LFA-1 expression defines a unique WBC population in patients with allergic asthma, whereas depleting WBCs expressing active LFA-1 causes resolution in an experimental mouse model.
Keywords: leukotoxin, LtxA, Leukothera, house dust mite, inflammation, CD11a
Abstract
Allergic asthma is a chronic respiratory disease that results from an exaggerated inflammatory response in the airways. Environment stimuli, such as pollen and HDM, cause activation and migration of inflammatory WBCs into the respiratory tract, where they cause lung damage. Migration of these WBCs is dependent on the active configuration of the β2 integrin LFA-1. The experimental therapeutic agent LtxA specifically targets active LFA-1 and causes cell death. We investigated the association between LFA-1 and allergic asthma and hypothesized that targeting LFA-1 with LtxA could be an attractive strategy for treatment of the condition. We examined LFA-1 (CD11a) levels on PBMCs from patients with allergic asthma compared with healthy controls. Patients exhibited a significantly higher percentage of PBMCs expressing LFA-1 than healthy controls. Furthermore, the level of LFA-1 expression on patient PBMCs was greater than on healthy PBMCs. We identified a unique cellular population in patients that consisted of CD4– CD11ahi cells. We also evaluated LtxA in a HDM extract-induced mouse model for allergic asthma. LtxA caused resolution of disease in mice, as demonstrated by a decrease in BALF WBCs, a reduction in pulmonary inflammation and tissue remodeling, and a decrease in proinflammatory cytokines IL-4, IL-5, IL-9, IL-17F, and IL-23α in lung tissue. LFA-1 may serve as an important marker in allergic asthma, and the elimination of activated WBCs by use of LtxA could be a viable therapeutic strategy for treating patients with this condition.
Introduction
The pathology that is observed in patients with inflammatory and autoimmune diseases is mediated by an overt immune response and damage caused by immune cells migrating to the site of inflammation in response to signaling molecules, such as cytokines. One of the key molecules responsible for mediating this migration is LFA-1. LFA-1 is a β-2 integrin, consisting of an α chain (CD11a) and a β chain (CD18), and is crucial for migration of WBCs across the endothelial barrier into the surrounding tissues [1, 2]. LFA-1 undergoes an inducible conformational change from a nonadhesive, inactive state to an adhesive, active state upon activation by a stimulus [1, 3]. Active LFA-1 then binds to ICAM-1 on endothelial cells, which acts as a ligand for LFA-1. Formation of the activated LFA-1/ICAM-1 complex then leads to the migration of WBCs across the endothelial barrier. Allergic conditions and autoimmune/inflammatory diseases, such as asthma, psoriasis, multiple sclerosis, lupus, rheumatoid arthritis, Crohn’s disease, ulcerative colitis, and type I diabetes, are characterized by the infiltration of highly active WBCs to the involved tissue. LFA-1 has been shown to be crucial for the initiation and perpetuation of these immune-mediated diseases [4]. Therefore, the targeting of WBCs expressing active LFA-1 is a rational therapeutic strategy with diverse clinical applications [5]. Indeed, therapeutics have been developed that target the LFA-1/ICAM-1 interaction in the form of statins, cinnamides, peptides, and mAb.
Asthma is an inflammatory disease of the airways, affects ∼300 million people worldwide, and causes 250,000 annual deaths [6–8]. Approximately 34 million Americans have been diagnosed with asthma. The pathogenesis of asthma involves infiltration of activated WBCs into the airways and release of inflammatory mediators, which cause bronchial epithelium damage [8]. Allergic asthma is IgE mediated and involves initial exposure to the inhaled allergen and subsequent antigen presentation to Th2 lymphocytes, which secrete IL-4 and IL-13 [9]. These cytokines lead to Ig class-switching in B lymphocytes, resulting in IgE production. Subsequent binding of IgE molecules to FcεRI on mast cells and basophils results in sensitization to allergen exposure [10].
Inflammatory effects that are characteristic of asthma depend on the migration of WBCs into the airways, which is mediated by LFA-1. Various studies have shown that there is overexpression of LFA-1 on the immune cells involved in asthma with negligible contribution from other integrins [11]. In addition to its role in cellular adhesion and migration, LFA-1 acts as a costimulatory molecule for T cell sensitization [12]. Therefore, LFA-1 appears to be an ideal marker for activated immune cells, contributing to the pathogenesis of asthma. Previous studies using simvastatin to target LFA-1 in murine models of allergic asthma have shown that the drug can reduce Th2 cytokine levels [13]. In addition, mAb against CD11a (efalizumab) in randomized controlled trials of asthma patients was shown to decrease airway and sputum eosinophilia [14].
LtxA is a unique LFA-1-targeting agent that causes rapid death of WBCs expressing active LFA-1 [15–19]. LtxA is a natural protein that is produced by the oral bacterium Aggregatibacter actinomycetemcomitans [17, 20]. LtxA is known to target specifically WBCs expressing active LFA-1 involved in disease, while sparing healthy, resting WBCs, thus the minimization of the possibility of general immunosuppression. The exquisite specificity and activity of LtxA have formed the basis for evaluating LtxA as a targeted biotherapy for WBC diseases characterized by overexpression of LFA-1, such as hematologic malignancies and autoimmune/inflammatory diseases. Our previous studies have demonstrated significant in vitro and in vivo therapeutic efficacy of LtxA in models of leukemia, lymphoma, and psoriasis [18, 21, 22].
In this report, we examined the levels of LFA-1 on WBCs from patients with allergic asthma and demonstrate a unique cellular population that is characteristic of patients with the condition. We also show that LtxA causes significant resolution of disease in a mouse model for allergic asthma.
MATERIALS AND METHODS
LtxA purification
LtxA was purified from culture supernatants of A. actinomycetemcomitans strain 4500, as described previously [23, 24].
Isolation of PBMCs from blood
Whole blood was collected from 8 allergic asthma patients and 11 nonallergic healthy controls, who did not exhibit any allergies. All of the allergic asthma patients (ages 5–50) were recruited from the Emergency Room, the Adult Allergy and Immunology Clinic, or the Pediatric Pulmonary Clinic at University Hospital/New Jersey Medical School. Patients had recent evidence of reversible airway disease on spirometry and allergy to dust mite, as determined by skin testing. Patients were allowed to be on any combination of short-acting bronchodilators, inhaled corticosteroids, and long-acting bronchodilators. Patients were excluded if they were pregnant; taking omalizumab (Xolair) or other immunotherapy treatment, systemic corticosteroids, or antibiotics within 30 days; or had significant comorbidities, such as HIV, heart disease, diabetes, and cancer. The control group had no history of asthma or allergic conditions. The collection protocol was approved by the Rutgers Institutional Review Board, and written, informed consent was obtained from all study subjects. PBMCs were isolated by use of Ficoll density gradient separation (Corning cellgro, Mediatech, Manassas, VA, USA). Viable cells were counted by use of Vi-CELL viability instrument.
