M-MDSCs inhibit autoreactive T and B cell function; reconstitution of CCR2-deficient or wild-type mice with M-MDSCs ameliorates autoimmune arthritis.
Keywords: autoimmune arthritis, collagen-induced arthritis, rheumatoid arthritis, immunotherapy, chemokine receptor
Abstract
MDSCs are a heterogeneous group of myeloid cells that suppress T cell activity in cancer and autoimmune disease. The effect of MDSCs on B cell function is not clear. Using the CIA model of autoimmune disease, we found an increase in M-MDSCs in the periphery of WT mice with CIA compared with naïve mice. These MDSCs were absent from the periphery of CCR2−/− mice that developed exacerbated disease. M-MDSCs, isolated from immunized mice, inhibited autologous CD4+ T cell proliferation. The M-MDSC-mediated suppression of T cell proliferation was NO and IFN-γ dependent but IL-17 independent. Furthermore, we demonstrated for the first time that M-MDSCs from CIA mice also inhibited autologous B cell proliferation and antibody production. The suppression of B cells by M-MDSCs was dependent on the production of NO and PGE2 and required cell–cell contact. Administration of M-MDSCs rescued CCR2−/− mice from the exacerbated CIA phenotype and ameliorated disease in WT mice. Furthermore, adoptive transfer of M-MDSCs reduced autoantibody production by CCR2−/− and WT mice. In summary, M-MDSCs inhibit T cell and B cell function in CIA and may serve as a therapeutic approach in the treatment of autoimmune arthritis.
Introduction
MDSCs are a diverse group of immature cells that suppress T cell responses [1]. MDSCs are derived from the bone marrow and arise from a delay in maturation, prompted by pathologic conditions. Initially, MDSCs were defined as cells expressing CD11b and Gr-1 [2, 3]. As the Gr-1+ population of cells is composed of monocytic and granulocytic cells, MDSCs are now divided into 2 subsets: M-MDSCs, identified as CD11b+Ly6C+Ly6G− cells, and G-MDSCs, described as CD11b+Ly6C+Ly6G+ cells [4, 5]. M-MDSC-mediated inhibition of T cell activities commonly results from up-regulation of iNOS expression, whereas G-MDSCs inhibit T cell function mainly through arginase-1 enzyme activity [6].
Although much of what is known about the suppressive function of MDSCs was derived from studies of cancer patients and animal tumor models [7], researchers have recently shown that MDSCs also play a role in autoimmune diseases. In an autoimmune hepatitis model, CD11b+Gr-1+ cells accumulated in the liver and suppressed CD4+ T cell responses [8]. An inflammatory bowel disease model showed that CD8+ T cell responses were inhibited in the intestines of diseased animals after administration of splenic MDSCs [9]. In EAE, the mouse model of multiple sclerosis, M-MDSCs and G-MDSCs were able to inhibit CD4+ T cell proliferation in vitro and ameliorated disease upon adoptive transfer [10, 11]. Whereas T cells are critical in autoimmune pathogenesis, B cells play a fundamental role in the initiation and perpetuation of autoimmunity [12, 13]. However, the impact of MDSCs on B cell function during the autoimmune process is not clear. Unraveling contributions of MDSCs to the regulation of autoimmunity will further the understanding of disease mechanisms and aid in the discovery of alternative approaches to treat autoimmune diseases.
RA is an autoimmune disease that causes joint inflammation, cartilage damage, and bone erosion [14]. Our previous data revealed that a monocyte subset is absent in the periphery of CCR2−/− mice with CIA, an animal model of RA [15]. These mice developed an exacerbated arthritis phenotype compared with WT controls [15, 16]. Therefore, we hypothesized that the monocytes absent from CIA CCR2−/− mice are MDSCs that regulate autoimmune arthritis. To test this hypothesis and further study the role of MDSCs in autoimmune disease, we examined the phenotype and function of MDSCs in WT and CCR2−/− mice with CIA. Our data demonstrate that M-MDSCs isolated from collagen-immunized mice are able to suppress CD4+ T cell proliferation. We also show for the first time that M-MDSCs inhibit B cell proliferation and antibody production during autoimmune arthritis. Finally, adoptive transfer of M-MDSCs rescued CCR2−/− mice from exacerbated arthritis and ameliorated CIA in WT mice.
MATERIALS AND METHODS
Animals
DBA/1J mice (WT mice) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred and maintained in a pathogen-free facility. CCR2−/− mice on a DBA/1J background were generated by back-crossing CCR2−/− C57BL/6J mice with WT DBA/1J mice for 12 generations. Male WT mice and CCR2−/− DBA/1J mice (CCR2−/− mice), 8–10 weeks of age, were used in the experiments. All experimental protocols were in compliance with the IACUC guidelines and were approved by the IACUC at the University of North Carolina at Chapel Hill (ID: 12-298; NC, USA).
CIA induction and evaluations
CIA was induced by immunization with CII (Chondrex, Redmond, WA, USA), emulsified with CFA (Sigma-Aldrich, St. Louis, MO, USA). The animals were given a booster dose of CII in IFA (Sigma-Aldrich) at Day 21 postimmunization. The immunization and disease evaluation methods were performed according to protocols validated previously by our group [15] with minor revisions. In brief, CII was dissolved in 0.1 M acetic acid and emulsified in an equal volume of CFA. On Day 0, mice were immunized by an s.c. injection of 100 µl emulsion containing 100 µg CII, ∼1 cm from the base of the tail at Day 0. The clinical disease of arthritis was evaluated as follows: 0 = normal; 1 = swelling in 1 joint; 2 = swelling in >1 joint; 3 = swelling of the entire paw. Paw swelling was measured in the millimeter range with a dial thickness gauge (Geneva Gage, Albany, OR, USA). Arthritis scoring and measurement were performed in a blinded manner.
Bone marrow transplantation assay
Recipient DBA/1J mice (WT or CCR2−/−) received 9 Gy irradiation from a cesium source, 4 h before the adoptive transfer of 5 × 106 total bone marrow cells derived from WT or CCR2−/− DBA/1J mice. Bone marrow was harvested by flushing femurs with RPMI media containing 5% FBS and 2 mM EDTA. RBCs were lysed with lysis buffer (0.14 M NH4CI, 0.017 M Tris-HCl, adjust to pH 7.2), and the remaining cells were washed with PBS (pH 7.4) and passed through a 40 µm nylon cell strainer. One month post-transfer, the spleens of recipient mice were harvested and analyzed by flow cytometry. The remaining recipient mice were immunized with collagen/CFA emulsion without boosting. Swelling and arthritis score were measured once/wk after the initial immunization and twice/wk after receiving a booster immunization.
Flow cytometry
A single-cell suspension of splenocytes was prepared by use of a manual tissue homogenizer. Whole blood was collected in heparinized tubes. RBCs were lysed with lysis buffer, and the cells were washed with RPMI containing 2 mM HEPES and 1% BSA before passing through a 40 µm nylon cell strainer. A CyAn flow cytometer (Cyan, Dako, Carpinteria, CA, USA) and FlowJo software (Tree Star, Ashland, OR, USA) were used to analyze cell-surface markers that were stained with fluorophore-conjugated primary and isotype control antibodies (eBioscience, San Diego, CA, USA: BioLegend, San Diego, CA, USA; and Invitrogen, Carlsbad, CA, USA). Antibodies used in the experiments include anti-CD3, -CD4, -CD8, -CD45R/B220, -CD11b, -CD11c, -CCR2, Gr-1, F4/80, Ly6C, Ly6G, -CD115, and all appropriate isotype controls. These antibodies were conjugated with FITC, PE, PerCP, PerCP-Cy5.5, Pacific Blue, Pacific Orange, APC, or Alexa Fluor-APC-Cy7.
