Abstract
Introduction
It is unclear whether or to what degree literacy, aging, and other neurologic abnormalities relate to cognitive deficits among people living with HIV/AIDS in the combined antiretroviral therapy (CART) era. The primary aim of this study was to simultaneously examine the association of age, HIV-associated motor abnormalities, major depressive disorder, and reading level with information processing speed, learning, memory, and executive functions, and to determine if processing speed mediated any of the relationships between cognitive and non-cognitive variables.
Method
Participants were 186 racially and ethnically diverse men and women living with HIV/AIDS who underwent comprehensive neurological, neuropsychological, and medical evaluations. Structural equation modeling was utilized to assess the extent to which information processing speed mediated the relationship between age, motor abnormalities, major depressive disorder, and reading level with other cognitive abilities.
Results
Age, motor dysfunction, reading level, and current major depressive disorder were all significantly associated with information processing speed. Information processing speed fully mediated the effects of age on learning, memory, and executive functioning, and partially mediated the effect of major depressive disorder on learning and memory. The effect of motor dysfunction on learning and memory was fully mediated by processing speed.
Conclusions
These findings provide support for information processing speed as a primary deficit which may account, at least in part, for many of the other cognitive abnormalities recognized in complex HIV/AIDS populations. The association of age and information processing speed may account for HIV/aging synergies in the generation of CART-era cognitive abnormalities.
Keywords: information processing speed, memory, executive function, structural equation model, human immunodeficiency virus
It is postulated that the rate at which an individual processes external stimuli mediates other cognitive abilities, and that when processing speed deficits reach a critical threshold other neuropsychological impairments become evident. Before the combined antiretroviral therapy (CART) era, psychomotor speed was described as a prominent component of HIV-related cognitive impairment; both as a manifestation of fully evolved fronto-striatal pathology, as well as an early predictor of progression to future HIV-associated dementia. The concept of a stereotypical subcortical dementia was predicated both on the pattern of cognitive abnormality, as well as the subcortical neuropathologies observed in brains of untreated individuals dying with HIV-associated neurocognitive disorders (HAND).
In the pre-CART era, there was evidence that processing speed contributed to performance in a variety of other neuropsychological domains among HIV-infected individuals (Becker et al., 1997; Becker & Salthouse, 1999). There was also recognition that processing speed could contribute to performance in other cognitive domains in seronegative adults (Salthouse, 1996a, 1996b). The CART-era has resulted in aging HIV populations with greater medical and socio-demographic complexity than historically untreated groups; there is now increased awareness of the contribution that other comorbidities may play in the generation of HAND. As complex HIV populations age, diffuse impairments in processing speed, learning, memory, motor ability, and executive functioning are being described (Baldewicz et al., 2004; Heaton et al., 2011; Reger, Welsh, Razani, Martin, & Boone, 2002). Although processing speed is still an important aspect of HAND, CART-era cognitive impairments may lack a distinctive diagnostic pattern (Dawes et al., 2008) and clear pathophysiologic mechanism (Lindl, Marks, Kolson, & Jordan-Sciutto, 2010; McArthur, Steiner, Sacktor, & Nath, 2010). Thus, understanding the genesis of diffuse HIV-associated cognitive abnormalities, and the potential relatedness of components of the cognitive syndromes, is important in advancing our understanding of CART-era impairments, as it may provide insights as to whether one, or multiple neural insults instigate the patterns of deficits observed in HIV populations.
Given that reading level is such a strong predictor of neuropsychological test performance, it is particularly important to explore the contribution of reading level to cognitive test performance in low literacy populations, as education corrections may not provide sufficient normative estimates of ability (e.g., Manly et al., 2011). Parsing the variability in test performance attributable to reading level may provide a more accurate estimate of HIV disease related cognitive deficits. This becomes particularly salient as HIV populations age with a wide range of literacy levels, important factors in predicting neuropsychological test performance.
Of relevance to aging CART-era HIV populations, among seronegative adults, increasing age is associated with declines in processing speed (Salthouse, 1994; Salthouse, 1996b). Additionally, in seronegative adults, dopaminergic abnormalities in the basal nuclei are associated with older age, and consequent motor dysfunction (Wang et al., 1998). In the context of HIV, motor deficits have been associated with neuronal loss in the basal nuclei (Itoh, Mehraein, & Weis, 2000; Kuper et al., 2011), incremental cognitive deficits (Robinson-Papp et al., 2008; Valcour et al., 2008), and an increased risk for developing HIV-associated dementia (HAD; Stern et al., 2001). As mild neurological abnormalities persist in the CART era, more research is needed to examine the association between motor deficits and cognitive impairment among people living with HIV/AIDS.
There is evidence that older age may exacerbate HIV-associated cognitive deficits (Valcour et al., 2004) and HIV-related extra pyramidal neurological abnormalities (Valcour et al., 2008), although some research indicates that the impacts of aging and HIV on the central nervous system are mostly independent, unrelated processes (Ances et al., 2010; Ances, Ortega, Vaida, Heaps, & Paul, 2012; Becker et al., 2011; Kissel, Pukay-Martin, & Bornstein, 2005; Valcour, Paul, Neuhaus, & Shikuma, 2011). As CART has significantly increased life expectancy for people living with HIV/AIDS, it is necessary to identify the independent, additive, or synergistic contributions of increased age and motor dysfunction on cognitive ability, to accurately identify the etiology of neurocognitive disorders.
