ADAM17 regulates CXCR2 surface levels in circulating neutrophils and their infiltration into sites of inflammation.
Keywords: inflammation, neutrophil, chemokines
Abstract
The chemokine receptor CXCR2 is expressed at high levels on circulating neutrophils and is critical for directing their migration to sites of inflammation. CXCR2 surface levels are rapidly modulated by 2 mechanisms—cell internalization and recycling upon ligand binding—and by a metalloprotease activity following overt neutrophil activation by nonligand stimuli. The latter process has only been described in human neutrophils, and essentially, nothing is known about its functional relevance and the specific protease involved. We show that targeting ADAM17 in mouse and human neutrophils blocks CXCR2 down-regulation induced by nonligand stimuli but not by chemokine ligands. This was determined by use of a selective ADAM17 inhibitor, an ADAM17 function-blocking antibody, and ADAM17 gene-targeted mice. CXCR2 is known to undergo a marked down-regulation during various inflammatory disorders, and this is associated with impaired neutrophil recruitment. We show that blocking ADAM17 activity reduced CXCR2 down-regulation on circulating neutrophils and enhanced their recruitment during acute inflammation, which was reversed by a CXCR2 inhibitor. Taken together, our findings demonstrate that unlike CXCR2 internalization, ADAM17 induction down-regulates the receptor in an irreversible manner and may serve as a master switch in controlling CXCR2 function, but may also contribute to neutrophil dysfunction during excessive inflammation.
Introduction
Neutrophils are the most abundant leukocyte population in the blood of humans and are the first to infiltrate sites of acute inflammation during bacterial infection [1, 2]. The migration of circulating neutrophils across the vascular endothelium at sites of inflammation is heavily dependent on host-derived CXC chemokines containing the tripeptide ELR. The primary receptors of these chemokines expressed by human neutrophils are CXCR1, which binds to CXCL6 and CXCL8, and CXCR2, which binds to CXCL1–3 and CXCL5–8 [3]. CXCR2, in particular, has been examined extensively in various animal models and in the clinic, as reviewed by Stadtmann and Zarbock [4]. CXCR2 in mouse neutrophils is very similar to human CXCR2 and binds to the chemokines KC, MIP-2, and LIX, functional homologs of the human ELR CXC chemokines [3, 5, 6]. CXCR1 expression has been reported in mice as well [7, 8], but whether this receptor is a functional analog of human CXCR1 is expressed by circulating neutrophils, and its physiologic ligand(s) are not well understood at this time.
Neutrophil recruitment can become impaired during various inflammatory disorders, including sepsis, trauma, and obesity, which correspond with a dramatic reduction in CXCR2 surface levels on circulating neutrophils [9–11]. CXCR2 undergoes a rapid down-regulation in expression by 2 mechanisms. The receptor is internalized via clatherin-coated vesicles upon ligand binding, which is then compartmentalized for degradation or recycled back to the cell surface for continued ligand binding, as reviewed in Stillie et al. [12]. In human neutrophils, CXCR2 also undergoes down-regulation upon cell activation by nonligand stimuli, and this process can be blocked by broad-spectrum metalloprotease inhibitors [13–15]. CXCR2 down-regulation in this manner, however, is poorly characterized, including the primary protease involved and its role in regulating neutrophil recruitment.
We show that ADAM17, a member of the ADAM family [16–18], is a key protease involved in CXCR2 down-regulation by mouse and human neutrophils following their activation with nonligand stimuli. This membrane-associated protease appears to regulate the cell-surface density of several receptors [19], and its enzymatic activity is rapidly induced upon neutrophil activation and apoptosis [20–24]. Our findings reveal for the first time that ADAM17 regulates CXCR2 surface levels in circulating neutrophils and their infiltration into sites of inflammation.
MATERIALS AND METHODS
Human subjects and animals
Peripheral blood collection from healthy individuals was performed in accordance with protocols approved by the Institutional Review Board at the University of Minnesota. Mice were housed in a specified pathogen-free facility, and procedures performed were done in accordance with protocols approved by the Animal Care and Use Committee of the University of Minnesota.
Mice used in this study were C57BL/6J mice, Adam17flox/flox (Adam17tm1.2Bbl/J) mice, and Vav1-Cre mice [B6.Cg-Tg(Vav1-cre)A2Kio/J] from The Jackson Laboratory (Bar Harbor, ME, USA). The Adam17flox/flox and Vav1-Cre mice were crossed to the C57BL/6J genetic background (both ≥98.4%) and then crossed together to generate Adam17flox/flox/Vav-Cre mice and littermate Adam17flox/flox mice. C57BL/6J, Adam17flox/flox, and Adam17flox/flox/Vav-Cre mice are referred to below as wild-type, control, and conditional ADAM17 knockout mice, respectively. ADAM17 knockout mice are embryonic or perinatal lethal [25, 26], whereas Adam17flox/flox/Vav-Cre mice, lacking ADAM17 in all leukocytes, are viable and lack any obvious developmental abnormalities [27–29].
Endotoxemia was induced in mice by administering Pseudomonas LPS (Sigma, St. Louis, MO, USA) i.p. at a dose of 5 mg/kg. After 4 h, mice were euthanized, and peritoneal lavage and blood samples were collected, as described previously [27, 29]. For blocking CXCR2 in vivo, mice were administered i.v. the selective inhibitor SB265610 (R&D Systems, Minneapolis, MN, USA) at a dose of 3 mg/kg or carrier alone (DMSO at an equal volume and dilution). For systemic inhibition of ADAM17, wild-type mice were administered the selective ADAM17 inhibitor BMS566394 (Bristol-Myers Squibb, Princeton, NJ, USA; referred to as inhibitor 32 in ref. [30]) at 33 mg/kg or an equal volume of carrier [10% N,N-dimethylacetamide (Sigma), 30% propylene glycol (Sigma), and 60% sterile water] by oral gavage. Both inhibitors were administered 30 min before LPS treatment.
