ABSTRACT
Dendritic cells (DCs) are the most efficient antigen-presenting cells, playing a key role in the adaptive immune responses to viral infections. Our studies demonstrate that wild-type (wt) rabies virus (RABV) does not activate DCs. Adoptive transfer of DCs primed with wt RABV did not activate DCs, stimulate virus neutralizing antibodies (VNA), or protect recipients against challenge. However, adoptive transfer of DCs primed with laboratory-attenuated RABV resulted in DC activation, production of VNA, and protection against challenge. In vitro studies with recombinant RABV (laboratory-attenuated RABV expressing the glycoprotein or the phosphoprotein from wt RABV) demonstrate that DC activation is dependent on the glycoprotein and involves the IPS-1 pathway. Furthermore, binding to and entry into DCs by wt RABV is severely blocked, and the copy number of de novo-synthesized leader RNA was two logs lower in DCs infected with the wt than in DCs treated with laboratory-attenuated RABV. However, transient transfection of DCs with synthesized leader RNA from either wt or attenuated RABV is capable of activating DCs in a dose-dependent manner. Thus, the inability of wt RABV to activate DCs correlates with its low level of the de novo-synthesized leader RNA.
IMPORTANCE Rabies remains a public health threat, with more than 55,000 fatalities each year around the world. Since DCs play a key role in the adaptive immune responses to viral infections, we investigated the ability of rabies virus (RABV) to activate DCs. It was found that the adoptive transfer of DCs primed with wt RABV did not activate DCs, stimulate VNA, or protect mice against lethal challenge. However, laboratory-attenuated RABV mediates the activation of DCs via the IPS-1 pathway and is glycoprotein dependent. We further show that wt RABV evades DC-mediated immune activation by inefficient binding/entry into DCs and as a result of a reduced level of de novo-synthesized leader RNA. These findings may have important implications in the development of efficient rabies vaccines.
INTRODUCTION
Despite the fact that rabies is one of the oldest human infectious diseases, it continues to present a public health threat by causing more than 55,000 human deaths every year around the globe (1). Its causative agent, rabies virus (RABV), belongs to the Rhabdoviridae family, and its genome encodes five structural proteins in the order of nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and RNA-dependent RNA polymerase (RdRp; also termed large protein [L]) (2). Among these, RABV G is the only viral protein that is glycosylated and exposed on the surface of the virion (3). RABV G is responsible for binding to neurospecific receptors, such as the acetylcholine receptor and neural cell adhesion molecule (NCAM), for invasion into the nervous system (4, 5). Moreover, RABV G is the only protein capable of inducing virus-neutralizing antibodies (VNA) that are protective against rabies (6–8).
It has been known for a long time that most of the human rabies patients (>70%) do not develop VNA at the time of death (9). The inability of wild-type (wt) RABV to induce VNA responses also has been reported in other animal species, such as mice (10), dogs (11), and skunks (12). On the other hand, experimental infection with laboratory-attenuated RABV induces VNA responses in laboratory animals (10, 13–17). Although the mechanism(s) by which different RABVs induce different VNA responses are unknown, recent studies (18–21) indicate that laboratory-attenuated RABV activates, while wt RABV evades, the host innate immune responses, particularly interferon (IFN) and chemokines, in the central nervous system (CNS).
Innate immune genes, such as chemokines, have been cloned into RABV vectors to enhance the immune responses (10, 14, 15, 22). It was found that the overexpression of these innate immune genes stimulated higher levels of VNA production and provided better protection by activating more dendritic cells (DCs) than the parental virus (10, 15) (14, 17). DCs are the most efficient antigen-presenting cells (APC), which play a key role in both innate and adaptive immune responses to viral infections (23–25). Immature DCs reside in almost all peripheral tissues as sentinels of the immune system. Once encountering infectious antigens, DCs begin to mature and lose their ability to take up antigens (26, 27). During their maturation, DCs undergo significant phenotypic changes by upregulation of major histocompatibility complex class II (MHC-II) and costimulatory molecules, such as CD40, CD80, and CD86 (28). It has been shown that infection with laboratory-attenuated but not wt RABV leads to strong activation of NF-κB and maturation of DCs (28). It has been reported that RABV activates DCs and induces the production of type I IFN in an IPS-1-dependent manner (29). Most likely it is the viral leader RNA that triggers IFN production in the infected cells (30). However, these studies were performed with laboratory-attenuated RABV.
