 176 | 0 | 0 |
| X (disialo?) | 103 | 0 | 0 |
| GT1b | 368 | 0 | 0 |
| GQ | 53 | 0 | 0 |
|
| |||
| TOTAL | 1766 | 2281 | 2321 |
Fig. 1.
Thin-layer chromatogram of brain gangliosides. Technical details are described in the text. A: Stained with the resorcinol spray; B: Stained with specific mouse monoclonal antibodies. Lane I: GalNAcT−/− mouse preparation after saponification; Lane II: GalNAcT−/− mouse preparation before saponification; Lane III: Preparation from Niemann-Pick type C brain as a control (This is only to show thin-layer chromatographic mobility of multiple gangliosides of known structures. The normal control brain used for quantitative analyses of gangliosides (Table I) was a wild-type mouse brain, which is not on this chromatogram.) Lane IV: Same as Lane II but stained with specific monoclonal antibody against O-acetylated GD3; Lane V: Same as Lane II but stained with specific monoclonal antibody against GD3. GalNAcT−/− brain contains essentially GD3, O-acetyl-GD3 and GM3 gangliosides. Upon saponification O-acetyl-GD3 disappears and the GD3 band becomes heavier (see Table I). We tentatively identify the band that runs slightly ahead of GD1b in Lane I as GT3, and the band running slightly ahead of GD1a in Lane II as O-acetylated GT3. Then, the mutant brain also contains detactable amounts of GT3 and O-acetylated GT3, and the latter is hydrolyzed to GT3 by saponificaiton. This interpretation can also be understood from the metabolic block in the GalNAcT−/− mouse.
Another quantitative finding of interest was that the total molar amount of brain gangliosides was approximately 30% greater in the mutant mouse brain than that in the wild-type control mouse brain despite the block in the synthetic pathway of major brain gangliosides (Table I). Although only one control brain was included in this particular series of analytical study, all quantitative values for the control brain matched closely to previous control values from our laboratory.
Immunohistochemistry (Table II, Fig. 2). As expected from the quantitative data, immunohistochemical staining of the wild-type brain was very weak with either GD3-specific or O-acetyl-GD3-specific monoclonal antibody (Fig. 2), while the GalNAcT−/− mice brain stained quite strongly. While the O-acetyl-GD3-specific antibody gave no staining with the wild-type brain, the anti-GD3 monoclonal antibody stained the Purkinje cells of the wild-type brain positively, albeit weakly. Table II gives the results of semi-quantitative evaluation. It should be kept in mind that, by its nature, immunohistochemistry is never precisely quantitative and even a semi-quantitative comparison is possible only within a single antibody but not across different antibodies.
Table II.
Semiquantitative distribution of GD3 and O-acetylated GD3 in the GalNAcT−/− mouse brain detected by specific monoclonal antibodies
| Brain region | Ganglioside | |
|---|---|---|
|
| ||
| GD3 | O-acetyl-GD3 | |
| Cerebellar cortex | ||
| Molecular layer | + | + |
| Purkinje cell layer | ++ | − |
| Granular layer | ++ | +++ |
| White matter | ± | − |
| Cerebral cortex | ||
| Molecular layer (I) | + | + |
| External granular layer (II) | − | − |
| External pyramidal layer (III) | + | + |
| Internal granular layer (IV) | + | ++ |
| Internal pyramidal layer (V) | + | − |
| Polymorphic cell layer (VI) | + | − |
| White matter | ± | − |
| Hippocampal formation | ||
| Hippocampus | ||
| Alveus | ++ | ++ |
| Stratum oriens | ± | + |
| Stratum pyramidale | ++ | − |
| Stratum radiatum | + | + |
| Stratum lacunosum moleculare | + | ++ |
| Dentate gyrus | ||
| Polymorphic layer | + | + |
| Granular layer | + | − |
| Molecular layer | + | + |
Fig. 2.