Cell isolation and treatment
Human and mouse neutrophils were isolated as described previously [27, 29, 31]. Mouse leukocytes (0.5 × 106/ml in PBS without Ca+2 and Mg+2) were stimulated at the indicated concentrations with PMA (Sigma), formyl peptide receptor-like 1 agonist (EMD Millipore, Billerica, MA, USA), Pseudomonas LPS (Sigma), KC, or MIP-2 (PeproTech, Rocky Hill, NJ, USA). Human leukocytes (0.5 × 106/ml in PBS) were stimulated with formyl peptide receptor-like 1 agonist, Pseudomonas LPS, or IL-8/CXCL8 (PeproTech). Cell stimulation occurred for 30 min at 37°C in 5% CO2, which was stopped by extensive cell washing with PBS at 4°C. Human neutrophil apoptosis was induced by anti-human Fas mAb CH-11 (500 ng/ml), as described previously [22, 24, 31]. Some cells were preincubated for 30 min with the broad-spectrum metalloprotease inhibitor BB94 (Abcam, Cambridge, MA, USA) at 10 μM, the selective ADAM17 inhibitor BMS566394 at 5 μM, the anti-human ADAM17 function-blocking antibody D1(A12) at 35 nM (kindly provided by Dr. Gillian Murphy, University of Cambridge, Cambridge, United Kingdom), isotype-matched negative control antibody, or the selective CXCR2 inhibitor SB265610 (200 nM). To induce CXCR2 re-expression by recycling of the internalized receptor after neutrophil stimulation, cells (2 × 105/ml) were washed extensively with PBS and then incubated in recycling media, consisting of DMEM, 2% FBS, and 35 μM cycloheximide (Calbiochem, San Diego, CA, USA) to block de novo protein synthesis plus 10 μM BB94 to block metalloprotease activity for 180 min at 37°C in 5% CO2.
Flow cytometry
Flow cytometric analyses were performed on a FACSCanto instrument (BD Biosciences, San Jose, CA, USA), as described [24, 31]. Mouse CXCR2 was detected by the mAb TG11, and human CXCR1 and CXCR2 were detected by the mAb 8F1 and 5E8, respectively (BioLegend, San Diego, CA, USA). Mouse neutrophils were detected by their characteristic staining with mAb to Mac-1 (M1/70) and Ly-6G (RB6-8C5; BioLegend), as described previously [27, 29]. The appropriate isotype-matched negative control antibodies were purchased from the same source. Human neutrophils were identified based on their forward and side light-scattering characteristics, as performed previously [31, 32]. Externalized phosphatidylinositol on apoptotic cells was detected by fluorochrome-conjugated Annexin V, as per the manufacturer’s instructions (BD Biosciences).
Statistical analyses
Statistical analyses were performed by use of GraphPad Prism (GraphPad Software, La Jolla, CA, USA). After assessing for approximate normal distribution, all variables were summarized as mean ± sd, unless otherwise indicated. Comparison between 2 groups was done with Student’s t-test, with P < 0.05 taken as statistically significant.
RESULTS
Role of ADAM17 in regulating CXCR2 surface levels on neutrophils
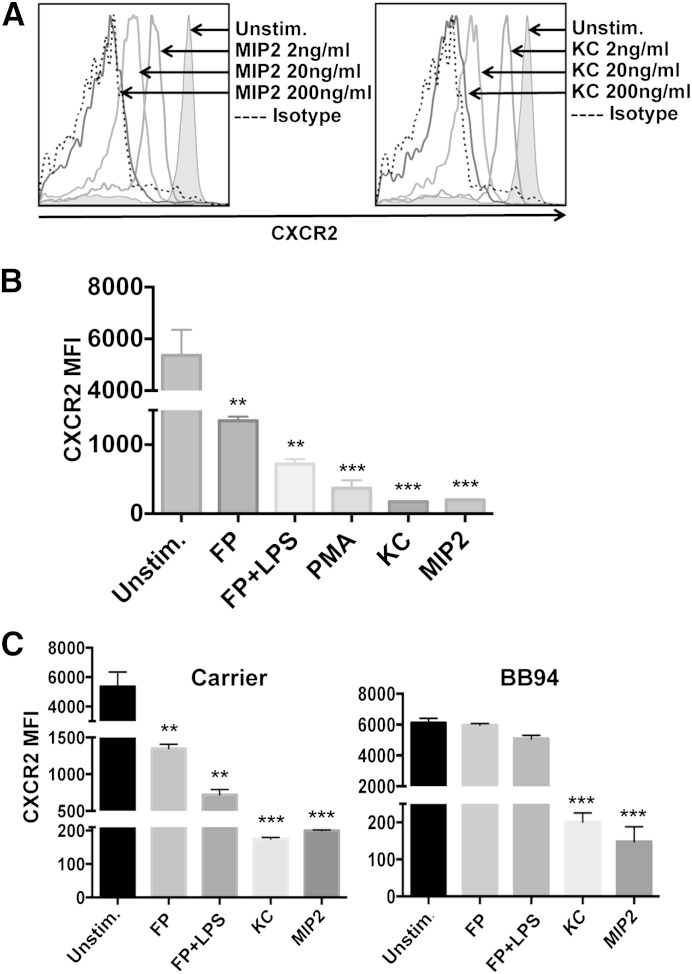
It is well described that ligand binding to mouse and human CXCR2 induces internalization of the receptor [12, 33–36]. Indeed, CXCR2 down-regulation from the surface of mouse neutrophils was very apparent by flow cytometry following their treatment with the chemokines KC and MIP-2 at various concentrations (Fig. 1A). Nonligand stimuli, including PMA and the PAMPs LPS and formyl peptide, also induced a significant down-regulation in CXCR2 surface levels (Fig. 1B). Such stimuli have been reported to induce CXCR2 down-regulation in human neutrophils by a metalloprotease activity [13–15]. We found that the broad-spectrum metalloprotease inhibitor BB94 significantly blocked CXCR2 down-regulation in mouse neutrophils when treated with nonligand stimuli but not with ligand stimuli (Fig. 1C). This is the first demonstration that we are aware of that mouse CXCR2 is regulated by a metalloprotease as well.