ELISA
Serum antigen-specific antibody levels were measured by ELISA by use of the Mouse anti-Bovine Type II Collagen IgG Assay Kit (Chondrex), according to the manufacturer’s specifications. In brief, collagen precoated wells were washed and blocked with blocking buffer before incubation with mouse serum samples with a 1:10,000 dilution. After incubation with an HRP-labeled polyclonal secondary antibody, color was developed with the tetramethylbenzidine solution. After color development, the reaction was stopped with 2 N H2SO4, and OD values were read at 450 nm on an Emax Precision Microplate Reader. Total IgM and total IgG in B cell culture supernatants were measured by ELISA (BioLegend), per the manufacturer’s instructions. PGE2 levels were measured by use of a competition ELISA (Enzo Life Sciences, Farmingdale, NY, USA), according to the manufacturer’s instructions, and the 4 parameter logistic curve fit was used to calculate PGE2 concentrations from the standard curve.
Magnetic cell isolation
Single-cell suspensions of the spleen or bone marrow were prepared as described above. All cell enrichments were achieved by use of magnetic separation with microbeads. CD4+ T cells and CD19+ B cells were isolated from the spleens of immunized mice by negative selection and positive selection, respectively. Ly6G+ cells were positively selected from the bone marrow of immunized mice. The Ly6G− fraction was then used to isolate Ly6C+ cells by positive selection. All separation reagents were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany) . All cells were cultured in RPMI (Sigma-Aldrich) containing 10% heat-inactivated FBS, 2 mM L-glutamine, and 1% antibiotic/antimycotic.
T and B cell proliferation
Freshly isolated T cells or B cells were incubated in the presence of CFSE (Invitrogen) for 30 min at 37°C, followed by a 5 min quenching step in 5 vol ice-cold culture media. Stimulation of CD4+ T cells was achieved by plating 200 K cells/well of a 96-well plate precoated with 5 µg/ml anti-CD3 antibody. The cells were incubated in the presence of 2 µg/ml anti-CD28 antibody (BioLegend and eBioscience). B cells were plated at 200 K cells/well and stimulated with 7–10 µg/ml mouse rCD40L (R&D Systems, Minneapolis, MN, USA) and 200 ng/ml IL-4 (BioLegend). For both T and B cell assays, an equal number of M-MDSCs were added, and the cells were cocultured for 72 h, after which time, the cells were harvested and stained for CD4, B220, CD11b, and 7-amino actinomycin D before being analyzed by flow cytometry. In some assays, NO inhibitors were added, as well as 10 µM manganese (III) tetrakis (4-benzoic acid) porphyrin chloride and 0.5 mM L-NG-monomethylarginine, acetate salt. Other assays included antibody treatment with anti-IL-6, anti-TNF-α, anti-IL-12p70, anti-IFN-γ, anti-IL-17, or IgGκ isotype control antibody. PGE2R antagonists for EP2 (AH6809) and EP4 (AH23848) were purchased from Sigma-Aldrich. For Transwell assays, M-MDSCs were added to the Transwell inserts to separate from B cells. Transwell plates were purchased from EMD Millipore (Billerica, MA, USA).
Griess assay
NO concentrations were determined for cell supernatants collected from CD4+ T or B cell cultures. The nitrite concentration in the culture medium, indicative of NO production, was measured by use of a Griess reagent kit (Invitrogen), according to the manufacturer's specifications. After 30 min of incubation at room temperature, the absorbance was measured at 560 nm. Sodium nitrite was used to prepare a standard curve for calculation of the nitrite concentration in culture medium.
Analysis of systemic cytokine profile
Systemic cytokine profiles of IL-1β, IL-6, IL-17A, TNF-α, and IFN-γ were determined by Luminex assay by use of serum collected from CCR2−/−and WT mice with CIA. Serum cytokine levels were measured with the Bio-Plex Pro mouse Th17 6-plex Luminex panel and analyzed by a Magpix Luminex reader (Bio-Rad Laboratories, Hercules, CA, USA). The 5-parameter regression formula was used to calculate cytokine concentrations from the standard curves.
Adoptive transfer experiment
Collagen-immunized WT or CCR2−/− DBA/1J mice were administered with M-MDSCs isolated from the bone marrow of collagen-immunized WT or CCR2−/− mice, which were administered 2.50 × 105 M-MDSCs by i.v. or 1.5 × 106 M-MDSCs by i.p., starting at 14 days postimmunization, followed by treatments every 5 days for a total of 5 treatments/mouse. Swelling and arthritis score were measured, and serum was collected over the course of the disease.
qRT-PCR
The expression of inflammatory cytokine mRNA in the joint tissues was measured by qRT-PCR. In brief, Trizol (Invitrogen) was used to isolate total RNA from the wrist joints of CIA mice, and cDNA was generated by use of the First-Strand cDNA Synthesis SuperScript II RT (Invitrogen). Primers used for the amplification of murine IL-17A, IFN-γ, IL-6, IL-1β, TNF-α, and 18s are as follows:
IL-17A forward TCTCTGATGCTGTTGCTGCT , reverse CTCCAGAAGGCCCTCAGACTAC ;
IFN-γ forward ACTGGCAAAAGGATGGTGAC , reverse ACCTGTGGGTTGTTGACCTC ;
IL-6 forward TTCCATCCAGTTGCCTTCTT , reverse CAGAATTGCCATTGCACAAC ;
IL-1β forward GGTCAAAGGTTTGGAAGCAG , reverse TGTGAAATGCCACCTTTTGA ;
TNF-α forward CCTTCACAGAGCAATGACTC , reverse GTCTACTCCCAGGTTCTCTTC ;
18S forward GACCATAAACGATGCCGACT , reverse GTGAGGTTTCCCGTGTTGAG
qRT-PCR was performed by use of a SYBR Green Master Mix (Bio-Rad Laboratories), and reactions were performed by an iCycler instrument (Bio-Rad Laboratories). The 2−ΔΔcomparative threshold method was used for data analysis.
Statistical analysis
Numerical data presented in the text and figures are expressed as mean ± sem. Statistical significance was established at P ≤ 0.05. For clinical disease assessment, separate general, linear-mixed effects models were used to determine significant differences in arthritis scores and paw swelling, respectively, between the treated and control mice over time. The overall group effect was assessed by use of a LRT. Analyses were conducted by use of SAS v9.2. All other statistical significance was determined by Student's unpaired 2-sample t-test.
RESULTS
Collagen immunization results in expansion of a monocyte population displaying the M-MDSC phenotype
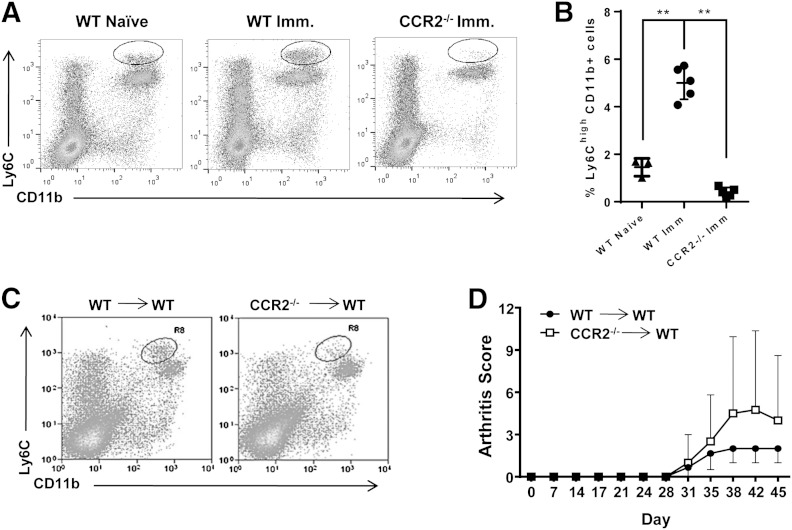
Exacerbated autoimmune arthritis in CCR2−/− mice is, at least in part, a result of an increase in Th17 cell responses [15]. IL-17-neutralizing antibody treatment of CCR2−/− mice was only partially effective in ameliorating CIA, indicating that additional mechanisms contribute to the exacerbated disease phenotype of CCR2−/− mice. In collagen-immunized WT mice, CD11b+Ly6ChighLy6G− monocytes that were missing from the periphery of collagen-immunized CCR2−/− mice markedly expanded in the blood compared with naïve mice (Fig. 1A and B). We hypothesized that this subset of monocytes acts as M-MDSCs, and the absence of these M-MDSCs in the periphery of CCR2−/− mice compromises tolerance, which results in the development of more severe autoimmune arthritis. To test this hypothesis, we first determined whether the disease susceptibility in CCR2−/− was a result of a hematopoietic defect by transferring the bone marrow of CCR2−/− mice or WT mice into lethally irradiated WT mice. After bone marrow reconstitution, CIA was induced in these mice. Examination of the spleens by flow cytometry showed that M-MDSCs were absent from the WT mice that received CCR2−/− bone marrow. In contrast, M-MDSCs were readily identified in the WT mice that received WT bone marrow (Fig. 1C). Moreover, WT mice that received CCR2−/− bone marrow developed exacerbated disease (Fig. 1D), which is consistent with disease progression in CCR2−/− mice compared with WT mice, although the differences are not significant due to the loss of animals during bone marrow transfer (LRT = 6.07; df = 4; P = 0.19). These results demonstrate that hematopoietic cells of the bone marrow are responsible for the severe autoimmune arthritis in CCR2−/− mice and suggest that M-MDSCs may be important in controlling CIA disease progression.
