Abstract
Exposure to indoor allergens represents a significant risk factor for allergies and asthma in several parts of the world. In Mexico, few studies have evaluated indoor allergens, including cat, dog, and mouse allergens and the factors that predict their presence. This study evaluates the main environmental and household predictors of high prenatal allergen levels and multiple allergen exposures in a birth cohort from Mexico City. A cross-sectional study was conducted as part of a birth cohort study of 1094 infants recruited during pregnancy and followed until delivery. We collected dust samples in a subset of 264 homes and assessed environmental factors. Der p 1, Der f 1, dust mite group 2, Fel d 1, Can f 1, Rat n 1, Mus m 1, and Bla g 2 concentrations in dust samples were measured using immunoassays. To define detectable allergen levels, the lowest limits of detection for each allergen were taken as cutoff points. Overall allergen exposure was considered high when four or more allergens exceeded detectable levels in the same household. Logistic regression was used for predictive models. Eighty-five percent of homes had at least one allergen in dust over the detection limit, 52.1% had high exposure (four or more allergens above detectable limits), and 11.7% of homes had detectable levels for more than eight allergens. Der p 1, Der p 2, Mus m 1, and Fel d 1 were the most frequent allergens detected. Each allergen had both common and distinct predictors. The main predictors of a high multiple allergen index were the size of the home, pesticide use, mother's age, mother as homemaker, and season. Increased indoor environmental allergen exposure is mainly related to sociodemographic factors and household cleaning.
Keywords: Cat, environment, indoor exposure, Mexico, mouse
Over the past few decades, asthma and other allergic diseases have been increasing worldwide. These diseases are responsible for significant morbidity and high health-care costs1 and constitute an important public health problem in Latin America and Mexico.2,3 A prevalence of 7% has been reported for allergic rhinitis among adults in Latin America,4 and 12% for asthma5 and 26% for allergic rhinitis among children in Mexico.6,7
These diseases are responsible for a significant use of medical resources,8 school absenteeism,9 poor quality of life, and high socioeconomic costs.10 The National Institute of Respiratory Diseases (Instituto Nacional de Enfermedades Respiratorias) reported that asthma is the principal cause of medical attention, accounting for 32.5% of total consultations for all ages.5 Better prevention and treatment strategies need to be implemented because of the negative impact of atopic disease on quality of life and the high cost of long-term treatment of symptoms.11 It is therefore important to identify modifiable risk factors that could be targeted to preventive efforts. Exposure to indoor allergens is a significant risk factor for allergies and asthma in several parts of the world,12–15 and temporal and geographic variations in exposure levels are thought to partially account for variations in asthma prevalence and morbidity.16–18 Although numerous studies of indoor allergens have been conducted in countries with large populations, such as the United States, Australia, and France, many have focused on single allergens and/or selected populations.12–15,19 Furthermore, allergens have been shown to come from a variety of both indoor and outdoor sources. Common indoor allergen sources include animal dander, cockroaches, house-dust mites, and molds. In addition, prior work suggests that allergen levels vary according to socioeconomic, racial/ethnic, and environmental factors.20 Salo and colleagues21 assessed exposure to multiple indoor allergens in the United States and observed that exposure was strongly influenced by race and socioeconomic status (SES) and that the presence of environmental factors such as smoking, pets, and pests were important determinants of high allergen exposure. Furthermore, these authors reported that exposure to all allergens increases the risk of asthma symptoms in atopic individuals.1
In Mexico, few studies have evaluated indoor allergens and their predictive factors.2 As part of a birth cohort study, we had the opportunity to examine allergen levels in the homes of mothers who were recruited in a single urban area and followed during pregnancy until delivery. The present study was undertaken to determine the household and environmental factors that are predictive of the presence of allergens in homes in Cuernavaca, Mexico.
MATERIALS AND METHODS
Design and Study Population
The women participating in the present study were a subsample of those enrolled in the randomized clinical trial, “Effect of Prenatal Supplementation with Omega 3 Fatty Acids in the Infant's Neurobehavioral Development,” conducted by Mexico's National Institute of Public Health in collaboration with the Mexican Social Security Institute (Instituto Mexicano del Seguro Social [IMSS]) in Cuernavaca, Mexico. The women were recruited during prenatal care visits at 18–22 weeks of gestation from February 2005 to February 2007. These women were 18–35 years old and planned to deliver at the IMSS General Hospital in Cuernavaca. More details on the methods of the clinical trial can be found in previous publications.22,23 All procedures were explained to the participants, who signed informed consent forms. The study protocol was approved by Emory University's Human Investigations Board, the Ethics Committee at the National Institute of Public Health and the IMSS General Hospital's Human Subjects Board.
