Abstract
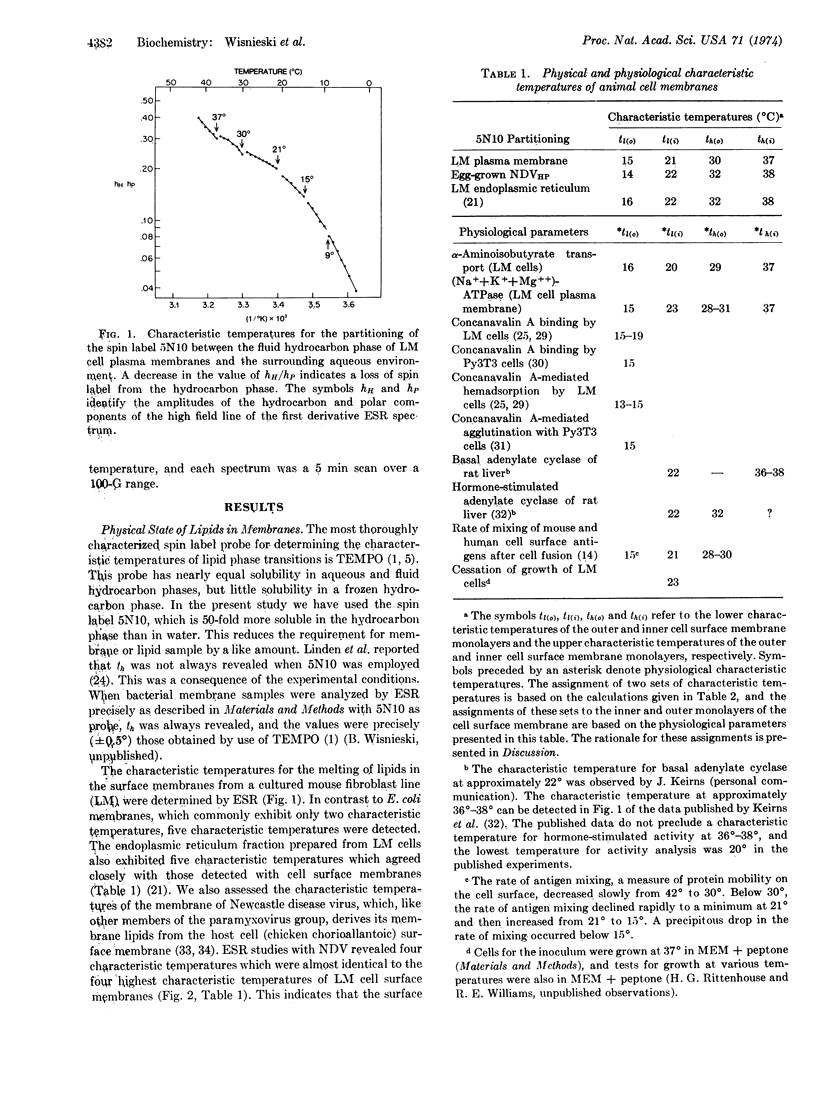
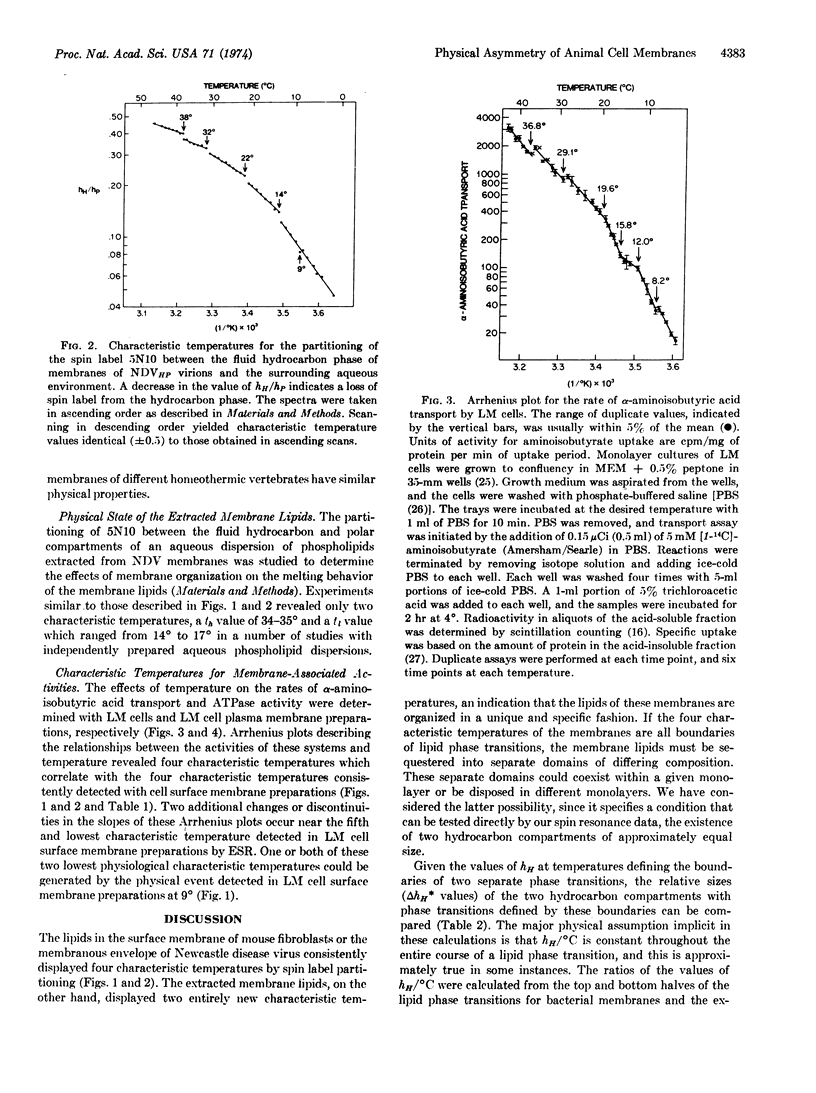
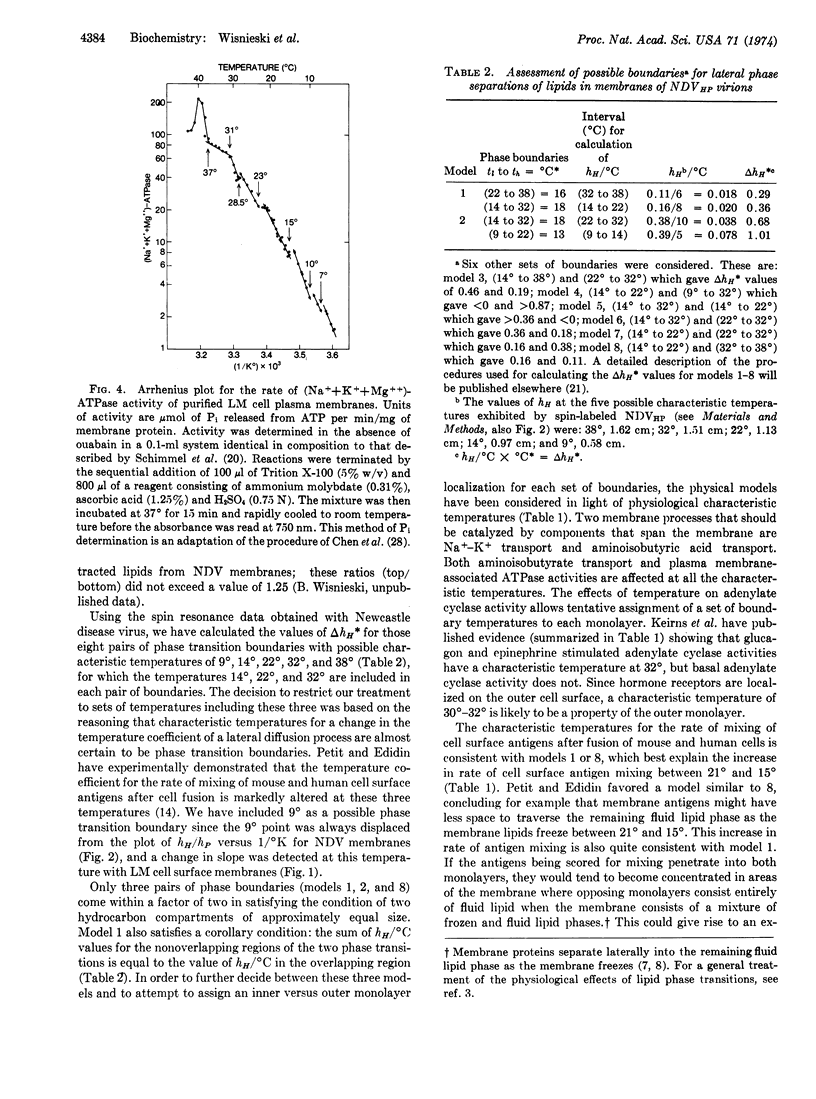
Electron spin resonance analysis of suspensions of animal cell plasma membranes consistently reveals four characteristic temperatures for lateral phase separations in the membrane lipids. Similar analysis of an aqueous dispersion of lipids extracted from these membranes reveals only two characteristic temperatures, indicating that some aspect of lipid organization in membranes is destroyed by the extraction procedure. The characteristic temperatures for surface membranes from two different species of homeothermic animals were nearly identical and were approximately 37°, 31°, 21°, and 15°. A treatment of the physical data revealed that these temperatures could identify independent phase transitions for two hydrocarbon compartments of approximately equal size with lower and upper characteristic temperatures of 21° and 37°, and of 15° and 31°. The analysis of the effects of temperature on a number of physiological parameters indicates that 21° and 37° are likely to define the boundaries for lateral phase separations in the inner monolayer and 15° and 31° the boundaries for lateral phase separations in the outer monolayer.