Figure 1. Collagen immunization results in expansion of a monocyte population that displays an MDSC phenotype. (A) Whole blood was collected from naïve WT, immunized (Imm.) WT, or immunized CCR2−/− mice and analyzed by flow cytometry to identify CD11b+Ly6ChighLy6G− cells. (B) Average percentage of CD11b+Ly6Chigh cells presented in each group was compared. **P ≤ 0.01. (C) A bone marrow transplantation experiment was performed in lethally irradiated WT mice by transfer of total bone marrow cells isolated from WT (WT→WT) or CCR2−/− (CCR2−/−→WT) mice. Splenocytes were isolated from recipient mice immunized with CIA and analyzed by flow cytometry. (D) Disease progression after bone marrow transfer was monitored in WT→WT and CCR2−/−→WT mice.
To further define the nature of this M-MDSC population in autoimmune arthritis, we isolated these cells from the bone marrow of collagen-immunized WT mice and determined the phenotype by flow cytometry (Supplemental Fig. 1A). M-MDSCs are CD11b+ and Gr-1 moderate, as well as Ly6Chigh, Ly6G−, CCR2+, CD115+, F4/80low, and CD11c−. As the classic CD11b+Gr-1+ MDSC population also includes Ly6C+Ly6G+ neutrophils, Ly6G+ cells isolated from the bone marrow were determined as CD11b+, Gr-1high, Ly6C+, Ly6G+, CCR2−, CD115low, F4/80−, and CD11c−. Morphologic analysis confirmed that the M-MDSCs isolated from CIA mice are immature and monocyte-like, whereas Ly6G+ cells are neutrophil-like (Supplemental Fig. 1B).
M-MDSC-mediated inhibition of CD4+ T cell proliferation is iNOS and IFN-γ dependent but IL-17 independent
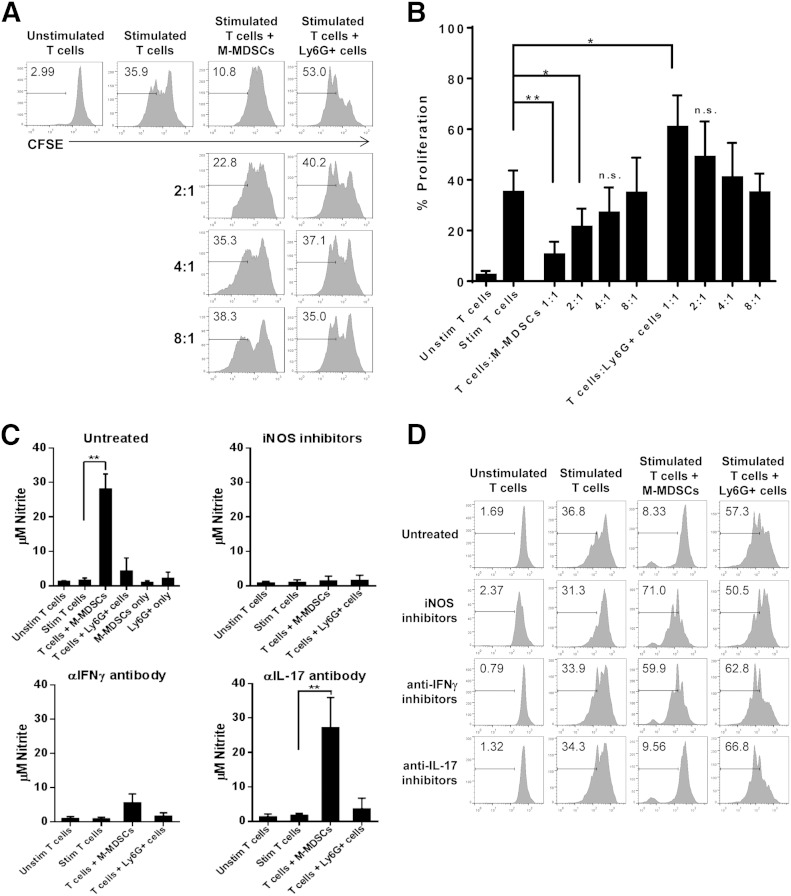
To determine the function of M-MDSCs in CIA, T cell proliferation was measured with a CFSE dilution assay. CD4+ T cells were isolated from the spleen and M-MDSCs (Ly6C+Ly6G−) from the bone marrow of a collagen-immunized mouse. Ly6G+ neutrophils were also isolated from the bone marrow and used as a control for the M-MDSCs. CD4+ T cells were stained with CFSE and then left unstimulated or were stimulated with anti-CD3/anti-CD28 antibodies. The stimulated CD4+ T cells were incubated alone or were cocultured with M-MDSCs or Ly6G+ cells in a 1:1, 2:1, 4:1, or 8:1 ratio for 72 h. At the end of incubation, cells were harvested and analyzed by flow cytometry. Unstimulated CD4+ T cells did not proliferate after 72 h, however, with stimulation ∼30% of the CD4+ T cells proliferated. When stimulated CD4+ T cells were incubated with M-MDSCs, T cell proliferation was markedly inhibited (Fig. 2A and B). The inhibition of CD4+ T cell proliferation by M-MDSCs was concentration dependent, whereas a T cell:M-MDSC ratio of 1:1 was the most effective, and the inhibitory function was lost at a 4:1 ratio. On the contrary, Ly6G+ cells did not suppress T cell proliferation under same conditions but instead, seemed to facilitate T cell proliferation (Fig. 2A and B). Together, these data indicate that M-MSDCs, but not Ly6G+ cells, are effective suppressors of CD4+ T cell proliferation under conditions of autoimmune arthritis.
Figure 2. M-MDSCs suppress autologous CD4+ T cell proliferation in CIA in an iNOS- and IFN-γ-dependent but IL-17-independent manner. CD4+ T cells were isolated from the spleens of collagen-immunized WT mice. CFSE-labeled CD4+ T cells were incubated in the absence or presence of varying ratios of M-MDSCs or Ly6G+ cells isolated from the bone marrow and stimulated with anti-CD3 and anti-CD28 antibodies. (A) T cell proliferation after culturing for 72 h was analyzed by flow cytometry. (B) Average percentage of T cell proliferation in various culture conditions was demonstrated in bar graphs. (C) NO production was measured by Griess assay in cell-culture supernatants in the absence or presence of iNOS inhibitors, anti (α)-IFN-γ antibody, and anti-IL-17 antibody. (D) T cell proliferation was measured after incubation in the absence or presence of iNOS inhibitors, anti-IFN-γ antibody, and anti-IL-17 antibody. Representative data of at least 3 independent experiments or averaged data from at least 3 independent experiments are shown. **P < 0.01, and *P < 0.05.