The sample for this study included 264 women from the larger sample of 1094 women recruited during the third trimester of pregnancy, of which only 519 had agreed to participate in the sampling of dust inside the home before the end of pregnancy. For purposes of this study and to optimize financial resources, the sample was trimmed to those who also simultaneously had cord blood samples to quantify IgE and sufficient dust samples to determine allergens.
Household Environment Data Collection
Information about home environmental exposure was collected by interviews of the mother during a home visit conducted in the second trimester of pregnancy. The questionnaire included information about parental education, family income, and characteristics of the home (structural aspects, overall home environment, and environmental factors known to be associated with allergen exposure).
Collection and Measurement of Allergen Levels
Dust samples were collected from a subset of 264 homes at the time the questionnaire was administered (within the first 6 months of pregnancy). The samples were taken from the mother's bed and her bedroom floor, as described by Vojta et al.,24 using a vacuum cleaner (Miele 516; Miele, Princeton, NJ), sieved through a 425-μM mesh screen and extracted in phosphate-buffered saline containing 0.05% Tween and 1% BSA. Mothers were asked not to clean (vacuum, sweep, or mop) for 3 days before the visit. Concentrations of indoor allergens were analyzed at the National Institute of Environmental Health Sciences (Research Triangle Park, NC) with Multiplex Array for Indoor Allergens (MARIA; Indoor Biotechnology, Charlottesville, VA)25 using the Indoor Biotechnologies 8-plex assay with Universal Allergen Standards (UAS)26 for dust mite (Der p 1, Der f 1, Mite group 2), cat (Fel d 1), dog (Can f 1), rat (Rat n 1), mouse (Mus m 1), and cockroach (Bla g 2) allergens. Results were reported as nanograms of allergen per gram of sieved dust. The lower limits of detection were 12 ng/g for Der p 1, Der f 1, and Can f 1; 4 ng/g for Der p 2Fel d 1, and Rat n 1; 1.2 ng/g for Mus m 1; and 98 ng/g for Bla g 2. Each allergen was dichotomized using the respective lowest limit of detection as the cutoff point, such that concentrations higher than the limit were considered “exposure” and vice versa. In addition, overall allergen exposure was considered “high” when four or more allergens exceeded detectable levels in the same household.21
Statistical Analysis
Frequency distributions for each demographic and housing variable were computed. Two different strategies to assess the main predictors of indoor dust allergen levels were used. First, potential predictors for each allergen were evaluated separately and, subsequently, a variable encompassing the overall exposure to allergens in the dust sample was used. Allergen concentrations were dichotomized using the lowest limit of detection for each allergen as a cutoff point. With regard to overall allergen exposure, the number of allergens that exceeded detection limits and the allergen-specific thresholds for detectable levels (high levels) in each home were assessed. Exposure to multiple allergens in the home was dichotomized to reflect high (four or more allergens, exceeding detectable levels) versus low levels (zero to three allergens, exceeding detectable levels), thereby generating an index of high exposure to multiple allergens.21
Bivariate and multiple logistic regression analyses were performed to identify factors that predicted the presence or absence of each allergen (odds ratio with 95% CIs). All potential sociodemographic and housing-related variables were first evaluated using bivariate analyses. Variables with values of p ≤ 0.20 in the bivariate analysis were selected for inclusion in the multiple regression models. To select the best models, the backward stepwise method was used with values of p ≤ 0.05. Some of the variables (mother as homemaker and season) were used to adjust the final model despite not being significant. All analyses were performed using STATA Version 12.0 (StataCorp LP, College Station, TX).
RESULTS
A total of 264 households was included in this study. The average age of the mothers at the time of the study was 26 years. Most of the participants lived in multiunit dwellings (67.1%). Fifty percent of the participants had been living in their current house for ≥5 years, 43% had less than a high school education, 9.5% of mothers and 6.1% of fathers reported having atopy by questionnaire, and 34.5% of women were classified as atopic based on IgE levels (Table 1).
Table 1.
Indoor exposure (characteristics of study population, Cuernavaca, Morelos, Mexico)

The value of n = 264.
*Socioeconomic status was defined by tertiles of score generated using principal component analysis, using variables for household characteristics, basic services, and use of appliances in the home.