Given that HIV preferentially involves sub-cortical structures (Becker et al., 2011; Itoh, et al., 2000; Nosheny, Bachis, Aden, De Bernardi, & Mocchetti, 2006), it may be hypothesized that processing speed deficits are still central to HAND in the CART era. HIV infection is associated with reduced frontostriatal connectivity (Melrose, Tinaz, Castelo, Courtney, & Stern, 2008), which is the neural circuitry that regulates information processing through neurotransmission (Tekin & Cummings, 2002). HIV-related disturbances in this neural circuitry are characterized by dopaminergic dysfunction and dopamine receptor loss, which has been implicated in abnormal processing speed in the CART era (e.g., Agrawal et al., 2010; Aksenova et al., 2006; Chang et al., 2008; Kumar et al., 2009; Kumar, Ownby, Waldrop-Valverde, Fernandez, & Kumar, 2011; Fitting, Booze, & Mactutus, 2006; Gelman et al., 2012; Hinkin et al., 2001). As studies continue to implicate reduced speed and cognitive slowing as core components of HIV-related neurological dysfunction (Cysique, Maruff, & Brew, 2004; Cysique, Maruff, Darby, & Brew, 2006; Reger et al., 2002; Lopez, Wess, Sanchez, Dew, & Becker, 1998; Paul, Cohen, & Stern, 2002), examining other neuropsychological domains and important noncognitive factors while accounting for processing speed may be one way to help to elucidate the etiology of these diffuse impairments.
The primary objectives of the current study were to 1) Identify the amount of variability in processing speed, learning, memory, and executive functioning that were attributable to age, motor abnormalities, MDD and reading level in a CART era, HIV cohort; and 2) Examine whether processing speed mediated any of the effects of age, reading level, and motor abnormalities on learning, memory and executive functioning.
Method
Participants
A total of 186 participants enrolled in the Manhattan HIV Brain Bank (MHBB; U01MH083501 and U24MH100931) were selected for the current study. The MHBB is an ongoing prospective observational study of people living with HIV/AIDS, which is approved by the local Institutional Review Board. Detailed information can be found in a previously published article (Morgello et al., 2004). Upon study enrollment, participants undergo comprehensive neurological, neuromedical, and neuropsychological evaluation. Baseline data collected from 1999 to 2012 were used in the current study. The primary inclusion criteria for the present investigation were confirmed HIV infection and complete neurological and neuropsychological data pertinent to the study aims. Participants were excluded from the current study if they reported a head injury with a loss of consciousness greater than 30 minutes, CNS opportunistic infection, cerebrovascular accident, active psychosis or other severe neurological condition (e.g., blindness). Other exclusion criteria included nonstandard assessment procedures or incomplete evaluation. Participant characteristics for baseline assessments are presented in Table 1.
Table 1. Participant Characteristics (N = 186).
| Mean (SD) or % | Range | |
|---|---|---|
| Age (years) | 45.3 (6.9) | 28 - 64 |
| Age 50+ (years) | 24.7 | - |
| Sex (% male) | 65.1 | - |
| Education (years) | 12.7 (2.8) | 3 - 20 |
| WRAT-3 RR (Scaled Score) | 88.6 (16.7) | 47 - 118 |
| Race/Ethnicity (%) | ||
| Non-Hispanic Black | 47.3 | - |
| Hispanic/Latino | 25.8 | - |
| Non-Hispanic White | 25.3 | - |
| Non-Hispanic Biracial | 1.6 | - |
| Duration of HIV Infection (years) | 11.3 (5.3) | 0 - 25 |
| CD4 cell count (n = 158) | 292.4 (314.2) | 1 - 1885 |
| Nadir CD4 cell count (n = 184) | 209.4 (272.1) | 1 - 1885 |
| Undetectable viral load (%; n = 143) | 28.7 | - |
| Viral load log10 transformed (n = 143) | 2.8 (2.1) | 0 – 5.9 |
| Current antiretroviral therapy (%) | 76.9 | - |
| Current or past antiretroviral therapy (%) | 96.3 | - |
| Hepatitis C (%; n = 182) | 43.4 | |
| HDMS Impaired (%) | 62.9 | - |
| Peripheral neuropathy (%) | 50.0 | - |
| Major depressive disorder (current; %) | 27.4 | - |
| Any substance use disorder (% lifetime history) | 79.0 | |
Note. WRAT-3 RR = Wide Range Achievement Test, Third Edition – Reading Recognition; HDMS = HIV-Dementia Motor Scale. HDMS impairment was determined using published criteria (Robinson-Papp et al., 2008).
Procedure
Neuropsychological and Psychiatric Assessment
The neuropsychological tests used for this study were selected from a larger battery of tests sensitive to HIV-infection (Antinori et al., 2007; Woods et al., 2004). The three tests comprising the information processing speed domain were WAIS-III Digit Symbol (DS), Symbol Search (SS), and Trail Making Test, Part A (TMT A); two measures of learning were the total recall scores of the Hopkins Verbal Learning Test (HVLT) and the Brief Visual Memory Test (BVMT); and two measures of memory were the delayed recall scores from the HVLT and BVMT; three measures of executive functions were the Wisconsin Card Sorting Test – Perseverative Responses (WCST-PR), Controlled Oral Word Association Test (COWAT), and the Trail Making Test, Part B (TMT B). In order to minimize the processing speed component, TMT B was regressed on TMT A and the residual values were used for analyses. In addition, the Wide Range Achievement Test, Third Edition - Reading Recognition (WRAT-3 RR) subtest scaled scores were used to estimate reading level because there is evidence that years of education are not an adequate measure of premorbid intellectual ability in racially/ethnically diverse populations, which may in turn bias neurocognitive diagnoses (Chin, Negash, Xie, Arnold, & Hamilton 2012; Manly, Jacobs, Touradji, Small & Stern, 2002; Rohit et al., 2007; Ryan et al., 2005). Details of these tests can be found in a published text (Strauss, Sherman, & Spreen, 2006). Although raw scores were used in for the structural equation models, T-scores are presented in Table 2 for descriptive purposes. Two domains that are part of the MHBB neuropsychological protocol, working memory and motor, were not included in this investigation because the data was not appropriate for structural equation models (i.e., single test domain or missing data). Mood disorder symptoms were assessed with the Psychiatric Research Interview for Substance and Mental Disorders (PRISM; Hasin et al., 1996), based on DSM-IV diagnostic criteria. Current major depressive disorder (MDD) was included in the analyses based on prior evidence of an association with cognitive impairment among individuals with HIV/AIDS (Fellows, Byrd, & Morgello, 2013).
