Abstract
Catecholamines [dopamine, noradrenaline (norepinephrine), and adrenaline (epinephrine); CAs] are neurotransmitters in the central and peripheral nervous systems as well as hormones in the endocrine system. CAs in the brain play a central role in versatile functions as slow-acting neurotransmitters functioning in synaptic neurotransmission, modulating the effects of fast-acting neurotransmitters such as glutamate and γ-aminobutyric acid (GABA). In this review, I focus on recent advances in the biochemistry and molecular biology of the CA system in humans in health and disease, especially in neuropsychiatric diseases such as Parkinson’s disease (PD), in relation to the biosynthesis of CAs regulated by a pteridine-dependent monooxygenase, tyrosine 3-monooxygenase (tyrosine hydroxylase, TH) and its pteridine cofactor, tetrahydrobiopterin (BH4).
Keywords: Catecholamines, GTP cyclohydrolase I, Parkinson’s disease, tetrahydrobiopterin, tyrosine 3-monooxygenase
1. Introduction
Biogenic amines that possess a 3,4-dihydroxyphenyl (catechol) nucleus are generally called catecholamines (CAs). Three CAs exist in vivo, i.e., dopamine, noradrenaline (norepinephrine), and adrenaline (epinephrine), all of which are derivatives of 3,4-dihydroxyphenylethylamine. CAs are synthesized from L-tyrosine by the following pathway: L-tyrosine → L-3,4-dihydroxyphenylalanine (L-DOPA) → dopamine → noradrenaline → adrenaline (Fig. 1). Some CAs are neurotransmitters in the central and peripheral nervous systems and also function as hormones of the adrenal medulla in the endocrine system (Fig. 2). Historically, around 1900, Abel, Takamine, and Aldrich established the nature of an active CA compound in the adrenal medulla. Abel named this substance EPINEPHRINE, whereas Takamine named it ADRENALIN. Later, ADRENALINE became a systematic name. In 1946, Euler discovered another CA as a neurotransmitter lacking the N-methyl group of adrenaline/epinephrine in the peripheral sympathetic nerves and named it NORADRENALINE (NOR = Stickstoff-N-Ohne Radikal), which is also called NOREPINEPHRINE. Later noradrenaline and adrenaline were also found to be neurotransmitters in the brain. In 1958 Carlsson discovered DOPAMINE (3-hydroxytyramine) as yet another CA neurotransmitter in the brain.
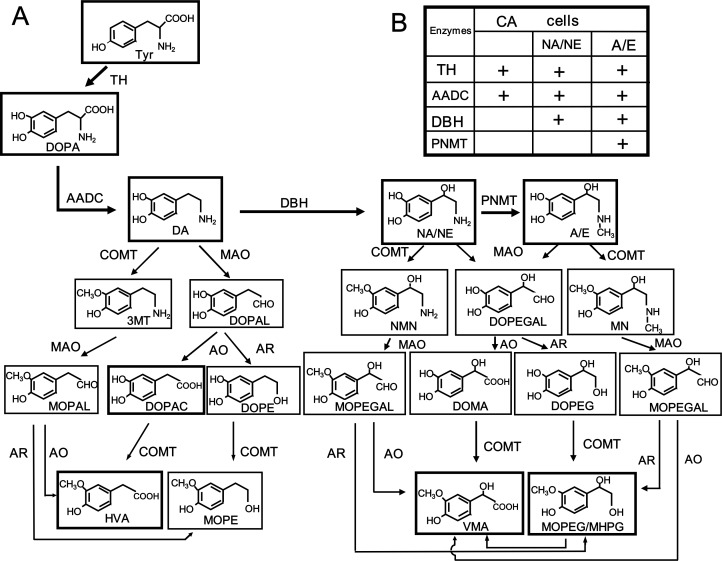
Fig. 1.
A Synthesis and metabolism of catecholamines (dopamine, noradrenaline, and adrenaline) and the related enzymes. A, adrenaline (E, epinephrine); AADC, aromatic L-amino acid decarboxylase; AO, aldehyde oxidase; AR, aldehyde reductase; CA, catecholamine; COMT, catechol O-methyltransferase; DA, dopamine; DBH, dopamine β-monooxygenase (dopamine β-hydroxylase); DOMA, 3,4-dihydroxymandelic acid; DOPA, 3,4-dihydroxyphenylalanine; DOPAC, 3,4-dihydroxyphenylacetic acid; DOPAL, 3,4-dihydroxyphenylacetaldehyde; DOPE, 3,4-dihydroxyphenylethanol; DOPEGAL, 3,4-dihydroxyphenylethyleneglycolaldehyde; HVA, homovanillic acid; MAO, monoamine oxidase; MN, metanephrine; MOPAL, 3-methoxy-4-hydroxyphenylacetaldehyde; MOPE, 3-methoxy-4-hydroxyphenylethanol; MOPEG/MHPG, 3-methoxy-4-hydroxyphenylethyleneglycol; MOPEGAL, 3-methoxy-4-hydroxyphenylethyleneglycolaldehyde; 3MT, 3-methoxytyramine; NA, noradrenaline (NE, norepinephrine); NMN, normetanephrine; PNMT, noradrenaline (phenylethanolamine) N-methyltransferase; TH, tyrosine 3-monooxygenase (tyrosine hydroxylase); VMA, vanillylmandelic acid.
B Catecholamine-synthesizing enzymes in dopamine, noradrenaline, and adrenaline cells.
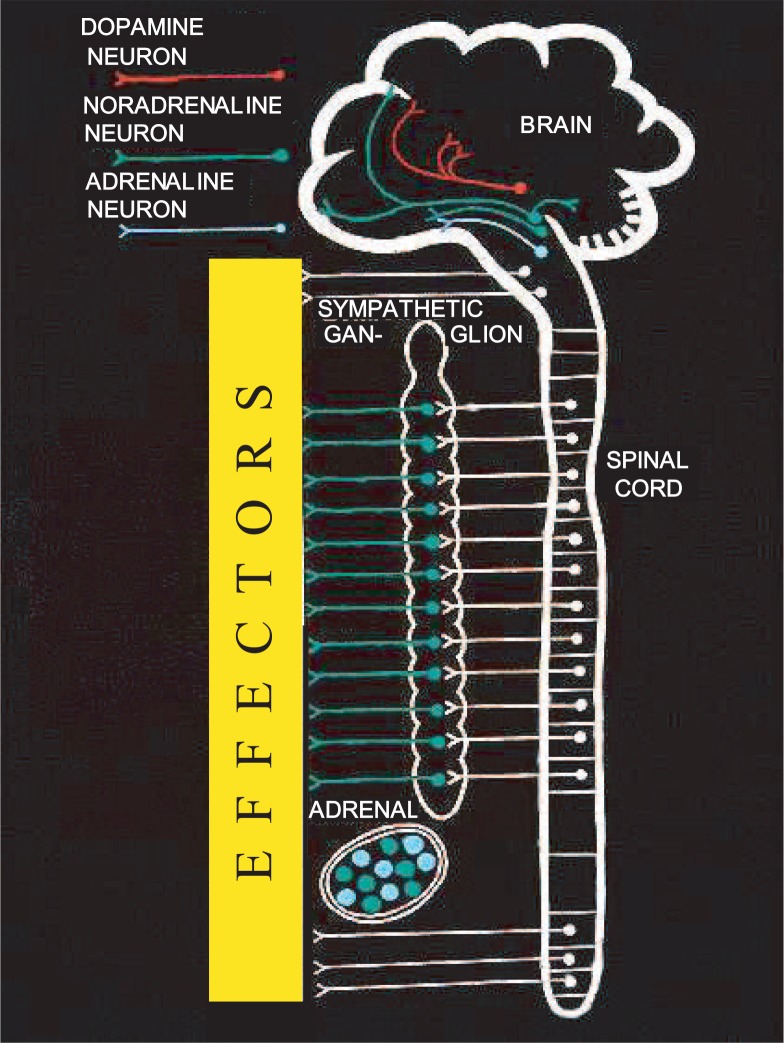
Fig. 2.
Schematic representation of the distribution of catecholamine systems in the brain, periphery, and adrenal medulla.
The three CAs, i.e., dopamine, noradrenaline, and adrenaline, are similar in their structures. However, their distributions in the central and peripheral nervous systems and in the endocrine system (Fig. 2) as well as their functions in the brain and peripheral tissues are quite different and versatile. In the brain the cell bodies of CA-producing neurons are localized mainly in the brain stem; and their axons are widely distributed in all brain regions including the cerebral cortex and cerebellum, as elucidated by histochemical studies done in Sweden.1) Noradrenaline neurons (cell bodies) in the medulla oblongata and pons are termed A1 to A7 neurons; dopamine neurons in the midbrain, hypothalamus, and olfactory bulb, A8 to A16 neurons; dopamine neurons in the retina, A17 neurons; and adrenaline neurons in the medulla oblongata located near to noradrenaline A1∼A3 neurons, C1 to C3 neurons. (Serotonin neurons are termed B1 to B9 neurons.)
CA research has advanced simultaneously in the following three fields, interacting with each other: new biochemical and molecular-biological technology; basic science on the structures of the genes and proteins of the enzymes, receptors, transporters in the plasma membrane or synaptic vesicles, the signal transducers, and animal and human molecular genetics; and clinical medicine of CAs in health and disease in the area of the molecular pathogenesis of stress reactions, cardiovascular diseases, hypertension, Parkinson’s disease (PD), depression, and schizophrenia. As a typical example, the clinical medicine of PD has made remarkable progress since the 1960’s, after the discovery of dopamine deficiencies in the nigro-striatal dopamine (A9) neurons in post-mortem brains. This progress may be measured in terms of diagnosis by molecular imaging, i.e., positron emission tomography (PET) and single photon emission computed tomography (SPECT), therapies using new drugs such as LDOPA, dopamine agonists, and monoamine oxidase (MAO)-B inhibitors for dopamine supplementation and possible protection of neurons, and clinical trials of transplantation and gene therapies. Thus CAs are said to be the molecules bridging basic science to clinical medicine.
In this review, I focus on the development of advances in the biochemistry and molecular biology of CAs mainly in humans in health and disease, especially in relation to CA biosynthesis regulated by tyrosine 3-monooxygenase (tyrosine hydroxylase, TH), a pteridine-dependent monooxygenase. The molecular mechanisms of DOPA-responsive or -non-responsive dystonia and PD caused by dysfunction of the dopamine system are also described.
2. Biosynthesis and metabolism of catecholamines (CAs)
The pathway of the biosynthesis of CAs from tyrosine by the related CA-synthesizing enzymes was established by the 1960’s (for reviews, see refs. 2)–5)). L-3,4-Dihydroxyphenylalanine (DOPA) decarboxylase (DDC), which catalyzes the second step of CA-biosynthesis from tyrosine, decarboxylating L-DOPA to dopamine, was the first enzyme discovered by Holz among the CA-synthesizing enzymes, and the following biosynthetic pathway of CAs was proposed by Blaschko in 1939: tyrosine → DOPA → dopamine → noradrenaline → adrenaline. This hypothetical pathway was later confirmed in the 1950’s by isotope experiments conducted by Udenfriend et al. The DDC enzyme was later isolated, and characterized, and designated as aromatic L-amino acid decarboxylase (AADC) by Lovenberg et al.,6) because it had both L-DOPA decarboxylase activity to produce dopamine in CA cells and L-5-hydroxytryptophan decarboxylase activity to produce serotonin (5-hydroxytryptamine, 5-HT) in serotonin cells. Serotonin, which is synthesized from tryptophan by tryptophan 5-monooxygenase (tryptophan hydroxylase, TPH)7)–9) and AADC, is another slow-acting neurotransmitter in the brain, a local hormone produced by enterochromaffin cells and mast cells in peripheral tissues, and also the precursor of melatonin (N-acetyl-5-methoxytryptamine), which is the pineal hormone produced in the pineal gland. Thus AADC is a common enzyme active in both the CA system and the serotonin system. We confirmed human L-DOPA decarboxylase and L-tryptophan decarboxylase to be the same enzyme, i.e., AADC, by cDNA cloning of human AADC.10), 11) The enzyme at the fourth step of the biosynthesis of adrenaline from tyrosine, noradrenaline (phenylethanolamine) N-methyltransferase (PNMT), catalyzes the N-methylation of noradrenaline to adrenaline by using S-adenosyl-L-methionine as the methyl donor. This enzyme was characterized by Axelrod.12) We identified this enzyme in the human brain,13) proving the presence of adrenaline neurons in the human brain. Dopamine β-monooxygenase (dopamine β-hydroxylase, DBH), which catalyzes the third step of CA biosynthesis from dopamine to noradrenaline, was isolated as a Cu-containing and ascorbate-dependent monooxygenase by Levin et al.14)
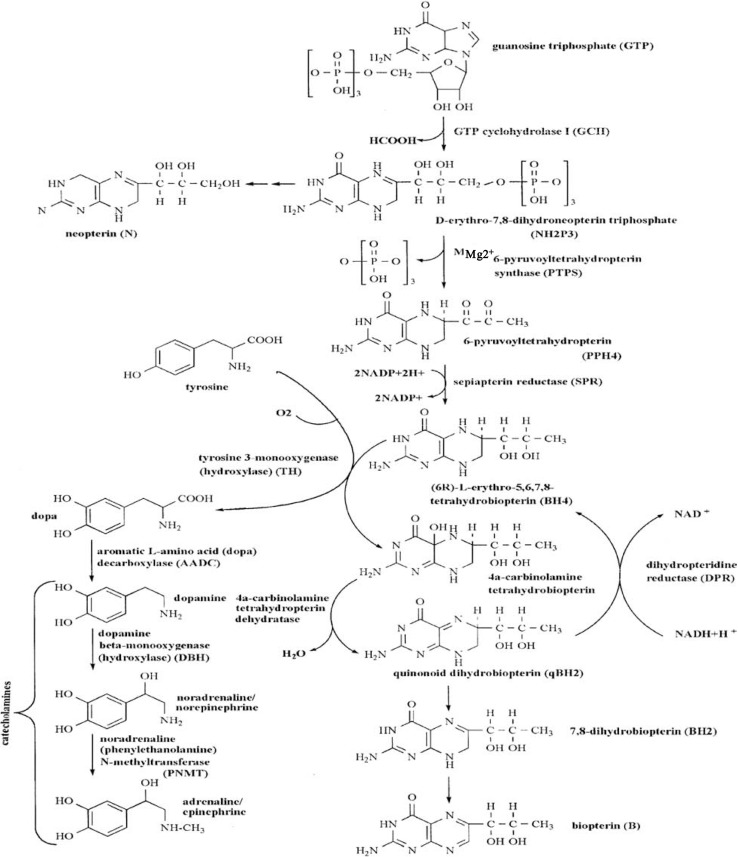
The last enzyme to be discovered was tyrosine 3-monooxygenase (tyrosine hydroxylase, TH), a new pteridine-dependent monooxygenase that converts L-tyrosine to L-DOPA as the first and regulatory step of CA biosynthesis. It was discovered by Nagatsu et al.15) The natural form of the pteridine cofactor of pteridine-dependent monooxygenases is (6R)-L-erythro-5,6,7,8-tetrahydrobiopterin (tetrahydrobiopterin, BH4), as described below. This reduced biopterin was discovered by Kaufman16) as the cofactor of phenylalanine 4-monooxygenase (phenylalanine hydroxylase, PAH), which converts phenylalanine to tyrosine in the liver, an essential step for complete degradation of phenylalanine, and causes hyperphenylalaninemia and phenylketonuria in its mutated form. BH4 is synthesized de novo in the CA-producing cells from guanosine triphosphate (GTP) by the following pathway (Fig. 3): GTP → D-erythro-6, 7-dihydroneopterin triphosphate → 6-pyruvoyltetrahydropterin → BH4. Three enzymes are required for the biosynthesis of BH4: GTP cyclohydrolase I (GCH1), pyruvoyltetrahydropterin synthase (PTPS), and sepiapterin reductase (SPR).17) GCH1 is the regulatory enzyme for the biosynthesis of BH4. As described below, in the hydroxylation reaction catalyzed by pteridine-dependent monooxygenases such as in the hydroxylation of L-tyrosine to L-DOPA by TH, BH4 is oxidized to 4-α-carbinolamine tetrahydropterin, which is then converted to quinonoid L-erythro-7,8-dihydrobiopterin (qBH2) by pterin-4-α-carbinolamine dehydratase. qBH2 is reduced back to BH4 by dihydropteridine reductase (DPR). We found that DPR coupled with TH is NADH-dependent.18) BH4 or qBH2 is easily non-enzymatically oxidized to 7,8-dihydrobiopterin (BH2), which is reduced back to BH4 by dihydrofolate reductase (a salvage pathway). As the reduction by DPR of oxidized qBH2 produced by TH reaction is not complete, BH4 in CA-producing cells is constantly supplied by its de novo biosynthesis from GTP. BH4 is an essential cofactor regulating the activity of TH, which in turn regulates the biosynthesis of CAs. BH4 also regulates the stability of the TH protein.19) Thus CA biosynthesis is regulated by TH activity and the content of BH4. The stereochemical structure of BH4, the cofactor of pteridine-dependent monooxygenase was determined to be (6R)-L-erythro-tetrahydrobiopterin by Matsuura, Sugimoto et al. in 1985.20)
Fig. 3.