Figure 1. CXCR2 regulation in neutrophils by ligand and nonligand stimuli. (A) Peripheral blood neutrophils from wild-type mice were unstimulated (Unstim.) or treated with KC or MIP-2 at the indicated concentrations for 30 min at 37°C. Isotype-negative control antibody staining is indicated by a dotted line. The x-axis = Log 10 fluorescence, and the y-axis = cell number. Flow cytometric data are representative of 3 independent experiments by use of leukocytes from separate mice. (B) Neutrophils were treated with or without KC (200 ng/ml), MIP-2 (200 ng/ml), PMA (200 ng/ml), formyl peptide (FP; 1 μg/ml) or formyl peptide and LPS (1 μg/ml each) for 30 min at 37°C. (C) Neutrophils were stimulated as indicated in the presence of the broad-spectrum metalloprotease inhibitor BB94 (10 μM) or carrier (appropriate DMSO dilution) for 30 min at 37°C. Relative cell-staining levels of CXCR2 antibodies were determined by flow cytometry. MFI, Mean fluorescence intensity. The bar graphs show mean ± sd for at least 3 mice/treatment. Statistical significance is indicated as **P < 0.01, and ***P < 0.001 vs. unstimulated.
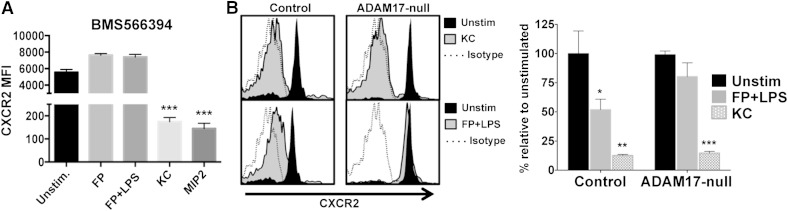
The membrane-associated metalloprotease ADAM17 modulates the surface density of several receptors on neutrophils [20, 23, 28, 31, 37]. Therefore, we examined the effects of blocking its function on CXCR2 down-regulation upon mouse neutrophil activation. Of interest is that the ADAM17 inhibitor BMS566394, which has a potency orders of magnitude higher for ADAM17 than other metalloproteases [30], effectively blocked CXCR2 down-regulation upon neutrophil activation but not with ligand stimuli (Fig. 2A). We also examined neutrophils from ADAM17 conditional knockout mice that lack the protease in all leukocytes [28, 29]. We found that ADAM17-null neutrophils and control neutrophils down-regulated their CXCR2 in an equivalent manner following ligand stimulation, whereas only the control neutrophils down-regulated CXCR2 upon their activation with nonligand stimuli (Fig. 2B).
Figure 2. ADAM17 inhibition blocks CXCR2 down-regulation by nonligand stimuli. (A) Peripheral blood neutrophils from wild-type mice were stimulated as indicated in the presence of the selective ADAM17 inhibitor BMS566394 (5 μM) for 30 min at 37°C. (B) Peripheral blood neutrophils from control and ADAM17 conditional knockout mice were stimulated as indicated for 30 min at 37°C. (Left histograms) Representative data; (right bar graph) cumulative data. For the histograms, negative control antibody staining is indicated by a dotted line, the x-axis = Log 10 fluorescence, and the y-axis = cell number. The bar graphs show mean ± sd for at least 3 mice/treatment. Statistical significance is indicated as *P < 0.05, **P < 0.01, and ***P < 0.001 vs. unstimulated.
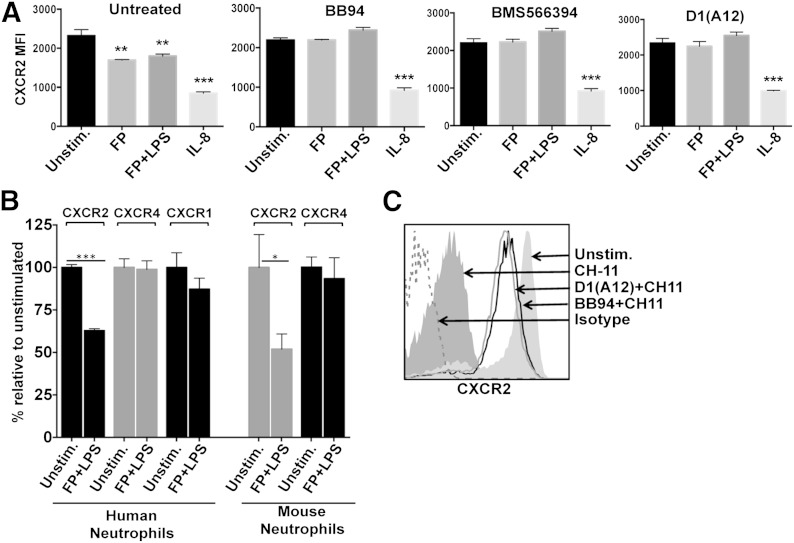
We next examined whether ADAM17 regulated CXCR2 in human neutrophils. In Fig. 3A we show that ligand (IL-8/CXCL8) and nonligand (LPS and formyl peptide) stimuli induced CXCR2 down-regulation and that only the latter was blocked significantly by a broad-spectrum metalloprotease inhibitor. We also found that the ADAM17 inhibitor BMS566394 blocked CXCR2 down-regulation upon neutrophil activation (Fig. 3A). This occurred at a level equivalent to the broad-spectrum inhibitor (Fig. 3A), suggesting that ADAM17 is the primary metalloprotease involved. To assess more directly the role of ADAM17, we treated neutrophils with D1(A12), the only published function-blocking antibody to human ADAM17, generated by phage display, which is highly selective as a result of being engineered to recognize 2 distinct epitopes in ADAM17 [31, 38]. D1(A12) blocked CXCR2 down-regulation upon neutrophil activation similar to the inhibitors (Fig. 3A).