Analysis of PBMCs
PBMCs (106 cells/ml) from patients and controls were stained with antibodies (BioLegend, San Diego, CA, USA) to the following markers: CD4 (Th cells), CD11a and CD14 (monocytes), and CD3 (T cells). PBMCs from asthma patients were also incubated overnight with buffer or LtxA (500 ng/ml) in RPMI-1640 medium. The treated PBMCs were washed with PBS and stained with Annexin V, along with the above antibodies, and analyzed through flow cytometry to measure LtxA-mediated cell death.
Animal study
Female BALB/c mice (6–8 weeks, 15–20 g; The Jackson Laboratory, Bar Harbor, ME, USA) were housed under specific pathogen-free conditions and a 12-h light/dark cycle with access to food and water. Under isoflurane anesthesia, mice were exposed to HDM extract (Dermatophagoides pteronyssinus; Greer Laboratories, Lenoir, NC, USA) i.n. (25 μg in 10 μl saline/nostril). Control (nonasthmatic) animals were administered an equal volume of saline alone. The frequency of exposure for HDM/saline was 5 days/week for a total of 5 weeks. Power calculations from pilot studies indicated that 4 animals/group were sufficient to detect a significant difference between control and experimental groups.
After 2 weeks, HDM-exposed mice were divided into 4 groups of 4 mice/group and treated with the following: 1) dexamethasone vehicle (saline) s.c. once daily, 5 days/week; 2) dexamethasone (1.25 mg/kg; Sigma, St. Louis, MO, USA) s.c. once daily, 5 days/week; 3) LtxA vehicle (20 mM Tris-HCl, pH 6.8, 250 mM NaCl, and 0.2 mM CaCl2) i.p., 3 days/week; and 4) LtxA (0.5 mg/kg) i.p., 3 days/week. HDM exposure was continued throughout the 3-week treatment period.
At the end of the study, mice were euthanized by giving a fatal dose of avertin. Isolation of BALF was performed by first making a transverse incision in the trachea below the larynx and filling the space with 0.5 ml PBS. BALF from each mouse was subjected to RBC lysis for 10 min at room temperature, followed by washing twice at 400 g for 5 min and resuspending the cell pellet in PBS. Total BALF cell number was counted by use of a hemocytometer. Immunophenotypic analysis of BALF cells was performed by antibody staining and flow cytometry. For each antibody stain, 106 cells were first blocked with a Fc blocker (rat anti-mouse CD16/CD32; BD Biosciences, San Jose, CA, USA) for 10 min at room temperature, followed by incubation with mAb at 4°C for 30 min and analysis on an LSR II flow cytometer (BD Biosciences). Antibodies (BD Biosciences) to the following markers were used to identify the various cellular subtypes: CD3e (T cells), CD45R/B220 (B cells), Ly6G and CCR3 (neutrophils), CCR3 and Ly6G (eosinophils), and MHC II (macrophages). Relevant isotype controls were included with each experiment.
Blood from the mice was collected by ileac vein puncture immediately following the BALF isolation. Blood was collected in anticoagulant tubes and centrifuged at 400 g for 10 min. The use of animals was approved by the Rutgers Institutional Animal Care and Use Committee, and “Principles of Laboratory Animal Care” (National Society for Medical Research) was followed for the care and use of animals.
Lung tissue histology
After collecting BALF and blood, lungs were removed through dissection and stored in 10% neutral-buffered formalin at room temperature until microscopic analysis. Sections of paraffin-embedded, fixed lung tissues were stained with H&E to analyze total lung inflammation. Sections were also stained with PAS reagent to identify mucous and goblet cell hyperplasia and Sirius Red for eosinophils. The tissue preparation and examination were carried out at the New Jersey Medical School Histology Core Facility. Samples were examined by a board-certified pathologist.
Cytokine analysis
qRT-PCR was used to determine the expression levels of proinflammatory cytokines (IL-4, IL-5, IL-9, IL-17F, and IL-23α) in the lungs of mice. Total RNA from the lung tissue was extracted with Trizol reagent (Life Technologies, Grand Island, NY, USA). Relative mRNA levels were determined by qRT-PCR. One microgram of total RNA was reverse transcribed by use of the High-Capacity cDNA Reverse Transcription Kit (Life Technologies). Amplification was carried out by use of TaqMan Fast Universal PCR Master Mix (Life Technologies). The data were normalized to GAPDH. Gene expression was calculated by use of the ΔΔ comparative threshold method relative to naïve sample.
Statistical analysis
BALF cell counts, differential cell counts, and cytokine levels were compared by Student's t-test. P ≤ 0.05 was considered significant.
RESULTS
Expression of LFA-1 on WBCs from allergic asthma patients and healthy controls
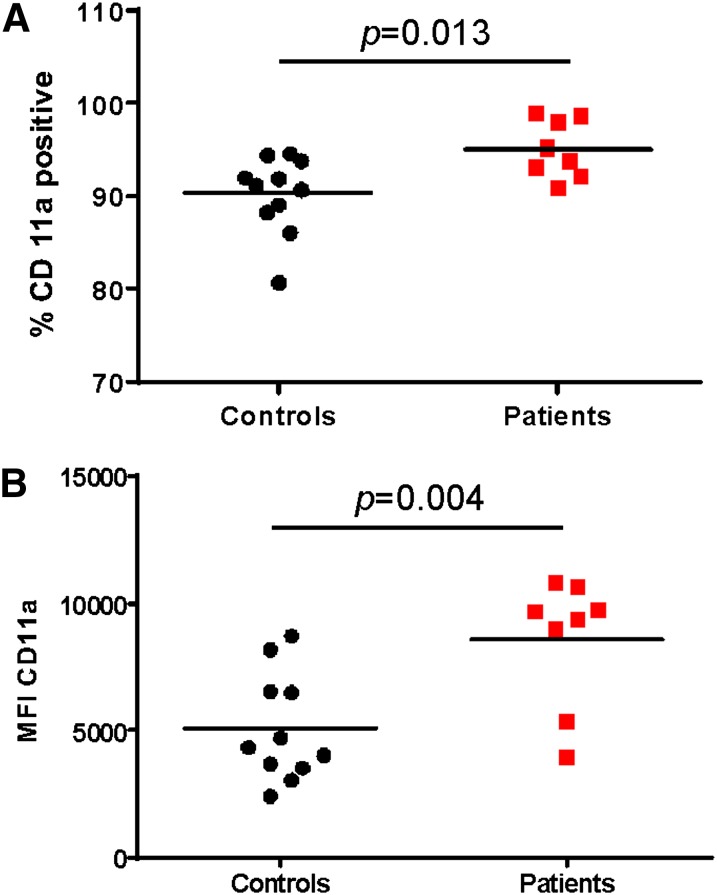
We analyzed PBMCs from the blood of 8 allergic asthma patients and 11 matched healthy controls. All of the allergic asthma patients (ages 5–50) were recruited from the Emergency Room, the Adult Allergy and Immunology Clinic, or the Pediatric Pulmonary Clinic at University Hospital/New Jersey Medical School. Patients diagnosed with asthma tested positive for an allergic reaction to HDM. We chose to examine the CD11a component of LFA-1 rather than CD18, as CD18 can be paired with other α chains, such as CD11b, CD11c, and CD11d [25]. Thus, CD11a uniquely represents LFA-1. From the total PBMC population, the percentage of CD11a-positive cells from patients was significantly higher than from the healthy controls (Fig. 1A). Patients had 95.1 ± 3.14% CD11a-positive cells, whereas healthy controls had 90.3 ± 4.11%. In addition, the number of LFA-1 molecules on the surface of CD11a+ WBCs from allergic asthma patients (8605 ± 2519) was significantly greater than on the surface of healthy control WBCs (5089 ± 2107). as indicated by the MFI; Fig. 1B).