Enzymes related to the biosynthetic pathway of catecholamines from tyrosine regulated by tyrosine 3-monooxygenase (tyrosine hydroxylase, TH) and those of the pteridine cofactor [(6R)-L-erythro-5,6,7,8-tetrahydrobiopterin (BH4)] from guanosine triphosphate (GTP): tyrosine 3-monooxygenase (tyrosine hydroxylase, TH), aromatic L-amino acid decarboxylase (AADC), dopamine β-monooxygenase (dopamine β-hydroxylase, DBH), noradrenaline (phenylethanolamine) N-methyltransferase (PNMT); GTP cyclohydrolase I (GCH1), pyruvoyltetrahydropterin synthase (PTPS), sepiapterin reductase (SPR), 4a-carbinolamine tetrahydropterin dehydratase, dihydropteridine reductase (DPR).
All CA-synthesizing enzymes except DBH are soluble and present in the cytoplasm in CA-producing cells, and only DBH is membrane-bound and localized in the synaptic vesicles. These enzymes are synthesized in the cell bodies of neurons and transported in axons by axonal flow to the nerve terminals.
CAs are inactivated by MAO and catechol O-methyltransferase (COMT), i.e., by oxidative deamination and O-methylation (Fig. 1). In humans phenolsulfotransferase forms inactive CA sulfates, which can be converted back to active CAs by sulfatase. Two isoforms, MAO-A and MAO-B, encoded by different genes, have been found to have different physiological and pathological functions such as in PD. The enzymatic O-methylation of CAs by COMT was discovered by Axelrod in 1957. Both MAO and COMT act on dopamine, noradrenaline, or adrenaline in turn to produce various metabolites, as shown in Fig. 1. The main final metabolites of dopamine are 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA); and those of noradrenaline and adrenaline are identical due to loss of the amino group by oxidative deamination by MAO; i.e., 3-methoxy-4-hydroxyphenylethyleneglycol (MHPG/MOPEG) and vanillylmandelic acid (VMA).2)
In CA neurons, dopamine, noradrenaline, or adrenaline is synthesized not only in the cell body, where the neurotransmitter is stored in synaptic vesicles and transported with CA-synthesizing enzymes through the axon to the nerve terminals by axonal flow, but also in the nerve terminals, where the neurotransmitter is locally synthesized and stored in synaptic vesicles. CA molecules that are newly synthesized locally at the pre-synaptic nerve terminals are preferentially released by exocytosis from synaptic vesicles into the synapse as neurotransmitters, diffuse across the synaptic cleft, and react with CA receptors (dopamine D1–D5 receptors, adrenergic α-1,2 or β-1,2,3 receptors) at the plasma membrane of post-synaptic cells to transmit chemical information; and then they are mainly re-taken up back into cytoplasm of pre-synaptic CA nerve terminals by CA transporters (dopamine or noradrenaline transporters, DAT or NAT) located in the plasma membrane and then into synaptic vesicles by vesicular monoamine transporter (VMAT). This re-uptake phenomenon of CAs was first discovered by Axelrod et al. in 1959, and since then the re-uptake of neurotransmitters has been recognized as a general principle for termination of neurotransmission in the synapse. DBH in peripheral or central noradrenaline or adrenaline neurons is localized in the synaptic vesicles, is released into the synaptic cleft together with CAs, and appears in blood or cerebrospinal fluid.21) CA molecules released at synapse also act on CA receptors located in the pre-synaptic nerve terminal (pre-synaptic receptor, also called autoreceptor), and inhibit both CA biosynthesis by TH and CA release by exocytosis. CA neurons have extrasynaptic varicosities along their axonal terminals, where extrasynaptic axonal and somatodendritic release occurs in the absence of postsynaptic cells. Such CA molecules released from these varicosities act by diffusion on CA receptors of surrounding neurons or glial cells that have CA receptors (paracrine function or volume transmission). CAs that are released but not taken-up into the presynaptic neurons by CA transporters are metabolized by MAO and COMT in turn. CAs produced in the periphery and released into blood cannot get into the brain due to the blood-brain barrier. CAs and their metabolites in the brain are transferred into cerebrospinal fluid and then their metabolites into blood, and those from the CA-producing cells in the periphery pass directly into blood. In humans they are mostly conjugated to sulfates by sulphotransferase and are excreted from blood into urine by the kidney.2)
3. Genes for human and mouse catecholamine-synthesizing enzymes
We aimed at elucidating the physiological and pathological functions of CAs by using genetically engineered mice and in patients with CA dysfunction, based on the structures of the genes and deduced proteins of CA-synthesizing enzymes. Therefore, we cloned the genes of humans and mice for the enzymes related to the biosynthesis of CAs and the BH4 cofactor of TH. A summary of the structures of these genes and CA- and BH4-synthesizing enzymes in humans are shown in Table I. (see reviews 3), 22)) Multiple mRNAs are produced in humans by alternative mRNA processing from a single gene in the case of TH, AADC, DBH, and PNMT. However, except for the human TH gene, which produces 4 isoform proteins, the other enzymes are composed of a single protein.
Table I.
Genes and proteins of catecholamine- and tetrahydrobiopterin-synthesizing enzymes
A) Human Catecholamine-Synthesizing Enzymes*)
| Enzyme (EC number) | Gene | mRNA | Alternative mRNA processing | Protein | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Chromosome | Exons | Species | Coding region (bases) | Mr/subunit | Amino acid residues/subunit | Subunit number | ||
| Tyrosine 3-monooxygenase (Tyrosine hydroxylase) (TH) (1. 14. 16. 2) |
11p15.5 | 14 | type 1 | 1491 | Alternative mRNA splicing | 55533 | 497 | 4 |
| type 2 | 1503 | 55973 | 501 | 4 | ||||
| type 3 | 1572 | 58080 | 524 | 4 | ||||
| type 4 | 1584 | 58521 | 528 | 4 | ||||
| Aromatic L-amino acid decarboxylase (Dopa decarboxylase) (AADC) (4. 1. 1. 28) |
7p12.1–p12.2 | 16 | type N type L |
1440 | Alternative promoter | 53891 | 480 | 2 |
| Dopamine β-monooxygenase (Dopamine β-hydroxylase) (DBH) (1. 14. 17. 1) |
9q34 | 12 | type A type B |
1809 | Alternative polyadenylation | 64862 | 578 (603)**) | 4 |
| Noradrenaline N-methyltransferase (Phenylethanolamine N-methyltransferase) (PNMT) (2. 1. 1. 28) |
17q21–q22 | 3 | type A type B |
846 | Alternative promoter | 30853 | 282 | 1 |
Reference, Review 3).
The number in parentheses for the amino acid residues of DBH includes the signal sequences.
B) Human Tetrahydrobiopterin-Synthesizing*)
| Enzyme (EC number) | Gene | Protein | |||
|---|---|---|---|---|---|
|
| |||||
| Chromosome | Exons | kDa/subunit | Amino acid residues/subunit | Subunit number | |
| Guanosine triphosphate (GTP) cyclohydrolase I (GCH1) (3. 5. 4. 16) |
14q22.1–q22.2 | 6 | 30 | 250 | 10 |
| Pyruvoyl-tetrahydropterin synthase (PTPS) (4. 6. 1. 10) |
11q22.3–q23.3 | 6 | 17 | 145 | 6 |
| Sepiapterin reductase (SPR) (1. 1. 1. 153) |
2q13 | 3 | 28 | 261 | 2 |
| Pterin-4 α-carbinolamine dehydratase (PCBD) (4. 2. 1. 96) |
10q22 | 4 | 11 | 103 | 2 |
| Dihydropteridine reductase (DPR) (1. 6. 99. 7) |
4q15.3 | 7 | 25 | 244 | 2 |
References, Review 22).
3.1. Tyrosine 3-monooxygenase (TH): the human gene, human multiple isoforms, and regulation
In early 1964, among the four enzymes involved in CA biosynthesis, only the enzyme responsible for converting tyrosine to DOPA still remained elusive. Then in that year we discovered a pteridine-dependent monooxygenase as this elusive enzyme.15), 23) Until the discovery of TH in 1964, there were three hypotheses for the conversion of tyrosine to DOPA in CA-producing cells: a non-enzymatic reaction; monophenol monooxygenase (tyrosinase) as the possible enzyme; or the presence of an unknown enzyme. Tyrosine can be easily converted to DOPA non-enzymatically in vitro, but not in vivo; and tyrosinase produces DOPA via dopaquinone and leucodopachrome from tyrosine in melanin-producing melanocytes,24) but not in CA-producing cells. Assuming that an unknown enzyme to convert tyrosine to DOPA may exist in CA-containing tissues, at the NIH we started to work to discover such enzyme. We first developed a highly sensitive isotopic assay to detect the assumed enzyme activity; L-[14C] tyrosine with high specific radioactivity was used as a substrate, and L-[14C] DOPA, if enzymatically formed, was isolated on an alumina column and assayed by the use of a liquid scintillation counter. We started our initial work to discover the enzyme in tissue slices and minces of the rat brain stem, where the dopamine content is high and the tissue should contain the enzyme and all of the necessary cofactors. We found the absolute stereo-specificity of this enzyme, which permitted the use of D-[14C] tyrosine as a control, and we became convinced that we were really detecting a new enzyme. We found that the bovine adrenal medulla contained a large amount of the enzyme in a soluble fraction, and developed a new and rapid assay for the activity by using L-[3,4-3H] tyrosine as substrate to determine the amount of 3H released in the hydroxylation reaction at the 3-position.25) Thus we could partially purify the enzyme. After testing many probable cofactor substances, the preparations were shown to require a tetrahydropteridine and molecular oxygen for the enzyme activity. Thus the systematic name of the enzyme is tyrosine 3-monooxygenase. Ferrous iron was also found to be another essential cofactor.15) This enzyme was later found by Levitt et al. to be rate-limiting in vivo in the biosynthesis of CAs.
TH has been shown to be regulated by complex mechanisms (see reviews 26)–28)). The enzyme is inhibited by dopamine and various catechol compounds, and so we proposed feedback inhibition as the mechanism for the short-term regulation of CA biosynthesis.15) Dopamine not only acutely inhibits TH activity competitively with a pteridine cofactor, but also inactivates the enzyme activity by binding to the protein.28) Among various catechol- and tyrosine-derivative inhibitors, L-α-methyl-p-tyrosine is the most potent competitive inhibitor toward the substrate L-tyrosine29); and it has been widely used to inhibit the enzyme in vivo in experimental animals. Several natural inhibitors of TH were found to be produced by microorganisms in the search for microbial enzyme inhibitors by Umezawa et al.; and oudenone was one of them.30) It inhibits the enzyme competitively with respect to the pteridine cofactor.
TH is regulated in the short term not only by feed-back inhibition but also by phosphorylation and dephosphorylation by a complex mechanism, as described below in more detail. It is phosphorylated at Ser8, Ser19, Ser31 and Ser40. Activation of TH by phosphorylation of the enzyme is mainly catalyzed by protein kinase A, Ca2+/calmodulin-dependent protein kinase II (Ca/CaMPK II), and protein kinase C.27), 28) These protein kinases activate TH by phosphorylation in the short term, and also increase production of the protein in the long term. Medium-to long-term regulation of the enzyme activity occurs at various phases of gene expression, such as transcription, alternative RNA processing, RNA stability, and translation. The enzyme activity can also be regulated at the level of protein stability, which is increased by an increase in the intracellular concentration of BH4.19)
Under stress this enzyme protein is increased by induction. The first indication of the induction of TH was found by the increased turn over of noradrenaline and the increased maximum velocity of this enzyme in the sympathetic nerves in the heart of sino-aortic denervated rabbits.31) Induction of TH was also confirmed after chemical sympathectomy as a compensatory mechanism of noradrenaline depletion.32) TH is induced under chronic stress together with DBH and PNMT.
Purification of TH was difficult, but was finally achieved in early 1980’s from various tissues, e.g., bovine adrenal medulla,33) rat adrenals,34) and human brain and adrenals.35)
The primary structure of the enzyme from various species including humans was determined by cloning of cDNA after the 1980’s (see reviews 3), 36), 37)). TH from various mammalian species is a 240-kDa homotetramer composed of four identical 60-kDa subunits. Each subunit has a C-terminal catalytic domain that binds the substrates tyrosine and molecular oxygen and the tetrahydropteridine cofactor with ferrous iron, and an N-terminal regulatory domain containing phosphorylated serine residues (Fig. 4). TH from non-primate animals such as mice38) is a single protein. Only human TH has four isoform types1–4 (hTH1, hTH2, hTH3, and hTH4), due to alternative mRNA splicing.39)–42) By comparison of the genomic DNA sequences of various primates, we found that non-human primates such as Macaca irus and Macaca fuscata (Japanese monkeys),43) gibbon, orangutan, gorilla, and chimpanzee44) produce only two of the TH isoforms, corresponding to human TH types 1 and 2 (hTH1 and hTH2). In contrast to humans, monkeys, like non-primate mammals, lack exon 2 in humans, but they have two isoforms corresponding to hTH1 and hTH2 by alternative mRNA splicing of exon 1. The expression of human TH types 1–4 and monkey TH types 1 and 2 was proved immunohistochemically by Haycock.45)
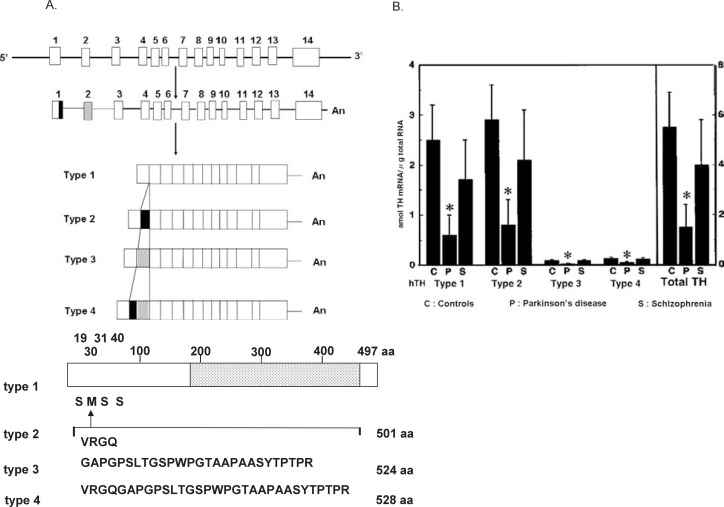
Fig. 4.