#Atopic mother according to specific IgE levels: maternal atopy was defined by testing positive to at least one of the tests for specific IgE at a detection level of 0.35 IU/mL for any of the allergens (milk, cat, egg, dust mite, wheat, fungus, Bermuda grass, timothy grass, mountain cedar, and ragweed).
§Atopic status was classified according to the question, “Has a doctor diagnosed you with any of the following conditions: asthma, rhinitis, conjunctivitis, or allergic dermatitis?”
m = meters.
Eighty-five percent of homes had at least one allergen in dust over the detection limit. Der p 1, Der p 2, Mus m 1, and Fel d 1 were the most commonly detected allergens and Rat n 1 was the least common allergen (Fig. 1 A). There were no significant differences between sampling seasons.
Figure 1.

Prevalence (%) of allergen types in home dust samples from Cuernavaca, Morelos Mexico. (A) Proportion of allergens above of the detectable level. The cutoff point was based on the lowest limit of detection for each allergen: 12 ng/g for Der p 1, Der f 1, and Can f 1; 4 ng/g for Der p 2, Fel d 1, and Rat n 1; 1.2 ng/g for Mus m 1; and 98 ng/g for Bla g 2. (B) Number of allergens exceeding detectable levels in homes. For the overall allergen exposure, we assessed how many allergens exceeded detection limits and allergen-specific thresholds for detectable levels in each home.
Exposure to multiple allergens was common (Fig. 1 B), with all eight allergens detectable. A total of 51.5% (136 of the 264 houses) had four or more allergens above detectable limits and, therefore, were classified as having “high” exposure to multiple allergens. Of these high exposure households, 100% had detectable Der p 1, 98.5% had Der p 2, 96.3% had Mus m 1, 93.4% had Fel d 1, 78.7% had Der f 1, 62.5% had Can f 1, 39.7% had Bla g 2, and 24% had Rat n 1.
Tables 2–4 list the main home environmental predictors of each allergen present in the households, derived from the multivariate analysis. Overall, the positive predictors of the presence of allergens included having pets in the home (cats or dogs), having pests (mice, rats, and cockroaches) or specific household characteristics such as size of the house (the smaller the home the greater the possibility of more allergens), and housing type (multiunit dwellings increase the risk). Other predictors associated with sociodemographic and environmental characteristics included having children <12 years of age in the home; age of the mattress (older); visible dampness in the home in the last 12 months (protective); and certain household practices such as cleaning frequency, use of certain deodorants and chemicals to clean the house (caustic soda and multipurpose products), use of pesticides or insecticides, and whether the mother was primarily responsible for household cleaning (protective).
Table 2.
Multiple logistic regression for main predictors of dust-mite allergens in home dust samples from Cuernavaca, Morelos, Mexico

All allergens were dichotomized using as cutoff points the lowest limit of detection for each allergen: 12 ng/g for Der p 1, Der f 1; 4 ng/g for Der p 2. All models were adjusted by season.
Effect by interquartile range (+by IQR = 20 m2; #by IQR = 5 yr).
*Mean, range.
Table 4.
Multiple logistic regression for main predictors of pest or cockroach allergens in home dust samples from Cuernavaca, Morelos, Mexico
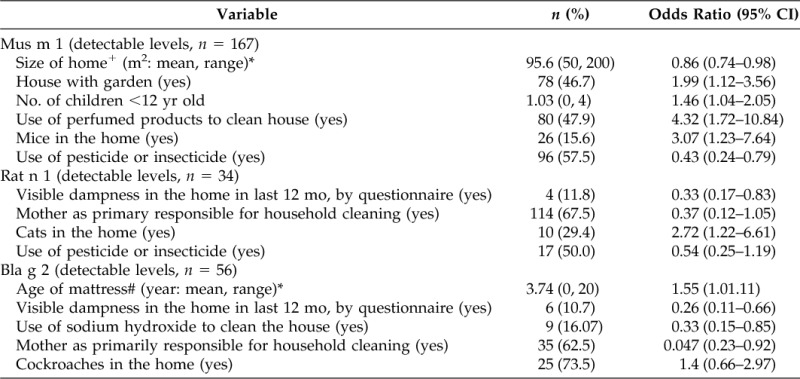
All allergens were dichotomized using as cutoff points the lowest limit of detection for each allergen: 4 ng/g for Rat n 1; 1.2 ng/g for Mus m 1; and 98 ng/g for Bla g 2. All models were adjusted by season.