Figure 1. PBMCs from allergic asthma patients express high levels of LFA-1. PBMCs were isolated from whole blood by use of Ficoll hypaque density gradient separation and stained with antibody against CD11a. The samples were analyzed by flow cytometry. The percent of CD11a-positive cells (A) and MFI (geometric mean) of CD11a (B) were measured. The significance of the differences was determined by use of Student’s t-test.
WBCs stained with anti-CD4 and anti-CD11a antibodies revealed a unique cellular population in allergic asthma patients that consisted of CD4–CD11ahi cells, which were absent from the healthy control samples (Fig. 2A and B). The CD11ahi population was defined as cells that had a CD11a MFI of >104. Immunophenotypic analysis revealed that this CD11ahi population in asthma samples consisted primarily of CD14+ monocytes and CD3+ non-Th cells (Supplemental Fig. 1). Thus, high LFA-1 expression defines a unique cellular population that is present in patients with allergic asthma.
Figure 2. PBMCs from allergic asthma patients constitute a unique population. PBMCs were isolated from whole blood by use of Ficoll hypaque density gradient separation and stained with antibody against CD4 (Th2) and CD11a. The samples were analyzed by flow cytometry. (A) PBMCs from asthma patients showed a distinct population of CD4–CD11ahi cells (boxed in red) that was absent in the healthy subjects. (B) Samples were analyzed for the presence of the CD4–CD11ahi population among the CD11a-positive cells in allergic asthma patients and healthy controls. The significance of the differences was determined by use of Student’s t-test.
Effects of LtxA on PBMCs from allergic asthma patients
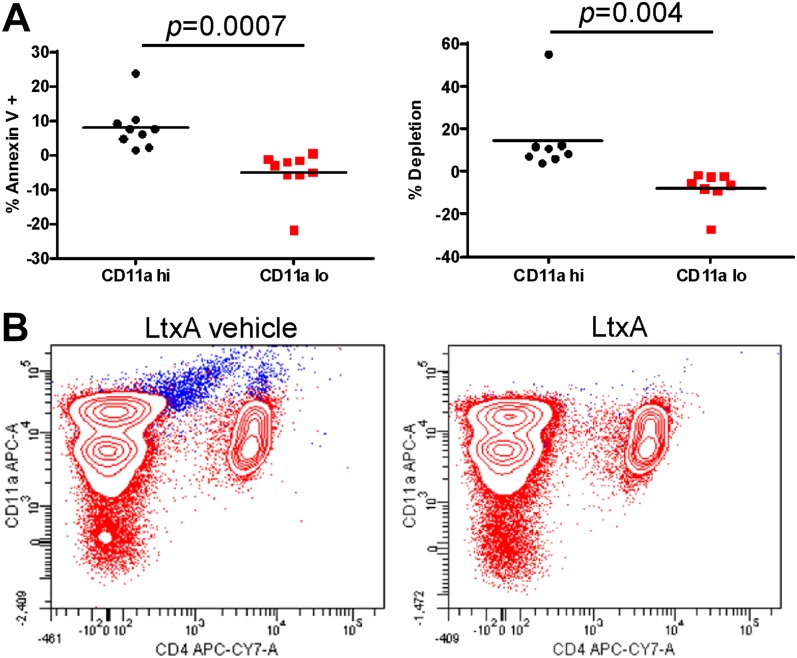
To determine which cells from patients are targeted by LtxA, PBMC samples were treated with LtxA for 24 h and then stained with Annexin V and analyzed by flow cytometry (Fig. 3A). Of the CD11a+ PBMCs, LtxA killed only the CD11ahi cells and did not affect cells that had a low expression of LFA-1. Cells were killed by apoptosis (Annexin V positive) and necrosis/depletion (Fig. 3A). The cells that were depleted by LtxA express active state LFA-1, as revealed by staining with an antibody (mAb24) that recognizes LFA-1 specifically in the active conformation (Fig. 3B). The majority of these cells also stained positive for CD14, indicating that they were monocytes.
Figure 3. Preferential targeting of CD11ahi cells by LtxA. PBMCs from allergic asthma patients were treated in vitro with LtxA (500 ng/mL) or buffer for 24 h. The treated PBMCs were then stained with anti-CD11a antibody and Annexin V and analyzed through flow cytometry. LtxA-mediated cytotoxicity was measured in terms of apoptosis (Annexin V positive) compared with buffer treated and depletion (buffer-treated PBMCs − LtxA-treated PBMCs). (A) LtxA preferentially killed cells with high LFA-1 (defined as CD11a-positive cells with an MFI between 104 and 105) and had a negligible effect on PBMCs with low LFA-1. The significance of the differences was determined by use of Student’s t-test. (B) LtxA causes depletion of cells that express the active conformation of LFA-1 (shown in blue). PBMCs were stained with mAb24 and CD11a antibodies to identify the cells with active LFA-1. APC-A, Allophycocyanin-area; CY7, cyanine 7.
Evaluation of LtxA in a mouse model for allergic asthma
Given the potential role that LFA-1 plays in the pathogenesis of allergic asthma and the ability for LtxA to target specifically the LFA-1hi WBCs ex vivo that are unique to allergic asthma patients, we performed an initial proof-of-principle evaluation of LtxA in a mouse model for allergic asthma. Mice were administered HDM extract or saline i.n., 5 days/week for 5 weeks. After 2 weeks of administration, HDM-exposed mice were subdivided into 4 groups of 4 mice/group and received the following treatments for an additional 3 weeks: dexamethasone vehicle, s.c., 5 days/week; dexamethasone (1.25 mg/kg), s.c., 5 days/week; LtxA vehicle, i.p., 3 days/week; and LtxA (0.5 mg/kg), i.p., 3 days/week.