In the present study, activation of DCs and induction of protective immune responses were investigated after infection with wt and laboratory-attenuated RABV. It was found that wt RABV does not induce efficient DC activation. Adoptive transfer of DCs primed with wt RABV did not activate DCs, stimulate VNA, or protect mice against lethal challenge. However, laboratory-attenuated RABV activated DCs via the IPS-1 pathway and is G dependent. Further investigation indicated that wt RABV is inefficient in binding and entry into DCs; consequently, the level of de novo-synthesized leader RNA is limited.
MATERIALS AND METHODS
Mice.
BALB/c mice were purchased from Harlan Sprague-Dawley Inc. B6/129-Mavstm1Zjc/J (IPS-1−/−) mice and B6129SF2/J (IPS-1+/+) mice were purchased from Jackson Laboratory and housed under specific-pathogen-free conditions in biosafety level 2 containment.
Ethics statement.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals from the NIH (31). All animal experiments were carried out as approved by the Institutional Animal Care and Use Committee, University of Georgia, on 11 July 2012 (AUP A2012 05-007). All efforts were made to minimize animal suffering. The Research Animal Resources unit in the University of Georgia is fully accredited by the Association of Assessment and Accreditation of Laboratory Animal Care International (AAALAC-I). The registration number from the U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Animal Care, is USDA APHIS-AC. We have an assurance on file with the NIH-Office of Laboratory Animal Welfare (NIH-OLAW) and are in compliance with the PHS policy on humane care and use of laboratory animals and the 8th edition of the Guide for the Care and Use of Laboratory Animals (31).
Cells and viruses.
Mouse neuroblastoma (NA) cells were maintained in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY). BSR cells, a cloned cell line derived from BHK-21 cells, were maintained in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Herndon, VA) containing 10% FBS. Myeloid DCs were generated as previously described (32). Briefly, bone marrow was removed from tibias and femur bones of BALB/c mice. Following lysis of red blood cells, progenitor cells were plated in RPMI 1640 medium (Invitrogen, USA) supplemented with 10% FBS, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Biosource, Camarillo, CA) in 6-well plates at 4 × 106/well. Cells were supplemented with fresh DC culture medium every other day. On day 8, DCs were positively selected for surface CD11c expression using magnetic beads (Stemcell Tech, Vancouver, Canada) to obtain a more than 95% pure population of CD11c+ cells. Flow-cytometric analysis of purified DCs displayed low levels of CD80, CD86, and MHC-II expression, which are characteristics of immature DCs. Purified CD11c+ DCs were cultured in fresh medium with FBS and GM-CSF and used in subsequent experiments. CVS-B2c is a laboratory-attenuated RABV derived from CVS-24 by serial passaging in BHK-21 cells (33). DRV-Mexico is a wt RABV isolated from a rabid dog in Mexico in the 1990s (34). Recombinant CVS-B2c expressing G [B2c(DRV-G)] or P [B2c(DRV-P)] from DRV-Mexico was constructed as described previously (35, 36). Virus stocks were prepared in 1-day-old suckling mice as described previously (37). When moribund, mice were euthanized and brains removed. A 10% (wt/vol) suspension was prepared by homogenizing the brain in DMEM. The homogenate was centrifuged to remove debris, and the supernatant was collected and stored at −80°C.
Trans-complementation assay (DRV expressing B2c-G).