A Structures of the human tyrosine 3-monooxygenase (tyrosine hydroxylase, TH) genes, indicating the alternative splicing pathway producing the four types of human tyrosine 3-monooxygenase (hTH1–hTH4) mRNAs from a single gene. The 3′-terminal portion of exon 1, which corresponds to the 12-bp insertion sequence, is indicated by a filled box. The hatched box shows exon 2 that encodes the 81-bp insertion sequence. Schematic diagram of the protein structure and the main phosphorylation sites (Ser19, Ser31, and Ser40) of hTH1 is shown below. The open area shows the regulatory N-terminal domain. The dotted area shows the catalytic C-terminal domain. Ser19, Ser31, and Ser40 are the main phosphorylation sites activating the enzyme. The insertion sequences of 4, 27, and 31 amino acid residues correspond to hTH2, hTH3 and hTH4. From Kaneda et al.40) and Kobayashi et al.42)
B mRNA contents of the four types of human tyrosine 3-monooxygenase (tyrosine hydroxylase, TH) in the striatum of the brain from controls (C), patients with Parkinson’s disease (P), and patients with schizophrenia (S). * Significantly different from controls. From Ichinose et al.114)
Crystallization of TH protein has been difficult. Goodwill et al.46) succeeded by removing the N-terminal regulatory domain: the crystal structure of the C-terminal catalytic and tetramerization domains of rat TH in the presence of the cofactor analogue 7,8-dihydrobiopterin and iron showed the mode of the pteridine cofactor binding and the proximity of its hydroxylated 4a carbon of the pteridine ring to the required iron.
The human TH gene42) is composed of 14 exons interrupted by 13 introns, spanning approximately 8.5 kb; whereas the genes of the enzyme of non-human primates and non-primate mammals lack exon 2, and consist of 13 exons. The 12-bp insertion sequence is derived from the 3′-terminal portion of exon 1 and the 81-bp insertion sequence is encoded by exon 2, which is specific in the human gene. The N-terminal region is encoded by the 5′-portion of exon 1, and the remaining region from exon 3 to exon 14 is common to all four kinds of TH mRNA. Thus, these four mRNAs differ by the presence of 12 (hTH2), 81 (hTH3), or 93 (12 plus 81, hTH4) additional nucleotides between nucleotide 90 and 91 of hTH1 (Fig. 4).3), 39)–42) Dumas et al.47) further reported three more isoforms of human TH produced by skipping of exon 3; and they found higher levels of these isoforms in the adrenal medulla of patients with progressive supranuclear palsy (PSP). We also looked for these new isoforms in the brain, but could not detect them in the brain of controls or patients with PSP. Instead we found a new splicing variant in the human adrenal medulla of a normal control; the mRNA lacked exon 4, resulting in a premature stop codon at amino acid 147.48) Although hTH1–hTH4 are the major isoforms of human TH, yet more isoforms of mRNA may exist in humans. As mentioned above (shown in the schematic diagram of Fig. 4), TH is activated by phosphorylation of mainly Ser19, Ser31, and Ser40 among the 4 Ser residues in the N-terminal regulatory domain, and deactivated by dephosphorylation via protein phosphatase (type 2A). Ser40 is mainly phosphorylated by protein kinase A, and Ser19 mainly by Ca/CaMPK II. We found that Ca/CaMPK II may phosphorylate and activate TH of PC 12h cells when they are depolarized by high K+, because a selective inhibitor of Ca/CaMPK II, KN-62 (1-[N,O-bis(5-isoquinolinsulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine) inhibits this TH phosphorylation and reduces dopamine synthesis.49) Itagaki et al.50) found that the chaperon 14-3-3 protein binds and activates TH at Ser19 phosphorylated by Ca/CaMPK II, indicating depolarization-evoked activation of TH in vivo; these results agree with the fact that Ca/CaMPK II mediates phosphorylation of TH by hormonal and electrical stimuli, which leads to elevation of Ca2+ levels. Lehmann et al.51) proposed human TH isoforms (hTH1–hTH4) to be differentially regulated via hierarchical phosphorylation. They reported that phosphorylation of the human enzyme type 1 (hTH1) at Ser31 by extracellular signal-regulated protein kinase (ERK; hTH2 was not phosphorylated by ERK) produced a 9-fold increase in the rate of phosphorylation of Ser40, whereas it had little effect on that rate in the TH types 3 and 4 (hTH3, hTH4). Phosphorylation of the Ser19 of hTH2 increased the phosphorylation of Ser40 more strongly than did that of the same Ser of hTH1. Thus, hTH1 might be regulated by an ERK pathway; and, hTH2, by a Ca/CaMPK II pathway. Ota with Nakashima et al.52), 53) prepared various deletion mutants of the regulatory N-terminus domain of human TH type 1 (hTH1) and found that deletion of N-terminus of the enzyme enhances the stability of the enzyme.
As described above, TH is regulated in the long term under stress by enzyme induction at the transcriptional level. Transcription of the gene is regulated by several transcription factors such as cAMP-response element binding protein (CREB), AP1, Egrl1, AP2, dyad, SP1, ATF-2, hypoxiainducible transcription factor, and Nurr1 (see review 26)). The expression of TH in cultured cells and tissues producing CAs is regulated by various first messengers: e.g., dopamine, dopamine agonists and antagonists, nicotine, vasoactive intestinal polypeptide (VIP), secretin, angiotensin II, bradykinin, neurotensin, hypoxia-inducible protein, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), ciliary neurotrophic factor (CNTF), insulin-like growth factor-I (IGF-I), and retinoic acid receptor. V-1/myotrophin containing cdc10/SWI6 motifs is unique since it induces not only TH, but also the pteridine cofactor-synthesizing enzyme, GCH1, in PC12 cells.54) Insulin-like growth factor-I (IGF-I) also induces not only TH, but also DBH and PNMT in bovine adrenomedullary chromaffin cells. These various messengers may coordinate TH induction with the induction of other CA-synthesizing enzymes under stress conditions.
3.2. Human aromatic L-amino acid decarboxylase (AADC) gene: Tissue-specific alternative splicing generates neuronal and non-neuronal types of mRNA in human AADC, but produces a single enzyme protein
AADC is the only one enzyme among the CA-synthesizing enzymes that is expressed in both neuronal CA cells and serotonin cells in the brain, and also in non-neuronal cells in the periphery such as those in the liver and kidney. Thus this enzyme relates to the biosynthesis of two important slow-acting neurotransmitters, i.e., CAs and serotonin, both of which play important roles in emotion, memory, and other higher brain functions in human behaviour. AADC requires pyridoxal phosphate as a cofactor. The enzyme is a homodimer composed of two identical 50-kDa subunits (Table I). We cloned cDNA10) of the human AADC and the genomic DNA,55) and assigned the gene to chromosome 7p12.1–p.12.3.55) The human gene, being approximately 100 kb in size and composed of 16 exons, is expressed in both CA and serotonin neurons as well as in non-neuronal tissues such as the liver. We (Ichinose et al.) found that an alternative usage of the non-neuronal (L1) and neuronal (N1) first exons in the 5′-untranslated regions of the human gene produces neuronal (CA and serotonin type) and non-neuronal (liver type) mRNAs encoding the same single protein.56), 57)
Furthermore, some neurons called D neurons in the brain express AADC only without expression of TH. The substrate of the enzyme in vivo and physiological and pathological functions of these D neurons are not clear, but they are distributed mainly near the ventricular system in the brain.58)–60) Nagatsu, I. et al.60) found that the nerve terminals of some D-neurons face the cerebral ventricle between the ependymal cells, suggesting that some monoamine neurotransmitter synthesized in the D-neurons may be released directly into cerebrospinal fluid. They also reported that, although D-neurons should synthesize some monoamine including dopamine or serotonin from various aromatic L-amino acids as the substrate, neither dopamine nor serotonin was identified in the D-neurons in the mouse and rat spinal cord by immunohitochemistry using dopamine- or serotonin-specific antibody. Thus, the physiological significance of dopamine-neurons remains to be determined by further investigation. A possible candidate of the neurotransmitter might be a trace amine in the brain such as tyramine or octopamine.
3.3. Human dopamine β-monooxygenase (DBH) gene: Alternative polyadenylation of human DBH gene produces two mRNAs, but the gene encodes a single glycoprotein with high concentrations in human blood
DBH is a copper-containing, ascorbate-requiring monooxygenase that catalyzes the hydroxylation of a phenylethylamine (mainly dopamine in vivo) at the β-C of the side chain for a phenylethanolamine (i.e., noradrenaline from dopamine), using molecular oxygen and ascorbic acid as an electron donor.14), 61) Human DBH is a 290-kDa homotetramer consisting of four subunits of Mr 64862 with 578 amino acids (603 amino acids including the signal peptide) and containing 2 atoms of Cu per subunit (Table I). DBH is a glycoprotein, as it contains carbohydrate side chains that may influence the stability of the enzyme. DBH is specifically localized in noradrenaline and adrenaline neurons (A1–A7 neurons and C1–C3 neurons) of the brain as well as in noradrenaline neurons of the peripheral sympathetic nerves and in adrenaline and noradrenaline cells of the adrenal medulla. Therefore, this monooxygenase is a marker of noradrenaline and adrenaline cells. Also DBH is the only CA-synthesizing enzyme localized in synaptic vesicles in noradrenaline and adrenaline neurons and in chromaffin granules containing adrenaline or noradrenaline in the adrenal medulla. About 50% of the activity is tightly bound to the vesicular membranes, and the rest of the activity can be easily released by hypotonic treatment of the vesicles. The soluble form of the enzyme is secreted into cerebrospinal fluid in the brain and into blood in the periphery together with noradrenaline or adrenaline as neurotransmitter or hormone.21), 62), 63) Interestingly only humans among primate and non-primate mammals have high DBH activity in their blood.62), 63); rats have very low activity.64) This may be due to a standing position of humans requiring high sympathetic nerve activity. In the blood or crude extracts of tissues, the activity is inhibited by the endogenous inhibitors that is sulfhydryl compounds like glutathione and cysteine;65) but for the assay of the activity the inhibition can be removed by N-ethylmaleimide or Cu, either of which binds with sulfhydryl groups.62), 63) Among natural inhibitors, Hidaka et al.66) discovered fusaric acid (5-butylpicolinic acid) as a specific and potent inhibitor of DBH, which have hypotensive activity in vivo.
We (Kobayashi et al.67)) isolated two different cDNAs (named types A and B based on the differences in their 3′-terminal regions) and the genomic DNA of human DBH. We showed that the two mRNAs are generated through alternative polyadenylation from a single gene. Our type A cDNA was identical to a cDNA encoding human DBH isolated by Lamouroux et al.68) The human DBH gene spans approximately 23 kb and consists of 12 exons interrupted by 11 introns. Exon 12 encodes the 3′-region of 1013 bp including the 300-bp sequence in type A. The 3′-untranslated region may be involved in mRNA stability and translational efficiency. The ratio of type A to type B mRNAs in human pheochromocytoma cells is approximately 1.0 to 0.2. We found possible transcription regulatory elements, including TATA, CCAAT, CAAAA, GC boxes, cyclic AMP response element (CRE), AP-2 element, and glucocorticoid response element (GRE), near the transcription initiation site of the human DBH gene. The cyclic AMP-mediated regulation of transcription from the DBH promoter is mediated by the AP1 proteins, i.e., c-Fos, c-Jun, and JunD. Cyclic AMP, diacylglycerol, and Ca2+ increase the transcription of both TH and DBH and lead to increased CA biosynthesis. We (Ishiguro et al.69)) identified and characterized a novel phorbol ester-response DNA sequence in the 5′-flanking region of the human DBH gene. The data suggest that transcriptional up-regulation of the human DBH gene in response to TPA (12-O-tetradecanoylphorbol-13-acetate) requires coordination among this novel TPA-response element (TRE), cyclic AMP-response element (CRE), and the YY1 binding site. We also cloned mouse DBH cDNA and the genomic DNA.70) The mouse DBH gene was composed of 12 exons about 17 kb in length, encoding a protein of 621 amino acids with Mr 70189. Typical TATA and CCAAT boxes were observed in the 5′-upstream region of the mouse gene. Northern blot analysis of adrenal gland detected a single size species of the mouse DBH mRNA.
The protein content and activity of DBH in human blood vary widely between individuals and are remarkably constant in each individual62), 63) and genetically determined. Although the activity of DBH in human plasma is specifically high among mammals, a small subgroup of the population has low activity levels. The activity in blood has been measured in various diseases. Linkage and association studies on human plasma DBH by Cubells et al.with us71) indicated the structural gene encoding DBH (locus name, DBH) to be a major quantitative trait locus for plasma DBH activity, and also to influence DBH protein levels in cerebrospinal fluid. Zabetian et al.72) further identified a new polymorphism (−1021 C→T) in the 5′-flanking region of the DBH gene as a major genetic marker for plasma DBH activity, which provides a new tool for investigation of the role of both DBH protein and the DBH gene in human diseases.
3.4. Human noradrenaline (phenylethanolamine) N-methyltransferase (PNMT) gene
PNMT is the terminal enzyme in CA biosynthesis, and catalyzes the methylation of noradrenaline to adrenaline, using S-adenosyl-L-methionine as the methyl donor.12) The enzyme is found in adrenaline cells of the adrenal medulla where adrenaline is synthesized, stored, and secreted as adrenomedullary hormone. PNMT is also localized in adrenaline (C1–C3) neurons in the medulla oblongata,1), 13) which neurons send their axons to various regions of the brain such as the hypothalamus, striatum73) and amygdala. These adrenaline neurons are supposedly involved in some important neuro-physiological functions such as cardiovascular and neuroendocrine regulation of the brain. PNMT is a 30-kDa monomeric enzyme and requires various phenylethanolamines including noradrenaline as substrates to form N-methylphenylethanolamines such as adrenaline. We (Kaneda et al.74)) cloned the full-length cDNA of the human PNMT and found it to encode a protein consisting of 282 amino acids with Mr 30853, and to reside on chromosome 17. We (Sasaoka et al.75)) also cloned the genomic DNA of the human enzyme, which consists of 3 exons and 2 introns and encodes a single protein. We observed a minor mRNA (named type B), besides the major mRNA (named type A). The type B mRNA carries an approximately 700-nucleotide-long untranslated region in its 5′ terminus, suggesting that the two types of mRNA are produced from a single gene through the use of two alternative promoters. Consensus sequences for glucocorticoid response element (GRE) and Sp1 binding sites are observed in the 5′-flanking region of the gene. We also cloned the mouse PNMT genomic DNA and cDNA for application to studies on transgenic mice. The genomic DNA spanned about 1.8 kb, and consisted of 3-exons; and the typical TATA, GC, and CACCC boxes as well as several sequences homologous to the glucocorticoid response element (GRE) were located in the 5′-flanking region.76)
3.5. Human genes for biosynthetic enzymes of tetrahydrobiopterin (BH4), the cofactor of tyrosine 3-monooxygenase (TH)
BH4 is a cofactor of TH; and it indirectly regulates the biosynthesis of CAs by regulating the activity of TH, the rate-limiting enzyme. As described above, there exist three enzymes for the biosynthesis of BH4 and two recycling enzymes during its oxidation in the TH reaction (Fig. 3, see reviews 4), 17), 22)). We isolated and characterized the human genes of GTP cyclohydrolase I (GCH1), the first and regulatory enzyme, and sepiapterin reductase (SPR), the last enzyme, of BH4 biosynthesis. Human GCH1 is encoded by three distinct mRNAs, hGCH-1, -2, and -3; and hGCH-1 is the most abundant of them in human liver.77) The full-length cDNA for mouse GCH178) encodes a protein of 241 amino acid sequence that is highly homologous to that of the human type 1 enzyme. We further characterized human and mouse GCH1 genes.79) As described below, the mutation of the human genes causes autosomal dominant GTP cyclohydrolase I deficiency/DOPA responsive dystonia (DRD)/Segawa’s disease and autosomal recessive GTP cyclohydrolase I deficiency/atypical phenylketonuria. The human GCH1 gene is composed of six exons spanning approximately 30 kb. The structural heterogeneity of human GCH1 mRNAs is caused by an alternative usage of the splicing acceptor site at the sixth exon. We also cloned cDNA80) and genomic DNA81) of the human SPR. The human cDNA encoded a protein of 261 amino acids with Mr 28047. The predicted amino acid sequence of human SPR showed a 74% identity with the sequence of the completely purified mature rat enzyme,82) the structure of which was determined by amino acid sequencing and began with an N-acetyl methionyl residue at its N-terminus. GCH1 is distributed in mice in CA neurons in the brain, adrenal medulla, and liver where BH4 is synthesized.83) SPR was proved by confocal microscopy to be colocalized with TH in the CA neurons of the human brain.84)
4. Elucidation of physiological functions of catecholamines (CA) in genetically engineered mice
Various physiological and pathological functions of CAs in vivo have been elucidated from studies on genetically engineered mice. Phenotypes of genetically modified mice carrying TH mutations are especially valuable as animal models of human diseases (Table II).