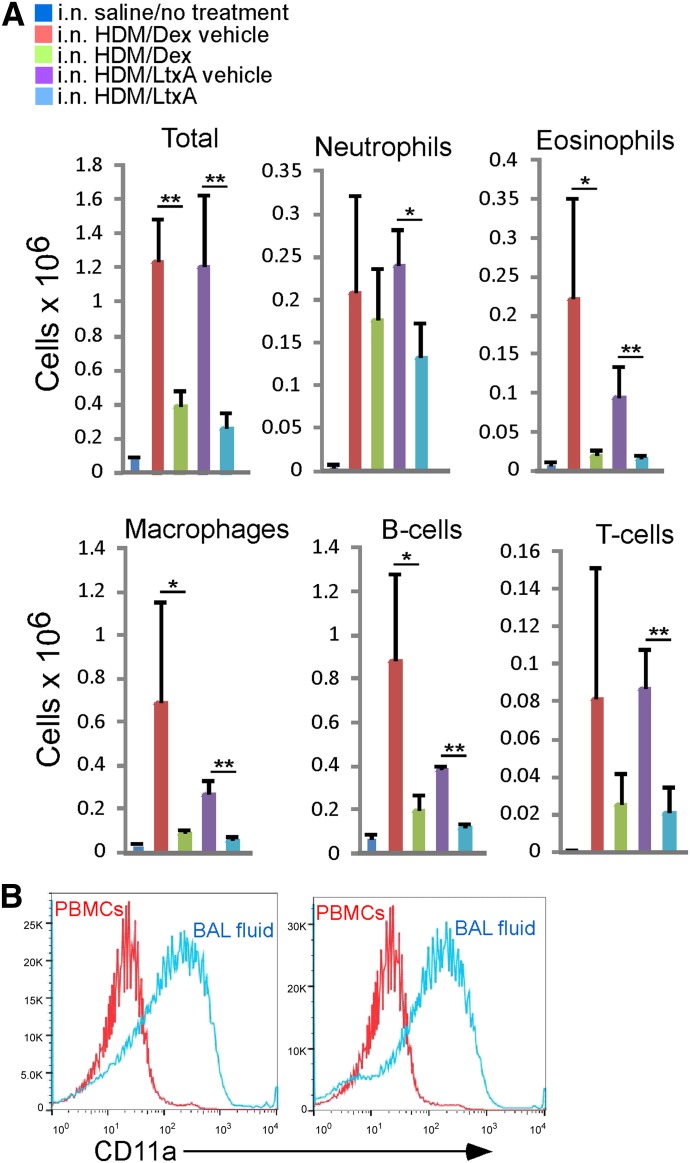
At the end of the study, BALF, lung tissue, and blood were collected from all mice for further evaluation. Examination of WBCs in the BALF revealed that HDM-exposed mice, treated with the dexamethasone vehicle or LtxA vehicle, had significantly higher levels of all WBC subsets than nonasthmatic mice that were given only saline (Fig. 4A). Treatment of HDM-exposed mice with dexamethasone or LtxA caused a significant reduction in the numbers of WBCs in the BALF (Fig. 4A).
Figure 4. Effects of experimental treatment on WBCs in the BALF in a mouse model for allergic asthma. Saline-treated mice were given saline i.n., whereas all of the other groups were administered HDM extract i.n. HDM extract-exposed mice were then treated with dexamethasone or LtxA or the respective vehicles, as described in the text. (A) Cells in the BALF were stained with mAb for specific cell types and then analyzed by use of flow cytometry. Each bar represents the average from 4 mice. Error bars show sd. The significance of the differences was determined by use of Student’s t-test (*P ≤ 0.05; **P ≤ 0.01). (B) High expression of LFA-1 characterizes cells that have migrated into the airways. PBMCs and BALF WBCs from 2 HDM extract-exposed mice that were treated with LtxA vehicle were stained with anti-CD11a antibody and analyzed by use of flow cytometry.
To determine if LFA-1 is involved in the migration of WBCs to the lung tissue in this animal model, we examined the levels of LFA-1 on PBMCs and BALF WBCs in two HDM-exposed mice that were treated with LtxA vehicle (Fig. 4B). We found that the migrated WBCs that were present in the BALF had significantly higher levels of LFA-1 than on the WBCs in the peripheral blood of the same animal. This finding suggests that there is a selection for the LFA-1hi subset of WBCs in the lung tissue in this mouse model for allergic asthma.
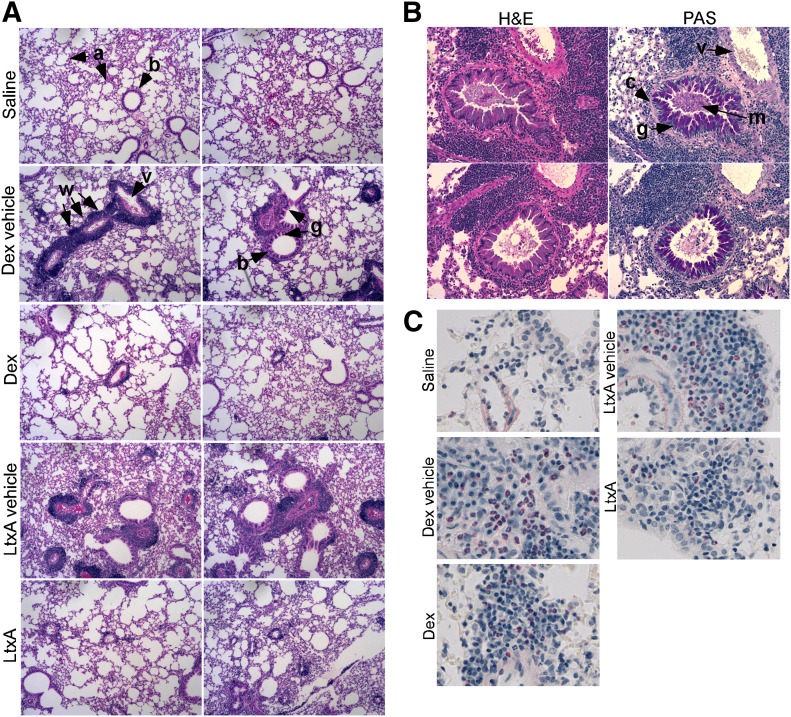
Lung tissue was sectioned and stained with H&E, PAS, or Sirius Red (Fig. 5). H&E staining revealed a large infiltration of WBCs in the lung tissue of HDM-exposed mice treated with dexamethasone vehicle or LtxA vehicle. Infiltration was not evident in saline-exposed mice. The infiltration of WBCs in HDM-exposed mice was most evident surrounding the blood vessels and bronchioles. We also observed significant goblet cell hyperplasia surrounding many of the bronchioles in the vehicle-treated controls but not in the other samples (Fig. 5A and B). Staining of polysaccharides with PAS in the lung tissue from LtxA vehicle-treated mice confirmed the presence of mucin-producing goblet cells and subepithelial accumulation of collagen. Sirius Red staining of sections revealed pink-staining eosinophils in the vehicle-treated mice but not in the LtxA-treated mice (Fig. 5C). Mice that were treated with dexamethasone had a reduced number of eosinophils compared with the vehicle control but still greater than LtxA-treated mice.