Table 2. Neuropsychological Test T-scores, Standard Deviations, and Normative Data Sources.
| T-Score | SD | Normative Source | |
|---|---|---|---|
| Processing Speed | |||
| WAIS-III Digit Symbol Coding | 41.1 | 9.8 | Wechsler (1997)1 |
| WAIS-III Digit Symbol Searching | 42.9 | 10.1 | Wechsler (1997)1 |
| Trail Making Test, Part A | 41.3 | 11.3 | Heaton et al. (1991)1,2,3 |
| Executive Function | |||
| WCST-PR | 38.8 | 8.7 | Kongs et al. (2000)1,2 |
| Trail Making Test, Part B | 41.3 | 12.3 | Heaton et al. (1991)1,2,3 |
| COWAT | 47.2 | 11.5 | Gladsjo et al. (1999)1,2,4 |
| Learning | |||
| HVLT-R Total Recall | 33.7 | 14.3 | Benedict (1998)1 |
| BVMT Total Recall | 34.7 | 12.2 | Benedict (1997)1 |
| Memory | |||
| HVLT-R Delayed Recall | 32.9 | 15.9 | Benedict (1998)1 |
| BVMT Delayed Recall | 36.3 | 13.1 | Benedict (1997)1 |
Note. WCST-PR is the Wisconsin Card Sorting Test – perseverative responses; COWAT is the Controlled Oral Word Association Test; HVLT is the Hopkins Verbal Learning Test; BVMT is the Brief Visuospatial Memory Test. Normative data provides adjustments for the following demographic characteristics, as indicated:
Age;
Education;
Gender;
Ethnicity.
Neurological Examination
All study participants underwent comprehensive standardized neurological examinations performed by neurologists experienced in evaluating movement disorders related to infectious disease. The HIV-Dementia Motor Scale (HDMS; Robinson-Papp et al., 2008), derived from standardized protocol, was used to produce a motor score for each participant. This scale produces a total score (range 0 - 20) based on five domains of neurological function: strength, tone, reflexes, coordination, and gait. Hyper-reflexia (i.e., biceps, patellar) and pathologic reflexes (i.e., plantar, glabellar, snout) contributed to the reflex portion of the scale. HDMS total scores were converted into z-scores based on sample means and standard deviations. The HDMS has been shown to be sensitive to HIV-associated neurocognitive impairment (Byrd et al., 2013; Robinson-Papp et al., 2008).
Statistical Analyses
Structural equation modeling (SEM) was used to identify the independent effects of age, reading level, motor abnormalities, and MDD on information processing speed, learning, memory, and executive functions. The direct and indirect impact of age, WRAT-3 RR, MDD, and HDMS total score on each of the four cognitive domains was examined. In addition, the mediatory effect of information processing speed on other cognitive abilities was evaluated, while accounting for the impact of participant characteristics. The covariance estimates for age, WRAT-3 RR, MDD, and HDMS were calculated. Only significant paths (p < .05) were retained in the final models. Multiple variables were considered for inclusion into the models but were not retained due to either missing data, non-significant contribution, or could not be fit without violating model fit parameters (i.e., hepatitis C, HIV viral load, current CD4, nadir CD4, lifetime history of substance use disorder, peripheral neuropathy). Of note, exploratory analyses revealed no correlation between the cognitive domains and HIV viral load, current CD4, or nadir CD4 (all p-values > 0.3). Bootstrapping was performed to obtain bias-corrected indirect effect confidence intervals (Cheung, 2009), and indices to assess model fit were selected from previously established criteria (Hu & Bentler, 1999).
Results
Model 1: Mediating Effect of Speed on Learning
This model was designed to examine the direct and indirect effect of age, reading level, MDD, and motor abnormalities on encoding efficiency. The model parameters indicated an acceptable fit for the data, χ2(13, N = 186) = 25.84, p = .308, RMSEA = 0.026, GFI = .970. The HVLT Total Recall and BVMT Total Recall have similar positive loadings on the latent learning variable (.72 and .67, respectively). This model shows that reading level (β = .17, p = .034) and MDD (β = -.16, p = .044), but not age or motor abnormalities, have a significant direct effect on learning. Table 3 shows the significant indirect effect of age, reading level, MDD, and motor abnormalities on learning, memory, and executive functioning mediated by speed. Reading level and MDD have both a significant direct effect and a speed mediated indirect effect on learning. The indirect effect of age on learning suggests that even though age is not directly correlated with learning, age-related slowed processing speed impacts encoding efficiency. The moderate indirect effect of motor abnormalities on learning mediated by speed supports the concept that HDMS-associated encoding deficits are related to processing speed. In this model, age and motor abnormalities associated with learning are completely mediated by speed.