Table II.
Phenotypes of genetically modified mice carrying tyrosine 3-monooxygenase (tyrosine hydroxylase, TH) mutations
| Genotype | Enzyme activity | Phenotype | |
|---|---|---|---|
| Knockout (homozygous mutation) | TH (−/−) mice | Complete loss | Abnormal electrocardiography, perinatal lethality |
| Heterozygous mutation | TH (+/−) mice | Reduction to 45% of control | Defect in latent learning, defect in conditioned learning (cued fear conditioning, taste aversion), defect in operant conditioning |
| Conditional knockout in dopamine (DA) neurons | DA (−/−) mice | Loss in dopamine neurons | Akinesia, cataleptic behaviour, loss of drug response, defect in operant conditioning, adipsia, aphagia, postnatal lethality, growth retardation |
Reference, Kobayashi and Nagatsu.37)
4.1. Human tyrosine 3-monooxygenase (TH) transgenic mice
As described above, mice contain a single form of TH,38) whereas only humans contain 4 TH isoforms.40), 42) It is an interesting question if multiple isoforms of human TH can be expressed in the CA neurons and adrenomedullary cells in transgenic mice that have a single enzyme protein and if the transgenic mice show changes in phenotypes including their behavioural one. To investigate ciselements responsible for CA neuron-specific expression of the human TH gene, we (Sasaoka et al.85)) produced lines of transgenic mice carrying 5.0-kb, 2.5-kb, and 0.2-kb fragments from the 5′-flanking region of the human TH gene fused to a reporter gene, chloramphenicol acetyltransferase (CAT). The results indicated that the 5.0-kb DNA fragment of the TH gene upstream region contained activity to express CAT in CA neurons, but lacked some regulatory elements attenuating ectopic expression, suggesting that the exon-intron structure and/or 3′-flanking region may also function in CA neuron-specific expression. Therefore, we (Kaneda, et al.86)) produced transgenic mice having the entire human TH gene. We employed an 11-kb fragment of the human TH gene consisting of 2.5 kb of its 5′-upstream region containing promoter information, the entire exon-intron structure, and 0.5 kb of the 3′-flanking region for the production of transgenic mice. The human transgene was transcribed correctly and expressed specifically in the brain and adrenal gland. The results show that the fundamental cellular machinery necessary for the alternative splicing pathway producing the multiple isoforms from the human TH transgene is present and functioning in the mouse brain and adrenal gland. The level of human TH mRNA in the brain was about 50-fold higher than that of endogenous mouse TH mRNA. In situ hybridization demonstrated a very strong region-specific expression of the transgene in the substantia nigra and ventral tegmental area. TH immunoreactivity in these regions was definitely increased, but only about 5 fold. Thus, a large difference was observed between the amount of TH mRNA and the enzyme protein. The enzyme activity and CA levels in the transgenics were also slightly increased, but not significantly. The transgenic mice exhibited no significant phenotypic abnormalities in blood pressure, circadian rhythms, or behavioural activity. These results suggest the existence of some unknown regulatory mechanisms for human TH gene expression and for the CA levels in transgenic mice. We (Ikuko Nagatsu, unpublished results) have noticed that the transgenic mice tended to live longer than the wild-type mice, although this must be further confirmed in a larger number of animals.
4.2. Tyrosine 3-monooxygenase (TH) gene knockout mice
When we (Kobayashi et al.87)) disrupted the TH locus in mice, the homozygous mice died at a late stage of embryonic development or shortly after birth. Both mRNA and enzyme activity were lacking with severe depletion of CAs. These changes, however, did not affect gross morphological development of the cells that normally express high CA levels. Analysis of electrocardiograms of surviving embryos and newborn mutants showed that an alteration of the sympathetic noradrenaline neurons and resultant cardiac dysfunction in the homozygous mice may lead to the lethality of this mutation. This agrees with the report by Thomas et al.,88) which showed that mice lacking DBH by gene targeting die in utero and that mortality can be rescued with L-threo-3,4-dihydroxyphenylserine (L-threo-DOPS), which is directly decarboxylated to noradrenaline by AADC.11) The transfer of a human TH transgene into the homozygous mice corrected the mutant phenotype, showing recovery of TH activity by expression of the human enzyme. These results indicate that TH is essential for survival of the animals during late gestational development and after birth. Zhou et al.89) also disrupted the TH gene in mice and proved that CAs are required for mouse fetal development. They rescued mutant mice in utero by administering L-DOPA to pregnant females. Kobayashi et al.90) also found that in the heterozygous TH(+/−) mice with a single mutated allele of the TH gene, in which the enzyme activity in tissues was reduced to about 40% of the wild-type activity, noradrenaline accumulation in brain regions was moderately decreased to 73–80% of the wild-type value. The heterozygous mutant mice displayed impairment in the water-finding task associated with latent learning performance. They also exhibited mild impairment in long-term memory formation in three forms of associative learning, including active avoidance, cued fear conditioning, and conditioned taste aversion. These deficits were restored by the drug-induced stimulation of noradrenergic activity. In contrast, the spatial learning and hippocampal long-term potentiation were normal in the mutants. These results indicate that the central noradrenergic system plays an important role in memory formation, particularly in the long-term memory of conditioned learning.
4.3. Dopamine-deficient mice
Dopamine-deficient mice (DA−/− mice) were prepared by Zhou and Palmiter91) and by us (Nishii et al.92)). We introduced the human TH gene specifically into noradrenaline and adrenaline cell types of TH knockout mice by using the DBH gene promoter, because we previously proved that the 4-kb DNA flanking region of the human DBH gene promoter can specifically express the transgenes in noradrenaline-and adrenaline-producing cells.93) The dopamine deficient-mice displayed growth retardation beginning from postnatal week 2 and then died until postnatal week 4. These mice showed a reduction in spontaneous locomotion, cataleptic behaviour, and blockade of dopamine receptor agonist-induced motor activation. They also showed defective acquisition of operant conditioning including the active avoidance. All these results indicate that knockout of TH function in dopamine neurons impairs motor control, feeding, and operant learning during postnatal development.
4.4. Genetic alteration of catecholamine specificity from noradrenaline cell type to adrenaline cell-type in transgenic mice
We (Kobayashi et al.94)) aimed at changing the noradrenaline phenotype to the adrenaline one by producing transgenic mice carrying a chimeric gene containing human PNMT cDNA fused to the 4-kb fragment of the human DBH gene promoter. The additional PNMT expression specifically in noradrenaline-producing cells in the adrenal gland, sympathetic ganglia, and brain converted these noradrenaline cells to adrenaline cells, suggesting that noradrenaline cells normally possess the basic machinery required for the synthesis of adrenaline except for PNMT. The conversion of noradrenaline cells to adrenaline cells was nearly complete in the adrenal gland, but was partial in the sympathetic ganglia and brain, resulting in the mixed production of noradrenaline and adrenaline. Significant phenotypic changes including those in locomotor activity, blood glucose, and blood pressure were not observed in these transgenic mice. Interestingly, alteration of CA specificity in the transgenic sympathetic neurons led to down-regulation of β2-adrenergic receptor, suggesting that agonist-induced regulation of the β-adrenergic receptor subtypes might be one of the mechanisms to control cellular functions in response to CA specificity that occurs in these transgenic mice.95)
4.5. Conditional disruption of catecholamine (CA) cells by immunotoxin-mediated cell targeting
In 1995 we (Kobayashi et al.96)) developed, in collaboration with Pastan of the NIH, a new transgenic approach, termed immunotoxin-mediated cell targeting (IMCT) for spatially and temporarily specific abration of neurons in the brain or in the periphery with the cytotoxic activity of immunotoxins. Noradrenaline neurons in the brain96) or sympathetic noradrenaline neurons97) were conditionally and specifically disrupted by the IMCT method. At the first step of this IMCT method, such transgenic mice were created that expressed the human interleukin-2 receptor α subunit (IL-2Rα) under the control of the DBH gene promoter. At the second step, for disrupting noradrenaline neurons in the brain, the animals were treated intracerebroventricularly with a recombinant immunotoxin, anti-Tac(Fv)-PE40, which selectively kills animal cells bearing human IL-2Rα. The immunotoxin caused a characteristic behavioural abnormality only in the transgenic mice. This abnormality was accompanied by a dramatic loss of DBH-containing neurons and a significant decrease in DBH activity and noradrenaline levels in various regions of the brain. Likewise, peripheral sympathetic noradrenaline neurons were conditionally disrupted by injecting the recombinant immunotoxin intravenously. Targeting of the peripheral CA cells resulted in severe and progressive phenotypic abnormalities mainly characterized by cardiac dysfunction, hypoactivity, and hypothermia, which may explain the development of autonomic neuropathy in humans. The result on cardiac dysfunction agrees with the phenotype observed in TH gene knock-out mice.87), 89) This IMCT method can be applied to disrupt conditionally any type of cells by producing transgenic mice with a cell type-specific gene promoter. Nakanishi’s group successfully applied this IMCT method for elucidating the functions of cerebellar Golgi cells98) and cholinergic interneurons in basal ganglia.99)
5. Diseases with dysfunction of the catecholamine (CA) system
Dysfunction of the CA system results in a wide range of diseases such as neurological, psychiatric, endocrine, cardiovascular or metabolic diseases, which are caused by genetic mutations or by stress reactions to environmental factors.
5.1. Genetic diseases caused by dysfunction of the tyrosine 3-monooxygenase (TH) system
As predicted by the phenotype changes of genetically engineered mice, genetic changes in the TH system in humans, resulting in CA system abnormalities, have been found to produce inherited neurological or psychiatric diseases.
5.1.1. Autosomal dominant GTP cyclohydrolase I (GCH1) deficiency/Autosomal dominant DOPA responsive dystonia(DRD)/Segawa’s disease
Dystonia is a movement disorder with a persistent posture produced by contraction of muscles. In 1971, Segawa described a childhood-onset dystonia, which is an autosomal dominant inherited disease and is completely controllable by L-DOPA administration. Segawa100) proposed the term “hereditary progressive dystonia with marked diurnal fluctuation (HPD)” as a new type of dystonia distinct from other types of dystonia and Parkinson’s disease (PD). Nygaard et al. proposed the term “Dopa Responsive Dystonia (DRD)” to describe dystonia responding to L-DOPA. The marked and sustained alleviation of dystonia by L-DOPA, which supplements dopamine in the brain, suggests that HPD/DRD is caused by dopamine deficiency in the nigro-striatal (A9) dopamine neurons that regulate muscle contraction. As described above, TH activity is partly regulated by the cofactor BH4, the synthesis of which is regulated by GCH1 in the same CA cells. We (Ichinose et al.79), 101), 102)) cloned the human GCH1 gene and determined the chromosomal localization to 14q22.1–q22.2, and proved, in collaboration with Segawa and Tsuji, that the GCH1 gene is the causative gene for HPD/DRD. The reduced dopamine content in the striatum in the HPD/DRD brain is caused by mutated GCH1 with low activity, resulting in low BH4 content and low TH activity.101)–103) TH in the nigrostriatal dopamine (A9) neurons is the most sensitive to a reduction in the BH4 level. Patients with HPD/DRD have one mutated allele of the GCH1 gene and one wild-type allele, resulting in a partial decrease (down to 2%–20% of the normal value) in the GCH1 activity in their mononuclear blood cells.101) The amount of GCH1 mRNA is also reduced in HPD/DRD patients.104) A reduced BH4 level also causes a selective reduction in TH protein content.19) According to Segawa et al.,105) nearly 100 independent mutations have been identified in the coding region of the GCH1 gene in 60% of HPD/DRD patients, but approximately 40% of HPD/DRD patients did not have an identified mutation in the coding region or in the exon-intron boundaries of the GCH1 gene, which remains to be further examined.
5.1.2. Autosomal recessive GTP cyclohydrolase I (GCH1) deficiency/Atypical phenylketonuria
We (Ichinose et al.79), 102)) in collaboration with Blau et al. found mutations in both alleles of the GCH1 gene in autosomal recessive, homozygous GCH1 deficiency, resulting in a nearly complete loss of BH4. In contrast to autosomal dominant GCH1 deficiency/DRD/Segawa’s disease with a partial (2%– 20%) decrease in the enzyme activity and dopamine deficiency only in the nigro-striatal dopamine neurons, the patients with autosomal recessive, homozygous GCH1 deficiency show severe clinical symptoms such as hyperphenylalaninemia (due to decreased phenylalanine 4-monooxygenase activity), parkinsonism, epileptic seizure, or fever episode (due to decreased TH, tryptophan 5-monooxygenase, and possibly nitric oxide synthase activities), indicating that nearly complete depletion of BH4 may cause a reduction in the activities of all the pteridine-dependent monooxygenases, i.e., TH, tryptophan 5-monooxygenase, phenylalanine 4-monooxygenase, and nitric oxide synthase, and thus a resultant reduction in the contents of dopamine, noradrenaline, adrenaline, serotonin, and nitric oxide. In accordance with this pathogenesis, combined treatment with BH4 and DOPA plus 5-hydroxytryptophan (CA and serotonin supplementations) has been reported to be clinically effective.
5.1.3. Tyrosine 3-monooxygenase (TH) deficiency
Mutations in the human TH have been reported in some inherited neurological diseases as shown in Table III (see review 37)). A missense point mutation (C to A at nucleotide 1234 in exon 11, which is numbered on the basis of the human TH type 4 (hTH4) mRNA sequence in Kobayashi et al.,42) and causes the amino acid change of Gln412 to Lys412), was reported in Germany in 1995 by Knappskog et al.106) The clinical pheno-type of this disease was reported to be autosomal recessive DOPA responsive dystonia or Segawa’s syndrome. The recombinant protein carrying the mutation, when expressed in Escherichia coli, showed a reduced affinity for tyrosine and the activity of the mutant enzyme was approximately 15% of the corresponding wild-type activity. Another homozygous missense mutation (A to G at nucleotide 698 in exon 6) was found to result in the clinical phenotype of progressive severe motor retardation with predominant extrapyramidal symptoms by van den Heuvel et al. and Bräutigam et al. in 1998. This mutation causes a substitution of the amino acid at residue 233 from Arg to His. One patient was compound heterozygous for the same mutation (G to A at nucleotide 698; Arg233His) and a novel truncating mutation in exon 3 (a deletion of a single nucleotide C at nucleotide 291 that generates a truncated form of the protein).107) The patient appeared hypokinetic with a mask face, and displayed rigidity of arms and legs and truncal hypotonia, without diurnal fluctuation in the signs; and there was a clear improvement of symptoms by L-DOPA treatment. These symptoms were DOPA responsive but different from those seen in DRD/Segawa’s disease. The missense point mutation (T to C at nucleotide 707) in the TH gene was also reported in a patient showing parkinsonism in early infancy. The symptoms were accompanied by sympathetic dysfunction (ptosis) and were responsive to L-DOPA.108) This mutation caused an amino acid substitution (Leu to Pro) at residue 236. The recombinant mutant protein possessed a lower TH activity relative to that of the wild-type protein, and the activity ranged from 1.5% to 16% depending on the expression system used for the recombinant enzyme. In addition, other point mutations were reported to cause progressive encephalopathy and DOPA-nonresponsive dystonia.109) One mutant allele was a missense point mutation, by which G at nucleotide 1076 in exon 10 was converted to T, causing an amino acid substitution (Cys to Phe) at residue 359. Another mutant allele was a point mutation around the splice acceptor site in intron 11, resulting in alternative splicing to generate an aberrant mRNA.110) Similarly, a branch site mutation of intron 11 leading to aberrant splicing of the TH gene in a child was found to cause a severe extrapyramidal movement disorder.110) These point mutations in the TH gene appear to reduce partially the enzyme activity in the patients carrying the homozygous or compound heterozygous mutations, and the clinical symptoms may be variable depending upon the degree of reduction in the activity.