Figure 3. ADAM17 regulates human CXCR2. (A) Isolated human peripheral blood neutrophils were unstimulated or treated with IL-8 (200 ng/ml), formyl peptide (1 μg/ml), or formyl peptide and LPS (1 μg/ml each) in the presence or absence of the broad-spectrum metalloprotease inhibitor BB94 (10 μM), the selective ADAM17 inhibitor BMS566394 (5 μM), or the ADAM17 antibody D1(A12) (35 nM) for 30 min at 37°C. (B) Mouse bone marrow neutrophils and human peripheral blood neutrophils were unstimulated or treated with formyl peptide and LPS for 30 min at 37°C. Relative cell-staining levels of CXCR1, CXCR2, or CXCR4 antibodies were determined by flow cytometry. (C) Human neutrophils were treated with the anti-Fas antibody CH-11 (500 ng/ml) in the presence or absence of BB94 (10 μM) or D1(A12) (35 nM) for 6 h at 37°C. Cell staining with antibody to detect CXCR2 was examined by flow cytometry. Cell apoptosis was assessed by Annexin V reactivity (data not shown). Negative control antibody staining is indicated by a dotted line. The x-axis = Log 10 fluorescence, and the y-axis = cell number. Data are representative of 3 independent experiments by use of leukocytes from separate donors. The bar graphs show mean ± sd of at least 3 mice or humans/treatment. Statistical significance is indicated as *P < 0.05, **P < 0.01, and ***P < 0.001 vs. unstimulated.
CXCR1 on human neutrophils has also been reported to be down-regulated upon neutrophil activation [14]. Similar to CXCR2, CXCR1 was greatly down-regulated in expression when neutrophils were treated with IL-8 (data not shown); however, CXCR1 down-regulation was very inconsistent when neutrophils were activated by nonligand stimuli (Fig. 3B). Others have reported the involvement of serine proteases in cleaving CXCR1 from the surface of neutrophils in the lungs of patients with cystic fibrosis [39], indicating that this receptor is regulated by proteases other than ADAM17. The activation of mouse and human neutrophils also did not cause a rapid down-regulation in the surface levels of CXCR4 (Fig. 3B), and thus, ADAM17 induction does not broadly promote chemokine receptor down-regulation.
In addition to neutrophil activation, engagement of the death receptor Fas on these cells induces ADAM17 activity [22–24], which involves a distinct cell-signaling pathway [24]. We show that Fas engagement caused a rapid down-regulation in CXCR2 expression, and this was largely blocked by ADAM17 inhibition (Fig. 3C). The blocking of ADAM17, however, did not impede neutrophil apoptosis, as reported previously [22–24]. Taken together, the above findings reveal that ADAM17 participates in regulating the surface density of CXCR2 on mouse and human neutrophils. In contrast, some ADAM17 substrates are human specific, including CD16 and IL-6R [28, 31, 40].
Reversible and nonreversible CXCR2 regulation
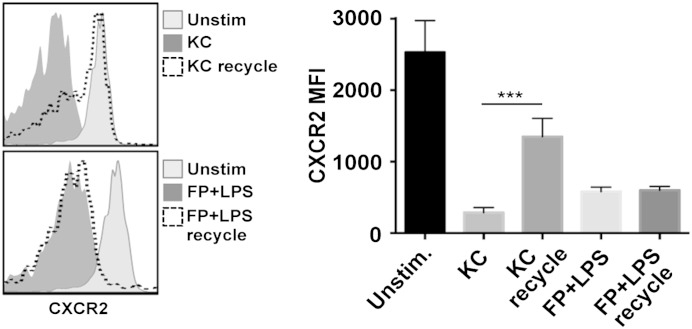
CXCR2 internalization following ligand binding results in its degradation and/or transport to a recycling compartment for re-expression [3]. CXCR2 recycling back to the cell surface can be assessed by washing neutrophils to remove ligand stimuli and then incubating the cells in the presence of cycloheximide to block de novo protein synthesis [41, 42]. Following this procedure, we observed considerable CXCR2 re-expression by mouse neutrophils treated with chemokine stimuli, whereas CXCR2 re-expression did not occur in neutrophils treated with nonligand stimuli (Fig. 4). This was observed in human neutrophils as well (data not shown). Hence, nonligand and ligand stimuli both promote CXCR2 down-regulation, but only the latter process is reversible.
Figure 4. Cell-surface CXCR2 undergoes recycling with ligand stimuli but not nonligand stimuli. Mouse peripheral blood neutrophils were stimulated with KC or formyl peptide and LPS for 30 min at 37°C. Stimuli were removed by extensive cell washing, and the cells were then cultured in the presence of cycloheximide (35 μM) and 10 μM BB94 for 180 min at 37°C for CXCR2 re-expression from the recycling compartment (recycle), as described in Materials and Methods. (Left histograms) Representative data; (right bar graph) cumulative data. Relative cell-staining levels of CXCR2 antibodies were determined by flow cytometry. For the histograms, the x-axis = Log 10 fluorescence, and the y-axis = cell number. The bar graph shows mean ± sd of 4 mice/treatment. Statistical significance is indicated as ***P < 0.001.