We next investigated the mechanism by which M-MDSCs inhibit CD4+ T cell functions in CIA. As NO production is one of the most common suppressive mechanisms used by M-MDSCs [1], levels of NO in the supernatants of T cell cultures were measured. The amount of NO increased significantly when T cells were cocultured with M-MDSCs but not when T cells were cultured alone or with Ly6G+ cells. The sharp increase in NO production was abolished when T cells and M-MDSCs were coincubated in the presence of iNOS inhibitors. Additionally, the increased NO production in the coculture supernatant was diminished by antibody neutralization of IFN-γ but not of IL-17 (Fig. 2C). Consistently, IFN-γ levels were significantly increased in cultures of activated T cells alone and activated T cells incubated with M-MDSCs (Supplemental Fig. 2). Thus, IFN-γ produced by activated T cells induces NO production by M-MDSCs. To confirm that the IFN-γ-driven production of NO by M-MDSCs was responsible for suppression of CD4+ T cell proliferation, CD4+ T cell proliferation was measured in cocultures with M-MDSCs in the presence of iNOS inhibitors, IFN-γ-neutralizing antibody, or IL-17-neutralizing antibody. The inhibition of CD4+ T cell proliferation by M-MDSCs was ablated upon the addition of iNOS inhibitors or anti-IFN-γ antibody; however, the anti-IL-17 antibody did not show any effect (Fig. 2D). This indicates that M-MDSCs but not Ly6G+ cells inhibit autologous CD4+ T cell proliferation in CIA in an iNOS- and IFN-γ-dependent but IL-17-independent manner.
M-MDSCs inhibit B cell proliferation and antibody production
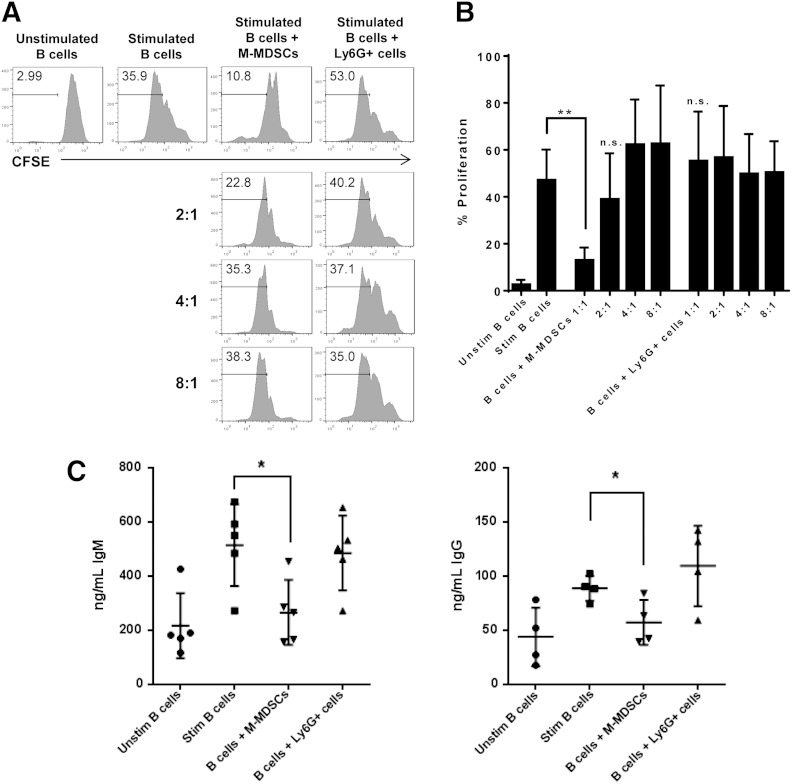
Autoimmunity results from abnormal B and T cell recognition of self-antigens, which leads to autoantibody production. Therefore, autoantibodies produced by B cells are pivotal to the induction and sustained escalation of autoimmune inflammation seen in many autoimmune diseases, including autoimmune arthritis [17]. To determine if M-MDSCs inhibit B cell responses during autoimmune disease, M-MDSCs were cocultured with B cells stimulated with CD40L and IL-4. As B cell receptor ligation is an early event in B cell proliferation, CD40L and IL-4 treatment will mimic the effects of B and T cell interactions [18–20]. B cells were isolated from the spleen of a collagen-immunized mouse and cocultured with various ratios of M-MDSCs or Ly6G+ cells isolated from the bone marrow of the same mouse. B cell proliferation was significantly reduced in the presence of M-MDSCs compared with stimulated B cells alone or stimulated B cells cocultured with Ly6G+ cells. This suppression of B cell proliferation by M-MDSCs was concentration dependent and most effective at a 1:1 ratio (Fig. 3A and B). To examine whether M-MDSCs affect B cell function, we measured antibody production and found that IgM and IgG production were substantially reduced in cocultures of B cells and M-MDSCs compared with cultures of B cells alone or B cells with Ly6G+ cells (Fig. 3C). In summary, M-MDSCs are capable of suppressing autologous B cell proliferation and inhibiting antibody production in CIA.
Figure 3. M-MDSCs inhibit B cell proliferation and antibody production. CD19+ B cells were isolated from the spleens of collagen-immunized WT mice, labeled with CFSE, and incubated in the absence or presence of varying ratios of M-MDSCs or Ly6G+ cells. CD19+ B cells were left unstimulated or stimulated with CD40L and IL-4 for 72 h. (A) B cell proliferation was analyzed by flow cytometry. (B) Average percentage of B cell proliferation in various culture conditions was demonstrated in bar graphs. (C) IgM and IgG antibody production in B cell culture supernatants was determined by ELISA. Representative data of at least 3 independent experiments or averaged data from at least 3 independent experiments are shown. **P < 0.01, and *P < 0.05.
M-MDSC-mediated suppression of B cell responses is mediated by iNOS and PGE2
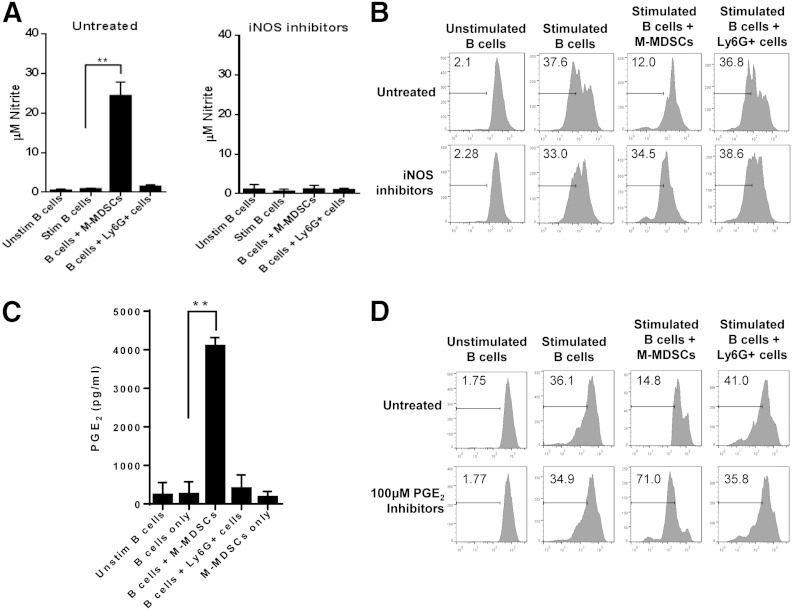
Identification of the mechanism by which M-MDSCs mediate B cell suppression during CIA is essential for understanding the role of these cells in autoimmune disease. Given the importance of M-MDSC-derived NO in T cell inhibition, the concentration of NO in supernatants of B cell cultures stimulated with CD40L and IL-4 was measured (Fig. 4A). As observed in T cell cultures, the levels of NO increased significantly when M-MDSCs were added to the B cell cultures. This increase was abolished in the presence of iNOS inhibitors. Levels of NO in cultures of stimulated B cells only or stimulated B cells cultured with Ly6G+ cells remained unchanged. Furthermore, the inhibition of B cell proliferation by M-MDSCs was abolished in the presence of iNOS inhibitors (Fig. 4B). These data demonstrate that M-MDSC-mediated inhibition of B cell proliferation is NO dependent.