Table III.
Tyrosine 3-monooxygenase (tyrosine hydroxylase, TH) mutations in human inherited diseases
| Symptom | Mutation*) | Amino acid sequence | Population |
|---|---|---|---|
| l-DOPA-responsive dystonia (Segawa’s disease) | Missense C → A (1234) in exon 11 | Gln → Lys (412) | Germany |
| Missense G → A (698) in exon 6 | Arg → His (233) | Netherlands | |
| Deletion delC (291) in exon 3**) | Frame shift (Leu 205 Ter) | Netherlands | |
| Parkinsonism in infancy | Missense T → C (707) in exon 6 | Leu → Pro (236) | Greek |
| Progressive infantile encephalopathy with l-DOPA-nonresponsive dystonia | Missense G → T (1076) in exon 10 | Cys → Phe (359) | Germany |
| Splice T → A (2 bp upstream of splice accepter site in intron 11)**) | Skipping exon 12 | Germany |
5.1.4. Tyrosine 3-monooxygenase (TH) mutations and neuropsychiatric diseases
There has been much debate as to whether mutations in the human tyrosine TH gene are associated with the pathogenesis of neuropsychiatric diseases. Linkage of bipolar affective disorder to the TH locus has been suggested.111) Another study suggests an association of the DNA polymorphism in the TH locus with disturbances in the CA system in schizophrenia.112) Mallet et al. have called special attention to a tetranucleotide polymorphic microsatellite, located in the first intron of the TH gene (HUMTH01 microsatellite alleles), which is generated by 5–10 repetitions of the core motif TCAT and acts as a transcription regulatory element.113) Since clinically effective drugs against schizophrenia are mostly blockers of mesocortical dopamine (A10) neurons, hyper-active dopamine hypothesis has been proposed in schizophrenia. As described below, we measured the contents of all four specific types of human TH mRNAs (hTH1–hTH4) in the substantia nigra (dopamine A9 neuron) in schizophrenia patients and compared them with those in Parkinson’s disease (PD) (Fig. 4). In contrast to dramatic decreases in all mRNA levels in PD, no significant differences from the controls were observed in the schizophrenia samples.114) Our studies suggest that a dysfunction in TH at least in the substantia nigra dopamine A9 neurons in schizophrenia is unlikely, but examination of mesocortical dopamine A10 neurons in the ventral tegmental area should be made.
5.2. Dopamine β-monooxygenase (DBH) deficiency
DBH deficiency is a very rare form of failure of the noradrenaline and adrenaline system in the sympathetic nerves and adrenal medulla, which is characterized by an absence of DBH activity and protein and noradrenaline and adrenaline in blood and probably in the brain and adrenal medulla with increased dopamine blood levels. The main symptoms are cardiovascular disorders and severe hypotension caused by standing.115) Restoration of plasma noradrenaline to the normal range and control of symptoms can be achieved by oral administration of a synthetic precursor of noradrenaline, L-threo-DOPS, which is converted to noradrenaline by AADC.11) DOPS was developed for supplementation of noradrenaline in Parkinson’s disease (PD) by Narabayashi et al.116) The rather mild symptoms in human DBH deficiency stand in contrast to the lethality seen in DBH gene knockout mice,88) in which the mice can survive only by in utero administration of L-threo-DOPS to the mother mice and continued treatment with L-threo-DOPS after birth. In human DBH deficiency high dopamine levels in blood and probably in the brain may compensate for noradrenaline and adrenaline deficiencies. The nora-drenaline and adrenaline cells without DBH protein probably by some mutation of the DBH gene are speculated to produce only dopamine. As described above, Zebetian et al. with us.72) found a functional −1021C → T polymorphism in the DBH gene to cause a very low plasma DBH protein and activity in the individuals with T/T genotype. This finding may give a clue to elucidate the changes in the DBH gene in DBH deficiency.
5.3. Parkinson’s disease (PD)
PD is the second most common aging-related neurodegenerative disease after Alzheimer’s disease (AD). The main symptoms are movement disorders called parkinsonism; i.e., slowness of movement, tremor, and muscular rigidity. A small percentage of PD is familial, but most PD is sporadic. PD has been characterized as dopamine deficiency and the presence of intracellular inclusion bodies called Lewy bodies in the nigro-striatal dopamine (A9) neurons in the brain, although recent pathological studies by Braak suggest that the disease processes start before parkinsonism from the lower brain stem. Dopamine deficiency and the dopamine supplementation therapy by L-DOPA in PD were predicted in animal experiments by Carlsson in 1958. Sano in Japan in 1960 first reported a decreased content of dopamine in the nigro-striatal region of a postmortem brain and clinical trial of L-DOPA therapy. At the same time deficiencies of dopamine and noradrenaline in the postmortem brain were confirmed by Ehringer and Hornykiewicz. Clinical trial of L-DOPA in PD started in 1961, established in 1970, and is still the gold standard for the treatment. The pathogenesis of sporadic PD is still enigmatic, but three groups of causative factors have been under investigation.117) (1) Free radicals produced by mitochondrial dysfunction and oxidative stress, which are detected in the post-mortem PD brain and progress in aging of the brain, are thought to play an important role.118) This concept is supported by accidental discovery around 1980 that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is an analogue of meperidine (a synthetic heroin) and lipophilic precursor neurotoxin, produced PD in humans. MPTP crosses the blood brain barrier from blood into the brain, is converted to 1-methyl-4-phenyl-pyridinium (MPP+) by MAO-B in glial cells; then MPP+ is taken up by dopamine transporter in the nigrostriatal dopamine (A9) neurons, inhibits complex I in the mitochondrial electron transport system, thereby causing mitochondrial dysfunction and oxidative stress, and finally causes apoptotic cell death. (2) Recent molecular genetical studies on mutations of the causative genes of familial PD (PARK), starting from the discoveries of α-synuclein, which is a main component of Lewy bodies, in PARK 1 in 1997 and parkin in PARK 2 in 1998, which is an E3 ubiquitin ligase and causes endoplasmic reticulum (ER) stress, suggest that dysfunction of the intracellular proteinase systems, such as ubiquitin-proteasome system or lysosomal system, which removes unnecessary toxic proteins in cells and prevents the resultant accumulation of misfolded proteins, may lead to programmed cell death, i.e., apoptosis or autophagy, of the dopamine neurons. (3) The presence of activated microglia that produce various cytokines and accompany the inflammatory process in the PD brain may promote progression of the disease.119) All these data indicate that dopamine deficiency in the nigrostriatal region in PD is caused by degeneration of dopamine A9 neurons.
The main question is why dopamine neurons specifically degenerate in PD and what changes may occur in the dopamine-synthesizing machinery during the neurodegeneration. There are several indications. Dopamine A9 neurons contain neuromelanin. Neuromelanin is composed of granules consisting of pheomelanin (a benzothiazine-based molecule formed through the incorporation of cysteine with dopamine) at the core and eumelanin (an indole based molecule that is believed to be formed through oxidation of dopamine) at the surface.120) Neuromelanin has iron-binding ability, which may act for neuroprotection. But higher iron levels in the substantia nigra in PD may saturate neuromelanin, causing an increase in cytosolic iron to produce reactive oxygen species leading to a vicious circle of neurodegeneration. As another implication on the selective degeneration of dopamine neurons in PD, Ischiropoulos’s group recently found by using hTH1 mutants created by us (Ota et al.) in a cell culture system that intraneuronal dopamine levels can be a major regulator of aggregation and inclusion formation of α-synuclein, which is the main component of Lewy bodies and causes familial PD1 (PARK1).121)
Since 1975 in collaboration with Narabayashi, Yoshida, Kanazawa, Mizuno and Riederer, and other neurologists, we worked on molecular pathogenesis of PD by studying post-mortem human brains and animal PD models produced by MPTP. We found that the activities of all of the enzymes related to CA synthesis (TH, AADC, DBH, PNMT, and GCH1) and the bioptein cofactor level were significantly decreased in the postmortem PD brain,13), 122), 123) indicating that not only nigro-striatal dopamine neurons but also noradrenaline and adrenaline neurons may also be impaired in PD. Remarkable reductions (to less than 20% of control values) in the activity and protein content of TH were found in the nigro-striatal region in postmortem PD brains, but the decrease in the protein was about 3-fold greater than that in the activity, resulting in increased molecular activity (enzyme activity/enzyme protein).124) Interestingly, activity and protein levels of DBH in cerebrospinal fluid in PD were also decreased to less than 20% of the control values with constant molecular activity.125) These results suggest a compensatory activation of TH in PD following the reduction in the protein content. We followed the changes in TH in a mouse model of PD produced by MPTP, in which nigro-striatal dopamine neurons specifically degenerate.126) Following a single intraperitoneal injection of MPTP, TH activity was acutely decreased only in tissue slices from the striatum but not after extraction of the enzyme from the tissue. This reduction in TH activity in tissues may have been due to acute inhibition of phosphorylation and inhibition by released dopamine.127) After repeated administration of MPTP to mice, TH protein was inactivated probably by oxidative or nitrative damage; and then both activity and protein of TH markedly decreased specifically in the striatum, as observed in human postmortem brains.128) Humans and monkeys are known to be highly susceptible to MPTP. Monkeys have TH types 1 and 2. Monkeys (Macaca fascicularis) with MPTP-produced PD showed a marked decrease in the content of both types of mRNAs specifically in the substantia nigra.129) A marked decrease in the levels of all four types of human TH mRNA (hTH1–hTH4) and in the mRNA content of AADC was confirmed in post mortem brains in PD, in contrast to no significant changes in schizophrenia (Fig. 4).114) These results showing decreases in mRNA, protein, and activity of TH in human post-mortem brain in PD patients and in the brains of MPTP-PD mice and monkeys suggest that some unknown MPTP-like neurotoxins in the environment might cause neurodegeneration and changes in TH. Thus we and other workers searched for MPTP-like neurotoxins in the postmortem PD brain. Two groups of MPTP-like compounds have been identified in human PD brains: isoquinolines (e.g., 1-benzyl-tetrahydroisoquinoline, R-N-methyl-salsolinol) and β-carbolines.130), 131) All of these neurotoxins inhibit complex I, causing mitochondrial dysfunction, oxidative stress, and apoptotic cell death of dopamine neurons in vitro; they also produce PD in animals. However, it is not yet clear whether or not these neurotoxins, except MPTP, produce PD in humans. We found that all of these compounds, like MPTP, acutely inhibit activity of the TH system in slices of the striatal tissue. The relationship between neurodegeneration of dopamine neurons and changes in the TH system remains for further study. Ozawa, Nakano, Muramatsu and their collaborators132) have been working on gene therapy on PD by using human genes cloned by us carried in adeno-associated virus (AAV) vectors for treatment of MPTP-induced parkinsonian monkeys. The results have been highly successful. For example, triple transduction with AAV vector expressing TH, AADC, and GCH1 into the striatum produced long-persisting remarkable behavioural recovery from parkinsonism without any side effects. We hope that gene therapy will become a safe and efficient therapy for PD in the future.
6. Future prospects of catecholamine (CA) research
The genes and proteins of all enzymes related to the biosynthesis of CAs including those of tyrosine 3-monooxygenase (TH) have been elucidated. However, there still remain several interesting unsolved problems.
First, why does only human TH have 14 exons with human-specific exon 2 in a single gene and produce four protein isoforms (hTH1–hTH4, Kobayashi et al.42))? Why does the gene of monkeys including chimpanzees have 2 mRNAs and produce 2 protein isoforms in contrast to a single mRNA and single protein from a single gene without alternative mRNA splicing in non-primate mammals? Our human TH gene transgenic mice express the four types of human mRNA, but do not show significant changes in the phenotype including their behaviour.86)
Second, Ikuko Nagatsu et al.59) and other workers have found by immunohistochemical means that in the brain of animals during development or in the adult some neurons express only TH, AADC (D-neurons58)), PNMT, or GCH1. The functions of these neurons and their possible neurotransmitters are not clear. Misu et al.133) proposed that the neurons that contain only TH without AADC produce DOPA as a neurotransmitter.
Third, even though we could not identify human tyrosinase protein in the nigro-striatal dopamine (A9) neurons containing neuromelanin by immunohistochemistry using human tyrosinase-specific antibody,134) some portion of dopamine may be produced from L-DOPA by tyrosinase in pigment cells. Our TH knockout mice lost noradrenaline and adrenaline completely, but about 30% of the dopamine remained, suggesting formation of dopamine from DOPA by tyrosinase.87)
Fourth, although changes in the CA system and in TH are similar between sporadic PD and in MPTP-induced PD, what are the differences in the molecular mechanism between PD and MPTP-PD in relation to brain aging?135)
Fifth, CA systems in the brain, sympathetic nerves, and adrenal medulla are important in connecting the endocrine system with the immune system, especially in stress reactions in humans. Stress in humans is mainly psychological and starts from the cerebral cortex. The neuronal circuits between CRF/CRH (corticotropin-releasing factor/hormone) neurons in the hypothalamus and noradrenaline (A6) neurons in the locus coeruleus, and other dopamine, noradrenaline and adrenaline neurons in the brain stem and mesolimbic-cortical dopamine neurons in the central nervous system, as well as the peripheral hypothalamus-pituitary-adrenal axis and sympathetic nerve-noradrenaline/adrenomedullary-adrenaline components have links with the immune system. Adrenaline, along with glucocorticoids, cortisol and corticosterone, is a major stress hormone; and PNMT plays important roles for biosynthesis of adrenaline in stress. A better understanding of the molecular mechanisms involved in the interaction among these neuro, endocrine, and immune systems is important for explaining the cause of and prevention of various diseases.
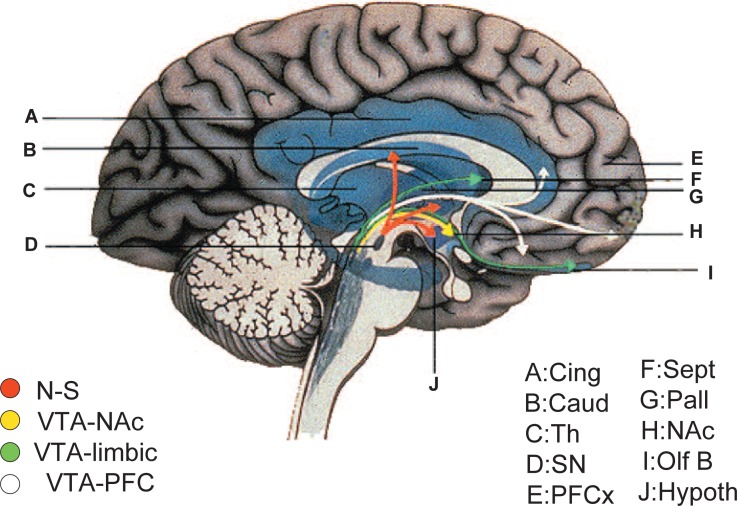
Finally, mesolimbic-cortical dopamine (A10) neurons (Fig. 5) are thought to play a central role in human behaviour in the neural circuits together with other neurotransmitters such as glutamate, GABA, acetylcholine, serotonin, histamine, and neuropep-tides like orexin and corticotropin-releasing hormone. This neuronal system is related to most important brain functions such as emotion, motivation, reward, novelty-seeking, addiction, memory or learning in health and disease. Further research on molecules, especially TH, related to the dopamine A10 neuron system is expected to provide us with much better insight for analyzing human behaviour and neuropsychiatric disorders. From this view point studies on CA/dopamine systems in mammalian midbrain, especially in the human brain, would be of great importance.136)
Fig. 5.