Effect by interquartile range (+IQR = 20 m2; #IQR = 5 yr).
*Mean, range.
Table 3.
Multiple logistic regression for main predictors of pet allergens in home dust samples from Cuernavaca, Morelos, Mexico

All allergens were dichotomized using as cutoff points the lowest limit of detection for each allergen: 12 ng/g for Can f 1 and 4 ng/g for Fel d 1. All models were adjusted by season.
Effect by interquartile range (+by IQR = 20 m2).
*Mean, range.
The principal predictors of high exposure to multiple allergens more than four allergens) were the size of the home (large home protective), age of the mattress (older as risk), pesticide use (protective), pets in the home, and whether the mother was primarily responsible for household cleaning (protective; Table 5).
Table 5.
Multiple logistic regression for main predictors of high allergen index* in home dust samples from Cuernavaca, Morelos, Mexico

For overall allergen exposure, we assessed how many allergens exceeded detection limits and allergen-specific thresholds for detectable levels in each home. Exposure to multiple allergens in the home was dichotomized to reflect high (four or more allergens exceeding detectable levels) versus low medium levels (zero to three allergens exceeding detectable levels).
Effect by interquartile range (+IQR = 20 m2, §IQR = 5 yr)
#Mean, range.
DISCUSSION
We found that the presence of detectable allergen levels in indoor dust was common in the homes in our study population. Approximately one-half of households had high exposure to multiple allergens, the most common being Der p 1, Der p 2, Mus m 1, Fel d 1, and Der f 1. Many environmental factors related to household and hygiene can influence detectable allergen levels in the house. The main predictors of a high allergen index in the home (after adjustment for season) were the size of the home, pesticide or insecticide use, age of the mattress, whether pets are in the home, and whether the mother was responsible for household cleaning. However, we could also observe that each allergen appeared to have a distinct set of predictors, which is similar to what has been found by studies conducted in the United States and other countries.21,27
Previous studies have found that family income and race/ethnicity are the main factors that predict variations in allergen levels. In our study, we did not observe a difference in exposure with SES, probably because our population was quite homogeneous. However, we found an association with certain sociodemographic characteristics and slight reductions in the presence of detectable allergen levels when the mother was primarily responsible for cleaning and in larger homes.
Local factors are also important to determine the presence of indoor allergens. Some studies have observed that high-poverty regions (considered low income) are more likely to have high levels of allergens.17,28 Kitch et al.17 found that although cockroaches were most frequently found in poor houses, they were less likely to have house-dust mites and cat allergens. In the present study, cat allergens and dust mites were detected more often than other allergens; cockroaches and rats were the least frequent allergens found. This is important because indoor aeroallergens such as house-dust mites, molds, and pets have been associated with allergic sensitivity in children while results obtained from skin testing for aeroallergens have been negative.29
As has been mentioned, allergens have been shown to come from a variety of indoor sources, including animal dander, cockroaches, house-dust mites, and molds. These may vary according to socioeconomic and demographic characteristics as well as racial/ethnic and environmental factors. A study conducted in Dutch homes and schools found that exposure to allergens and β-(1,3)-glucans both at school and at home varied according to the SES of the children.20 All of the aforementioned factors together can have a significant impact on health, increasing the risk of asthmatic symptoms in atopic individuals.21
As expected, the presence of pets in the home increased the likelihood of having a high allergen index. Although having a cats or dogs in the home were significant factors for detectable levels of allergens in dust samples, allergens were not detected in every home with pets. In addition, 32% of people with pets did not keep them indoors and because the question related to pets was not specific for kind of pet, drawing a conclusion was not possible because of this limitation.
Furthermore, it is important to note that we did not find any significant association between the question about the presence of rodents or pests in the home and a high allergen index, while the use of pesticides or insecticides was associated with a decreased risk of high exposure to multiple allergens. The use of pesticides mainly reduced the presence of Der f 1, Fel d 1, Mus m 1, and Rat n 1, as shown in Tables 2–4. Although the frequency of housecleaning was not a significant predictor in the multiple allergens model, it was a predictive factor in models for each independent allergen. Based on our data, we observed that the use of some chemicals such as sodium hydroxide, known as caustic soda, and deodorants reduced the presence of some allergens, while the use of certain multipurpose products appeared to increase allergens.