In order to generate chimeric wt-DRV-Mexico expressing G from attenuated RABV CVS-B2c, BSR cells were transfected with 5 μg of CVS-B2c-G plasmid using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol. Twenty-four h posttransfection, cells were infected with DRV-Mexico at a multiplicity of infection (MOI) of 0.1. After 1 h postinfection, the culture medium was removed and fresh medium was added to the cells. After incubation for another 3 days, the virus in the culture supernatant was collected, and the genomic RNA gene copy number was calculated using qRT-PCR. As a control, Western blotting was performed to confirm the expression of CVS-B2c-G in BSR cells after 4 days of transfection (data not shown).
Viral infection in DCs.
DCs were plated in 6-well plates at 5 × 105 cells/well and then incubated with different viruses or mock treated with RPMI 1640 medium for 2 h. After 2 h of incubation, cells were washed with phosphate-buffered saline (PBS), resuspended in RPMI 1640 supplemented with 10% FBS and 20 ng/ml GM-CSF, and cultured for another 24 h. For the analysis of DC activation, cells were harvested and stained with specific antibodies against CD80, CD86, and MHC-II, and the expression of these molecules was analyzed by flow cytometry.
Adoptive transfer of DCs.
CD11c+ DCs were isolated and purified using CD11c magnetic beads according to the manufacturer's protocol (Stemcell Tech, Vancouver, Canada) and then primed with CVS-B2c or DRV. After treatment for 24 h, cells were collected and washed twice with PBS by centrifugation and then injected intraperitoneally (i.p.) into naive mice three times, on days 1, 3, and 5. The virus titration assay was performed to confirm that no free viruses remained in the DC preparations. As expected, we could not detect any virus in the DC preparations (data not shown). On day 6, the mice were challenged with wt DRV and monitored for 21 days for rabies-related clinical signs, and their survival was recorded. To analyze the immune responses, adoptive transfer was performed as described above. On days 3, 6, and 9 after the last transfer, mice were anesthetized and mesenteric lymph nodes, spleens, and sera were collected for analysis of immune cell activation and VNA in the sera.
Virus binding assay.
DCs as well as NA and BSR cells were mock treated with medium or incubated with different RABVs at 4°C. After 2 h postinfection, unbound viruses were washed three times using PBS. Cells were fixed with 80% acetone, stained with fluorescein isothiocyanate (FITC)-labeled anti-RABV N monoclonal antibodies, and subjected to fluorescence-activated cell sorter (FACS) analysis. Alternatively, cells were harvested for RNA isolation and for qRT-PCR analysis.
Virus entry assay.
For virus entry, cells were incubated with different viruses at 4°C for 2 h, washed thrice with PBS, and then cultured for another 4 h at 37°C. After being treated with 0.25% trypsin without EDTA for 10 min, the cells were washed with PBS 3 times to remove bound viruses on the cell surface, fixed with 80% acetone, and stained with FITC-labeled anti-RAV N antibodies for FACS. Unfixed cells were harvested for qRT-PCR analysis.
Virus titration.
NA cells cultured in a 96-well plate were inoculated with serial 10-fold dilutions of virus and incubated at 34°C for 48 h. The cells then were fixed with 80% ice-cold acetone for 30 min, washed twice with PBS, and stained with FITC-conjugated anti-RABV N antibodies. Antigen-positive foci were counted under a fluorescence microscope (Zeiss, Germany), and virus titer was calculated as focus-forming units per milliliter (FFU/ml). All titrations were carried out in quadruplicate.
qRT-PCR.