The human nigrostriatal (A9) and mesocorticolimbic (A10) dopamine systems. Cing, cingulate; Caud, caudate; PFCx, prefrontal cortex; Hypoth, hypothalamus; NAc, nucleus accumbens; N-S, nigro-striatum; Olf B, olfactory bulb; Pall, pallidum; Sept, septum; SN, substantia nigra; Th, thalamus; VTA-NAc, ventral tegmental area-nucleus accumbens; VTA-PFC, ventral tegmental area-prefrontal cortex.
Acknowledgements, and apology.
I would like to thank Dr. Tamio Yamakawa, m. j. a., for encouraging me to write this review. In light of their encouragement of me over the years to pursue my research in the field of catecholamine neurotransmitters, I would like to dedicate this manuscript to the memory of the late Dr. Sidney Udenfriend of Roche Institute of Molecular Biology and the late Dr. Julius Axelrod of the National Institutes of Health. I also thank all of my former colleagues, especially Drs. Makoto Sawada, Kazuto Kobayashi, Hiroshi Ichinose, Takahide Nomura, Akira Ota, and my wife Ikuko Nagatsu for their collaboration over these many years. This review was written mainly based on experiments conducted in my laboratory. I apologize for not having had the space to refer to the many other important contributions to this field made by various other investigators around the world. Several international authors contributed to a monograph of tyrosine 3-monooxygenase.137) The main parts of the work reviewed here were supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and from the Ministry of Health, Labor, and Welfare of Japan.
Profile
Toshiharu Nagatsu was born in 1930 and started his research career in 1956 with studies on the biochemistry of catecholamines at the Department of Biochemistry, Nagoya University School of Medicine. Shortly after having received his doctoral degree, he traveled to the United States and worked at the National Institutes of Health as a Public Health Service International Postdoctoral Research Fellow in the laboratory of Dr. Sidney Udenfriend. In 1964, during his two-year appointment, he published his landmark paper, in which he announced the discovery of tyrosine 3-monooxygenase (tyrosine hydroxylase), the first and rate-limiting enzyme in the biosynthesis of the catecholamine neurotransmitters. This paper provided the starting point for virtually all work since then on the biochemistry and molecular biology of the catecholamine pathway. After having returned to Japan at the conclusion of his stay with Dr. Udenfriend, he continued his singularly prolific work on catecholamines, delving into their metabolism and characterizing their related enzymes in health and disease, especially in Parkinson’s disease. In 1985 he started studies on the molecular biology of catecholamine-synthesizing enzymes. In these studies he cloned and characterized the human tyrosine 3-monooxygenase gene, discovering the existence of 4 isoforms of the human enzyme. He also discovered the causative gene of DOPA-responsive dystonia (Segawa’s disease) to be that encoding GTP cyclohydrolase I, the enzyme that synthesizes tetrahydrobiopterin, the cofactor of tyrosine 3-monooxygenase. Studying tyrosine 3-monooxygenase gene knockout mice, he also found noradrenaline to be essential for heart function. Over the years, he has held the position of professor at Aichi-Gakuin University, at the Tokyo Institute of Technology, at the Department of Biochemistry of Nagoya University School of Medicine, and served as Professor and Director of the Institute of Comprehensive Medical Science of Fujita Health University. During various sabbaticals, he also carried out research at the University of Southern California School of Medicine as Visiting Professor in 1968, at Roche Institute of Molecular Biology as Visiting Scientist in 1972, and at the National Institutes of Health as a Forgarty Scholar-in-Residence in 1999. In recognition of his many contributions to biochemistry, he was awarded the Erwin von Bärz Prize in 1988, the Uehara Prize in 1993, the Japan Medical Science Prize in 1993, the Purple Ribbon Medal in 1995, the Julius Axelrod Medal in 2001, and the WFN (World Federation of Neurology) Award in 2005.

References
- 1).Hökfelt, T., Martensson, O., Björklund, A., Kleinau, S., and Goldstein, M. (1984) Distribution maps of tyrosine-hydroxylaseimmunoreactive neurons in the rat brain. InHandbook of Chemical Neuroanatomy (eds. Björklund A., and Hökfelt T.). Classical Transmitters in the CNS, Part 1, Vol. 2, Elsevier, Amsterdam, pp. 277–379. [Google Scholar]
- 2).Nagatsu, T. (1973) Biochemistry of Catecholamines. University of Tokyo Press, Tokyo, and University Park Press, Baltimore. [Google Scholar]
- 3).Nagatsu, T. (1991) Genes for human catecholamine-synthesizing enzymes. Neurosci. Res. 12, 315–345. [DOI] [PubMed] [Google Scholar]
- 4).Nagatsu, T., and Ichinose, H. (1999) Regulation of pteridine-requiring enzymes by the cofactor tetrahydrobiopterin. Mol. Neurobiol. 19, 79–96. [DOI] [PubMed] [Google Scholar]
- 5).Nagatsu, T., and Stjärne, L. (1998) Catecholamine synthesis and release. InCatecholamines: Bridging Basic Science with Clinical Medicine (eds. Goldstein D. S., Eisenhofer G., and McCarty R.). Adv. Pharmacol. Vol. 42, Academic Press, New York, pp. 1–14. [PubMed] [Google Scholar]
- 6).Lovenberg, W., Weissbach, H., and Udenfriend, S. (1962) Aromatic l-amino acid decarboxylase. J. Biol. Chem. 237, 89–93. [PubMed] [Google Scholar]
- 7).Lovenberg, W., Jequir, E., and Sjoerdsma, A. (1967) Tryptophan hydroxylase: Measurement in pineal gland, brain stem, and carcinoid tumor. Science 155, 217–219. [DOI] [PubMed] [Google Scholar]
- 8).Ichiyama, A., Nakamura, S., Nishizuka, Y., and Hayaishi, O. (1970) Enzymatic studies on the biosynthesis of serotonin in mammalian brain. J. Biol. Chem. 245, 1699–1709. [PubMed] [Google Scholar]
- 9).Hasegawa, H., and Ichiyama, A. (2005) Distinct iron requirement of tryptophan 5-monooxygenase: TPH1 requires dissociable ferrous iron. Biochem. Biophys. Res. Commun. 338, 277–284. [DOI] [PubMed] [Google Scholar]
- 10).Ichinose, H., Kurosawa, Y., Titani, K., Fujita, K., and Nagatsu, T. (1989) Isolation and characterization of a cDNA clone encoding human aromatic L-amino acid decarboxylase. Biochem. Biophys. Res. Commun. 164, 1024–1030. [DOI] [PubMed] [Google Scholar]
- 11).Sumi, C., Ichinose, H., and Nagatsu, T. (1990) Characterization of recombinant human aromatic amino acid decarboxylase expressed in COS cells. J. Neurochem. 55, 1075–1078. [DOI] [PubMed] [Google Scholar]
- 12).Axelrod, J. (1962) Purification and properties of phenylethanolamine N-methyltransferase. J. Biol. Chem. 237, 1657–1660. [PubMed] [Google Scholar]
- 13).Nagatsu, T., Kato, T., Numata-Sudo, Y., Ikuta, K., Sano, M., Nagatsu, I., Kondo, S., Inagaki, R., Iizuka, R., Hori, A., and Narabayashi, H. (1977) Phenylethanolamine N-methyltransferase and other enzymes of catecholamine metabolism in human brain. Clin. Chim. Acta 75, 221–232. [DOI] [PubMed] [Google Scholar]
- 14).Levin, E. Y., Levenberg, B., and Kaufman, S. (1960) The enzymatic conversion of 3,4-dihydroxyphenylethanolamine to norepinephrine. J. Biol. Chem. 235, 2080–2086. [PubMed] [Google Scholar]
- 15).Nagatsu, T., Levitt, M., and Udenfriend, S. (1964) Tyrosine hydroxylase: The initial step in norepinephrine biosynthesis. J. Biol. Chem. 239, 2910–2917. [PubMed] [Google Scholar]
- 16).Kaufman, S. (1963) The structure of the phenylalanine-hydroxylation cofactor. Proc. Natl. Acad. Sci. USA 50, 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Nichol, C. A., Smith, G. K., and Duch, D. S. (1985) Biosynthesis and metabolism of tetrahydrobiopterin and molybdopterin. Ann. Rev. Biochem. 54, 729–762. [DOI] [PubMed] [Google Scholar]
- 18).Togari, A., Kano, H., Oka, K., and Nagatsu, T. (1983) Simultaneous simple purification of tyrosine hydroxylase and dihydropteridine reductase. Anal. Biochem. 132, 183–189. [DOI] [PubMed] [Google Scholar]
- 19).Sumi-Ichinose, C., Urano, F., Kuroda, R., Ohye, T., Kojima, M., Tazawa, M., Shiraishi, H., Hagino, Y., Nagatsu, T., Nomura, T., and Ichinose, H. (2001) Catecholamines and serotonin are differentially regulated by tetrahydrobiopterin. J. Biol. Chem. 276, 41150–41160. [DOI] [PubMed] [Google Scholar]
- 20).Matsuura, S., Sugimoto, T., Murata, S., Sugawara, Y., and Iwasaki, H. (1985) Stereochemistry of biopterin cofactor and facile methods for the determination of the stereochemistry of a biologically active 5,6,7,8-tetrahydrobiopterin. J. Biochem. 98, 1341–1348. [DOI] [PubMed] [Google Scholar]
- 21).Nagatsu, T. (1977) Dopamine β-hydroxylase in blood and cerebrospinal fluid. Trends Biochem. Sci. 2, 217–219. [Google Scholar]
- 22).Thöny, B., Auerbach, G., and Blau, N. (2000) Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 347, 1–16. [PMC free article] [PubMed] [Google Scholar]
- 23).Nagatsu, T. (1995) Tyrosine hydroxylase: human isoforms, structure and regulation in physiology and pathology. InEssays in Biochemistry (eds. Apps D. K., and Tipton K. S.). Portland Press, London, pp. 15–35. [PubMed] [Google Scholar]
- 24).Cooksey, C. J., Garratt, P. J., Land, E. J., Pavel, S., Ramsden, C. A., Riley, P. A., and Smit, N. P. M. (1997) Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 272, 26226–26232. [DOI] [PubMed] [Google Scholar]
- 25).Nagatsu, T., Levitt, M., and Udenfriend, S. (1964) A rapid and simple radioassay for tyrosine hydroxylase activity. Anal. Biochem. 9, 122–126. [DOI] [PubMed] [Google Scholar]
- 26).Kumer, S. C., and Vrana, K. E. (1996) Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 67, 443–462. [DOI] [PubMed] [Google Scholar]
- 27).Dunkley, P. R., Bobrovskaya, L., Graham, M. E., von Nagy-Felsobuki, E. I., and Dickson, P. W. (2004) Tyrosine hydroxylase phosphorylation: regulation and consequences. J. Neurochem. 91, 1025–1034. [DOI] [PubMed] [Google Scholar]
- 28).Fujisawa, H., and Okuno, S. (2005) Regulatory mechanism of tyrosine hydroxylase activity. Biochem. Biophys. Res. Commun. 338, 271–276. [DOI] [PubMed] [Google Scholar]
- 29).Udenfriend, S., Zalzman-Nirenberg, P., and Nagatsu, T. (1965) Inhibitors of purified tyrosine hydroxylase. Biochem. Pharmacol. 14, 837–845. [DOI] [PubMed] [Google Scholar]
- 30).Nagatsu, T., Takeuchi, T., and Umezawa, H. (1988) Inhibitors and stimulators of monoamine-synthesizing enzymes from microbe. InHorizons on Antibiotic Research (eds. Davis B. D., Ichikawa T., Maeda K., and Mitsher L. A.). Japan Antibiotics Res. Ass., Tokyo, pp. 121–137. [Google Scholar]
- 31).DeQuattro, V., Nagatsu, T., Maronde, R., and Alexander, N. (1969). Catecholamine synthesis in rabbits with neurogenic hypertension. Circ. Res. 24, 545–555. [DOI] [PubMed] [Google Scholar]
- 32).Müller, R. A., Thönen, H., and Axelrod, J. (1969) Adrenal tyrosine hydroxylase: compensatory increase in activity after chemical sympathectomy. Science 163, 468–469. [DOI] [PubMed] [Google Scholar]
- 33).Nagatsu, T., and Oka, K. (1987) Tyrosine 3-monooxygenase from bovine adrenal medulla. InMethods in Enzymology (ed. Kaufman S.). Vol. 142, Academic Press, New York, pp. 56–62. [DOI] [PubMed] [Google Scholar]
- 34).Fujisawa, H., and Okuno, S. (1987) Tyrosine 3-monooxygenase from rat adrenals. InMethods in Enzymology (ed. Kaufman S.). Vol. 142, Academic Press, New York, pp. 63–71. [DOI] [PubMed] [Google Scholar]
- 35).Mogi, M., Kojima, K., and Nagatsu, T. (1984) Detection of inactive and less active forms of tyrosine hydroxylase in human brain and adrenals by a sandwich enzyme immunoassay. Anal. Biochem. 138, 125–132. [DOI] [PubMed] [Google Scholar]
- 36).Nagatsu, T., and Ichinose, H. (1991) Comparative studies on the structure of human tyrosine hydroxylase with those of the enzyme of various mammals. Comp. Biochem. Physiol. 98C, 203–210. [PubMed] [Google Scholar]
- 37).Kobayashi, K., and Nagatsu, T. (2005) Molecular genetics of tyrosine 3-monooxygenase and inherited diseases. Biochem. Biophys. Res. Commun. 338, 267–270. [DOI] [PubMed] [Google Scholar]
- 38).Ichikawa, S., Sasaoka, T., and Nagatsu, T. (1991) Primary structure of mouse tyrosine hydroxylase deduced from its cDNA. Biochem. Biophys. Res. Commun. 176, 1610–1616. [DOI] [PubMed] [Google Scholar]
- 39).Grima, B., Lamouroux, A., Boni, C., Julien, J.-F., Javoy-Agid, F., and Mallet, J. (1987) A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature 326, 707–711. [DOI] [PubMed] [Google Scholar]
- 40).Kaneda, N., Kobayashi, K., Ichinose, H., Kishi, F., Nakazawa, A., Kurosawa, Y., Fujita, K., and Nagatsu, T. (1987) Isolation of a novel cDNA clone for human tyrosine hydroxylase: alternative mRNA splicing produces four kinds of mRNA from a single clone. Biochem. Biophys. Res. Commun. 146, 971–975. [DOI] [PubMed] [Google Scholar]
- 41).Kobayashi, K., Kaneda, N., Ichinose, H., Kishi, F., Nakazawa, A., Kurosawa, Y., Fujita, K., and Nagatsu, T. (1987) Isolation of a full-length cDNA clone encoding human tyrosine hydroxylase type 3. Nucleic Acids Res. 15, 6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Kobayashi, K., Kaneda, N., Ichinose, H., Kishi, F., Nakazawa, A., Kurosawa, Y., Fujita, K., and Nagatsu, T. (1988) Structures of the human tyrosine hydroxylase gene: alternative splicing from a single gene accounts for generation of four mRNA types. J. Biochem. 103, 907–912. [DOI] [PubMed] [Google Scholar]