Contrary to what was expected, visible dampness in the home over the last 12 months was found to be a protective factor for pet allergens and pests. Nevertheless, the data showed that all households that had visible dampness also always kept a window open. Similar findings were obtained for living in a high rise building. This may be why dampness was protective for household pet allergens and pests.
Having the window open was not a consistent predictor of detectable levels of allergens. In addition, we found it protective for mite group 2 but not for others. Some studies had reported that Dermatophagoides pteronyssinus is dominant in interiors and depends on external climatological characteristics27,30,31 such as temperature and humidity. In this study, although moisture or open windows were significant for some allergens, these factors were not significant for mite allergens—neither was season, probably because the temperature is warm most of the year.
Indoor allergen concentrations in the carpet or bed dust were lower in this study than in reports from other parts of the world where mite infestation is greater and asthma prevalences are highest.14 This finding might be caused by preventive measures by parents or more efforts to clean the house before the visit to collect the sample. However, we do not think these factors biased our results. In addition, risk factors may have been underreported because of prior knowledge that they are risk factors and because they are strongly associated with the cleanliness of the house.
Although the sampling for the study was conducted throughout all the seasons to capture seasonal variations in the data, we were not able to assess the seasonal variability in allergen levels in individual homes. We acknowledge that the cutoff points reported by the literature to assess the allergen burden are somewhat arbitrary and need to be interpreted with caution.18
A potential problem may be the study sample size, which could have limited power for some of the comparisons performed, and the possibility of type 2 statistical errors can not be excluded. However, we were able to detect significant predictive factors for the primary exposures studied (size of home, pesticide or insecticide use [protective], age of the mattress, pets in the home, and mother's responsibility for household chores) and thus these associations had sufficient power.
Several studies have used lower limits of detection to establish cutoffs for low and high exposure. In these studies, the higher the level of allergen exposure to house-dust mites and cockroaches the more likely patients were to have positive allergy skin test responses.32 Higher levels of allergens were also strongly associated with endotoxin levels and current asthma.21
CONCLUSION
In summary, our results confirm the presence of “high” exposure to multiple allergens in Mexican houses. Sociodemographic factors and certain environmental indoor characteristics were strong predictors of high multiple allergen exposures. The use of pesticides or insecticides decreased the presence of detectable allergen levels. An understanding of factors that increase the risk of aeroallergen exposure in homes is important to understand the relationship between exposure and atopic conditions such as asthma and allergic rhinitis.
ACKNOWLEDGMENTS
The authors thank all study staff who assisted in conducting the questionnaire and follow-up of participants.
Footnotes
Funded by the National Council of Sciences and Technology CONACYT (Grant 87121). The project described was funded by Award Number R01HD058818 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and, in part, by the Intramural Research Division of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01 ES025034)
ML Sever is presently affiliated with Rho, Chapel Hill, North Carolina
The authors have no conflicts of interest to declare pertaining to this article
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official view of the Eunice Kennedy Shiver National Institute of Child Health & Human Development or the National Institutes of Health
REFERENCES
- 1. Meltzer EO, Szwarcberg J, Pill MW. Allergic rhinitis, asthma, and rhinosinusitis: Diseases of the integrated airway. J Manag Care Pharm 10:310–317, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baena-Cagnani CE, Patiño CM, Cuello MN, et al. Prevalence and severity of asthma and wheezing in an adolescent population. Int Arch Allergy Immunol 118:245–246, 1999. [DOI] [PubMed] [Google Scholar]
- 3. Mallol J, Solé D, Asher I, et al. Prevalence of asthma symptoms in Latin America: The International Study of Asthma and Allergies in Childhood (ISAAC). Pediatr Pulmonol 30:439–444, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Meltzer EO, Blaiss MS, Naclerio RM, et al. Burden of allergic rhinitis: Allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc 33(suppl 1):S113–S141, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Sienra-Monge JJ, del Río-Navarro BE, Baeza-Bacab M. Asthma [in Spanish]. Salud Publica Mex 41:64–70, 1999. [PubMed] [Google Scholar]