Table 3. Standardized Indirect Effects of Age, Reading Level, MDD, and Motor Function on Learning, Memory, and Executive Function, Mediated by Information Processing Speed.
| Indirect effects | ||||
|---|---|---|---|---|
|
|
||||
| Construct | Age | WRAT-3 RR | HDMS | MDD |
| LRN | -.165 | .272 | -.228 | -.135 |
| SE | .044 | .057 | .064 | .053 |
| p | .001 | .001 | .001 | .007 |
| 95% CI | [-0.24, -0.10] | [0.18, 0.37] | [-0.34, -0.13] | [-0.24, -0.03] |
| MEM | -.174 | .306 | -.250 | -.145 |
| SE | .047 | .060 | .064 | .056 |
| p | .001 | .001 | .001 | .010 |
| 95% CI | [-0.27, -0.08] | [0.19, 0.42] | [-0.38, -0.13] | [-0.25, -0.03] |
| EXE | -.132 | .215 | -.180 | -.116 |
| SE | .043 | .046 | .054 | .045 |
| p | .001 | .001 | .001 | .006 |
| 95% CI | [-0.22, -0.05] | [.13, 0.32] | [-0.30, -0.08] | [-0.21, -0.03] |
Note. Indirect effect confidence intervals and corresponding p-values were determined by bootstrapping technique. LRN = learning; MEM = memory; EXE = executive functioning; WRAT-3 RR = Wide Range Achievement Test, Third Edition – Reading Recognition; HDMS = HIV-Dementia Motor Scale
Model 2: Mediating Effect of Speed on Memory
The model used to examine the effects of age, reading level, and motor abnormalities on memory is presented in Figure 2. The nonsignificant overall likelihood chi-square value indicates that the model is a good fit for the data, χ2(24, N = 186) = 27.77, p = .269. The RMSEA value of 0.029 and GFI value of .968 also indicate that model fits the data. The HVLT Delayed Recall and BVMT Delayed Recall scores have similar positive loadings on the latent memory variable (.69 and .68, respectively). This model shows that MDD (β = -.15, p = .009), but not age, reading level, or motor abnormalities, had a significant direct effect on memory retrieval. However, age, reading level, and motor abnormalities have a moderate indirect effect on memory mediated by speed.
Figure 2.
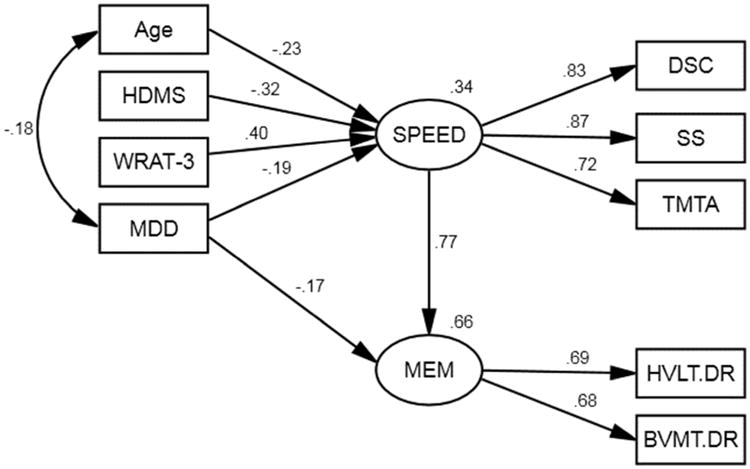
Structural model for reading level, age, MDD, and motor abnormalities on memory mediated by information processing speed. WRAT-3 = Wide Range Achievement Test, Third Edition – Reading Recognition; HDMS = HIV-Dementia Motor Scale; MDD = current major depressive disorder; DS = Wechsler Adult Intelligence Scale, Third Edition – Digit Symbol Coding; SS = Wechsler Adult Intelligence Scale, Third Edition – Symbol Search; TMTA = Trail Making Test, Part A; BVMT.DR = Brief Visuospatial Memory Test – Delayed Recall; HVLT.DR = Hopkins Verbal Memory Test – Dealyed Recall. All path estimates presented are significant at p < .05.
Model 3: Mediating Effect of Speed on Executive Functioning
The model used to examine the effects of age, reading level, and motor abnormalities on executive functioning is presented in Figure 3. The model is a good fit for the data, χ2(30, N = 186) = 29.20, p = .507, RMSEA = 0.000, GFI = .969. The COWAT had a higher loading on the executive functioning latent variable (.75), with lower loadings for WCST-PR (.50) and the Trail Making Test B residual score (.47). This model shows that reading level and motor abnormalities have direct and indirect effects on executive functions, whereas age and MDD have only indirect effects mediated by speed.
Figure 3.
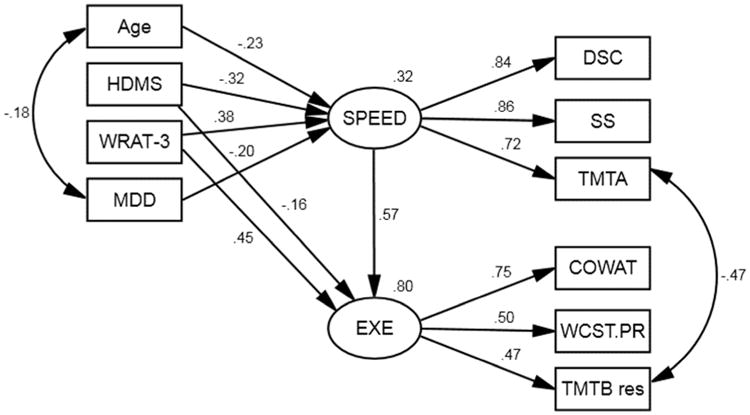
Structural model for reading level, age, MDD, and motor abnormalities on executive function mediated by information processing speed. WRAT-3 = Wide Range Achievement Test, Third Edition – Reading Recognition; HDMS = HIV-Dementia Motor Scale; MDD = current major depressive disorder; DS = Wechsler Adult Intelligence Scale, Third Edition – Digit Symbol Coding; SS = Wechsler Adult Intelligence Scale, Third Edition – Symbol Search; TMTA = Trail Making Test, Part A; WCST.PR = Wisconsin Card Sorting Test – Perseverative Responses; COWAT = Controlled Oral Word Association Test; TMT Bres = Trail Making Test B residual. All path estimates presented are significant at p < .05.