- 43).Ichikawa, S., Ichinose, H., and Nagatsu, T. (1990) Multiple mRNAs of monkey tyrosine hydroxylase. Biochem. Biophys. Res. Commun. 173, 1331–1336. [DOI] [PubMed] [Google Scholar]
- 44).Ichinose, H., Ohye, T., Fujita, K., Yoshida, M, Ueda, S., and Nagatsu, T. (1993) Increased heterogeneity of tyrosine hydroxylase in humans. Biochem. Biophys. Res. Commun. 195, 158–165. [DOI] [PubMed] [Google Scholar]
- 45).Haycock, J. W. (2002) Species differences in the expression of multiple tyrosine hydroxylase protein isoforms. J. Neurochem. 81, 947–953. [DOI] [PubMed] [Google Scholar]
- 46).Goodwill, K. E., Sabatier, C., Marks, C., Raag, R., Fitzpatrick, P. F., and Stevens, R. C. (1997) Crystal structure of tyrosine hydroxylase at 2.3 Å and its implications for inherited neurodegenerative diseases. Nature Struc. Biol. 4, 578–585. [DOI] [PubMed] [Google Scholar]
- 47).Dumas, S., Hir, H. L., Bodeau-Pean, S., Hirsch, E., Thermes, C., and Mallet, J. (1996) New species of human tyrosine hydroxylase mRNA are produced in variable amounts in adrenal medulla and are overexpressed in progressive supranuclear palsy. J. Neurochem. 67, 19–25. [DOI] [PubMed] [Google Scholar]
- 48).Ohye, T., Ichinose, H., Yoshizawa, T., Kanazawa, I., and Nagatsu, T. (2001) A new splicing variant for human tyrosine hydroxylase in the adrenal medulla. Neurosci. Lett. 312, 157–160. [DOI] [PubMed] [Google Scholar]
- 49).Ishii, A., Kiuchi, K., Kobayashi, R., Sumi, M., Hidaka, H., and Nagatsu, T. (1991) A selective Ca2+/calmodulin-dependent protein kinase II inhibitor, KN-62, inhibits the enhanced phosphorylation and the activity of tyrosine hydroxylase by 56 mM K+ in rat pheochromocytoma PC12 cells. Biochem. Biophys. Res. Commun. 176, 1051–1056. [DOI] [PubMed] [Google Scholar]
- 50).Itagaki, C., Isobe, T., Taoka, M., Natsume, T., Nomura, N., Horigome, T., Omura, S., Ichinose, H., Nagatsu, T., Greene, L. A., and Ichimura, T. (1999) Stimulus-coupled interaction of tyrosine hydroxylase with 14-3-3 proteins. Biochemistry 38, 15673–15680. [DOI] [PubMed] [Google Scholar]
- 51).Lehmann, I. T., Bobrovskaya, L., Gordon, S. L., Dunkley, P. R., and Dickson, P. W. (2006) Differential regulation of the human tyrosine hydroxylase isoforms via hierarchical phosphorylation. J. Biol. Chem. 281, 17644–17651. [DOI] [PubMed] [Google Scholar]
- 52).Ota, A., Yoshida, S., and Nagatsu, T. (1995) Deletion mutants of human tyrosine hydroxylase type 1 regulatory domain. Biochem. Biophys. Res. Commun. 213, 1099–1106. [DOI] [PubMed] [Google Scholar]
- 53).Nakashima, A., Mori, K., Suzuki, T., Kurita, H., Otani, M., Nagatsu, T., and Ota, A. (1999) Dopamine inhibition of human tyrosine hydroxylase type 1 is controlled by the specific portion in the N-terminus of the enzyme. J. Neurochem. 72, 2145–2153. [DOI] [PubMed] [Google Scholar]
- 54).Suzuki, T., Inagaki, H., Yamakuni, T., Nagatsu, T., and Ichinose, H. (2002) Enhanced expression of GTP cyclohydrolase I in V-1-overexpressing PC12D cells. Biochem. Biophys. Res. Commun. 293, 962–968. [DOI] [PubMed] [Google Scholar]
- 55).Sumi-Ichinose, C., Ichinose, H., Takahashi, E., Hori, T., and Nagatsu, T. (1992) Molecular cloning of genomic DNA and chromosomal assignment of the gene for human aromatic L-amino acid decarboxylase, the enzyme for catecholamine and serotonin biosynthesis. Biochemistry 31, 2229–2238. [DOI] [PubMed] [Google Scholar]
- 56).Ichinose, H., Sumi-Ichinose, C., Ohye, T., Hagino, Y., Fujita, T., and Nagatsu, T. (1992) Tissue-specific alternative splicing of the first exon generates two types of mRNAs in human aromatic L-amino acid decarboxylase. Biochemistry 31, 11546–11550. [DOI] [PubMed] [Google Scholar]
- 57).Sumi-Ichinose, C., Hasegawa, S., Ichinose, H., Sawada, H., Kobayashi, K., Sakai, M., Fujii, T., Nomura, H., Nomura, T., Nagatsu, I.et al. (1995) Analysis of the alternative promoters that regulate tissue-specific expression of human aromatic L-amino acid decarboxylase. J. Neurochem. 64, 514–524. [DOI] [PubMed] [Google Scholar]
- 58).Jaeger, C. B., Ruggiero, D. A., Albert, V. R., Park, D. H., Joh, T. H., and Reis, D. J. (1984) Immunohistochemical localization of aromatic-L-amino acid decarboxylase. InHandbook of Chemical Neuroanatomy, Classical Transmitters in the CNS (eds. Björklund A., and Hökfelt T.). Part 1, Vol. 2, Elsevier, Amsterdam, pp. 387–408. [Google Scholar]
- 59).Nagatsu, I., Yamada, K., Sakai, M., and Karasawa, N. (1993) Immunocytochemistry and in situ hybridization of catecholamine-synthesizing enzymes and the related neurotransmitters. InMethods in Neurotransmitter and Neuropeptide Research (eds. Parvez S. H., Naoi M., Nagatsu T., and Parvez S.). Elsevier, Amsterdam, pp. 151–183. [Google Scholar]
- 60).Nagatsu, I., Sakai, M., Yoshida, M., and Nagatsu, T. (1988) Aromatic l-amino acid decarboxylaseimmunoreactive neurons in and around the cerebrospinal fluid-contacting neurons of the central canal do not contain dopamine or serotonin in the mouse and rat spinal cord. Brain Res. 475, 91–102. [DOI] [PubMed] [Google Scholar]
- 61).Friedman, S., and Kaufman, S. (1965) 3,4-Dihydroxyphenylethylamine β-hydroxylase. Physical properties, copper content, and role of copper in the catalytic activity. J. Biol. Chem. 240, 4763–4773. [PubMed] [Google Scholar]
- 62).Weinshilboum, R., and Axelrod, J. (1971) Serum dopamine-β-hydroxylase activity. Circ. Res. 28, 307–315. [DOI] [PubMed] [Google Scholar]
- 63).Nagatsu, T., and Udenfriend, S (1972) Photometric assay of dopamine β-hydroxylase in human blood. Clin. Chem. 18, 980–983. [PubMed] [Google Scholar]
- 64).Nagatsu, T., Thomas, P., Rush, R, and Udenfriend, S. (1973) A radioassay for dopamine-β-hydroxylase activity in rat serum. Anal. Biochem. 55, 615–619. [DOI] [PubMed] [Google Scholar]
- 65).Nagatsu, T., Kuzuya, H., and Hidaka, H. (1967) Inhibition of dopamine β-hydroxylase by sulfhydryl compounds and the nature of the natural inhibitors. Biochem. Biophys. Acta 139, 319–327. [DOI] [PubMed] [Google Scholar]
- 66).Hidaka, H., Nagatsu, T., Takeya, K., Takeuchi, T., Suda, H., Kojiri, K., Matsuzaki, M., and Umezawa, H. (1969) Fusaric acid, a hypotensive agent produced by fungi. J. Antibiotics 22, 228–230. [DOI] [PubMed] [Google Scholar]
- 67).Kobayashi, K., Kurosawa, Y., Fujita, K., and Nagatsu, T. (1989) Human dopamine β-hydroxylase gene: two mRNA types having different 3′-terminal regions are produced through alternative polyadenylation. Nucleic Acids Res. 17, 1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Lamouroux, A., Vigny, A., Faucon Biguet, N., Darmon, M. C., Franck, R., Henry, J.-P., and Mallet, J. (1987) The primary structure of human dopamine-β-hydroxylase: insights into the relationship between the soluble and the membrane-bound forms of the enzyme. EMBO J. 6, 3931–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Ishiguro, H., Yamada, K., Ichino, N., and Nagatsu, T. (1998) Identification and characterization of a novel phorbol ester-responsive DNA sequence in the 5′-flanking region of the human dopamine β-hydroxylase gene. J. Biol. Chem. 273, 21941–21949. [DOI] [PubMed] [Google Scholar]
- 70).Nakano, T., Kobayashi, K., Saito, S., Fujita, K., and Nagatsu, T. (1992) Mouse dopamine β-hydroxylase: primary structure deduced from the cDNA sequence and exon/intron organization of the gene. Biochem. Biophys. Res. Commun. 189, 590–599. [DOI] [PubMed] [Google Scholar]
- 71).Cubells, J. F., van Kammen, D. P., Kelley, M. E., and Anderson, G. M., O’Connor, D. T., Price, L. H., Malison, R., Rao, P. A., Kobayashi, K., Nagatsu, T., and Gelerntner, J. (1998) Dopamine β-hydroxylase: two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotype variation. Human Genet. 102, 533–540. [DOI] [PubMed] [Google Scholar]
- 72).Zabetian, C. P., Anderson, G. M., Buxbaum, S. G., Elston, R. C., Ichinose, H., Nagatsu, T., Kim. K.-S., Kim, C.-H., Malison, R. T., Gelerntner, J., and Cubells, J. F. (2001) A quantitative-trait analysis of human plasma-dopamine β-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am. J. Hum. Genet. 68, 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Nagatsu, I., Ikemoto, K., Takeuchi, T., Arai, R., Karasawa, N., Fujii, T., and Nagatsu, T. (1998) Phenylethanolamine-N-methyltransferase-immunoreactive nerve terminals afferent to the mouse substantia nigra. Neurosci. Lett. 245, 41–44. [DOI] [PubMed] [Google Scholar]
- 74).Kaneda, N., Ichinose, H., Kobayashi, K., Oka, K., Kishi, F., Nakazawa, Y., Kurosawa, Y., Fujita, K, and Nagatsu, T. (1988) Molecular cloning of cDNA and chromosomal assignment of the gene for human phenylethanolamine N-methyltransferase, the enzyme for epinephrine biosynthesis.J. Biol. Chem. 263, 7672–7677. [PubMed] [Google Scholar]
- 75).Sasaoka, T., Kaneda, N., Kurosawa, Y., Fujita, K., and Nagatsu, T. (1989) Structures of human phenylethanolamine N-methyltransferase gene: existence of two types of mRNA with different transcription initiation sites. Neurochem. Int. 15, 555–565. [DOI] [PubMed] [Google Scholar]
- 76).Morita, S., Kobayashi, K., Hidaka, H., and Nagatsu, T. (1992) Organization and complete nucleotide sequence of the gene encoding mouse phenylethanolamine N-methyltransferase. Mol. Brain Res. 13, 313–319. [DOI] [PubMed] [Google Scholar]
- 77).Togari, A., Ichinose, H., Matsumoto, S., Fujita, K., and Nagatsu, T. (1992) Multiple mRNA forms of human GTP cyclohydrolase I. Biochem. Biophys. Res. Commun. 187, 359–365. [DOI] [PubMed] [Google Scholar]
- 78).Nomura, T., Ichinose, H., Sumi-Ichinose, C., Nomura, H., Hagino, Y., Fujita, K., and Nagatsu, T. (1993) Cloning and sequencing of cDNA encoding mouse GTP cyclohydrolase I. Biophys. Res. Commun. 191, 523–527. [DOI] [PubMed] [Google Scholar]
- 79).Ichinose, H., Ohye, T., Matsuda, Y., Hori, T., Blau, N., Burlina, A., Rouse, B., Matalon, R., Fujita, K., and Nagatsu, T. (1995) Characterization of mouse and human GTP cyclohydrolase I genes: Mutations in patients with GTP cyclohydrolase I deficiency. J. Biol. Chem. 270, 10062–10071. [DOI] [PubMed] [Google Scholar]
- 80).Ichinose, H., Katoh, S., Sueoka, T., Titani, K., Fujita, K., and Nagatsu, T. (1991) Cloning and sequencing of cDNA encoding human sepiapterin reductase. Biochem. Biophys. Res. Commun. 179, 183–189. [DOI] [PubMed] [Google Scholar]
- 81).Ohye, T., Hori, T., Katoh, S., Nagatsu, T., and Ichinose, H. (1998) Genomic organization and chromosomal localization of the human sepiapterin reductase gene. Biochem. Biophys. Res. Commun. 251, 597–602. [DOI] [PubMed] [Google Scholar]
- 82).Oyama, R., Katoh, S., Sueoka, T., Suzuki, M., Ichinose, H., Nagatsu, T., and Titani, K. (1990) The complete amino acid sequence of the mature form of rat sepiapterin reductase. Biochem. Biophys. Res. Commun. 173, 627–631. [DOI] [PubMed] [Google Scholar]
- 83).Nagatsu, I., Ichinose, H., Sakai, S., Titani, K., Suzuki, M., and Nagatsu, T. (1995) Immunohistochemical localization of GTP cyclohydrolase in the brain, adrenal gland, and liver of mice. J. Neural Transm. [Gen. Sec.] 102, 175–188. [DOI] [PubMed] [Google Scholar]
- 84).Ikemoto, K., Suzuki, T., Ichinose, H., Ohye, T., Nishimura, A., Nishi, K., Nagatsu, I., and Nagatsu, T. (2002) Localization of sepiapterin reductase in the human brain. Brain Res. 954, 237–246. [DOI] [PubMed] [Google Scholar]
- 85).Sasaoka, T., Kobayashi, K., Nagatsu, I., Takahashi, R., Kimura, M., Yokoyama, M., Nomura, T., Katsuki, M., and Nagatsu, T. (1992) Analysis of the human tyrosine hydroxylase promoter-chloramphenicol acetyltransferase chimeric gene expression in transgenic mice. Mol. Brain Res. 16, 274–286. [DOI] [PubMed] [Google Scholar]
- 86).Kaneda, N., Sasaoka, T., Kobayashi, K., Kiuchi, K., Nagatsu, I., Kurosawa, Y., Fujita, K., Yokoyama, M., Nomura, T., Katsuki, M., and Nagatsu, T. (1991) Tissue-specific and high-level expression of the human tyrosine hydroxylase gene in transgenic mice. Neuron 6, 583–594. [DOI] [PubMed] [Google Scholar]
- 87).Kobayashi, K., Morita, S., Sawada, H., Mizuguchi, T., Yamada, K., Nagatsu, I., Hata, T., Watanabe, Y., Fujita, K., and Nagatsu, T. (1995) Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J. Biol. Chem. 270, 27235–27243. [DOI] [PubMed] [Google Scholar]
- 88).Thomas, S. A., Matsumoto, A. M., and Palmiter, R. D. (1995) Noradrenaline is essential for mouse fetal development. Nature 374, 643–646. [DOI] [PubMed] [Google Scholar]
- 89).Zhou, Q.-Y., Quaife, C. J., and Palmiter, R. D. (1995) Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature 374, 640–643. [DOI] [PubMed] [Google Scholar]
- 90).Kobayashi, K., Noda, Y., Matsushita, N., Nishii, K., Sawada, H., Nagatsu, T., Nakahara, D., Fukabori, R., Yasoshima, Y., Yamamoto, T.et al. (2000) Modest neuropsychological deficits caused by reduced noradrenaline metabolism in mice heterozygous for a mutated tyrosine hydroxylase gene. J. Neurosci. 20, 2418–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Zhou, Q.-Y., and Palmiter, R. D. (1995) Dopamine deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209. [DOI] [PubMed] [Google Scholar]
- 92).Nishii, K., Matsushita, N., Sawada, H., Sano, H., Noda, Y., Mamiya, T., Nabeshima, T., Nagatsu, I., Hata, T., Kiuchi, K.et al. (1998) Motor and learning dysfunctions during postnatal development in mice defective in dopamine neuronal transmission. J. Neurosci. Res. 54, 450–464. [DOI] [PubMed] [Google Scholar]