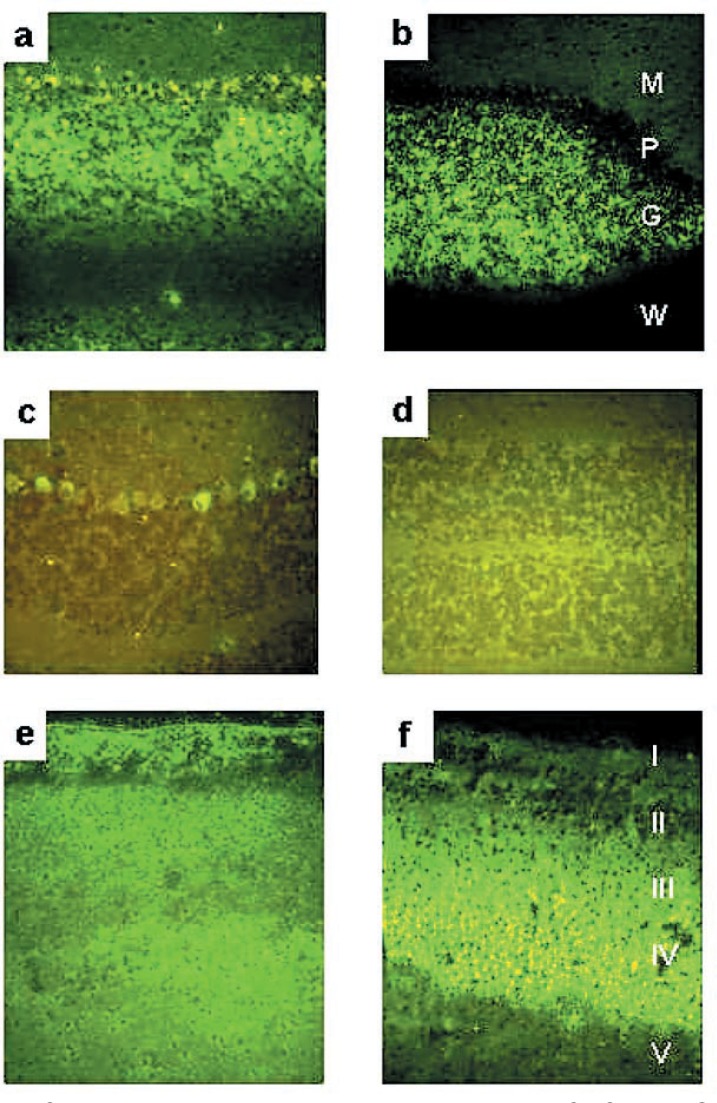
Immunohistochemical localization of GD3 and O-acetyl-GD3 gangliosides in the brain of GalNAcT−/− mouse (a, b, e, f) and wild-type mouse (c, d). Sections were treated with monoclonal antibodies specific for GD3 (a, c, e) and O-acetyl-GD3 (b, d, f) and visualized with FITC-conjugated donkey anti-mouse IgG (H+L) antibody. Technical details are described in the text. Cerebellar cortex (a–d); M: molecular layer, P: Purkinje cell layer, G: granular layer, W: white matter. Cerebral cortex (e, f); I: molecular layer, II: external granular layer, III: external pyramidal layer, IV: internal granular layer, V: internal pyramidal layer.
Cerebellum: In the GalNAcT−/− mouse, basically similar distributions of the GD3 and O-acetyl-GD3 were observed in the layers of the cerebellar cortex except for the Purkinje cells. With the anti-GD3 monoclonal antibody, the granular and molecular layers and the Purkinje cells stained positively, while the white matter did not stain. With the anti-O-acetyl-GD3 monoclonal antibody the results were similar with those of the anti-GD3 monoclonal antibody except that the Purkinje cells did not stain. In the wild type mouse brain, only the Purkinje cell bodies showed positive staining with the anti-GD3 monoclonal antibody. The anti-O-acetyl-GD3 monoclonal antibody gave no staining with the wild-type brain.
Cerebral cortex: The anti-GD3 monoclonal antibody stained layers I–VI diffusely and the white matter only very weakly. On the other hand, the anti-O-acetyl-GD3 monoclonal antibody stained layers I-IV, most strongly layer IV. Unlike the anti-GD3 monoclonal antibody, it did not stain layers V, VI nor the white matter.
Hippocampal formation: The anti-GD3 monoclonal antibody stained the alveus, the stratum pyramidale and the stratum lacunosum-moleculare and the granular layer of the dentate gyrus. The positive areas with the anti-O-acetyl-GD3 monoclonal antibody were basically similar to those of the anti-GD3, except that the stratum pyramidale and the granular layer of the dentate gyrus were negative.