Discussion
Although the strong correlation between cognitive domains is well established, the primary purpose of the current investigation was to test specific theoretical models in order to develop a more cohesive understanding of the diffuse pattern of deficits observed in laboratory and clinical evaluations of people living with HIV/AIDS. Pre-CART research has shown that that processing speed was the primary HIV-related cognitive deficit and was associated with other cognitive abilities. However, in the CART era, a more mild but diffuse pattern of cognitive impairment has emerged and the relative contribution of processing speed on other cognitive domains has been largely unexamined. Given that cognitive impairment in the CART era is likely multifactorial, re-examining factors that may contribute to neuropsychological test performance is warranted. The complexity of the relationships between predictor variables and neuropsychological performance, especially in ethnically diverse cohorts, requires a sophisticated analytical approach. We chose to utilize structural equation modeling in order to simultaneously examine not only the association between reading level, age, MDD, and neurological abnormalities, but also the potential influence of these factors on specific cognitive functions mediated by speed.
It is possible that information processing speed is a sensitive indicator of pathological progression in HIV infected persons because it has a broad impact on other cognitive processes. In the current study we found that processing speed accounted for a significant amount of variability in learning (71%), memory (74%), and executive functions (77%), which is consistent with prior research (Salthouse, 1996b). Therefore, it is reasonable to postulate that the diffuse cognitive deficits associated with HIV infection may be partially modulated by decreased information processing speed. This concept is particularly relevant to assessing HIV-associated neurocognitive impairments because if neuropsychological tests are selected based on sensitivity to HIV, the variance that processing speed shares with tests in other domains may exacerbate diffuse impairments, which would present a unique challenge for researchers and clinicians attempting to identify specific deficit patterns and pathologies. This issue becomes more salient as our recognition of co-morbid factors contributing to cognitive impairments increases, and the need to discern disease-specific manifestations becomes important. One method of ascertaining more distinct cognitive abilities is by statistically controlling for the variance attributable to processing speed, with the assumption that isolating the residual deficits will aid in the detection of pathology-specific cognitive patterns. However, this method is of limited clinical utility because of the interdependence between cognitive processes.
Although research has shown a clear connection between older age and decreased processing speed, studies investigating the interaction between age and HIV have yielded mixed results. In the current study, chronological age had a significant direct effect on speed only, but the magnitude of influence was smaller than reading level and motor abnormalities. Older age was not directly associated with learning, memory, or executive functions, which may be because the sample was relatively young (mean age = 45 years old), but was inversely related to current MDD. However, the indirect effect of increased age on other cognitive processes mediated by speed suggests that age-related differences in learning and executive abilities may be a function of age-related decreases in time-dependent processing, which is consistent with previous research (e.g., Salthouse, 1994; Salthouse, 1996b). These findings suggest that some learning and executive performance deficits exhibited by adults with HIV infection may stem from processing speed disturbances, rather than represent discrete cognitive deficits. Although the mechanism of age-related cognitive slowing among seronegative adults may be different from mechanisms of cognitive dysfunction in HIV-infected individuals, the cognitive effect appears to be similar: processing speed is an important factor in other cognitive abilities. This may partially explain the diffuse pattern of deficits identified in cognitive studies of people living with HIV infection.
Reading level, as indexed by the WRAT-3 RR, had a direct effect on speed, learning, and executive functioning. This supports previous studies that have shown that word recognition tests can be useful predictors of fluid cognitive abilities, particularly in populations with low literacy (Ryan et al., 2005). In addition, findings from the current study indicate that reading level is independent of variations in age and motor abnormalities, consistent with prior evidence that WRAT-3 RR performance remains stable over time, even in clinical populations with fluctuating cognitive patterns (Ashendorf, Jefferson, Green, & Stern, 2009). Moreover, the association between HIV-related motor abnormalities and all of the cognitive domains, but not reading level provides additional support for this test as an estimate of verbal intelligence separate from fluid cognitive abilities.
The influence of HIV-related motor abnormalities, as represented by the HDMS, on learning, memory, was fully mediated by processing speed. Whereas, processing speed partially mediated the effect of motor dysfunction on executive abilities. These findings not only provide additional support for the utility of the HDMS to bridge brain function and cognitive abilities through a targeted neurological assessment, but also suggest that neurological abnormalities often observed in people living with HIV/AIDS may be indicative of disturbances in brain regions that are also responsible for time-dependent cognitive processes. Given that memory impairments in people living with HIV may result from inefficient encoding, rather than amnestic processes characteristic of other cognitive disorders such as Alzheimer's disease, the indirect effect of neurological motor abnormalities on learning and memory suggests that these deficits are at least partially attributable to diminished processing speed. This finding is consistent with previous evidence that the direct effect of age-related neuroanatomical and neurophysiological abnormalities on memory is completely eliminated when the role of processing speed is considered (Rabbitt et al., 2007). Further research investigating the neural mechanisms of processing speed in the presence of other cognitive deficits is warranted.