Total RNA were extracted from the cells and the supernatant using the RNeasy kit and viral RNA minikit (Qiagen, USA), respectively. RNA was used for quantitative real-time PCR (qRT-PCR) in an Mx3000P apparatus (Stratagene, La Jolla, CA) as described previously (19). Each reaction was carried out in triplicate with approximately 100 ng of DNase-treated RNA and 5 nM each primer pair by using Brilliant II SYBR green qRT-PCR master mix kit (Stratagene, CA) according to the manufacturer's instructions. For absolute quantification of viral genomic RNA (vRNA), cDNA was synthesized by SuperScript III reverse transcriptase (Invitrogen) by following the manufacturer's instructions using rabies gene-specific primers (Table 1). Further, qRT-PCR was performed using primers v_1121F and v_1250R. A standard curve was generated from serially diluted RNA in vitro transcribed from plasmids expressing RABV N, and the copy numbers of viral RNA were normalized to 1 mg of total RNA. For chemokine and cytokine expression, cDNAs were synthesized with oligo(dT) primer, and mRNA copy numbers of a particular gene were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Levels of chemokine and cytokine expression in a test sample are presented as fold increase over that detected in mock-infected controls.
TABLE 1.
Oligonucleotides used for quantification of RABV genome, leader RNA, chemokines, and cytokines
| Primer name | Sequence (5′–3′) | Use |
|---|---|---|
| IFN-betaR | ACCCAGTGCTGGAGAAATTG | qRT-PCR |
| IFN-betaF | CCCTATGGAGATGACGGAGA | qRT-PCR |
| Beta-actinR | ACACAGAGTACTTGGGCTCAGGAGG | qRT-PCR |
| Beta-actinF | CCTTCTTGGGTATGGAATCCTGTGG | qRT-PCR |
| RantiseF | CACTCCCTGCTGCTTTG | qRT-PCR |
| RantiseR | CACTTGGCGGTTCCTTC | qRT-PCR |
| IP-10F | AGCCTATCCTGCCCACG | qRT-PCR |
| IP-10R | CAGCCCTTTTAGACCTT | qRT-PCR |
| IL-6F | GGCATAACGCACTAGGTTT | qRT-PCR |
| IL-6R | GCTGGAGTCACAGAAGGAG | qRT-PCR |
| v_1121F | GGAAAAGGGACATTTGAAAGAA | qRT-PCR |
| v_1250R | AGTCCTCGTCATC AGAGTTGAC | cDNA synthesis/qRT-PCR |
| Letag_49F | CCAGATGCTTGGCGTCCTCTTTGCAATTGACGCTGT | cDNA synthesis |
| Letag | CCAGATGCTTGGCGTCCT | qRT-PCR |
| Le_1R | ACGCTTAACAACAAAACC | qRT-PCR |
Synthesis of ssRNA and dephosphorylation of ssRNA.
Single-stranded leader RNAs (ssRNAs) were produced by in vitro transcription with T7 RNA polymerase (T7 RiboMAX express large-scale RNA production system; Promega). Templates for the leader RNA from CVS-B2c and DRV-Mexico were created by synthesizing a DNA oligonucleotide containing the T7 promoter followed by the RNA-encoding sequence and annealing it to a cDNA oligonucleotide. Enzymatically synthesized ssRNA molecules were isolated with TRIzol-chloroform (2:1) extraction, followed by precipitation with isopropyl alcohol. All RNAs were dissolved in sterile H2O. To remove 5′ triphosphates, enzymatically synthesized ssRNAs were treated with calf intestine alkaline phosphatase (New England BioLabs). The reactions were carried out at 37°C for 60 min. Dephosphorylated RNAs subsequently were purified as described above. To transfect the purified leader RNAs into DCs, 0.5 μl of Lipofectamine 2000 and 100 μl of Opti-MEM medium were used according to the manufacturer's protocol.
For the quantification of leader RNA, cDNAs were synthesized with tagged primer (Letag_49F) with an 18-nucleotide (nt) tag that was unrelated to RABV. qRT-PCR was performed using primers Letag and Le_1R. However, these primers also could amplify the antigenomic RNA, the level of which is negligible compared to that of genomic RNA (1:49) (38). Copy numbers of a leader RNA were calculated from the standard curve generated using a serially diluted, enzymatically synthesized leader RNA in vitro (as described above), and the copy numbers of viral RNA were normalized to 1 mg of total RNA. Table 1 provides primer sequence details.
Flow cytometry.