Discussion
This series of studies was initiated when one of us observed the extra sialic acid-containing band running between GM2- and GM1-gangliosides on a thin-layer chromatogram of the acidic lipid fraction from the brain of the GalNAcT−/− mouse (Fig. 1). The earlier report from either of the two laboratories that generated the mutant mouse independently did not indicate its existence.3),4) Its disappearance and apparent conversion to GD3 upon mild alkaline treatment as well as the known enzymological background of this knockout mouse strongly suggested that the extra band is O-acetylated GD3. Its quantitative conversion to GD3 upon saponification and its reactivity with the specific anti-O-acetyl GD3 monoclonal antibody further confirmed this initial impression. We believe this was not detected in the earlier reports because a step of mild alkaline treatment was included in their analytical procedures.
GM3 and GD3 were the main gangliosides in the brain of the GalNAcT−/− mice, as expected from the known biosynthetic pathways of gangliosides and substrate specificity of GM2 synthase. However, we had not expected that one-fourth of total GD3-ganglioside was O-acetylated. In the normal brain GD3 is a minor ganglioside and its O-acetylated derivative is nearly undetectable, and therefore, the proportion of O-acetylated GD3 is difficult to assess. Anecdotally, it is of interest that the liver of the rainbow trout appears to be a rich source of O-acetylated GD3-ganglioside.14) It is conceivable that the proportion of O-acetylated and non-acetylated GD3 might be similar in the normal and the mutant brains. The absence of O-acetylated GM3 in the mutant brain is consistent with the O-acetyl-group being only on the second sialic acid of GD3.
Another finding of potential interest is that the molar amount of total brain ganglioside was approximately 30% greater in the GalNAcT−/− mice compared to the wild-type mouse brain despite the block in the synthetic pathways for the normally major brain gangliosides and the normal complement of degrading capacity for GM3 and GD3 gangliosides. Possible functional compensation by the normally minor gangliosides in the absence of normally major ones was speculated earlier3),4) and our information that the amount of total brain gangliosides in the mutant brain is actually greater than normal provides an even firmer ground for such a speculation. Nevertheless, the idea remains a speculation.
Some immunohistochemical studies on GD3 distribution in the wild-type rodent brain have been reported.16)–22) While there are some discrepancies among the previous reports, our finding of a relatively intense GD3 expression in the granular layer of the cerebellum in the GalNAcT−/− mice is consistent with the previous reports in the wild-type mice brain. Since the O-acetylated GD3 is a very minor ganglioside in the wild-type mouse brain, there is no immunohistochemical study of its distribution in the mouse brain using a specific antibody. The high concentration of O-acetylated GD3 in the mutant brain allowed us to examine its distribution in the mutant brain. Generally, GD3 and its O-acetylated counterpart showed similar distributions among various regions of the brain. However, we did find some differential patterns of staining in Purkinje cells in the cerebellum and the pyramidal neurons in the cerebrum between GD3 and O-acetyl-GD3. Since immunohistochemistry is at best semi-quantitative and no quantitative interpretation is possible across different antibodies, it is difficult to interpret such apparently discrepant localization. We had hoped that possible differential distributions of GD3 and its O-acetylated derivative with respect to brain regions and/or neural cell types might shed some light on their physiological functions. However, the findings did not allow clear interpretations. These immunohistochemical findings must remain a piece of descriptive information at this time.
Although GD3 and O-acetylated GD3 gangliosides usually exist in very low amounts, their possible physiological functions have been attracting attention of many laboratories. Their association with diverse functions are implicated, for example, in apoptosis,23)–33) as tumor antigens,34)–38) in development, differentiation and cell migration,16),17),39)–44) and in immunity and other functions.45),46) In most instances, however, the relationship of these gangliosides and the implicated physiological functions remains that of an association rather than causal. Active participation of GD3 and its O-acetylated derivative in diverse physiological functions is expected to be an active area of future more mechanistic investigations. Presence of these normally minor constituents in high concentrations should make the GalNAcT−/− mice an attractive tool for such investigations into functions of GD3 ganglioside and its O-acetyl-derivative in normal and disease conditions.
Acknowledgments
This work was supported in part by research grants from the Japan Society for the Promotion of Science, Grant-in-aids for Scientific Research 16591032 to J.M. and 17300122 to K.S. and a research grant from Vaincre les Maladies Lysosomales to M.T.V. The work was initiated when J. M. and K. S. were at the Neuroscience Center, University of North Carolina School of Medicine, Chapel Hill, NC. U.S.A. and completed at the present location.