The executive functioning construct represents a complex collection of cognitive processes, which may partially explain why multiple variables had both direct and indirect effects. The independent direct effect of motor abnormalities on speed and executive functions, but not learning or memory (after controlling for speed) is consistent with frontal-subcortical dysfunction observed in HIV-infected adults. In contrast, age, motor abnormalities, MDD, and reading level all had a significant indirect effect on executive functions mediated by speed. In the current study, the executive functioning domain contains tests that require a variety of different skills including set-shifting, phonemic fluency, and visual processing. However, it is important to note that not all aspects of the executive functioning construct were represented with measures used (e.g., inhibition). Nevertheless, the indirect and direct effects of these variables on executive functioning may be interpreted to represent parallel mechanisms of neural involvement, in part mediated by speed of information processing, in part by intrinsic HIV motor/neurologic pathologies, which is expected given the diversity of cognitive processes required.
One of greatest difficulties in interpreting these results is the shared variance between cognitive domains. Although additional statistical methods (e.g., factor analysis) could have been employed to create domains that would maximize differences in shared variance, this would have been inherently problematic for the primary aims of the study. Given that cognitive processes do not occur in isolation, using statistical procedures to minimize the overlap in variance between domains might have maximized smaller differences in abilities and reduced the variance in speed that is shared by the other domains. As such, it was essential to the aims of this study to examine the influence, or shared variance, between processing speed and other cognitive functions.
The results of this study offer support for the mediatory effect of processing speed on select cognitive functions, but there are certainly limitations that must be considered. The MHBB cohort is comprised of participants with advanced HIV disease in which cognitive and motor abnormalities are common, which may make associations among these factors more prominent. It may be useful to examine the role of these factors in the neuropsychological performance of individuals with less advanced HIV disease. Although the tests utilized in the processing speed (DS, SS, TMT-A) domain are often used for this purpose in HIV research, these tests also require visual spatial and graphomotor skills. In order to further isolate the specific role of processing speed future studies may benefit from using more basic speed measures, such as simple reaction time tests, in order to minimize the influence of extraneous factors and better isolate the construct.
Using the total score from the HDMS in the model provided support for neuropathlogical contributions of HIV to cognitive performance, but did not provide details regarding the specific etiology of deficits in information processing speed, learning, memory, and executive functions. However, the effect of neurological motor abnormalities on learning and memory was fully mediated by speed, which supports the proposed theory that processing speed is central to HIV-associated cognitive impairment. Indeed, one study that matched HIV-infected participants to seronegative controls on reading level, methadone treatment, and urine toxicology for illicit substances found that HIV seropositivity predicted learning and processing speed impairments, with a trend towards memory impairment (Byrd et al., 2013). Moreover, in the HIV-infected group, neurologically assessed motor abnormalities were associated with cognition. This finding is consistent with numerous studies that have identified slowed processing speed as a core factor associated with age-related cognitive deficits. Slowed processing speed is implicated in mediating other cognitive processes in normal aging, but may also be relevant to other disorders with primary subcortical pathology (e.g., Parkinson's disease). Although the neurological abnormalities assessed in this study are sensitive to HIV-associated neurocognitive deficits, future research may benefit including an equally large sample of seronegative individuals as a control group to further isolate the mediatory effect of processing speed attributable to HIV infection. In addition, future research may benefit from examining the contribution of peripheral neuropathy on tests mediated by speed, given the evidence of an association between peripheral neuropathy and psychomotor test performance in people with HIV/AIDS (Fellows et al., 2012). Peripheral neuropathy was not included in the current study because it could not be fit to the models. It is important to note that the HDMS provides a more comprehensive assessment of HIV-related motor abnormalities than a diagnosis of peripheral neuropathy. In summary, information processing speed appears to fully mediate the influence of age-related changes in learning, memory, and executive functioning in this sample of adults living with HIV/AIDS. Motor abnormalities commonly observed in people with HIV/AIDS had a direct effect on processing speed and executive functions, and an indirect effect on learning, memory, and executive functions mediated by speed. These findings support information processing speed as an important factor in HIV-associated cognitive deficits.
Figure 1.
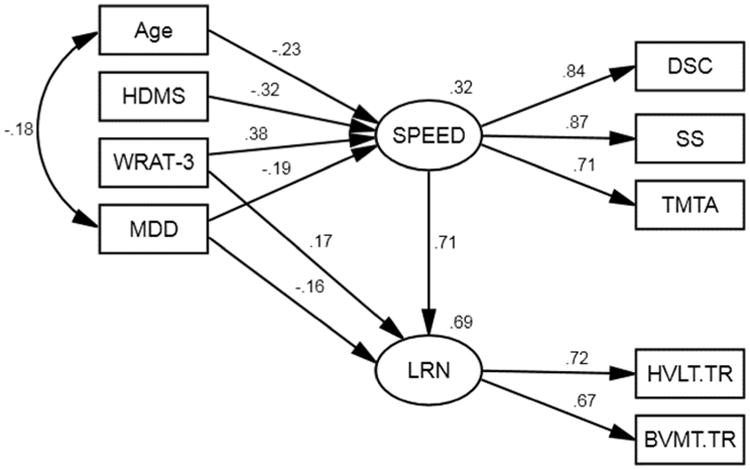
Structural model for reading level, age, MDD, and motor abnormalities on learning mediated by information processing speed. WRAT-3 = Wide Range Achievement Test, Third Edition – Reading Recognition; HDMS = HIV-Dementia Motor Scale; MDD = current major depressive disorder; DS = Wechsler Adult Intelligence Scale, Third Edition – Digit Symbol Coding; SS = Wechsler Adult Intelligence Scale, Third Edition – Symbol Search; TMTA = Trail Making Test, Part A; BVMT.TR = Brief Visuospatial Memory Test – Total Recall; HVLT.TR = Hopkins Verbal Memory Test – Total Recall. All path estimates presented are significant at p < .05.