Flow cytometry was carried out as described previously (15). For surface markers, cells were stained with CD3, CD4, CD8, CD19, CD40, CD138, CD11c, CD86, CD80, and major histocompatibility complex class II (MHC-II) antibodies and an isotype control (BD Pharmingen). For N protein, cells first were permeabilized by fixation and permeabilization solution (BD Cytofix/Cytoperm single) and then stained with FITC-conjugated anti-RABV N protein monoclonal antibody (FujiRebio Diagnostic Inc., Malvern, PA). After incubation on ice for 30 min, cells were washed twice in PBS containing 2% FBS and 0.02% NaN3 and then fixed with 1% paraformaldehyde. Data collection and analysis were performed using a BD LSR-II flow cytometer, BD FACSDiva software (BD Pharmingen), and FlowJo software (TreeStar, San Carlos, CA).
ELISA.
Cultured DCs were treated with different RABVs at 10 gene copies/cell, 2 μg lipopolysaccharide (LPS), 60 μg poly(I·C), or 80 ng tumor necrosis factor alpha (TNF-α). After incubation for 72 h, cell supernatants were harvested and the expression of cytokines, including IFN-α and interleukin-6 (IL-6), was analyzed using a mouse IFN-α enzyme-linked immunosorbent assay (ELISA) kit (PBL, USA) and IL-6 DuoSet (R&D Systems, USA), respectively. All assays were performed according to the manufacturer's instructions.
RFFIT.
VNA titers were measured using the rapid fluorescent focus inhibition test (RFFIT) as described previously (39). Briefly, 50 μl of serial 5-fold dilutions of serum were prepared in Laboratory-Tek chamber slides (Nalge Nunc International, Rochester, NY). Fifty percent fluorescent focus-forming doses (FFD50) of CVS-11 were added to each chamber and incubated at 37°C for 90 min. NA cells (5 × 105 cells/ml) were added to each chamber, and the slides were incubated at 37°C for 24 h. The slides then were fixed with ice-cold 80% acetone and stained with FITC-conjugated anti-RABV N antibodies. Twenty fields in each chamber were observed under a fluorescence microscope, and the 50% endpoint titers were calculated according to the Reed-Muench formula (40). The values were compared to those obtained with the reference serum (National Institute for Biological Standards and Control, Herts, United Kingdom) and normalized to international units (IU)/ml.
Statistics analysis.
The statistical significance of survival rates was determined by the log-rank test and Kaplan-Meier survival analysis. Virus titer, cytokine production, cell surface marker expression, RNA levels, and G incorporated into virions were evaluated by one-way analysis of variance (ANOVA). Asterisks denote statistical differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) between different groups. A P value of less than 0.05 was considered statistically significant.
RESULTS
Adoptive transfer of DCs primed with wt RABV failed to induce protective immune responses against lethal RABV infection in mice.