Figure 4. M-MDSC-mediated suppression of B cell proliferation is dependent on iNOS and PGE2 production. CD19+ B cells were isolated from the spleens of collagen-immunized WT mice, stained with CFSE, and incubated in the absence or presence of M-MDSCs or Ly6G+ cells. CD19+ B cells were left unstimulated or stimulated with CD40L and IL-4 for 72 h. (A) Culture supernatants were analyzed for NO levels in the presence of iNOS inhibitors by Griess assay. (B) B cell proliferation in the presence or absence of iNOS inhibitors was determined by flow cytometry. (C) PGE2 productions in B cell culture supernatants under various conditions were measured by ELISA. (D) B cell proliferation, in the absence or presence of PGE2 inhibitors, was determined by flow cytometry. Representative data of at least 3 independent experiments or averaged data from at least 3 independent experiments are shown. **P < 0.01.
PGE2 has been shown to promote MDSC function in the tumor microenvironment, as well as regulate B cell function during infection [21, 22]. Thus, we sought to determine whether PGE2 was also involved in the M-MDSC-mediated inhibition of B cell function during CIA. The level of PGE2 was markedly increased in the supernatants of cocultured B cells and M-MDSCs but not in the supernatants of B cells cultured alone, M-MDSC alone, or in the presence of Ly6G+ cells (Fig. 4C). The suppressive effects of M-MDSCs on B cell proliferation were specifically abolished by antagonists of the PGE2 receptors, EP2 and EP4 (Fig. 4D). These results suggest that M-MDSC-mediated suppression of B cell proliferation is also mediated by PGE2 production.
M-MDSC suppression of B cells is contact dependent
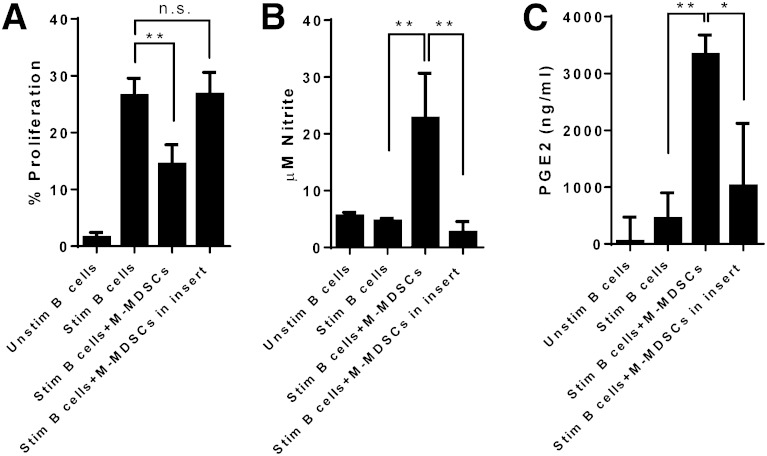
Whereas the suppressive function of MDSCs on T cells requires cell–cell contact [23], it is unknown whether this mechanism is required for B cell suppression. To examine this, we performed B cell suppression assays with Transwell inserts. Transwell separation of M-MDSCs from B cells abolished suppression of B cell proliferation by M-MDSCs (Fig. 5A). Additionally, separation of M-MDSCs from B cells by Transwell eliminated the enhanced production of NO and PGE2 in cocultures of M-MSDCs and B cells (Fig. 5B and C). Thus, NO- and PGE2-mediated suppression of B cell responses by M-MDSCs is contact dependent.
Figure 5. M-MDSC suppression of B cells is cell-contact dependent. CD19+ B cells were isolated from the spleens of collagen-immunized WT mice, labeled with CFSE, and cultured in the absence or presence of M-MDSCs added to the bottom chamber with B cells or the insert of a Transwell plate. CD19+ B cells were left unstimulated or stimulated with CD40L and IL-4 for 72 h. (A) B cell proliferation at various conditions was analyzed by flow cytometry. (B) NO production in B cell culture supernatants was determined by Griess assay. (C) Production of PGE2 in B cell culture supernatants was measured by ELISA. Data shown are averages of at least 3 independent experiments. **P < 0.01, and *P < 0.05.
Administration of M-MDSCs rescues CCR2−/− mice from the severe CIA phenotype
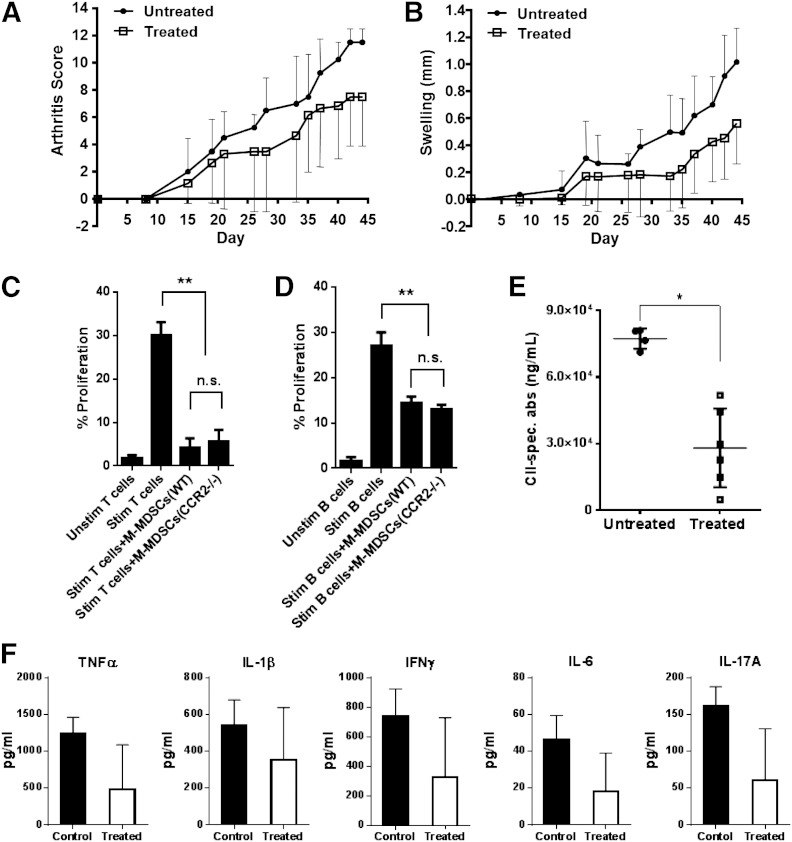
M-MDSCs were absent from the blood, spleen, and lymph nodes of CCR2−/− mice that developed more severe CIA [15]. To determine if the exacerbated CIA phenotype in CCR2−/− mice is a result of the absence of peripheral M-MDSCs, we reconstituted M-MDSCs in the periphery of CCR2−/− mice. We first assessed the suppressive ability of M-MDSCs derived from CCR2−/− mice compared with M-MDSCs derived from WT mice. Both T cell and B cell proliferation was inhibited by M-MDSCs isolated from immunized CCR2−/− mice, and the suppressive ability of M-MDSCs isolated from CCR2−/− was similar to that of M-MDSCs isolated from WT mice (Fig. 6C and D). Therefore, results from the reconstitution experiment would be based on the presence or absence of the M-MDSCs and not the level of their suppressive ability. The reconstitution efficiency of CCR2−/− mice with M-MDSCs was monitored 24 h after adoptive transfer. The number of M-MDSCs increased 8.9-fold in the spleen and 3.7-fold in the lymph node in mice receiving M-MDSCs compared with control mice, whereas bone marrow M-MDSCs only increased by 1.1-fold after adoptive transfer (Supplemental Fig. 3). Thus, peripheral reconstitution of M-MDSCs in CCR2−/− mice by adoptive transfer is efficient. Finally, the development of CIA in reconstituted CCR2−/− mice was examined. After monitoring disease progression for 45 days, the disease of CCR2−/− mice that received M-MDSCs was significantly mitigated as evidenced by decreased clinical arthritis scores (LRT = 14.4; df = 4; P = 0.006) and reduced paw swelling (LRT = 25.5; df = 4; P < 0.0001; Fig. 6A and B). Additionally, CII collagen-specific antibody production was markedly reduced in the serum of mice treated with M-MDSCs (Fig. 6E). Although not statistically significant, levels of proinflammatory cytokines, such as IL-6 and IL-17, in the serum of M-MDSC-treated mice were decreased compared with the control group (Fig. 6F). Together, these results suggest that the M-MDSCs absent from the periphery of CCR2−/− mice are immunosuppressive during CIA. Therefore, the absence of M-MDSCs from the periphery is one of the mechanisms contributing to the aggravated CIA phenotype of CCR2−/− mice.