- 93).Morita, S., Kobayashi, K., Mizuguchi, T., Yamada, K., Nagatsu, I., Titani, K., Fujita, K., Hidaka, H., and Nagatsu, T. (1993) The 5′-flanking region of the human dopamine β-hydroxylase gene promotes neuron subtype-specific gene expression in the central nervous system of transgenic mice. Mol. Brain Res. 17, 239–244. [DOI] [PubMed] [Google Scholar]
- 94).Kobayashi, K., Sasaoka, T., Morita, S., Nagatsu, I., Iguchi, A., Kurosawa, Y., Fujita, K., Nomura, T., Kimura, M., Katsuki, M., and Nagatsu, T. (1992) Genetic alteration of catecholamine specificity in transgenic mice. Proc. Natl. Acad. Sci. USA 89, 1631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Kobayashi, K., Ota, A., Togari, A., Morita, S., Mizuguchi, T., Sawada, H., Yamada, K., Nagatsu, I., Matsumoto, S., Fujita, K., and Nagatsu, T. (1995) Alteration of catecholamine phenotype in transgenic mice influences expression of adrenergic receptor subtypes. J. Neurochem. 65, 492–501. [DOI] [PubMed] [Google Scholar]
- 96).Kobayashi, K., Morita, S., Sawada, H., Mizuguchi, T., Yamada, K., Nagatsu, I., Fujita, K., Kreitman, R. J., Pastan, I., and Nagatsu, T. (1995) Immunotoxin-mediated conditional disruption of specific neurons in transgenic mice. Proc. Natl. Acad. Sci. USA 92, 1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97).Sawada, H., Nishii, K., Suzuki, T., Hasegawa, K., Hata, T., Nagatsu, I., Kreitman, R. J., Pastan, I., Nagatsu, T., and Kobayashi, K. (1998) Autonomic neuropathy in transgenic mice caused by immunotoxin targeting of the peripheral nervous system. J. Neurosci. Res. 51, 162–173. [DOI] [PubMed] [Google Scholar]
- 98).Watanabe, D., Inokawa, H., Hashimoto, K., Suzuki, N., Kano, M., Shigemoto, R., Hirano, T., Toyama, K., Kaneko, S., Yokoi, M.et al. (1998) Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell 95, 17–27. [DOI] [PubMed] [Google Scholar]
- 99).Kaneko, S., Hikida, T., Watanabe, D., Ichinose, H., Nagatsu, T., Kreitman, R. J., Pastan, I., and Nakanishi, S. (2000) Synaptic integration mediated by striatal cholinergic interneurons in basal ganglia function. Science 289, 633–637. [DOI] [PubMed] [Google Scholar]
- 100).Segawa, M., Hosaka, A., Miyagawa, F., Nomura, Y., and Imai, H. (1976) Hereditary progressive dystonia with marked diurnal fluctuation. InAdvances in Neurology (eds. Eldridge R., and Fahn S.). Vol. 14, Raven Press, New York, pp. 215–233. [PubMed] [Google Scholar]
- 101).Ichinose, H., Ohye, T., Takahashi, E., Seki, N., Hori, T., Segawa, M., Nomura, Y., Endo, K., Tanaka, H., Tsuji, S.et al. (1994) Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nature Gen. 8, 236–242. [DOI] [PubMed] [Google Scholar]
- 102).Ichinose, H., Suzuki, T., Inagaki, H., Ohye, T., and Nagatsu, T. (1999) Molecular genetics of dopa-responsive dystonia. Biol. Chem. 380, 1355–1364. [DOI] [PubMed] [Google Scholar]
- 103).Ichinose, H., Suzuki, T., Inagaki, H., Ohye, T., and Nagatsu, T. (2001) DOPA-responsive dystonia: From causative gene to molecular mechanism. InParkinson’s disease: Advances in Neurology (eds. Calne D., and Calne S.). Vol. 86, Lippincott Williams & Wilkins, Philadelphia, pp. 173–176. [PubMed] [Google Scholar]
- 104).Inagaki, H., Ohye, T., Suzuki, T., Segawa, M., Nomura, Y., Nagatsu, T., and Ichinose, H. (1999) Decrease in GTP cyclohydrolase I gene expression caused by inactivation of one allele in hereditary progressive dystonia with marked diurnal fluctuation (HPD). Biochem. Biophys. Res. Commun. 260, 747–751. [DOI] [PubMed] [Google Scholar]
- 105).Segawa, M., Nomura, Y., and Nishiyama, N. (2002) Autosomal dominant guanosine triphosphate cyclohydrolase I deficiency (Segawa disease). Ann. Neurol. 54 (Suppl. 6), S32–S45. [DOI] [PubMed] [Google Scholar]
- 106).Knappskog, P. M., Flatmark, T., Mallet, J., Lüdecke, B., and Bartholomé, K. (1995) Recessively inherited L-DOPA-responsive dystonia caused by a point mutation (Q381K) in the tyrosine hydroxylase gene. Hum. Mol. Genet. 4, 1209–1212. [DOI] [PubMed] [Google Scholar]
- 107).Wevers, R. A., de Rijk-van Andel, J. F., Bräutigam, C., Geurtz, B., van den Heuvel, L. P. W. J., Steenbergen-Spanjers, G. C. H., Smeitink, J. A. M., Hoffmann, G. F., and Gabreels, J. J. M. (1999) A review of biochemical and molecular genetic aspects of tyrosine hydroxylase deficiency including a novel mutation (291delC). J. Inher. Metab. Dis. 22, 364–373. [DOI] [PubMed] [Google Scholar]
- 108).Lüdecke, B., Knappskog, P. M., Clayton, P. T., Surtees, R. A. H., Clelland, J. D., Heales, S. J. R., Brand, M. P., Bartholomé, K., and Flatmark, T. (1996) Recessively inherited L-DOPA-responsive parkinsonism in infancy caused by a point mutation (L205P) in the tyrosine hydroxylase gene. Hum. Mol. Genet. 5, 1023–1028. [DOI] [PubMed] [Google Scholar]
- 109).Hoffmann, G. F., Assmann, B., Bräutigam, C., Dionisi-Vici, C., Häussler, M., de Klert, J. B. C., Naumann, M., Steenbergen-Spanjers, G. C. H., Strassburg, H.-M., and Wevers, R. A. (2003) Tyrosine hydroxylase deficiency causes progressive encephalopathy and dopa-nonresponsive dystonia. Ann. Neurol. 54, S56–S65. [DOI] [PubMed] [Google Scholar]
- 110).Janssen, R. J. R. J., Wevers, R. A., Häussler, M., Luyten, J. A. F. M., Steenbergen-Spanjers, G. C. H., Hoffmann, G. F., Nagatsu, T., and van den Heuvel, L. P. W. J. (2000) A branch site mutation leading to aberrant splicing of the human tyrosine hydroxylase gene in a child with a severe extrapyramidal movement disorder. Ann. Hum. Genet. 64, 375–382. [DOI] [PubMed] [Google Scholar]
- 111).Meloni, R., Leboyer, M., Bellivier, F., Barbe, B., Samolyk, D., Allilaire, J. F., and Mallet, J. (1995) Association of manic–depressive illness with tyrosine hydroxylase microsatellite marker. Lancet 345, 932. [DOI] [PubMed] [Google Scholar]
- 112).Thibaut, F., Ribeyre, J. M., Dourmap, N., Meloni, R., Laurent, C., Campion, D., Menard, J. F., Dollfus, S., Mallet, J., and Petit, M. (1997) Association of DNA polymorphism in the first intron of the tyrosine hydroxylase gene with disturbances of the catecholaminergic system in schizophrenia. Schizophr. Res. 23, 259–264. [DOI] [PubMed] [Google Scholar]
- 113).Meloni, R., Albanese, V., Ravassard, P., Treihou, F., and Mallet, J. (1998) A tetranucleotide polymorphic microsatellite, located in the first intron of the tyrosine hydroxylase gene, acts as a transcription regulatory element in vitro. Hum. Mol. Genet. 7, 423–428. [DOI] [PubMed] [Google Scholar]
- 114).Ichinose, H., Ohye, T., Fujita, K., Pantucek, F., Lange, K., Riederer, P., and Nagatsu, T. (1994) Quantification of mRNA of tyrosine hydroxylase in the substantia nigra in Parkinson’s disease and schizophrenia. J. Neural Transm. [P-D Sect] 8, 149–158. [DOI] [PubMed] [Google Scholar]
- 115).Robertson, D., Goldberg, M. R., Onrot, J., Hollister, A. S., Wiley, R., Thompson, J. G. Jr., and Robertson, R. M. (1986) Isolated failure of autonomic noradrenergic neurotransmission: Evidence for impaired β-hydroxylation of dopamine. New Engl. J. Med. 314, 1494–1497. [DOI] [PubMed] [Google Scholar]
- 116).Narabayashi, H., Kondo, T., Hayashi, A., Suzuki, T., and Nagatsu, T. (1981) L-Threo-3,4-dihydroxyphenylserine treatment for akinesia and freezing of parkinsonism. Proc. Jpn. Acad., Ser. B 57, 351–354. [Google Scholar]
- 117).Nagatsu, T., and Sawada, M. (2006) Cellular and molecular mechanisms of Parkinson’s disease: Neurotoxins, causative genes, and inflammatory cytokines. Cell. Mol. Neurobiol. 26, 779–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118).Mizuno, Y., Yoshino, H., Ikebe, S., Hattori, N., Kobayashi, T., Shimoda-Matsubayashi, S., Matsumine, H., and Kondo, T. (1998) Mitochondrial dysfunction in Parkinson’s disease. Ann. Neurol. 44 (Suppl. 1), S999–S1009. [DOI] [PubMed] [Google Scholar]
- 119).Nagatsu, T., and Sawada, M. (2005) Inflammatory process in Parkinson’s disease: role for cytokines. Current Pharmaceutical Design 11, 999–1016. [DOI] [PubMed] [Google Scholar]
- 120).Bush, W. D., Garguilo, J., Zecca, F. A., Albertini, A., Zecca, L., Edwards, G. S., Nemanich, R. J., and Simon, J. D. (2006) The surface oxidation potential of human neuromelanin reveals a spherical architecture with a pheomelanin core and a eumelanin surface. Proc. Natl. Acad. Sci. USA 103, 14785–14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121).Mizzulli, J. R., Mishizen, A. J., Giasson, B. I., Lynch, D. R., Thomas, S. A., Nakashima, A., Nagatsu, T., Ota, A., and Ischiropoulos, H. (2006) Cytosolic catechols inhibit α-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J. Neurosci. 26, 10068–10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122).Nagatsu, T., Yamaguchi, T., Rahman, M. K., Trocewicz, J., Oka, K., Hirata, Y., Nagatsu, I., Narabayashi, H., Kondo, T., and Iizuka, R. (1984) Catecholamine-related enzymes and the biopterin cofactor in Parkinson’s disease. InAdvances in Neurology (eds. Hassler R. G., and Christ J. F.). Vol. 40, Raven Press, New York, pp. 467–473. [PubMed] [Google Scholar]
- 123).Nagatsu, T, Horikoshi, T., Sawada, M., Nagatsu, I., Kondo, T., Iizuka, R., and Narabayashi, H. (1986) Biosynthesis of tetrahydrobiopterin in parkinsonian human brain. InAdvances in Neurology (eds. Yahr M. D., and Bergmannn K. S.). Vol. 45, Raven Press, New York, pp. 223–226. [PubMed] [Google Scholar]
- 124).Mogi, M., Harada, M., Kiuchi, K., Kojima, K., Kondo, T., Narabayashi, H., Rausch, D., Riederer, P., Jellinger, K., and Nagatsu, T. (1988) Homospecific activity (activity per enzyme protein) of tyrosine hydroxylase increases in Parkinsonian brain. J. Neural Transm. 72, 77–81. [DOI] [PubMed] [Google Scholar]
- 125).Mogi, M., Harada, M., Kojima, K., Inagaki, H., Kondo, T., Narabayashi, H., Arai, T., Teradaira, R., Fujita, K., Kiuchi, K., and Nagatsu, T. (1988) Sandwich enzyme immunoassay of dopamine-β-hydroxylase in cerebrospinal fluid from control and Parkinsonian patients. Neurochem. Int. 12, 187–191. [DOI] [PubMed] [Google Scholar]
- 126).Nagatsu, T. (1990) Changes of tyrosine hydroxylase in Parkinsonian brains and in the brains of MPTP-treated mice. InAdvances in Neurology (eds. Streifler M. B., Korczyn A. D., Melamed E., and Youdim M. B. H.). Vol. 53, Raven Press, New York, pp. 207–214. [PubMed] [Google Scholar]
- 127).Hirata, Y., and Nagatsu, T. (1986) Early and late effects of systemically administered 1-phenyl-4-hydroxy-1,2,3,6-tetrahydopyridine (MPTP) on tyrosine hydroxylase activity in vitro and on tyrosine hydroxyalse in tissue slices of mouse striatum. Neurosci. Lett. 68, 245–248. [DOI] [PubMed] [Google Scholar]
- 128).Mogi, M., Harada, M., Kojima, K., Kiuchi, K., and Nagatsu, T. (1988) Effects of systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine to mice on tyrosine hydroxylase, L-3,4-dihydroxyphenylalanine decarboxylase, dopamine β-hydroxylase, and monoamine oxidase activities in the striatum and hypothalamus. J. Neurochem. 50, 1053–1056. [DOI] [PubMed] [Google Scholar]
- 129).Ohye, T., Ichinose, H., Ogawa, M., Yoshida, M., and Nagatsu, T. (1995) Alterations in multiple tyrosine hydroxylase mRNAs in the substantia nigra, locus coeruleus and adrenal gland of MPTP-treated Parkinsonian monkeys. Neurodegeneration 4, 81–85. [DOI] [PubMed] [Google Scholar]
- 130).Nagatsu, T. (1997) Isoquinoline neurotoxins in the brain and Parkinson’s disease. Neurosci. Res. 29, 99–111. [DOI] [PubMed] [Google Scholar]
- 131).Storch A., and Collins M. A. (eds.) (2000) Neurotoxic Factors in Parkinson’s and Related Disorders. Kluwer Academic Publishing/Plenum, New York. [Google Scholar]
- 132).Shen, Y., Muramatsu, S., Ikeguchi, K., Fujimoto, K., Fan, D.-S., Ogawa, M., Mizutani, H., Urabe, M., Kume, A., Nagatsu, I.et al. (2000) Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic-L-amino-acid decarboxylase, and GTP cyclohydrolase I for gene therapy of Parkinson’s disease. Human Gene Therapy 11, 1509–1519. [DOI] [PubMed] [Google Scholar]
- 133).Misu Y., and Goshima Y. (eds.) (2005) Neurobiology of DOPA as a Neurotransmitter. CRC Press, Boca Laton, FL. [Google Scholar]
- 134).Ikemoto, K., Nagatsu, I., Ito, S., King, R. A., Nishimura, A., and Nagatsu, T. (1998) Does tyrosinase exist in neuromelanin-pigmented neurons in the human substantia nigra? Neurosci. Lett. 253, 198–200. [DOI] [PubMed] [Google Scholar]
- 135).Nagatsu, T. (1990) Normal brain aging versus pathological brain aging — similarity and dissimilarity between MPTP parkinsonism and Parkinson’s disease in relation to brain aging. InAging of the Brain: Cellular and Molecular Aspects of Brain Aging and Alzheimer’s Disease (eds. Nagatsu T., and Hayaishi O.). Japan Scientific Societies Press, Tokyo, and Karger, Basel, pp. 119–127. [Google Scholar]
- 136).Kitahama, K., Nagatsu, I., and Pearson, J. (1994) Catecholamine systems in mammalian midbrain and hindbrain: theme and variations. InPhylogeny and Development of Catecholamine Systems in the CNS of Vertebrates (eds. Smeets W. J. A. J., and Reiner A.). Cambridge University Press, Cambridge, pp. 183–295. [Google Scholar]
- 137).Naoi M., and Parvez S. H. (eds.) (1993) Tyrosine Hydroxylase: From Discovery to Cloning. VSP Press, Utrecht. [Google Scholar]