Acknowledgments
This research was supported by the National Institutes of Health (grant numbers U01MH083501 and U24MH100931). Statistical consultation was provided by Conduits, the Biostatistics, Ethics and Research Design Core at the Icahn School of Medicine at Mount Sinai. Conduits is supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1TR000067. The authors thank the staff and participants of the Manhattan HIV Brain Bank.
References
- Agrawal L, Louboutin JP, Marusich E, Reyes BA, Van Bockstaele EJ, Strayer DS. Dopaminergic neurotoxicity of HIV-1 gp120: Reactive oxygen species as signaling intermediates. Brain Research. 2010;1306:116–130. doi: 10.1016/j.brainres.2009.09.113. 10.1016/j.brainres.2009.09.113. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 tat neurotoxicity in primary cultures of rat midbrain fetal neurons: Changes in dopamine transporter binding and immunoreactivity. Neuroscience Letters. 2006;395(3):235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of Acquired Immune Deficiency Syndromes (1999) 2012;59(5):469–477. doi: 10.1097/QAI.0b013e318249db17. 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, et al. Ellis RJ. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. The Journal of Infectious Diseases. 2010;201(3):336–340. doi: 10.1086/649899. 10.1086/649899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashendorf L, Jefferson AL, Green RC, Stern RA. Test-retest stability on the WRAT-3 reading subtest in geriatric cognitive evaluations. Journal of Clinical and Experimental Neuropsychology. 2009;31(5):605–610. doi: 10.1080/13803390802375557. 10.1080/13803390802375557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldewicz TT, Leserman J, Silva SG, Petitto JM, Golden RN, Perkins DO, et al. Evans DL. Changes in neuropsychological functioning with progression of HIV-1 infection: results of an 8-year longitudinal investigation. AIDS and Behavior. 2004;8(3):345–355. doi: 10.1023/B:AIBE.0000044081.42034.54. [DOI] [PubMed] [Google Scholar]
- Becker JT, Salthouse TA. Neuropsychological test performance in the acquired immunodeficiency syndrome: Independent effects of diagnostic group on functioning. Journal of the International Neuropsychological Society: JINS. 1999;5(1):41–47. doi: 10.1017/s1355617799511065. [DOI] [PubMed] [Google Scholar]
- Becker JT, Sanchez J, Dew MA, Lopez OL, Dorst SK, Banks G. Neuropsychological abnormalities among HIV-infected individuals in a community-based sample. Neuropsychology. 1997;11(4):592–601. doi: 10.1037//0894-4105.11.4.592. [DOI] [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, et al. Multicenter AIDS Cohort Study. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging and Behavior. 2011;5(2):77–85. doi: 10.1007/s11682-011-9113-8. 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test–Revised. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. doi: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- Byrd DA, Robinson-Papp J, Mindt MR, Mintz L, Elliott K, Lighty Q, Morgello S. Isolating cognitive and neurologic HIV effects in substance-dependent, confounded cohorts: A pilot study. Journal of the International Neuropsychological Society: JINS. 2013;19(4):463–473. doi: 10.1017/S1355617712001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42(2):869–878. doi: 10.1016/j.neuroimage.2008.05.011. 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MW. Comparison of methods for constructing confidence intervals of standardized indirect effects. Behavior Research Methods. 2009;41(2):425–438. doi: 10.3758/BRM.41.2.425. 10.3758/BRM.41.2.425. [DOI] [PubMed] [Google Scholar]
- Chin AL, Negash S, Xie S, Arnold SE, Hamilton R. Quality, and not just quantity, of education accounts for differences in psychometric performance between african americans and white non-hispanics with alzheimer's disease. Journal of the International Neuropsychological Society: JINS. 2012;18(2):277–285. doi: 10.1017/S1355617711001688. 10.1017/S1355617711001688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficien cy virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: A combined study of two cohorts. Journal of Neurovirology. 2004;10(6):350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. The neuropsychological profile of symptomatic AIDS and ADC patients in the pre-HAART era: A meta-analysis. Journal of the International Neuropsychological Society: JINS. 2006;12(3):368–382. doi: 10.1017/s1355617706060401. [DOI] [PubMed] [Google Scholar]
- Dawes S, Suarez P, Casey CY, Cherner M, Marcotte TD, Letendre S, et al. Group H. Variable patterns of neuropsychological performance in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 2008;30(6):613–626. doi: 10.1080/13803390701565225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows RP, Byrd DA, Elliott K, Robinson-Papp J, Mindt MR, Morgello S. Distal sensory polyneuropathy is associated with neuropsychological test performance among persons with HIV. Journal of the International Neuropsychological Society: JINS. 2012;18(5):898–907. doi: 10.1017/S1355617712000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows RP, Byrd DA, Morgello S. Major depressive disorder, cognitive symptoms, and neuropsychological performance among ethnically diverse HIV+ men and women. Journal of the International Neuropsychological Society: JINS. 2013;19(2):216–225. doi: 10.1017/S1355617712001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: The role of dopaminergic alterations in prepulse inhibition in adult rats. The Journal of Pharmacology and Experimental Therapeutics. 2006;318(3):1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH, Jr, Soukup VM. Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology. 2012;7(3):686–700. doi: 10.1007/s11481-012-9345-4. 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6(2):147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric research interview for substance and mental disorders (PRISM): Reliability for substance abusers. The American Journal of Psychiatry. 1996;153(9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HNRC Group. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Farinpour R, Newton T, Singer E. Methylphenidate improves HIV-1-associated cognitive slowing. The Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13(2):248–254. doi: 10.1176/jnp.13.2.248. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. [Google Scholar]
- Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathologica. 2000;99(4):376–384. doi: 10.1007/s004010051139. [DOI] [PubMed] [Google Scholar]