Figure 6. Administration of M-MDSC rescues CCR2−/− mice from the severe CIA phenotype. CCR2−/− mice were immunized with collagen and boosted at Day 21. Purified M-MDSCs (2.5 × 105) were administered to CCR2−/− mice by i.v. injection at Day 14 postimmunization and then once every 5 days for a total of 5 treatments (untreated n = 4; treated n = 6). Arthritis score (A) and joint swelling (B) were monitored for 45 days. CD4+ T cells (C) and CD19+ B cells (D) were isolated from the spleens of collagen-immunized WT mice, stained with CFSE, and incubated in the absence or presence of M-MDSCs isolated from the bone marrow of immunized WT or CCR2−/− mice. After stimulation for 72 h, proliferation of T and B cells was determined by flow cytometry. (E) Levels of anti-CII antibodies [CII-specific antibodies (spec. abs)] in the serum of M-MDSC-treated and control CCR2−/− mice were determined by ELISA. (F) Cytokine levels were determined in the serum of M-MDSC-treated and control CCR2−/− mice by Luminex assay. **P < 0.01, and *P < 0.05.
M-MDSC treatment ameliorates CIA in WT mice
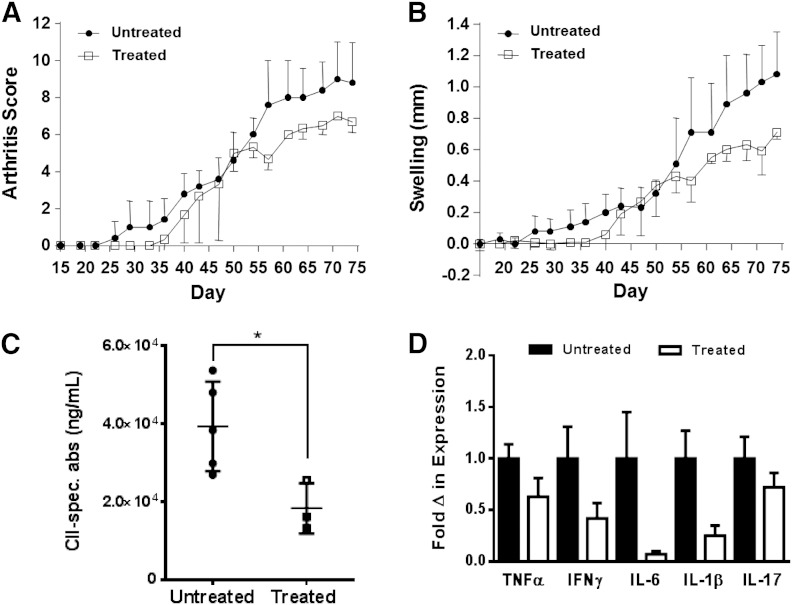
To test whether M-MDSCs have a suppressive function in autoimmune arthritis under WT conditions, the M-MDSC treatment experiment was repeated in WT mice. Compared with control mice, a delay of disease onset and a significant decrease in the progression of arthritis score (LRT = 16.9; df = 4; P = 0.002) and joint swelling (LRT = 30.8; df = 4; P < 0.0001) were observed in the M-MDSC-treated mice (Fig. 7A and B). Consistent with the B cell-suppressive function of M-MDSCs, we found a significant decrease in the level of CII collagen-specific antibodies in the serum of mice that received M-MDSC treatment (Fig. 7C). We also identified decreased expression of proinflammatory cytokines, including IL-6, IL-1β, and IFN-γ, in the joints of M-MDSC-treated mice (Fig. 7D). Thus, administration of M-MDSCs ameliorates CIA in WT mice, suggesting that M-MDSCs can potentially serve as a component of the cell-based therapy against autoimmune arthritis.
Figure 7. M-MDSC treatment ameliorates CIA in WT mice. WT mice were immunized with collagen and boosted at Day 21. Purified M-MDSCs (1.5 × 106) were administered to WT mice by i.p. injection at Day 14 postimmunization and then once every 5 days for a total of 5 treatments (untreated n = 5; treated n = 3). (A) Arthritis score and (B) joint swelling were monitored for 75 days. (C) Levels of anti-CII antibodies in the serum of M-MDSC-treated and control mice were determined by ELISA. (D) Cytokine mRNA levels in the joints of M-MDSC-treated and control mice were determined by qRT-PCR. Δ, Change. *P < 0.05.
DISCUSSION
MDSCs have been reported to regulate autoimmunity by suppressing T cell responses [24]. However, the effect of MDSCs on other cells critically involved in the autoimmune process, such as B cells, is unknown. In the current study, by use of the CIA model of autoimmune arthritis, we demonstrate that M-MDSCs increase in the periphery of WT CIA mice. M-MDSCs isolated from the bone marrow of collagen-immunized WT mice not only suppress CD4+ T cell proliferation but also inhibit proliferation and antibody production by B cells. Interestingly, M-MDSCs are absent from the periphery of CCR2−/− mice that develop exacerbated CIA. We hypothesized that the absence of M-MDSCs from the periphery was one of the mechanisms contributing to aggravated CIA in CCR2−/− mice. Indeed, adoptive transfer of M-MDSCs to collagen-immunized CCR2−/− mice resulted in attenuation of disease severity. Furthermore, adoptive transfer of M-MDSCs ameliorated CIA in WT mice. Consistent with our novel in vitro findings, M-MDSC administration significantly reduced autoantibody production in WT and CCR2−/− mice with CIA.
MDSCs suppress T cell functions via a number of different mechanisms. NO production through iNOS is one of the major mechanisms of M-MDSC-mediated T cell inhibition [6, 25, 26]. Consistent with data from an EAE model [27], our data demonstrate that M-MDSC-mediated inhibition of collagen-activated CD4+ T cells is NO dependent. We demonstrate further that the blocking of IFN-γ but not IL-17 abolishes M-MDSC-mediated CD4+ T cell suppression. This result is in contrast to earlier findings that IL-17 is required for activation of suppressive activity of MDSCs in a tumor model [28]. This discrepancy may be a result of the heterogeneous nature of MDSCs and suggests that the inhibitory functions of MDSCs may be dictated by the particular disease or microenvironment.
B cells are the origin of humoral immunity and precursors of antibody-producing cells. Recent success of anti-B cell therapy against several autoimmune diseases, including RA, confirms a pathologic role for B cells in autoimmune disorders [29–31]. Thus, it would be of interest to determine if MDSCs can inhibit B cell activities under autoimmune conditions. Our data show that the proliferation of CD40L/IL-4-stimulated B cells isolated from CIA mice is inhibited by autologous M-MDSCs. More importantly, we show that M-MDSCs not only inhibit B cell antibody production in vitro but also reduce serum levels of antigen-specific antibodies in CIA mice after adoptive transfer. To our knowledge, this is the first demonstration that MDSCs are capable of suppressing B cell function in autoimmune disease.