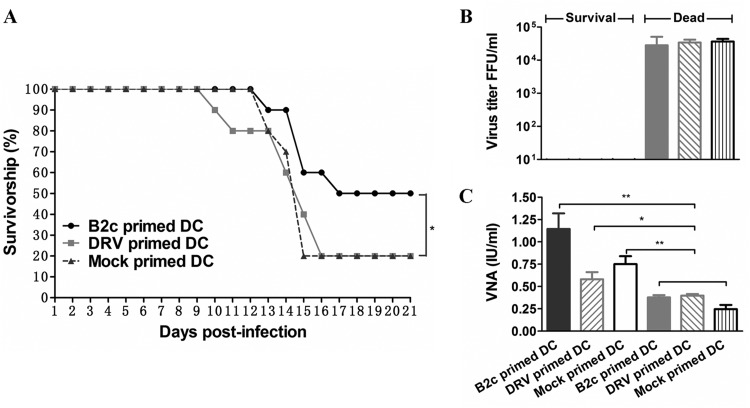
To determine the roles of DC activation in RABV immunogenicity, adoptive transfer of DCs treated with wt or laboratory-attenuated RABVs was carried out in mice. Briefly, 5 × 106 mock-primed DCs or DCs primed in vitro with wt DRV-Mexico or laboratory-adapted CVS-B2c (10 gene copies of virus/cell) were injected into the peritoneal cavity (i.p.) of each mouse (10/group) three times on days 1, 3, and 5. The gene copy number was used to standardize the virus dose, because virus titration in cells is not accurate, particularly with wt RABV. One day after the last adoptive transfer, mice were challenged intramuscularly with a lethal dose of DRV-Mexico (103.5 FFU) and observed for clinical signs and death for 21 days. As shown in Fig. 1A, significantly more mice (50%) transferred with DCs primed with CVS-B2c survived the challenge than mice transferred with mock-primed DCs (20%) or DCs primed with DRV-Mexico (20%) (P < 0.05). In mice that succumbed to rabies, virus titers reached 104.5 FFU/ml, and the VNA level was less than 0.5 IU/ml (0.23 to ∼0.4 IU) (Fig. 1B and C). In the mice passively transferred with DCs primed with CVS-B2c, an average VNA production of 1.125 IU/ml was detected, and no RABV virus was found in the brain of the survivors. These results demonstrate that the adoptive transfer of DCs primed with wt DRV-Mexico failed to induce the production of VNA and protect mice against challenge infections, while the adoptive transfer of DCs primed with laboratory-attenuated CVS-B2c resulted in the induction of VNA and, subsequently, protection against challenge.
FIG 1.
Adoptive transfer of DCs primed with wt RABV failed to induce protective immune responses against lethal RABV infection in mice. (A) Survivorship of mice after adoptive transfer with DCs primed with B2c, DRV, or medium by the i.p. route. The survival rates were analyzed for statistical significance by Kaplan-Meier plots (n = 10 in each group; P < 0.05 by log-rank test). (B) Virus titers in the brains of surviving versus dead mice. (C) VNA in the sera of dead and surviving mice.
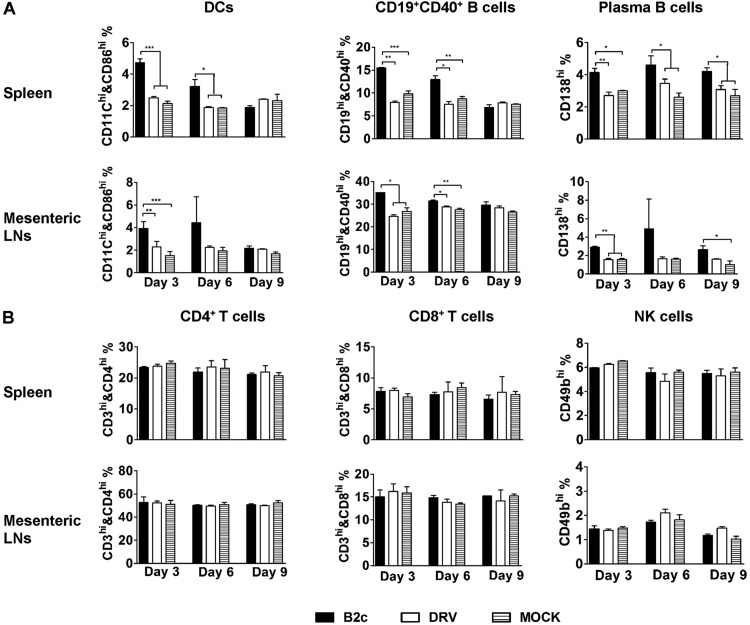
To assess the immune responses mediated by DC adoptive transfer, activation of DCs as well as NK, T, and B cells in spleen and mesenteric lymph nodes was analyzed at days 3, 6, and 9 after the last transfer. As shown in Fig. 2A, there was no significant difference in the number of activated DCs (CD11c+/CD86+), B cells (CD19+/CD40+), or plasma cells (CD138+) in the spleens and the mesenteric lymph nodes of mice transferred with DCs primed with DRV-Mexico compared to that in mice transferred with mock-primed DCs. On the other hand, significantly more activated DCs, B cells, and plasma cells were detected in the spleen and lymph nodes of mice transferred with DCs primed with CVS-B2c as early as 3 days after the last transfer compared to mice transferred with mock-primed DCs or DCs primed with DRV-Mexico (Fig. 2A). No significant differences were detected in the number of CD4+, CD8+, or NK (CD49+) cells between different groups (Fig. 2B). These results indicate that adoptive transfer of DCs primed with wt RABV failed to elicit innate or adaptive immune responses in the recipients, while the adoptive transfer of DCs primed with laboratory-attenuated RABV elicited these responses. These results demonstrate the critical role of DC activation in RABV immunogenicity and protection.