Regulation of CXCR2 by ADAM17 in vivo
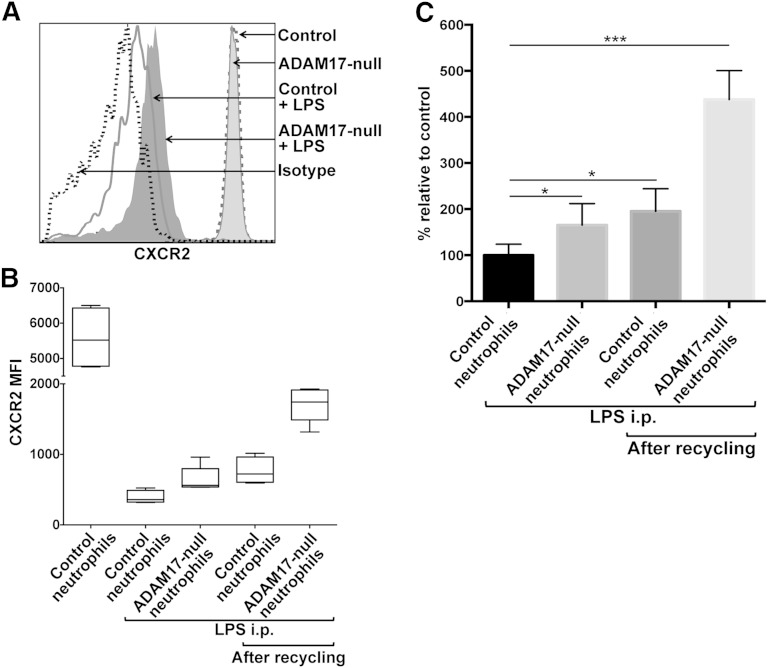
Cell-surface levels of CXCR2 on circulating neutrophils can undergo considerable down-regulation in expression during various inflammatory disorders, promoting neutrophil dysfunction [9–11]. We examined the down-regulation of CXCR2 on circulating neutrophils in conditional ADAM17 knockout and control mice during acute inflammation. Peripheral blood neutrophils from both groups of mice expressed similar levels of CXCR2 before LPS-induced inflammation (Fig. 5A). Following i.p. injection of LPS, CXCR2 surface levels underwent a marked down-regulation on circulating neutrophils in control mice (Fig. 5A and B), as others have reported [43]. Interestingly, circulating neutrophils in conditional ADAM17 knockout mice treated with LPS expressed higher levels of CXCR2 (Fig. 5A and B). Although CXCR2 surface levels were significantly higher on ADAM17-null neutrophils, the receptor still underwent a considerable down-regulation in expression during inflammation (Fig. 5A and B). In Fig. 2, we show that the blocking of ADAM17 does not affect CXCR2 down-regulation by ligand stimuli. KC and MIP-2 are produced in vivo within minutes following LPS administration [43], and their presence is going to confound evaluating the role of ADAM17 in regulating CXCR2 surface levels. We postulated that by blocking ADAM17, we should increase the levels of CXCR2 that undergo internalization upon binding ligand and thus, the levels of CXCR2 in the recycling compartment. To address this, peripheral blood leukocytes from control and conditional ADAM17 knockout mice following LPS administration were washed and incubated at 37°C in the presence of the protein synthesis inhibitor cycloheximide to facilitate CXCR2 re-expression by recycling. After this process, we observed that ADAM17-null neutrophils expressed substantially higher surface levels of CXCR2 than control neutrophils (Fig. 5B). In Fig. 5C, data are presented as a percent relative to control neutrophils before recycling. ADAM17-null neutrophils before recycling expressed 1.7-fold higher levels of CXCR2 than control neutrophils in LPS-treated mice, whereas after recycling, these cells expressed 4.4-fold higher levels of CXCR2. Control neutrophils after recycling also expressed higher levels of CXCR2, but this increase (1.9-fold) was much less than that for ADAM17-null neutrophils (Fig. 5C). Hence, circulating ADAM17-null neutrophils during inflammation maintained higher levels of CXCR2 on their surface and in the recycling compartment, which is available for re-expression and ligand binding.
Figure 5. ADAM17 regulates the surface levels of CXCR2 in vivo. (A) Control and conditional ADAM17-knockout mice were administered LPS i.p. (5 mg/kg). CXCR2 surface levels on peripheral blood neutrophils from both groups of mice were determined by flow cytometry, before and after (4 h) LPS administration. Isotype-negative control antibody staining is indicated by a dotted line, the x-axis = Log 10 fluorescence, and the y-axis = cell number. (B) Some cells were subjected to CXCR2 recycling, as described in Fig. 4. The mean fluorescence intensities of CXCR2 staining for control and ADAM17-null neutrophils are displayed as box-whisker plots of 5 independent experiments, which depict the smallest value, lower quartile, median, upper quartile, and largest value. (C) For mice that were administered LPS, CXCR2 mean fluorescence intensity data are presented as a percent change relative to control neutrophils before recycling. The bar graph shows mean ± sd of 5 mice/group (control or conditional ADAM17-knockout mice). Statistical significance is indicated as *P < 0.05, and ***P < 0.001.
The targeting of ADAM17 increases neutrophil recruitment and involves CXCR2
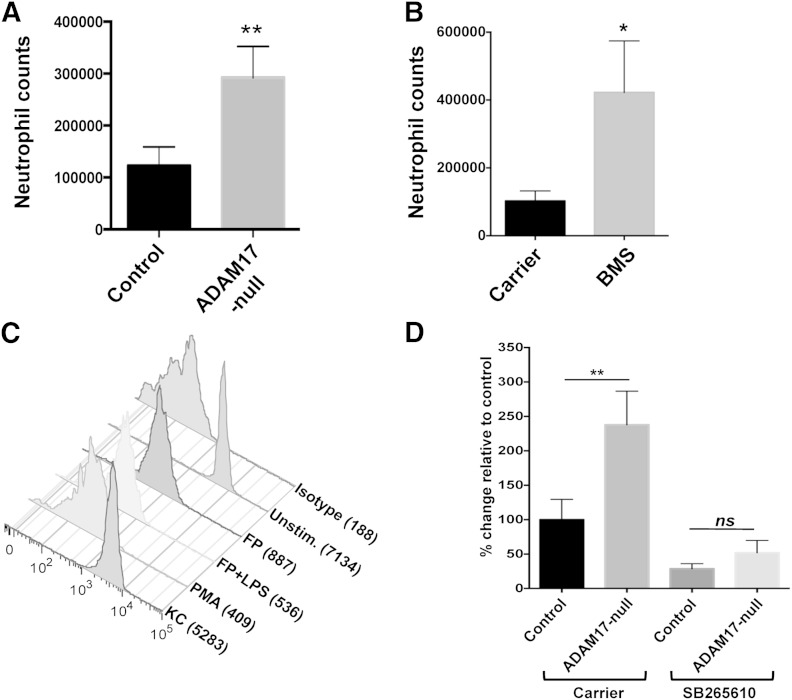
Consistent with the above results, we found that significantly greater numbers of neutrophils infiltrated the peritoneal cavity of conditional ADAM17 knockout mice than control mice following i.p. LPS administration (Fig. 6A). This was not the result of a greater proportion or number of circulating neutrophils in conditional ADAM17 knockout mice, as we and others have shown that these levels are similar in ADAM17-null and control mice [28, 32, 44]. Other potential developmental differences in conditional ADAM17 knockout mice are unlikely to have contributed to the enhanced recruitment of neutrophils, as short-term inhibition of ADAM17 by BMS566394 administration also significantly increased neutrophil infiltration into the peritoneal cavity following i.p. administration of LPS (Fig. 6B).