Macrophage-derived PGE2 was first described as a potent suppressor of splenic B cell colony formation over 30 years ago [32]. Further studies showed a direct effect of PGE2 on B cell proliferation [33], initiated via signaling through PGE2 receptors on the B cell [34]. Based on these findings, we measured the PGE2 levels in our cell-culture supernatants and found that they were strongly up-regulated in the coculture of B cells and M-MDSCs compared with cultures of B cells or M-MDSCs alone. The addition of Ly6G+ cells to the B cell culture did not increase PGE2 production, suggesting that activated M-MDSCs are the source of PGE2. The suppressive effect of M-MDSCs on B cell proliferation was ablated upon the addition of inhibitors blocking PGE2 binding to the EP2 and EP4. Together, our data suggest that PGE2 is required for M-MDSC-mediated inhibition of B cell proliferation.
Together, the different subsets of MDSCs exhibit a diverse range of functions. In our model of autoimmune arthritis, we showed that M-MDSCs but not Ly6G+ cells can inhibit T and B cell proliferation. Similar results were also observed in models of tumor and acute airway inflammation. M-MDSCs suppressed anti-tumor-immune responses in a B16 melanoma model, whereas G-MSDCs lacked this inhibitory function [35]. In mice with acute airway inflammation, whereas M-MDSCs (Ly6C+Ly6G− monocytes) suppressed T cell proliferation, G-MDSCs (Ly6C+Ly6G+ neutrophils) promoted airway inflammation in a superoxide-dependent manner [36]. Many studies have defined MDSCs as CD11b+Gr-1+ cells, but as the anti-Gr-1 antibody recognizes Ly6C and Ly6G myeloid surface antigens, the inhibitory effects observed in these studies could have originated from M-MDSC or G-MDSC subsets or perhaps both [37]. For example, Fujii et al. [38] have recently reported that MDSCs were beneficial to CIA. Similar to our data, they demonstrated CIA amelioration after adoptive transfer of MDSCs. These studies used the total Gr1+ cell population, and therefore, the inhibitory effect cannot be attributed to either subset specifically, leaving the possibility for Ly6G+ cell-mediated amelioration of CIA. However, other studies have shown that the depletion of Ly6G+ neutrophils by the anti-Ly6G antibody led to reduced CIA severity [39]; thus, the role of Ly6G+ cells in CIA needs further examination. Standardized methods for classifying MDSCs are essential for comparing results from different studies.
CCR2 is the major chemokine receptor driving monocyte and macrophage migration [40, 41]. Consistent with the finding that CCR2−/− results in exacerbated CIA, clinical trials of anti-CCR2 therapy against RA were not successful [42–44]. As monocytes and macrophages were thought to be detrimental to inflammatory tissue damage, the underlying mechanism contributing to these unexpected results could not be explained. Our data show that M-MDSCs express high levels of CCR2 and therefore, are absent from the periphery in CCR2−/− mice with CIA, as CCR2 is required for the egress of monocytes from the bone marrow [15, 45, 46]. M-MDSCs, isolated from the bone marrow of collagen-immunized CCR2−/− mice, suppress T and B cell proliferation as effectively as M-MDSCs from WT mice with CIA. Additionally, replenishing the periphery of CCR2−/− mice with M-MDSCs rescues these mice from exacerbated CIA. This indicates that the absence of M-MDSCs from the periphery of CCR2−/− mice contributes to the exacerbated phenotype of CIA in CCR2−/− mice. Furthermore, M-MDSC treatment is beneficial to CIA in WT mice, although it cannot eliminate the disease completely. Together, our results suggest that the failure of anti-CCR2 therapy in RA patients may be a result of disruption of M-MDSC regulatory functions.
In summary, we demonstrate that M-MDSCs are capable of suppressing T and B cell function, as well as disease progression in CIA, suggesting that the targeting of MDSCs can provide a potential therapeutic approach against autoimmune disease. However, this objective is complicated by evidence of regulatory and pathogenic roles for monocytes and macrophages during inflammation [47–49]. Whereas the targeting of MDSCs may be a potential therapeutic approach in treating autoimmune disease, careful consideration of different MDSC subsets and corresponding functions in autoimmunity is critical to the design of successful therapies.
ACKNOWLEDGMENTS
This work was funded by a grant (5R01AR063132; to P.L.) from the U.S. National Institutes of Health and support from the Thurston Arthritis Research Center at the University of North Carolina at Chapel Hill. The authors thank Dr. Carlton Anderson for assistance with the Luminex assays and Dr. June Brickey for helping with the lethal irradiations. The authors also thank Dr. Israel Charo for consulting on our data and Dr. Kathryn Pietrosimone for proofreading of the manuscript.
Glossary
- −/−
deficient
- APC
allophycocyanin
- CII
type II bovine collagen
- CD40L
CD40 ligand
- CIA
collagen-induced arthritis
- df
degree(s) of freedom
- EAE
experimental autoimmune encephalomyelitis
- EP2/4
E-prostanoid receptors 2 and 4
- G-MDSC
granulocytic myeloid-derived suppressor cell
- IACUC
Institutional Animal Care and Use Committee
- iNOS
induced nitric oxide synthase
- LRT
likelihood ratio test
- M-MDSC
monocytic myeloid-derived suppressor cell
- MDSC
myeloid-derived suppressor cell
- NO
nitric oxide
- qRT-PCR
quantitative real-time PCR
- RA
rheumatoid arthritis
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
AUTHORSHIP
K.R.C. contributed to experimental design and performance, data analysis, and manuscript writing. M.J., M.F.W., R.R.R., R.M.B., A.S.G., and Y.S. performed experiments. D.A.E., P.L., and T.A.S. contributed to statistical analysis. P.L. designed the overall project, analyzed data, and wrote the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusmartsev S. A., Li Y., Chen S. H. (2000) Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J. Immunol. 165, 779–785. [DOI] [PubMed] [Google Scholar]
- 3.Bronte V., Wang M., Overwijk W. W., Surman D. R., Pericle F., Rosenberg S. A., Restifo N. P. (1998) Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J. Immunol. 161, 5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 4.Youn J. I., Nagaraj S., Collazo M., Gabrilovich D. I. (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawanobori Y., Ueha S., Kurachi M., Shimaoka T., Talmadge J. E., Abe J., Shono Y., Kitabatake M., Kakimi K., Mukaida N., Matsushima K. (2008) Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111, 5457–5466. [DOI] [PubMed] [Google Scholar]
- 6.Peranzoni E., Zilio S., Marigo I., Dolcetti L., Zanovello P., Mandruzzato S., Bronte V. (2010) Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 22, 238–244. [DOI] [PubMed] [Google Scholar]
- 7.Marigo I., Dolcetti L., Serafini P., Zanovello P., Bronte V. (2008) Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 222, 162–179. [DOI] [PubMed] [Google Scholar]
- 8.Cripps J. G., Wang J., Maria A., Blumenthal I., Gorham J. D. (2010) Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology 52, 1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haile, L. A., von Wasielewski, R., Gamrekelashvili, J., Krüger, C., Bachmann, O., Westendorf, A. M., Buer, J., Liblau, R., Manns, M. P., Korangy, F., Greten, T. F. (2008) Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway, Gastroenterology 135, 871–881, 881.e1–881.e5.. [DOI] [PubMed]
- 10.Ioannou M., Alissafi T., Lazaridis I., Deraos G., Matsoukas J., Gravanis A., Mastorodemos V., Plaitakis A., Sharpe A., Boumpas D., Verginis P. (2012) Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J. Immunol. 188, 1136–1146. [DOI] [PubMed] [Google Scholar]