Figure 5. Histologic examination of lung tissue from mice. Mouse lungs were dissected at the end of the study after collecting blood and BALF. Lung tissue was fixed in 10% formalin and embedded in paraffin. Sections of the lung were stained with (A) H&E, (B) PAS reagent to stain polysaccharides, or (C) Sirius Red to stain eosinophils, which appear pink. a, Alveoli; b, bronchiole; c, collagen; w, WBC infiltration; v, blood vessel; g, goblet cell hyperplasia; m, mucous production. Original magnification, 100× (A and B) and 400× (C).
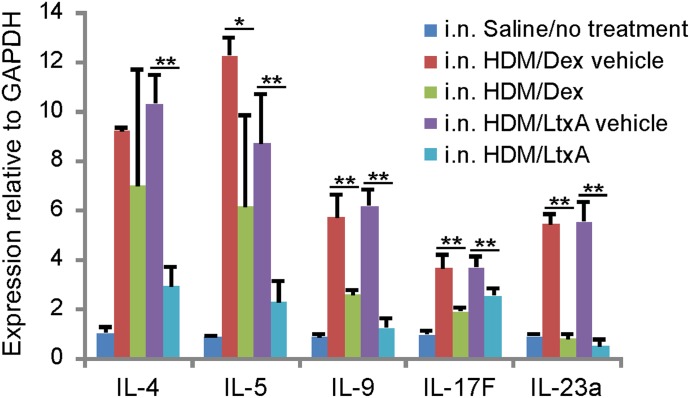
Proinflammatory cytokines play a crucial role in the pathogenesis of allergic asthma and other inflammatory conditions. In allergic asthma, IL-4, IL-5, IL-9, IL-17F, and IL-23α are the primary signaling molecules involved in disease. We evaluated the levels of IL-4, IL-5, IL-9, IL-17F, and IL-23α mRNA in the lung tissue from all mice (Fig. 6). We found that the vehicle-treated mice had significantly greater expression of the proinflammatory cytokines compared with saline-exposed mice. In addition, dexamethasone caused reduction of IL-9, IL-17F, and IL-23α, whereas LtxA treatment caused significant reduction of all of the cytokines that we examined.
Figure 6. Effects of experimental treatment on cytokine levels in mice. Mouse lungs were dissected at the end of the study, and the tissue was processed for cytokine analysis, as described in Materials and Methods. qRT-PCR was carried out to determine the mRNA expression levels of proinflammatory cytokines in lung tissue.
DISCUSSION
Asthma is a disease of increasing prevalence, with many patients developing resistance and intolerance to conventional therapy. To address this situation, there is a need to develop new treatments targeting novel markers. The β2-integrin, LFA-1, is expressed on the surfaces of WBCs and is crucial for the migration of cells out of the bloodstream and into the tissue [2, 26]. LFA-1 (CD11a and CD18) has been shown to play a significant role in the pathogenesis of asthma [11, 27, 28], as well as other autoimmune and inflammatory diseases [29–31]. In asthma, various inflammatory subtypes of WBCs show up-regulation and activation of LFA-1. This activated LFA-1 then mediates the migration of these cells into the airways. Once migrated, the inflammatory cells cause airway inflammation and remodeling, thereby leading to symptoms of asthma. The importance of LFA-1 has been demonstrated in animal models by use of mice, rats [32, 33], and rabbits [34, 35]. Human studies have also confirmed the important role that LFA-1 plays in asthma [11, 36]. More recently, Knight et al. [37] reported that CD11a polymorphisms in mice can affect Th2 cell homing and Th2-mediated allergic diseases and potentially play a role in human allergic diseases. Our findings in this report support the above paradigm.
We noted that PBMCs from allergic asthma patients had higher levels of LFA-1 compared with matched healthy controls. LFA-1 could possibly be used as a diagnostic marker for the disease by acting as a differentiating factor between asthmatic and healthy PBMCs. As a higher percentage of cells expressing LFA-1 would translate to an increase in the number of inflammatory cells infiltrating the airway tissue, LFA-1 levels may also be used as a measure of disease severity. In addition, reduction in LFA-1 levels following treatment could be a potential measure of treatment efficacy. Furthermore, the CD4– CD11ahi cellular population could serve as a diagnostic parameter for other allergic or inflammatory diseases, as they all have a similar pathogenesis. This work lays the foundation for ongoing studies to examine larger populations of patients.
Importantly, our work demonstrates why LFA-1 may represent an important therapeutic target for the treatment of asthma and related conditions. Indeed, other agents that target LFA-1 have been found to be effective in allergic diseases. For example, use of simvastatin, a small molecule drug that can target LFA-1, and mAb against LFA-1 (efalizumab) in randomized controlled trials of asthma patients was shown to reduce airway and sputum eosinophilia [14, 38]. In clinical trials, simvastatin was able to reduce the eosinophil count and improve the late airway response but did not affect airway hyper-responsiveness or inflammatory cytokines (IL-4, IL-5) compared with the placebo. One reason could be that although simvastatin decreased the number of airway eosinophils, it increased the number of lymphocytes in the sputum. An increase in the number of Th2 cells will cause further amplification of an inflammatory response. Furthermore, only subjects with eosinophilic asthma were included in the study. Simvastatin could have had a stronger effect in preventing migration of other inflammatory subtypes, which were not included in the study.
Treatment of asthma patients with efalizumab caused a decrease in the number of inflammatory cells, as well as a decrease in the late airway response compared with placebo. It did not have any effect on the early asthmatic response. This effect of simvastatin and efalizumab in reducing airway eosinophilia but not translating into an improvement in pulmonary function may be explained by studies showing dissociation between pulmonary function and airway eosinophilia [39, 40]. Thus, simply acting on the eosinophils is not necessarily sufficient to cause resolution of asthma.
LtxA differs in its mechanism of action from simvastatin and efalizumab in several important ways. Efalizumab binds LFA-1 and blocks interactions between WBCs and endothelial cells, thereby preventing the migration of inflammatory cells. Simvastatin primarily has a cholesterol-lowering effect with a recently recognized anti-inflammatory action. The mechanism of anti-inflammatory action for statins is varied, ranging from blockade of LFA-1, preventing neutrophilic migration, and release of inflammatory mediators to inducing efferocytosis (phagocytosis of apoptotic cells) [38, 41]. In contrast, LtxA causes a rapid depletion of activated inflammatory cells after binding to active LFA-1 [15, 16, 18, 22]. Simply blocking LFA-1, as efalizumab does, prevents migration of cells only while the drug is bound to the receptor, whereas depletion of activated inflammatory cells by LtxA allows for a long-term anti-inflammatory effect. Furthermore, LtxA targets all of the activated inflammatory cell types, providing a robust, anti-inflammatory effect, which is especially relevant in a heterogenous disease, such as asthma. Similar to other biologic drugs that are used to treat inflammatory conditions, such as asthma, LtxA administered s.c. is active in experimental animals [42]. In addition, LtxA does not elicit a neutralizing response in dogs or monkeys, even after repeat dosing over several weeks (unpublished data).