Figure 6. Enhanced recruitment of ADAM17-null neutrophils involves CXCR2. (A) Control and conditional ADAM17-knockout mice were administered LPS i.p. (5 mg/kg). (B) Wild-type mice were administered the ADAM17 inhibitor BMS566394 (BMS; 33 mg/kg) or carrier (see Materials and Methods) and then LPS i.p., 30 min later. After 4 h, neutrophil levels in the peritoneal cavity of all mice were enumerated. The bar graph shows mean ± sd of 3 mice/group or treatment. Control, conditional ADAM17-knockout, and wild-type mice had few, if any, peritoneal neutrophils before LPS administration (data not shown). (C) Mouse peripheral blood neutrophils were stimulated as described in Fig. 1B but in the presence of the CXCR2 inhibitor SB265610 (200 nM) for 30 min at 37°C, which selectively blocked CXCR2 down-regulation by KC-stimulated neutrophils. Negative control antibody staining of untreated cells is indicated (Isotype). Mean fluorescence intensity is indicated in parentheses. The y-axis = cell number. Flow cytometry data are representative of 3 independent experiments by use of leukocytes from separate mice. (D) Control and conditional ADAM17-knockout mice were administered the CXCR2 inhibitor SB265610 (3 mg/kg) or carrier (see Materials and Methods) i.v., 30 min before LPS treatment. After 4 h, peritoneal neutrophil levels were determined. Results are expressed as mean ± sd of 4 mice in each group/treatment. Statistical significance is indicated as *P < 0.05, and **P < 0.01.
To investigate the effects of higher levels of CXCR2 in ADAM17-null neutrophils on their augmented recruitment into the inflamed peritoneal cavity, we treated conditional ADAM17 knockout and control mice with the CXCR2 inhibitor SB265610, which blocks chemokine binding [12, 45]. Indeed, the efficient down-regulation of CXCR2 that occurs upon ligand binding, as shown in Fig. 1, was blocked when neutrophils were treated with SB265610, whereas CXCR2 down-regulation by nonligand stimuli was not (Fig. 6C). Conditional ADAM17 knockout and control mice were administered SB265610 i.v. and then LPS i.p., 30 min later. The CXCR2 inhibitor did not reduce circulating levels of neutrophils in either group of mice (data not shown), but it did decrease their infiltration into the inflamed peritoneal cavity (Fig. 6D). When mice received carrier and then LPS, peritoneal neutrophils levels were significantly higher in conditional ADAM17 knockout mice than control mice, whereas for mice treated with SB265610, peritoneal neutrophil levels were essentially equalized in both groups of mice (Fig. 6D). These findings indicate that the higher levels of CXCR2 in ADAM17-null neutrophils were a key factor in their enhanced recruitment at the inflammatory locus.
DISCUSSION
The surface levels of CXCR2 on circulating neutrophils can be down-regulated rapidly by internalization upon ligand binding [12] and by metalloprotease activity following neutrophil stimulation with various nonligand stimuli [13–15]. Our data demonstrate for the first time that ADAM17 is the primary metalloprotease involved in the latter process. Although a large number of ADAM17 substrates have been reported, only a small subset has been assessed in primary cells and their functional effects examined in vivo [19]. With the use of conditional ADAM17 knockout mice, we provide in vivo evidence that ADAM17 regulates the surface density of CXCR2 on circulating neutrophils and their recruitment during inflammation. Indeed, ADAM17-null neutrophils infiltrated the inflamed peritoneal cavity at much greater levels than control neutrophils, which was largely reversed by a CXCR2 inhibitor. An important distinction between CXCR2 down-regulation by internalization and ADAM17 induction is that the former process is transient, as the receptor can be recycled back to the cell surface, whereas the latter process is irreversible. Therefore, ADAM17-mediated CXCR2 down-regulation may serve as a master switch to diminish neutrophil stimulation by CXCR2 ligands. For instance, once neutrophils encounter bacteria at a site of infection, their overt activation (e.g., PAMP recognition) may promote CXCR2 down-regulation by ADAM17 to narrow the neutrophil response to pathogen chemoattractants. The induction of apoptosis is another example of when it may be beneficial to induce ADAM17 and CXCR2 down-regulation to impede neutrophil movement and facilitate their engulfment by macrophages. At this time, it is unclear whether ADAM17 mediates CXCR2 down-regulation by a direct or indirect process, which is a focus of our ongoing studies. If CXCR2 were cleaved directly by ADAM17, then the receptor would be an unusual substrate, considering it is a seven-transmembrane protein.
ADAM17 regulates the cell-surface density of other receptors on neutrophils that participate in directing these cells into sites of inflammation. The adhesion protein L-selectin is a well-described ADAM17 substrate in neutrophils [20, 25, 28], and blocking its shedding has also been shown to enhance neutrophil adhesion and accumulation in models of inflammation and bacterial infection [27, 29, 44]. Human CD16b has been reported recently to be an ADAM17 substrate as well [31], and others have reported the involvement of this receptor in neutrophil attachment to the vascular endothelium [46, 47]. We contend that the disruption of the down-regulation of L-selectin, CD16b, or CXCR2 upon targeting ADAM17 in activated neutrophils is not a mutually exclusive event in terms of enhancing neutrophil recruitment. The individual contribution of these receptors, however, will likely vary, depending on the inflammatory event and tissue involved. Thus, ADAM17 appears to regulate various aspects of the multistep process of neutrophil migration out of blood vessels at sites of inflammation.
In conclusion, CXCR2, on circulating neutrophils, undergoes a marked down-regulation in expression in humans and in mice during various inflammatory disorders, which is associated with impaired neutrophil function [9–11]. Our findings reveal that ADAM17 overactivation contributes to CXCR2 down-regulation and neutrophil dysfunction. In consideration of this and the other substrates of ADAM17, the protease may provide an important therapeutic target to bolster neutrophil infiltration and pathogen clearance during immunosuppressive inflammation.
ACKNOWLEDGMENTS
This work was supported by funding from the U.S. National Institutes of Health (Grants AI103328 and AI107543). The authors thank Dr. Yue Wang for her contributions to this study.