- 11.Zhu B., Kennedy J. K., Wang Y., Sandoval-Garcia C., Cao L., Xiao S., Wu C., Elyaman W., Khoury S. J. (2011) Plasticity of Ly-6C(hi) myeloid cells in T cell regulation. J. Immunol. 187, 2418–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards J. C., Cambridge G. (2006) B-Cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat. Rev. Immunol. 6, 394–403. [DOI] [PubMed] [Google Scholar]
- 13.Simmonds M. J. (2013) GWAS in autoimmune thyroid disease: redefining our understanding of pathogenesis. Nat. Rev. Endocrinol. 9, 277–287. [DOI] [PubMed] [Google Scholar]
- 14.McInnes I. B., Schett G. (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442. [DOI] [PubMed] [Google Scholar]
- 15.Rampersad R. R., Tarrant T. K., Vallanat C. T., Quintero-Matthews T., Weeks M. F., Esserman D. A., Clark J., Di Padova F., Patel D. D., Fong A. M., Liu P. (2011) Enhanced Th17-cell responses render CCR2-deficient mice more susceptible for autoimmune arthritis. PLoS ONE 6, e25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinones M. P., Ahuja S. K., Jimenez F., Schaefer J., Garavito E., Rao A., Chenaux G., Reddick R. L., Kuziel W. A., Ahuja S. S. (2004) Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J. Clin. Invest. 113, 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finnegan A., Ashaye S., Hamel K. M. (2012) B Effector cells in rheumatoid arthritis and experimental arthritis. Autoimmunity 45, 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehry M. R., Castle B. E. (1994) Regulation of CD40 ligand expression and use of recombinant CD40 ligand for studying B cell growth and differentiation. Semin. Immunol. 6, 287–294. [DOI] [PubMed] [Google Scholar]
- 19.Maliszewski C. R., Grabstein K., Fanslow W. C., Armitage R., Spriggs M. K., Sato T. A. (1993) Recombinant CD40 ligand stimulation of murine B cell growth and differentiation: cooperative effects of cytokines. Eur. J. Immunol. 23, 1044–1049. [DOI] [PubMed] [Google Scholar]
- 20.Noelle R. J., Roy M., Shepherd D. M., Stamenkovic I., Ledbetter J. A., Aruffo A. (1992) A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc. Natl. Acad. Sci. USA 89, 6550–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha P., Clements V. K., Fulton A. M., Ostrand-Rosenberg S. (2007) Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 67, 4507–4513. [DOI] [PubMed] [Google Scholar]
- 22.Grainger J. R., Wohlfert E. A., Fuss I. J., Bouladoux N., Askenase M. H., Legrand F., Koo L. Y., Brenchley J. M., Fraser I. D., Belkaid Y. (2013) Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat. Med. 19, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzoni A., Bronte V., Visintin A., Spitzer J. H., Apolloni E., Serafini P., Zanovello P., Segal D. M. (2002) Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695. [DOI] [PubMed] [Google Scholar]
- 24.Cripps J. G., Gorham J. D. (2011) MDSC in autoimmunity. Int. Immunopharmacol. 11, 789–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bronte V., Zanovello P. (2005) Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 5, 641–654. [DOI] [PubMed] [Google Scholar]
- 26.Movahedi K., Guilliams M., Van den Bossche J., Van den Bergh R., Gysemans C., Beschin A., De Baetselier P., Van Ginderachter J. A. (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111, 4233–4244. [DOI] [PubMed] [Google Scholar]
- 27.Zhu B., Bando Y., Xiao S., Yang K., Anderson A. C., Kuchroo V. K., Khoury S. J. (2007) CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 179, 5228–5237. [DOI] [PubMed] [Google Scholar]
- 28.He D., Li H., Yusuf N., Elmets C. A., Li J., Mountz J. D., Xu H. (2010) IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J. Immunol. 184, 2281–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dörner T. (2006) Crossroads of B cell activation in autoimmunity: rationale of targeting B cells. J. Rheumatol. Suppl. 77, 3–11. [PubMed] [Google Scholar]
- 30.Burmester G. R., Feist E., Dorner T. (2014) Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 10, 77–88. [DOI] [PubMed] [Google Scholar]
- 31.Wahren-Herlenius M., Dörner T. (2013) Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 382, 819–831. [DOI] [PubMed] [Google Scholar]
- 32.Kurland J. I., Kincade P. W., Moore M. A. (1977) Regulation of B-lymphocyte clonal proliferation by stimulatory and inhibitory macrophage-derived factors. J. Exp. Med. 146, 1420–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simkin N. J., Jelinek D. F., Lipsky P. E. (1987) Inhibition of human B cell responsiveness by prostaglandin E2. J. Immunol. 138, 1074–1081. [PubMed] [Google Scholar]
- 34.Murn J., Alibert O., Wu N., Tendil S., Gidrol X. (2008) Prostaglandin E2 regulates B cell proliferation through a candidate tumor suppressor, Ptger4. J. Exp. Med. 205, 3091–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleem S. J., Martin R. K., Morales J. K., Sturgill J. L., Gibb D. R., Graham L., Bear H. D., Manjili M. H., Ryan J. J., Conrad D. H. (2012) Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity. J. Immunol. 189, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deshane J., Zmijewski J. W., Luther R., Gaggar A., Deshane R., Lai J. F., Xu X., Spell M., Estell K., Weaver C. T., Abraham E., Schwiebert L. M., Chaplin D. D. (2011) Free radical-producing myeloid-derived regulatory cells: potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal Immunol. 4, 503–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleming T. J., Fleming M. L., Malek T. R. (1993) Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 151, 2399–2408. [PubMed] [Google Scholar]
- 38.Fujii W., Ashihara E., Hirai H., Nagahara H., Kajitani N., Fujioka K., Murakami K., Seno T., Yamamoto A., Ishino H., Kohno M., Maekawa T., Kawahito Y. (2013) Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J. Immunol. 191, 1073–1081. [DOI] [PubMed] [Google Scholar]
- 39.Eyles J. L., Roberts A. W., Metcalf D., Wicks I. P. (2006) Granulocyte colony-stimulating factor and neutrophils—forgotten mediators of inflammatory disease. Nat. Clin. Pract. Rheumatol. 2, 500–510. [DOI] [PubMed] [Google Scholar]
- 40.Boring L., Gosling J., Chensue S. W., Kunkel S. L., Farese R. V. Jr., Broxmeyer H. E., Charo I. F. (1997) Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 100, 2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tacke F., Randolph G. J. (2006) Migratory fate and differentiation of blood monocyte subsets. Immunobiology 211, 609–618. [DOI] [PubMed] [Google Scholar]
- 42.Horuk R. (2009) Chemokine receptor antagonists: overcoming developmental hurdles. Nat. Rev. Drug Discov. 8, 23–33. [DOI] [PubMed] [Google Scholar]
- 43.Vergunst C. E., Gerlag D. M., Lopatinskaya L., Klareskog L., Smith M. D., van den Bosch F., Dinant H. J., Lee Y., Wyant T., Jacobson E. W., Baeten D., Tak P. P. (2008) Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 58, 1931–1939. [DOI] [PubMed] [Google Scholar]
- 44.Kalinowska A., Losy J. (2008) Investigational C-C chemokine receptor 2 antagonists for the treatment of autoimmune diseases. Expert Opin. Investig. Drugs 17, 1267–1279. [DOI] [PubMed] [Google Scholar]
- 45.Serbina N. V., Pamer E. G. (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317. [DOI] [PubMed] [Google Scholar]
- 46.Tsou C. L., Peters W., Si Y., Slaymaker S., Aslanian A. M., Weisberg S. P., Mack M., Charo I. F. (2007) Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 117, 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon S., Plűddemann A. (2013) Tissue macrophage heterogeneity: issues and prospects. Semin. Immunopathol. 35, 533–540. [DOI] [PubMed] [Google Scholar]
- 48.Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. [DOI] [PubMed] [Google Scholar]
- 49.Gabrilovich D. I., Ostrand-Rosenberg S., Bronte V. (2012) Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]