In this study, we found that ex vivo treatment of PBMCs from allergic asthma patients with LtxA caused a preferential cell death of PBMCs with high LFA-1, whereas the PBMCs with low LFA-1 were not affected. This specificity of LtxA for cells expressing high LFA-1 allows the drug to target the disease-causing PBMCs, while sparing the resting PBMCs. This approach provides the benefit of resolving an immune-mediated disease without causing significant immunosuppression, which is a major drawback of the prevalent therapies for asthma and other autoimmune and inflammatory diseases that target components of the immune system nonspecifically. For example, corticosteroids, which are the standard of care for asthma, have a generalized immunosuppressive effect, whereby they decrease migration of leukocytes and reduce the number of immune cells and production of immunomodulators and cytokines. Steroids can be well tolerated for a brief duration but chronic use often leads to multiple side-effects, such as proximal myopathy, Cushingoid habitus, hyperglycemia, diabetes, infections, and osteoporosis [43, 44]. Chemotherapy immunosuppressants, such as cyclosporine and tacrolimus, are used after organ transplants and in myesthenia gravis. They act by preventing Th2 cell activation and are known to cause nephrotoxicity [45]. Targeted immune therapies, which are more similar to LtxA, include mAb, such as natalizumab and rituximab. These immune therapies do not differentiate between resting and activated immune cells and cause a more potent immunosupression. LtxA, however, preferentially targets the activated immune cells [15, 16, 18, 22] and therefore, should show minimal immunosupression and associated side-effects.
We tested LtxA in a validated, robust model of chronic allergic asthma [46], which is caused by chronic allergen exposure, triggering immunologic and biochemical pathways leading to airway changes, manifesting in asthmatic symptoms of wheezing and breathlessness. To reproduce this system, we used a murine model showing sustained airway eosinophilic inflammation caused by chronic exposure (5 weeks) to common allergen HDM extract. This model is characterized by airway inflammation in BALF and lung tissue, persistent Th2 response with increased cytokine production, progressive airway remodeling, and bronchial hyper-reactivity [46]. Importantly, we noted that the WBCs present in the BALF of asthmatic mice expressed significantly higher levels of LFA-1 than the peripheral WBCs in the same animals. This finding indicates that LFA-1 plays an important role in the migration of WBCs to the airways in mice and further validates the use of this model to evaluate a drug that targets LFA-1. As the saline-exposed mice did not exhibit pulmonary inflammation and therefore, did not contain inflammatory WBCs in the BALF, we were unable to compare levels of LFA-1 in these animals.
Treatment of asthmatic mice with LtxA caused significant reduction in the BALF cell count of all of the inflammatory WBC subtypes. This reduction was more than that caused by the positive-control treatment dexamethasone. Lungs of LtxA-treated mice also showed reduced inflammatory infiltrate, a decreased number of eosinophils, and negligible airway remodeling. We also found that LtxA treatment caused a significant reduction in levels of expression of IL-4, -5, -9, -17F, and -23α in the lung.
The role of Th2 cytokines, such as IL-4, IL-5, and IL-9, has been well defined in the pathogenesis of asthma, orchestrating the initiation and amplification of the inflammatory response. The decrease of Th2 cytokine levels is also a measure of efficacy of prevalent treatments, such as steroids. IL-4 plays a role in Th2 differentiation [47], IgE synthesis [48], transmigration of inflammatory cells, and mucous secretion [49]. IL-5 is important for terminal differentiation of eosinophils [40, 50]. IL-9 is crucial for airway inflammation and airway hyper-responsiveness [51]. In addition to these Th2 cytokines, IL-17F and IL-23α are important in asthma. IL-17F is produced by the Th17 subtype of CD4 T cells and is involved in autoimmune diseases. IL-17F causes induction of various cytokines, chemokines, and adhesion molecules and is responsible for neutrophil recruitment, airway hypersensitivity, and mucous secretion [52]. IL-23 is a novel IL-12α family cytokine member that is involved in the maintenance and function of Th17 cells. This IL-23/Th17 axis is responsible for neutrophilic and eosinophilic recruitment in asthma [53].
In conclusion, we demonstrated that high LFA-1 expression defines a unique population of WBCs in patients with allergic asthma. We also showed that a natural bacterial protein, LtxA, targets this specific activated population ex vivo. In a proof-of-principle study, LtxA also causes resolution of the disease in vivo by decreasing the number of inflammatory WBCs in BALF, reducing lung inflammation and remodeling, and lowering the levels of inflammatory cytokines in an allergic asthma mouse model. Given the effect of LtxA on activated WBCs and proinflammatory cytokines levels, it is feasible to test the experimental drug as an anti-inflammatory agent in other allergic diseases as well. Importantly, LtxA therapy may be an attractive alternative in patients where steroids are no longer effective or well tolerated. Ultimately, clinical studies will be required to elucidate the therapeutic potential of LtxA as a novel anti-inflammatory agent.
ACKNOWLEDGMENTS
This work was funded by grants from the Foundation of the University of Medicine and Dentistry of New Jersey/Rutgers and the Office of Technology Transfer and Business Development of Rutgers (to S.C.K.) and U.S. National Institutes of Health Grants R21CA167238 (to A.R.) and F31AI098408 (to V.E.). The authors thank Tetyana Khrabatyn, Jenny Matthew, Orchi Dutta, Raj Kaswala, and Rohan Prabhu for assistance with the animal studies and Luke Fritzky and David Lagunoff for assistance with histopathology services.
Glossary
- BALF
bronchoalveolar lavage fluid
- HDM
house dust mite
- i.n.
intranasally
- LFA-1
lymphocyte function-associated antigen 1
- LtxA
leukotoxin, Leukothera
- MFI
mean fluorescence intensity
- PAS
Periodic Acid Schiff
- qRT=PCR
quantitative RT-PCR
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
AUTHORSHIP
A.G. planned and carried out studies and wrote the manuscript. V.E. and L.E.G. planned and carried out studies. V.R., K.L.M., E.C., and H.A.A. planned studies and collected human blood samples. A.R-M. planned studies and edited the manuscript. C.K. planned studies and carried out experiments. S.C.K. planned studies and wrote the manuscript.
DISCLOSURES
S.C.K. is a consultant for Actinobac Biomed and owns stock in the company.