Glossary
- ADAM17
a disintegrin and metalloprotease-17
- ELR
Glu-Leu-Arg
- KC
keratinocyte-derived chemokine
- PAMP
pathogen-associated molecular pattern
AUTHORSHIP
H.K.M. designed and performed the research, analyzed data, and helped with manuscript preparation. C.L. and N.S.B. helped perform the research. B.W. designed research, analyzed and interpreted data, and wrote the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182. [DOI] [PubMed] [Google Scholar]
- 2.Mayadas T. N., Cullere X., Lowell C. A. (2014) The multifaceted functions of neutrophils. Annu. Rev. Pathol. 9, 181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadik C. D., Kim N. D., Luster A. D. (2011) Neutrophils cascading their way to inflammation. Trends Immunol. 32, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stadtmann A., Zarbock A. (2012) CXCR2: from bench to bedside. Front. Immunol. 3, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cacalano G., Lee J., Kikly K., Ryan A. M., Pitts-Meek S., Hultgren B., Wood W. I., Moore M. W. (1994) Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science 265, 682–684. [DOI] [PubMed] [Google Scholar]
- 6.Gonçalves A. S., Appelberg R. (2002) The involvement of the chemokine receptor CXCR2 in neutrophil recruitment in LPS-induced inflammation and in Mycobacterium avium infection. Scand. J. Immunol. 55, 585–591. [DOI] [PubMed] [Google Scholar]
- 7.Fu W., Zhang Y., Zhang J., Chen W. F. (2005) Cloning and characterization of mouse homolog of the CXC chemokine receptor CXCR1. Cytokine 31, 9–17. [DOI] [PubMed] [Google Scholar]
- 8.Fan X., Patera A. C., Pong-Kennedy A., Deno G., Gonsiorek W., Manfra D. J., Vassileva G., Zeng M., Jackson C., Sullivan L., Sharif-Rodriguez W., Opdenakker G., Van Damme J., Hedrick J. A., Lundell D., Lira S. A., Hipkin R. W. (2007) Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J. Biol. Chem. 282, 11658–11666. [DOI] [PubMed] [Google Scholar]
- 9.Quaid G. A., Cave C., Robinson C., Williams M. A., Solomkin J. S. (1999) Preferential loss of CXCR-2 receptor expression and function in patients who have undergone trauma. Arch. Surg. 134, 1367–1371, discussion 1371–1372. [DOI] [PubMed] [Google Scholar]
- 10.Alves-Filho J. C., Spiller F., Cunha F. Q. (2010) Neutrophil paralysis in sepsis. Shock 34 (Suppl 1), 15–21. [DOI] [PubMed] [Google Scholar]
- 11.Kordonowy L. L., Burg E., Lenox C. C., Gauthier L. M., Petty J. M., Antkowiak M., Palvinskaya T., Ubags N., Rincón M., Dixon A. E., Vemooy J. H., Fessler M. B., Poynter M. E., Suratt B. T. (2012) Obesity is associated with neutrophil dysfunction and attenuation of murine acute lung injury. Am. J. Respir. Cell Mol. Biol. 47, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stillie R., Farooq S. M., Gordon J. R., Stadnyk A. W. (2009) The functional significance behind expressing two IL-8 receptor types on PMN. J. Leukoc. Biol. 86, 529–543. [DOI] [PubMed] [Google Scholar]
- 13.Khandaker M. H., Xu L., Rahimpour R., Mitchell G., DeVries M. E., Pickering J. G., Singhal S. K., Feldman R. D., Kelvin D. J. (1998) CXCR1 and CXCR2 are rapidly down-modulated by bacterial endotoxin through a unique agonist-independent, tyrosine kinase-dependent mechanism. J. Immunol. 161, 1930–1938. [PubMed] [Google Scholar]
- 14.Khandaker M. H., Mitchell G., Xu L., Andrews J. D., Singh R., Leung H., Madrenas J., Ferguson S. S., Feldman R. D., Kelvin D. J. (1999) Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood 93, 2173–2185. [PubMed] [Google Scholar]
- 15.Doroshenko T., Chaly Y., Savitskiy V., Maslakova O., Portyanko A., Gorudko I., Voitenok N. N. (2002) Phagocytosing neutrophils down-regulate the expression of chemokine receptors CXCR1 and CXCR2. Blood 100, 2668–2671. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann M., Herrlich A., Herrlich P. (2013) Who decides when to cleave an ectodomain? Trends Biochem. Sci. 38, 111–120. [DOI] [PubMed] [Google Scholar]
- 17.Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S., Nelson N., Boiani N., Schooley K. A., Gerhart M., Davis R., Fitzner J. N., Johnson R. S., Paxton R. J., March C. J., Cerretti D. P. (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385, 729–733. [DOI] [PubMed] [Google Scholar]
- 18.Moss M. L., Jin S. L., Milla M. E., Bickett D. M., Burkhart W., Carter H. L., Chen W. J., Clay W. C., Didsbury J. R., Hassler D., Hoffman C. R., Kost T. A., Lambert M. H., Leesnitzer M. A., McCauley P., McGeehan G., Mitchell J., Moyer M., Pahel G., Rocque W., Overton L. K., Schoenen F., Seaton T., Su J. L., Warner J., Willard D., Becherer J. D. (1997) Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 385, 733–736. [DOI] [PubMed] [Google Scholar]
- 19.Scheller J., Chalaris A., Garbers C., Rose-John S. (2011) ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 32, 380–387. [DOI] [PubMed] [Google Scholar]
- 20.Li Y., Brazzell J., Herrera A., Walcheck B. (2006) ADAM17 deficiency by mature neutrophils has differential effects on L-selectin shedding. Blood 108, 2275–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Herrera A. H., Li Y., Belani K. K., Walcheck B. (2009) Regulation of mature ADAM17 by redox agents for L-selectin shedding. J. Immunol. 182, 2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Zhang A. C., Ni Z., Herrera A., Walcheck B. (2010) ADAM17 activity and other mechanisms of soluble L-selectin production during death receptor-induced leukocyte apoptosis. J. Immunol. 184, 4447–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalaris A., Adam N., Sina C., Rosenstiel P., Lehmann-Koch J., Schirmacher P., Hartmann D., Cichy J., Gavrilova O., Schreiber S., Jostock T., Matthews V., Häsler R., Becker C., Neurath M. F., Reiss K., Saftig P., Scheller J., Rose-John S. (2010) Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J. Exp. Med. 207, 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Robertson J. D., Walcheck B. (2011) Different signaling pathways stimulate a disintegrin and metalloprotease-17 (ADAM17) in neutrophils during apoptosis and activation. J. Biol. Chem. 286, 38980–38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., Boyce R. W., Nelson N., Kozlosky C. J., Wolfson M. F., Rauch C. T., Cerretti D. P., Paxton R. J., March C. J., Black R. A. (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi K., Kimura T., Miyamoto T., Takaishi H., Okada Y., Toyama Y., Blobel C. P. (2007) Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J. Immunol. 179, 2686–2689. [DOI] [PubMed] [Google Scholar]