- 6. Barraza-Villarreal A, Hernandez-Cadena L, Moreno-Macias H, et al. Trends in the prevalence of asthma and other allergic diseases in school children from Cuernavaca, Mexico. Allergy Asthma Proc 28:368–374, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Tatto-Cano MI, Sanín-Aguirre LH, González V, et al. Prevalence of asthma, rhinitis and eczema in school children in the city of Cuernavaca, Mexico. Salud Publica Mex 39:497–506, 1997. [PubMed] [Google Scholar]
- 8. Newacheck PW, Stoddard JJ. Prevalence and impact of multiple childhood chronic illnesses. J Pediatr 124:40–48, 1994. [DOI] [PubMed] [Google Scholar]
- 9. Romieu I, Lugo MC, Velasco SR, et al. Air pollution and school absenteeism among children in Mexico City. Am J Epidemiol 136:1524–1531, 1992. [DOI] [PubMed] [Google Scholar]
- 10. Rely K, McQuire SE, Alexandre PK, Escudero GS. Cost effectiveness of treatment with salmeterol/fluticasone compared to montelukast for the control of persistent asthma in children. Value Health 14:S43–S47, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Denburg JA, Hatfield HM, Cyr MM, et al. Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatr Res 57:276–281, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Gruchalla RS, Pongracic J, Plaut M, et al. Inner City Asthma Study: Relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol 115:478–485, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Langley SJ, Goldthorpe S, Craven M, et al. Exposure and sensitization to indoor allergens: Association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol 112:362–368, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Platts-Mills TA, Vervloet D, Thomas WR, et al. Indoor allergens and asthma: Report of the Third International Workshop. J Allergy Clin Immunol 100:S2–S24, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Lodge CJ, Allen KJ, Lowe AJ, et al. Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: A systematic review of longitudinal studies. Clin Dev Immunol 2012:176484, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arlian LG, Bernstein D, Bernstein IL, et al. Prevalence of dust mites in the homes of people with asthma living in eight different geographic areas of the United States. J Allergy Clin Immunol 90:292–300, 1992. [DOI] [PubMed] [Google Scholar]
- 17. Kitch BT, Chew G, Burge HA, et al. Socioeconomic predictors of high allergen levels in homes in the greater Boston area. Environ Health Perspect 108:301–307, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med 323:502–507, 1990. [DOI] [PubMed] [Google Scholar]
- 19. Crain EF, Walter M, O'Connor GT, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: The Inner-City Asthma Study. Environ Health Perspect 110:939–945, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krop EJ, Jacobs JH, Sander I, et al. Allergens and β-Glucans in Dutch homes and schools: Characterizing airborne levels. PLoS One 9:e88871, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salo PM, Arbes SJ, Jr, Crockett PW, et al. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol 121:678–684, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramakrishnan U, Stein AD, Parra-Cabrera S, et al. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: Randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr Bull 31:S108–S116, 2010. [DOI] [PubMed] [Google Scholar]
- 23. Imhoff-Kunsch B, Stein AD, Villalpando S, et al. Docosahexaenoic acid supplementation from mid-pregnancy to parturition influenced breast milk fatty acid concentrations at 1 month postpartum in Mexican women. J Nutr 141:321–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vojta PJ, Friedman W, Marker DA, et al. First National Survey of Lead and Allergens in Housing: Survey design and methods for the allergen and endotoxin components. Environ Health Perspect 110:527–532, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. King EM, Filep S, Smith B, et al. A multi-center ring trial of allergen analysis using fluorescent multiplex array technology. J Immunol Methods 387:89–95, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chapman MD, Filep S, Tsay A, et al. Allergen standardization: CREATE principles applied to other purified allergens. Arb Paul Ehrlich Inst Bundesinstitut Impfstoffe Biomed Arzneim Langen Hess 96:21–24, 2009. [PubMed] [Google Scholar]
- 27. Arbes SJ, Jr, Cohn RD, Yin M, et al. House dust mite allergen in US beds: Results from the First National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol 111:408–414, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Rosenfeld L, Chew GL, Rudd R, et al. Are building-level characteristics associated with indoor allergens in the household? J Urban Health 88:14–29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sahiner UM, Buyuktiryaki AB, Yavuz ST, et al. The spectrum of aeroallergen sensitization in children diagnosed with asthma during first 2 years of life. Allergy Asthma Proc 34:356–361, 2013. [DOI] [PubMed] [Google Scholar]
- 30. Diette GB, McCormack MC, Hansel NN, et al. Environmental issues in managing asthma. Respir Care 53:602–615, 2008. [PMC free article] [PubMed] [Google Scholar]
- 31. National Weather Service. JetStream—Online School for Weather. Available online at www.srh.noaa.gov/jetstream/global/climate_max.htm; Last date accessed July 30, 2013.
- 32. Huss K, Adkinson NF, Jr, Eggleston PA, et al. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol 107:48–54, 2001. [DOI] [PubMed] [Google Scholar]