References
- 1).Nagata, Y., Yamashiro, S., Yodoi, J., Lloyd, K. O., Shiku, H., and Furukawa, K. (1992) Expression cloning of β1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J. Biol. Chem. 267, 12082–12089. [PubMed] [Google Scholar]
- 2).Sango, K., Johnson, O. N., Kozak, C. A., and Proia, R. L. (1995) β-1,4-N-acetylgalactosaminyltransferase involved in ganglioside synthesis: cDNA sequence, expression, and chromosome mapping of the mouse gene. Genomics 27, 362–365. [DOI] [PubMed] [Google Scholar]
- 3).Takamiya, K., Yamamoto, A., Furukawa, K., Yamashiro, S., Shin, M., Okada, M., Fukumoto, S., and Haraguchi, M., Takeda, N., Fujimura, K.et al. (1996) Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl. Acad. Sci. USA 93, 10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Liu, Y. J., Wada, R., Kawai, H., Sango, K., Deng, C. X., Tai, T., McDonald, M. P., Araujo, K., Crawley, J. N., Bierfreund, U.et al. (1999) A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J. Clin. Invest. 103, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Sheikh, K. A., Sun, J., Liu, Y. J., Kawai, H., Crawford, T. O., Proia, R. L., Griffin, J. W., and Schnaar, R. L. (1999) Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc. Natl. Acad. Sci. USA 96, 7532–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Takamiya, K., Yamamoto, A., Furukawa, K., Yamashiro, S., Shin, M., Okada, M., Fukumoto, S., Haraguchi, M., Takeda, N., Fujimura, K.et al. (1996) Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl. Acad. Sci. USA 93, 10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Liu, Y. J., Wu, Y. P., Wada, R., Neufeld, E. B., Mullin, K. A., Howard, A. C., Pentchev, P. G., Vanier, M. T., Suzuki, K., and Proia, R. L. (2000) Alleviation of neuronal ganglioside storage does not improve the clinical course of the Niemann-Pick C disease mouse. Hum. Mol. Genet. 9, 1087–1092. [DOI] [PubMed] [Google Scholar]
- 8).Svennerholm, L., and Fredman, P. (1980) A procedure for the quantitative isolation of brain gangliosides. Biochim. Biophys. Acta 617, 97–109. [DOI] [PubMed] [Google Scholar]
- 9).Svennerholm, L., Boström, K., Helander, C. G., and Jungbjer, B. (1991) Membrane lipids in the aging human brain. J. Neurochem. 56, 2051–2059. [DOI] [PubMed] [Google Scholar]
- 10).Kyrklund, T. (1987) Two procedures to remove polar contaminants from a crude brain lipid extract by using prepacked reverse-phase columns. Lipids 22, 274–277. [DOI] [PubMed] [Google Scholar]
- 11).Svennerholm, L. (1957) Quantitative estimation of sialic acids. II. A colorimetric resorcinol- hydrochloric acid method. Biochim. Biophys. Acta 24, 604–611. [DOI] [PubMed] [Google Scholar]
- 12).Miettinen, T., and Takki-Luukkainen, I.-T. (1959) Use of butylacetate for determination of sialic acid. Acta Chem. Scand. 13, 856–858. [Google Scholar]
- 13).Cerato, E., Birkle, S., Portoukalian, J., Mezazigh, A., Chatal, J. F., and Aubry, J. (1997) Variable region gene segments of nine monoclonal antibodies specific to disialogangliosides (GD2, GD3) and their O-acetylated derivatives. Hybridoma 16, 307–316. [DOI] [PubMed] [Google Scholar]
- 14).Ostrander, G. K., Bozlee, M., Fukuda, M., Dell, A., Thomas-Oates, J. E., Levery, S. B., Eaton, H. L., Hakomori, S., and Holmes, E. H. (1991) Isolation and characterization of the major glycosphingolipids from the liver of the rainbow trout (Oncorhynchus mykiss): identification of an abundant source of 9-O-acetyl GD3. Arch. Biochem. Biophys. 284, 413–421. [DOI] [PubMed] [Google Scholar]
- 15).Portoukalian, J., and Bouchon, B. (1986) Hydrolysis of all gangliosides, including GM1 and GM2, on thin-layer plates by Vibrio cholerae neuraminidase. J. Chromatogr. 380, 386–392. [DOI] [PubMed] [Google Scholar]