- 27.Long C., Wang Y., Herrera A. H., Horiuchi K., Walcheck B. (2010) In vivo role of leukocyte ADAM17 in the inflammatory and host responses during E. coli-mediated peritonitis. J. Leukoc. Biol. 87, 1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arndt P. G., Strahan B., Wang Y., Long C., Horiuchi K., Walcheck B. (2011) Leukocyte ADAM17 regulates acute pulmonary inflammation. PLoS ONE 6, e19938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long C., Hosseinkhani M. R., Wang Y., Sriramarao P., Walcheck B. (2012) ADAM17 activation in circulating neutrophils following bacterial challenge impairs their recruitment. J. Leukoc. Biol. 92, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ott G. R., Asakawa N., Lu Z., Anand R., Liu R. Q., Covington M. B., Vaddi K., Qian M., Newton R. C., Christ D. D., Trzaskos J. M., Duan J. J. (2008) Potent, exceptionally selective, orally bioavailable inhibitors of TNF-alpha converting enzyme (TACE): novel 2-substituted-1H-benzo[d]imidazol-1-yl)methyl)benzamide P1′ substituents. Bioorg. Med. Chem. Lett. 18, 1577–1582. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Wu J., Newton R., Bahaie N. S., Long C., Walcheck B. (2013) ADAM17 cleaves CD16b (FcγRIIIb) in human neutrophils. Biochim. Biophys. Acta 1833, 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walcheck B., Herrera A. H., St Hill C., Mattila P. E., Whitney A. R., Deleo F. R. (2006) ADAM17 activity during human neutrophil activation and apoptosis. Eur. J. Immunol. 36, 968–976. [DOI] [PubMed] [Google Scholar]
- 33.Samanta A. K., Oppenheim J. J., Matsushima K. (1990) Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J. Biol. Chem. 265, 183–189. [PubMed] [Google Scholar]
- 34.Chuntharapai A., Kim K. J. (1995) Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J. Immunol. 155, 2587–2594. [PubMed] [Google Scholar]
- 35.Mueller S. G., White J. R., Schraw W. P., Lam V., Richmond A. (1997) Ligand-induced desensitization of the human CXC chemokine receptor-2 is modulated by multiple serine residues in the carboxyl-terminal domain of the receptor. J. Biol. Chem. 272, 8207–8214. [DOI] [PubMed] [Google Scholar]
- 36.Yang W., Wang D., Richmond A. (1999) Role of clathrin-mediated endocytosis in CXCR2 sequestration, resensitization, and signal transduction. J. Biol. Chem. 274, 11328–11333. [DOI] [PubMed] [Google Scholar]
- 37.Bell J. H., Herrera A. H., Li Y., Walcheck B. (2007) Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J. Leukoc. Biol. 82, 173–176. [DOI] [PubMed] [Google Scholar]
- 38.Tape C. J., Willems S. H., Dombernowsky S. L., Stanley P. L., Fogarasi M., Ouwehand W., McCafferty J., Murphy G. (2011) Cross-domain inhibition of TACE ectodomain. Proc. Natl. Acad. Sci. USA 108, 5578–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartl D., Latzin P., Hordijk P., Marcos V., Rudolph C., Woischnik M., Krauss-Etschmann S., Koller B., Reinhardt D., Roscher A. A., Roos D., Griese M. (2007) Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat. Med. 13, 1423–1430. [DOI] [PubMed] [Google Scholar]
- 40.Garbers C., Jänner N., Chalaris A., Moss M. L., Floss D. M., Meyer D., Koch-Nolte F., Rose-John S., Scheller J. (2011) Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J. Biol. Chem. 286, 14804–14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feniger-Barish R., Ran M., Zaslaver A., Ben-Baruch A. (1999) Differential modes of regulation of CXC chemokine-induced internalization and recycling of human CXCR1 and CXCR2. Cytokine 11, 996–1009. [DOI] [PubMed] [Google Scholar]
- 42.Zaslaver A., Feniger-Barish R., Ben-Baruch A. (2001) Actin filaments are involved in the regulation of trafficking of two closely related chemokine receptors, CXCR1 and CXCR2. J. Immunol. 166, 1272–1284. [DOI] [PubMed] [Google Scholar]
- 43.Kesteman N., Vansanten G., Pajak B., Goyert S. M., Moser M. (2008) Injection of lipopolysaccharide induces the migration of splenic neutrophils to the T cell area of the white pulp: role of CD14 and CXC chemokines. J. Leukoc. Biol. 83, 640–647. [DOI] [PubMed] [Google Scholar]
- 44.Tang J., Zarbock A., Gomez I., Wilson C. L., Lefort C. T., Stadtmann A., Bell B., Huang L.-C., Ley K., Raines E. W. (2011) Adam17-dependent shedding limits early neutrophil influx but does not alter early monocyte recruitment to inflammatory sites. Blood 118, 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auten R. L., Richardson R. M., White J. R., Mason S. N., Vozzelli M. A., Whorton M. H. (2001) Nonpeptide CXCR2 antagonist prevents neutrophil accumulation in hyperoxia-exposed newborn rats. J. Pharmacol. Exp. Ther. 299, 90–95. [PubMed] [Google Scholar]
- 46.D’Arrigo C., Candal-Couto J. J., Greer M., Veale D. J., Woof J. M. (1995) Human neutrophil Fc receptor-mediated adhesion under flow: a hollow fibre model of intravascular arrest. Clin. Exp. Immunol. 100, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuboi N., Asano K., Lauterbach M., Mayadas T. N. (2008) Human neutrophil Fcgamma receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity 28, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]