Keywords: hydrocarbon spin labels, amino-acid transport, membrane lipids, bilayer structure, (Na++K++Mg++)-ATPase, lateral phase separations, membrane asymmetry
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Lawson D. E. The lipids of paramyxoviruses: a comparative study of Sendai and Newcastle disease viruses. Virology. 1968 Oct;36(2):286–292. doi: 10.1016/0042-6822(68)90146-3. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol. 1972 Mar 1;236(61):11–12. doi: 10.1038/newbio236011a0. [DOI] [PubMed] [Google Scholar]
- Chen Y. S., Hubbell W. L. Temperature- and light-dependent structural changes in rhodopsin-lipid membranes. Exp Eye Res. 1973 Dec 24;17(6):517–532. doi: 10.1016/0014-4835(73)90082-1. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Revel J. P. Ultrastructural localization of rhodopsin in the vertebrate retina. J Cell Biol. 1974 Aug;62(2):257–273. doi: 10.1083/jcb.62.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirns J. J., Kreiner P. W., Bitensky M. W. An abrupt temperature-dependent change in the energy of activation of hormone-stimulated hepatic adenylyl cyclase. J Supramol Struct. 1973;1(4):368–379. doi: 10.1002/jss.400010415. [DOI] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Lateral phase separations in Escherichia coli membranes. Biochim Biophys Acta. 1974 Apr 29;345(2):220–230. doi: 10.1016/0005-2736(74)90260-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linden C. D., Keith A. D., Fox C. F. Correlations between fatty acid distribution in phospholipids and the temperature dependence of membrane physical state. J Supramol Struct. 1973;1(6):523–534. doi: 10.1002/jss.400010607. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtiger N. A., Fox C. F. Biochemistry of bacterial membranes. Annu Rev Biochem. 1973;42:575–600. doi: 10.1146/annurev.bi.42.070173.003043. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The effect of alterations in the physical state of the membrane lipids on the ability of Acholeplasma laidlawii B to grow at various temperatures. J Mol Biol. 1974 Mar 25;84(1):145–157. doi: 10.1016/0022-2836(74)90218-6. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Temperature-dependent mobility of concanavalin A sites on tumour cell surfaces. Nat New Biol. 1973 Jun 13;243(128):218–220. doi: 10.1038/newbio243218a0. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. Binding of ( 3 H)concanavalin A to normal and transformed cells. J Biol Chem. 1973 Jun 25;248(12):4286–4292. [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. The relationship of concanavalin A binding to lectin-initiated cell agglutination. J Cell Biol. 1973 Oct;59(1):134–142. doi: 10.1083/jcb.59.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit V. A., Edidin M. Lateral phase separation of lipids in plasma membranes: effect of temperature on the mobility of membrane antigens. Science. 1974 Jun 14;184(4142):1183–1185. doi: 10.1126/science.184.4142.1183. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Ladbrooke B. D., Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970 Jan 6;196(1):35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- Rittenhouse H. G., Fox C. F. Concanavalin A mediated hemagglutination and binding properties of LM cells. Biochem Biophys Res Commun. 1974 Mar 15;57(1):323–331. doi: 10.1016/s0006-291x(74)80393-1. [DOI] [PubMed] [Google Scholar]
- Rittenhouse H. G., Williams R. E., Wisnieski B., Fox C. F. Alterations of characteristic temperatures for lectin interactions in LM cells with altered lipid composition. Biochem Biophys Res Commun. 1974 May 7;58(1):222–228. doi: 10.1016/0006-291x(74)90915-2. [DOI] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson A. C., Fox C. F. Precursor protein for Newcastle disease virus. J Virol. 1973 Sep;12(3):579–587. doi: 10.1128/jvi.12.3.579-587.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel S. D., Kent C., Bischoff R., Vagelos P. R. Plasma membranes from cultured muscle cells: isolation procedure and separation of putative plasma-membrane marker enzymes. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3195–3199. doi: 10.1073/pnas.70.11.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., Kleemann W., Hubbell W. L., McConnell H. M. Lateral phase separations in membranes. J Supramol Struct. 1973;1(4):285–294. doi: 10.1002/jss.400010406. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Abortive assembly of the lactose transport system in Escherichia coli. Biochemistry. 1973 Jul 17;12(15):2816–2822. doi: 10.1021/bi00739a007. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Transport system assembly and the mobility of membrane lipids in Escherichia coli. Biochemistry. 1973 Jul 17;12(15):2822–2829. doi: 10.1021/bi00739a008. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Zwaal R. F., Roelofsen B., Comfurius P., Kastelijn D., van Deenen L. L. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta. 1973 Oct 11;323(2):178–193. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Wisnieski B. J., Williams R. E., Fox C. F. Manipulation of fatty acid composition in animal cells grown in culture. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3669–3673. doi: 10.1073/pnas.70.12.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]