- 16).Goldman, J. E., Hirano, A., Yu, R. K., and Seyfried, T. N. (1984) GD3 ganglioside is a glycolipid characteristic of immature neuroectodermal cells. J. Neuroimmunol. 7, 179–192. [DOI] [PubMed] [Google Scholar]
- 17).Schaal, H., Wille, C., and Wille, W. (1985) Changes of ganglio-side pattern during cerebellar development of normal and staggerer mice. J. Neurochem. 45, 544–551. [DOI] [PubMed] [Google Scholar]
- 18).Kotani, M., Kawashima, I., Ozawa, H., Ogura, K., Ishizuka, I., Terashima, T., and Tai, T. (1994) Immunohistochemical localization of minor gangliosides in the rat central nervous system. Glycobiology 4, 855–865. [DOI] [PubMed] [Google Scholar]
- 19).Kotani, M., Terashima, T., and Tai, T. (1995) Developmental changes of ganglioside expressions in postnatal rat cerebellar cortex. Brain Res. 700, 40–58. [DOI] [PubMed] [Google Scholar]
- 20).Kotani, M., and Tai, T. (1997) An immunohistochemical technique with a series of monoclonal antibodies to gangliosides: their differential distribution in the rat cerebellum. Brain Res. Brain Res. Protoc. 1, 152–156. [DOI] [PubMed] [Google Scholar]
- 21).Molander, M., Berthold, C. H., Persson, H., and Fredman, P. (2000) Immunostaining of ganglioside GD1b, GD3 and GM1 in rat cerebellum: Cellular layer and cell type specific associations. J. Neurosci. Res. 60, 531–542. [DOI] [PubMed] [Google Scholar]
- 22).Yasuda, Y., Naito, T., Watarai, S., Fujita, S., and Kitamura, T. (2000) Ganglioside GD3 immunochemistry does not visualize microglia but astroglia. Acta Histochem. Cytochem. 33, 253–258. [Google Scholar]
- 23).Bektas, M., and Spiegel, S. (2003) Glycosphingolipids and cell death. Glycoconjugate J. 20, 39–47. [DOI] [PubMed] [Google Scholar]
- 24).Colell, A., Morales, A., Fernández-Checa, J. C., and García-Ruiz, C. (2002) Ceramide generated by acidic sphingomyelinase contributes to tumor necrosis factor-β-mediated apoptosis in human colon HT-29 cells through glycosphingolipids formation - Possible role of ganglioside GD3. FEBS Lett. 526, 135–141. [DOI] [PubMed] [Google Scholar]
- 25).García-Ruiz, C., Colell, A., París, R., and Fernández-Checa, J. C. (2000) Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 14, 847–858. [DOI] [PubMed] [Google Scholar]
- 26).Malisan, F., Franchi, L., Tomassini, B., Ventura, N., Condò, I., Rippo, M. R., Rufini, A., Liberati, L., Nachtigall, C., Kniep, B.et al. (2002) Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J. Exp. Med. 196, 1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Malisan, F., and Testi, R. (2002) GD3 ganglioside and apoptosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1585, 179–187. [DOI] [PubMed] [Google Scholar]
- 28).Melchiorri, D., Martini, F., Lococo, E., Gradini, R., Barletta, E., De Maria, R., Caricasole, A., Nicoletti, F., and Lenti, L. (2002) An early increase in the disialoganglioside GD3 contributes to the development of neuronal apoptosis in culture. Cell Death Differ. 9, 609–615. [DOI] [PubMed] [Google Scholar]
- 29).Morales, A., Colell, A., Mari, M., García-Ruiz, C., and Fernández-Checa, J. C. (2003) Glycosphingolipids and mitochondria: Role in apoptosis and disease. Glycoconjugate J. 20, 579–588. [DOI] [PubMed] [Google Scholar]
- 30).Saqr, H. E., Omran, O., Dasgupta, S., Yu, R. K., Oblinger, J. L., and Yates, A. J. (2006) Endogenous GD3 ganglioside induces apoptosis in U-1242 MG glioma cells. J. Neurochem. 96, 1301–1314. [DOI] [PubMed] [Google Scholar]
- 31).Simon, B. M., Malisan, F., Testi, R., Nicotera, P., and Leist, M. (2002) Disialoganglioside GD3 is released by microglia and induces oligodendrocyte apoptosis. Cell Death Differ. 9, 758–767. [DOI] [PubMed] [Google Scholar]
- 32).Tomassini, B., Malisan, F., Franchi, L., Nicolo, C., Brea-Calvo, G., Saito, T., Testi, R. (2004) Calnexin suppresses GD3 synthase-induced apoptosis. FASEB J. 18, NIL75–NIL88. [DOI] [PubMed] [Google Scholar]