- Kissel EC, Pukay-Martin ND, Bornstein RA. The relationship between age and cognitive function in HIV-infected men. The Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17(2):180–184. doi: 10.1176/appi.neuropsych.17.2.180. [DOI] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. WCST-64: Wisconsin Card Sorting Test-64 Card Version Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. Journal of Neurovirology. 2009;15(3):257–274. doi: 10.1080/13550280902973952. 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: Relationship with neuropsychological performance. Journal of Neurovirology. 2011;17(1):26–40. doi: 10.1007/s13365-010-0003-4. 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, Obermann M. Structural gray and white matter changes in patients with HIV. Journal of Neurology. 2011;258(6):1066–1075. doi: 10.1007/s00415-010-5883-y. 10.1007/s00415-010-5883-y. [DOI] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: Pathogenesis and therapeutic opportunities. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology. 2010;5(3):294–309. doi: 10.1007/s11481-010-9205-z. 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Wess J, Sanchez J, Dew MA, Becker JT. Neurobehavioral correlates of perceived mental and motor slowness in HIV infection and AIDS. The Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10(3):343–350. doi: 10.1176/jnp.10.3.343. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between african american and white elders. Journal of the International Neuropsychological Society: JINS. 2002;8(3):341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Smith C, Crystal HA, Richardson J, Golub ET, Greenblatt R, et al. Young M. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV- women: The Women's Interagency HIV Study (WIHS) neurocognitive substudy. Journal of Clinical and Experimental Neuropsychology. 2011;33(8):853–863. doi: 10.1080/13803395.2010.547662. 10.1080/13803395.2010.547662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Annals of Neurology. 2010;67(6):699–714. doi: 10.1002/ana.22053. 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: An fMRI investigation of semantic event sequencing. Behavioural Brain Research. 2008;188(2):337–347. doi: 10.1016/j.bbr.2007.11.021. 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, et al. Manhattan HIV Brain Bank. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: The Manhattan HIV Brain Bank. Archives of Neurology. 2004;61(4):546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Aden SA, De Bernardi MA, Mocchetti I. Intrastriatal administration of human immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line derived neurotrophic factor levels and causes apoptosis in the substantia nigra. Journal of Neurobiology. 2006;66(12):1311–1321. doi: 10.1002/neu.20288. [DOI] [PubMed] [Google Scholar]
- Paul RH, Cohen RA, Stern RA. Neurocognitive manifestations of human immunodeficiency virus. CNS Spectrums. 2002;7(12):860–866. doi: 10.1017/s1092852900022471. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Mogapi O, Scott M, Thacker N, Lowe C, Horan M, et al. Lunn D. Effects of global atrophy, white matter lesions, and cerebral blood flow on age-related changes in speed, memory, intelligence, vocabulary, and frontal function. Neuropsychology. 2007;21(6):684–695. doi: 10.1037/0894-4105.21.6.684. [DOI] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society: JINS. 2002;8(3):410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Robinson-Papp J, Byrd D, Mindt MR, Oden NL, Simpson DM, Morgello S. Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Archives of Neurology. 2008;65(8):1096–1101. doi: 10.1001/archneur.65.8.1096. 10.1001/archneur.65.8.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohit M, Levine A, Hinkin C, Abramyan S, Saxton E, Valdes-Sueiras M, Singer E. Education correction using years in school or reading grade-level equivalent? comparing the accuracy of two methods in diagnosing HIV-associated neurocognitive impairment. Journal of the International Neuropsychological Society: JINS. 2007;13(3):462–470. doi: 10.1017/S1355617707070506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EL, Baird R, Mindt MR, Byrd D, Monzones J, Bank SM Manhattan HIV Brain Bank. Neuropsychological impairment in racial/ethnic minorities with HIV infection and low literacy levels: Effects of education and reading level in participant characterization. Journal of the International Neuropsychological Society: JINS. 2005;11(7):889–898. doi: 10.1017/S1355617705051040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Aging associations: Influence of speed on adult age differences in associative learning. Journal of Experimental Psychology Learning, Memory, and Cognition. 1994;20(6):1486–1503. doi: 10.1037//0278-7393.20.6.1486. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. General and specific speed mediation of adult age differences in memory. The Journals of Gerontology: Series B, Psychological Sciences and Social Sciences. 1996a;51(1):30–42. doi: 10.1093/geronb/51b.1.p30. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996b;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Stern Y, McDermott MP, Albert S, Palumbo D, Selnes OA, McArthur J, et al. Dana Consortium on the Therapy of HIV-Dementia and Related Cognitive Disorders. Factors associated with incident human immunodeficiency virus-dementia. Archives of Neurology. 2001;58(3):473–479. doi: 10.1001/archneur.58.3.473. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman E, Spreen O. A compendium of neuropsychological tests: Administration, norms and commentary. 3rd. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Valcour V, Paul R, Neuhaus J, Shikuma C. The Effects of Age and HIV on Neuropsychological Performance. Journal of the International Neuropsychological Society: JINS. 2011;17(1):190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, et al. Sacktor N. Higher frequency of dementia in older HIV-1 individuals: The hawaii aging with HIV-1 cohort. Neurology. 2004;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. Journal of Neurovirology. 2008;14(5):362–367. doi: 10.1080/13550280802216494. 10.1080/13550280802216494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, et al. Stoessl AJ. Age-dependent decline of dopamine D1 receptors in human brain: A PET study. Synapse (New York, N Y) 1998;30(1):56–61. doi: 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. doi:2-J. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–Third edition: Administration and scoring manual. San Antonio, TX: Harcourt Brace; 1997. [Google Scholar]
- Wilkinson G. Wide Range Achievement Test (3rd ed) administration manual. Wilmington, DE: Wide Range Inc; 1993. [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]