REFERENCES
- 1.Hogg N., Smith A., McDowall A., Giles K., Stanley P., Laschinger M., Henderson R. (2004) How T cells use LFA-1 to attach and migrate. Immunol. Lett. 92, 51–54. [DOI] [PubMed] [Google Scholar]
- 2.Kinashi T. (2005) Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 5, 546–559. [DOI] [PubMed] [Google Scholar]
- 3.Hogg N., Harvey J., Cabanas C., Landis R. C. (1993) Control of leukocyte integrin activation. Am. Rev. Respir. Dis. 148, S55–S59. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf-Makagiansar H., Anderson M. E., Yakovleva T. V., Murray J. S., Siahaan T. J. (2002) Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 22, 146–167. [DOI] [PubMed] [Google Scholar]
- 5.Yonekawa K., Harlan J. M. (2005) Targeting leukocyte integrins in human diseases. J. Leukoc. Biol. 77, 129–140. [DOI] [PubMed] [Google Scholar]
- 6.Holgate S. T. (2013) Mechanisms of asthma and implications for its prevention and treatment: a personal journey. Allergy Asthma Immunol. Res. 5, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambrecht B. N., Hammad H. (2013) Asthma: the importance of dysregulated barrier immunity. Eur. J. Immunol. 43, 3125–3137. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M., Ishigatsubo Y., Aoki I. (2013) Pathology of asthma. Front. Microbiol. 4, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt P. G., Macaubas C., Stumbles P. A., Sly P. D. (1999) The role of allergy in the development of asthma. Nature 402(6760 Suppl) B12–B17. [DOI] [PubMed] [Google Scholar]
- 10.Robinson D. S. (2004) The role of the mast cell in asthma: induction of airway hyperresponsiveness by interaction with smooth muscle? J. Allergy Clin. Immunol. 114, 58–65. [DOI] [PubMed] [Google Scholar]
- 11.Lantero S., Alessandri G., Spallarossa D., Scarso L., Rossi G. A. (1998) LFA-1 expression by blood eosinophils is increased in atopic asthmatic children and is involved in eosinophil locomotion. Eur. Respir. J. 12, 1094–1098. [DOI] [PubMed] [Google Scholar]
- 12.Steinman R. M., Koide S., Witmer M., Crowley M., Bhardwaj N., Freudenthal P., Young J., Inaba K. (1988) The sensitization phase of T-cell-mediated immunity. Ann. N. Y. Acad. Sci. 546, 80–90. [DOI] [PubMed] [Google Scholar]
- 13.McKay A., Leung B. P., McInnes I. B., Thomson N. C., Liew F. Y. (2004) A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J. Immunol. 172, 2903–2908. [DOI] [PubMed] [Google Scholar]
- 14.Gauvreau G. M., Becker A. B., Boulet L. P., Chakir J., Fick R. B., Greene W. L., Killian K. J., O’byrne P. M., Reid J. K., Cockcroft D. W. (2003) The effects of an anti-CD11a mAb, efalizumab, on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J. Allergy Clin. Immunol. 112, 331–338. [DOI] [PubMed] [Google Scholar]
- 15.DiFranco K. M., Gupta A., Galusha L. E., Perez J., Nguyen T. V., Fineza C. D., Kachlany S. C. (2012) Leukotoxin (Leukothera®) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. J. Biol. Chem. 287, 17618–17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hioe C. E., Tuen M., Vasiliver-Shamis G., Alvarez Y., Prins K. C., Banerjee S., Nádas A., Cho M. W., Dustin M. L., Kachlany S. C. (2011) HIV envelope gp120 activates LFA-1 on CD4 T-lymphocytes and increases cell susceptibility to LFA-1-targeting leukotoxin (LtxA). PLoS ONE 6, e23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kachlany S. C. (2010) Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J. Dent. Res. 89, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kachlany S. C., Schwartz A. B., Balashova N. V., Hioe C. E., Tuen M., Le A., Kaur M., Mei Y., Rao J. (2010) Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leuk. Res. 34, 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lally E. T., Kieba I. R., Sato A., Green C. L., Rosenbloom J., Korostoff J., Wang J. F., Shenker B. J., Ortlepp S., Robinson M. K., Billings P. C. (1997) RTX toxins recognize a beta2 integrin on the surface of human target cells. J. Biol. Chem. 272, 30463–30469. [DOI] [PubMed] [Google Scholar]
- 20.Lally E. T., Hill R. B., Kieba I. R., Korostoff J. (1999) The interaction between RTX toxins and target cells. Trends Microbiol. 7, 356–361. [DOI] [PubMed] [Google Scholar]
- 21.Gupta A., Le A., Belinka B. A., Kachlany S. C. (2011) In vitro synergism between LFA-1 targeting leukotoxin (Leukothera™) and standard chemotherapeutic agents in leukemia cells. Leuk. Res. 35, 1498–1505. [DOI] [PubMed] [Google Scholar]
- 22.Stenderup K., Rosada C., Dam T. N., Salerno E., Belinka B. A., Kachlany S. C. (2011) Resolution of psoriasis by a leukocyte-targeting bacterial protein in a humanized mouse model. J. Invest. Dermatol. 131, 2033–2039. [DOI] [PubMed] [Google Scholar]
- 23.Diaz R., Ghofaily L. A., Patel J., Balashova N. V., Freitas A. C., Labib I., Kachlany S. C. (2006) Characterization of leukotoxin from a clinical strain of Actinobacillus actinomycetemcomitans. Microb. Pathog. 40, 48–55. [DOI] [PubMed] [Google Scholar]
- 24.Kachlany S. C., Fine D. H., Figurski D. H. (2002) Purification of secreted leukotoxin (LtxA) from Actinobacillus actinomycetemcomitans. Protein Expr. Purif. 25, 465–471. [DOI] [PubMed] [Google Scholar]
- 25.Tan S. M. (2012) The leucocyte β2 (CD18) integrins: the structure, functional regulation and signalling properties. Biosci. Rep. 32, 241–269. [DOI] [PubMed] [Google Scholar]
- 26.Dransfield I., Cabañas C., Barrett J., Hogg N. (1992) Interaction of leukocyte integrins with ligand is necessary but not sufficient for function. J. Cell Biol. 116, 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloemen P. G., Buckley T. L., van den Tweel M. C., Henricks P. A., Redegeld F. A., Koster A. S., Nijkamp F. P. (1996) LFA-1, and not Mac-1, is crucial for the development of hyperreactivity in a murine model of nonallergic asthma. Am. J. Respir. Crit. Care Med. 153, 521–529. [DOI] [PubMed] [Google Scholar]
- 28.Buckley T. L., Bloemen P. G., Henricks P. A., van den Tweel M. C., Redegeld F. A., Koster A. S., Nijkamp F. P. (1996) LFA-1 and ICAM-1 are crucial for the induction of hyperreactivity in the mouse airways. Ann. N. Y. Acad. Sci. 796, 149–161. [DOI] [PubMed] [Google Scholar]