- 33).Castiglione, M., Spinsanti, P., Iacovelli, L., Lenti, L., Martini, F., Gradini, R., and Di, G. G., V., Caricasole, A., Caruso, A., De Maria, R.et al. (2004) Activation of Fas receptor is required for the increased formation of the disialoganglioside GD3 in cultured cerebellar granule cells committed to apoptotic death. Neuroscience 126, 889–898. [DOI] [PubMed] [Google Scholar]
- 34).Hamamura, K., Furukawa, K., Hayashi, T., Hattori, T., Nakano, J., Nakashima, H., Okuda, T., Mizutani, H., Hattori, H., Ueda, M.et al. (2005) Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc. Natl. Acad. Sci. USA 102, 11041–11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Hedberg, K. M., Dellheden, B., Wikstrand, C. J., and Fredman, P. (2000) Monoclonal anti-GD3 antibodies selectively inhibit the proliferation of human malignant glioma cells in vitro. Glycoconjugate J. 17, 717–726. [DOI] [PubMed] [Google Scholar]
- 36).París, R., Morales, A., Coll, O., Sánchez-Reyes, A., García-Ruiz, C., and Fernández-Checa, J. C. (2002) Ganglioside GD3 sensitizes human hepatoma cells to cancer therapy. J. Biol. Chem. 277, 49870–49876. [DOI] [PubMed] [Google Scholar]
- 37).Zeng, G. C., Gao, L. Y., Birklé, S., and Yu, R. K. (2000) Suppression of ganglioside GD3 expression in a rat F-11 tumor cell line reduces tumor growth, angiogenesis, and vascular endothelial growth factor production. Cancer Res. 60, 6670–6676. [PubMed] [Google Scholar]
- 38).Furukawa, K., Horie, M., Okutomi, K., Sugano, S., and Furukawa, K. (2003) Isolation and functional analysis of the melanoma specific promoter region of human GD3 synthase gene. Biochim. Biophys. Acta 1627, 71–78. [DOI] [PubMed] [Google Scholar]
- 39).Birklé, S., Ren, S., Slominski, A., Zeng, G., Gao, L., and Yu, R. K. (1999) Down-regulation of the expression of O-acetyl-GD3 by the O-acetylesterase cDNA in hamster melanoma cells: Effects on cellular proliferation, differentiation, and melanogenesis. J. Neurochem. 72, 954–961. [DOI] [PubMed] [Google Scholar]
- 40).Birklé, S., Gao, L. Y., Zeng, G. C., and Yu, R. K. (2000) Down-regulation of GD3 ganglioside and its O-acetylated derivative by stable transfection with antisense vector against GD3-synthase gene expression in hamster melanoma cells: Effects on cellular growth, melanogenesis, and dendricity. J. Neurochem. 74, 547–554. [DOI] [PubMed] [Google Scholar]
- 41).Negreiros, E. M. A., Leao, A. C. M., Santiago, M. F., and Mendez-Otero, R. (2003) Localization of ganglioside 9-O-acetyl GD3 in point contacts of neuronal growth cones. J. Neurobiol. 57, 31–37. [DOI] [PubMed] [Google Scholar]
- 42).Osanai, T., Kotani, M., Yuen, C. T., Kato, H., Sanai, Y., and Takeda, S. (2003) Immunohistochemical and biochemical analyses of GD3, GT1b, and GQ1b gangliosides during neural differentiation of P19 EC cells. FEBS Lett. 537, 73–78. [DOI] [PubMed] [Google Scholar]
- 43).Santiago, M. F., Berredo-Pinho, M., Costa, M. R., Gandra, M., Cavalcante, L. A., and Mendez-Otero, R. (2001) Expression and function of ganglioside 9-O-acetyl GD3 in postmitotic granule cell development. Mol. Cell. Neurosci. 17, 488–499. [DOI] [PubMed] [Google Scholar]
- 44).Santiago, M. F., Costa, M. R., and Mendez-Otero, R. (2004) Immunoblockage of 9-O-acetyl GD3 ganglioside arrests the in vivo migration of cerebellar granule neurons. J. Neurosci. 24, 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Wu, D. Y., Segal, N. H., Sidobre, S., Kronenberg, M., and Chapman, P. B. (2003) Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 198, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Inoki, Y., Miura, T., Kajimoto, T., Kawase, M., Kawase, Y., Yoshida, Y., Tsuji, S., Kinouchi, T., Endo, H., Kagawa, Y.et al. (2000) Ganglioside GD3 and its mimetics induce cytochrome c release from mitochondria. Biochem. Biophys. Res. Commun. 276, 1210–1216. [DOI] [PubMed] [Google Scholar]