- 29.McGregor J. M., Barker J. N., Ross E. L., MacDonald D. M. (1992) Epidermal dendritic cells in psoriasis possess a phenotype associated with antigen presentation: in situ expression of beta 2-integrins. J. Am. Acad. Dermatol. 27, 383–388. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi M., Mimura Y., Hayashi Y. (1996) Role of the ICAM-1/LFA-1 pathway during the development of autoimmune dacryoadenitis in an animal model for Sjögren's syndrome. Pathobiology 64, 269–274. [DOI] [PubMed] [Google Scholar]
- 31.Whitcup S. M., Chan C. C., Kozhich A. T., Magone M. T. (1999) Blocking ICAM-1 (CD54) and LFA-1 (CD11a) inhibits experimental allergic conjunctivitis. Clin. Immunol. 93, 107–113. [DOI] [PubMed] [Google Scholar]
- 32.Laberge S., Rabb H., Issekutz T. B., Martin J. G. (1995) Role of VLA-4 and LFA-1 in allergen-induced airway hyperresponsiveness and lung inflammation in the rat. Am. J. Respir. Crit. Care Med. 151, 822–829. [DOI] [PubMed] [Google Scholar]
- 33.Rabb H. A., Olivenstein R., Issekutz T. B., Renzi P. M., Martin J. G. (1994) The role of the leukocyte adhesion molecules VLA-4, LFA-1, and Mac-1 in allergic airway responses in the rat. Am. J. Respir. Crit. Care Med. 149, 1186–1191. [DOI] [PubMed] [Google Scholar]
- 34.Gascoigne M. H., Holland K., Page C. P., Shock A., Robinson M., Foulkes R., Gozzard N. (2003) The effect of anti-integrin monoclonal antibodies on antigen-induced pulmonary inflammation in allergic rabbits. Pulm. Pharmacol. Ther. 16, 279–285. [DOI] [PubMed] [Google Scholar]
- 35.Grunstein M. M., Hakonarson H., Maskeri N., Kim C., Chuang S. (2000) Intrinsic ICAM-1/LFA-1 activation mediates altered responsiveness of atopic asthmatic airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 278, L1154–L1163. [DOI] [PubMed] [Google Scholar]
- 36.Lantero S., Spallarossa D., Silvestri M., Sabatini F., Scarso L., Crimi E., Rossi G. A. (2002) In allergic asthma experimental exposure to allergens is associated with depletion of blood eosinophils overexpressing LFA-1. Allergy 57, 1036–1043. [DOI] [PubMed] [Google Scholar]
- 37.Knight J. M., Lee S. H., Roberts L., Smith C. W., Weiss S. T., Kheradmand F., Corry D. B. (2014) CD11a polymorphisms regulate TH2 cell homing and TH2-related disease. J. Allergy Clin. Immunol. 133, 189–197.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cowan D. C., Cowan J. O., Palmay R., Williamson A., Taylor D. R. (2010) Simvastatin in the treatment of asthma: lack of steroid-sparing effect. Thorax 65, 891–896. [DOI] [PubMed] [Google Scholar]
- 39.Koarai A., Ichinose M., Ishigaki-Suzuki S., Yamagata S., Sugiura H., Sakurai E., Makabe-Kobayashi Y., Kuramasu A., Watanabe T., Shirato K., Hattori, T., Ohtsu, H. (2003) Disruption of L-histidine decarboxylase reduces airway eosinophilia but not hyperresponsiveness. Am. J. Respir. Crit. Care Med. 167, 758–763. [DOI] [PubMed] [Google Scholar]
- 40.Leckie M. J., ten Brinke A., Khan J., Diamant Z., O’Connor B. J., Walls C. M., Mathur A. K., Cowley H. C., Chung K. F., Djukanovic R., Hansel T. T., Holgate S. T., Sterk P. J., Barnes P. J. (2000) Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356, 2144–2148. [DOI] [PubMed] [Google Scholar]
- 41.Morimoto K., Janssen W. J., Fessler M. B., Xiao Y. Q., McPhillips K. A., Borges V. M., Kench J. A., Henson P. M., Vandivier R. W. (2006) Statins enhance clearance of apoptotic cells through modulation of Rho-GTPases. Proc. Am. Thorac. Soc. 3, 516–517. [DOI] [PubMed] [Google Scholar]
- 42.Difranco K. M., Kaswala R. H., Patel C., Kasinathan C., Kachlany S. C. (2013) Leukotoxin kills rodent WBC by targeting leukocyte function antigen 1. Comp. Med. 63, 331–337. [PMC free article] [PubMed] [Google Scholar]
- 43.O’Byrne P. M., Rennard S., Gerstein H., Radner F., Peterson S., Lindberg B., Carlsson L. G., Sin D. D. (2012) Risk of new onset diabetes mellitus in patients with asthma or COPD taking inhaled corticosteroids. Respir. Med. 106, 1487–1493. [DOI] [PubMed] [Google Scholar]
- 44.Zöllner E. W., Lombard C. J., Galal U., Hough F. S., Irusen E. M., Weinberg E. (2012) Hypothalamic-pituitary-adrenal axis suppression in asthmatic school children. Pediatrics 130, e1512-e1519. [DOI] [PubMed] [Google Scholar]
- 45.De Mattos A. M., Olyaei A. J., Bennett W. M. (2000) Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am. J. Kidney Dis. 35, 333–346. [DOI] [PubMed] [Google Scholar]
- 46.Johnson J. R., Wiley R. E., Fattouh R., Swirski F. K., Gajewska B. U., Coyle A. J., Gutierrez-Ramos J. C., Ellis R., Inman M. D., Jordana M. (2004) Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 169, 378–385. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh C. S., Heimberger A. B., Gold J. S., O’Garra A., Murphy K. M. (1992) Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc. Natl. Acad. Sci. USA 89, 6065–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawankar R., Okuda M., Yssel H., Okumura K., Ra C. (1997) Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J. Clin. Invest. 99, 1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dabbagh K., Takeyama K., Lee H. M., Ueki I. F., Lausier J. A., Nadel J. A. (1999) IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J. Immunol. 162, 6233–6237. [PubMed] [Google Scholar]
- 50.Kips J. C., O’Connor B. J., Langley S. J., Woodcock A., Kerstjens H. A., Postma D. S., Danzig M., Cuss F., Pauwels R. A. (2003) Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am. J. Respir. Crit. Care Med. 167, 1655–1659. [DOI] [PubMed] [Google Scholar]
- 51.Dong Q., Louahed J., Vink A., Sullivan C. D., Messler C. J., Zhou Y., Haczku A., Huaux F., Arras M., Holroyd K. J., Renauld J. C., Levitt R. C., Nicolaides M. C. (1999) IL-9 induces chemokine expression in lung epithelial cells and baseline airway eosinophilia in transgenic mice. Eur. J. Immunol. 29, 2130–2139. [DOI] [PubMed] [Google Scholar]
- 52.Oda N., Canelos P. B., Essayan D. M., Plunkett B. A., Myers A. C., Huang S. K. (2005) Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am. J. Respir. Crit. Care Med. 171, 12–18. [DOI] [PubMed] [Google Scholar]
- 53.Wakashin H., Hirose K., Maezawa Y., Kagami S., Suto A., Watanabe N., Saito Y., Hatano M., Tokuhisa T., Iwakura Y., Puccelli P., Iwamoto I., Nakajima H. (2008) IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 178, 1023–1032. [DOI] [PubMed] [Google Scholar]