FIG 2.
Adoptive transfer of DCs primed with wt RABV fails to induce activation of DCs, B cells, and plasma B cells in the spleen and mesenteric lymph nodes. BALB/c mice were transferred i.p. with mock-primed DCs or DCs primed with CVS-B2c or DRV-Mexico (5 × 105 cells/mouse). Spleen and mesenteric lymph nodes were collected at 3, 6, and 9 days postinfection. Single-cell suspensions were prepared and stained with the indicated fluorescent antibodies. (A) Flow-cytometric analysis of DCs, B cells, and plasma B cells in the spleen and lymph nodes. (B) Flow-cytometric analysis of T cells and NK cells in the spleen and lymph nodes.
wt RABV fails to induce DC activation in vitro.
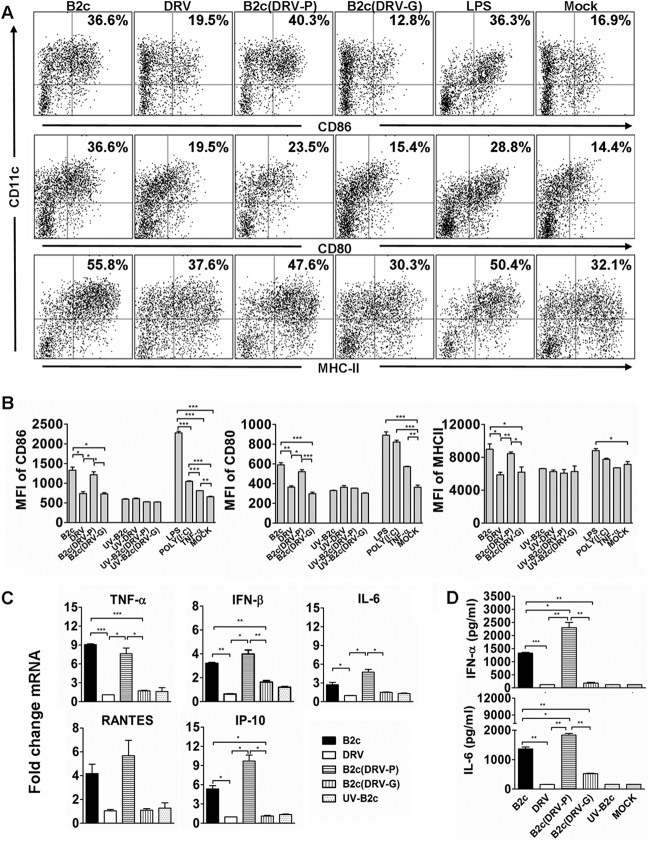
To investigate why DCs primed with wt RABV fail to stimulate immune responses and provide protection against lethal challenge, DCs were isolated from mouse bone marrow and cocultured with wt or laboratory-attenuated RABV. DCs treated with lipopolysaccharide (LPS), poly(I·C), or TNF-α were included as positive controls. The expression and number of CD86, CD80, and MHC-II on CD11c+ cells were measured using flow cytometry. Compared to mock-infected DCs, no increased expression of CD86, CD80, or MHC-II was detected in DCs infected with wt DRV-Mexico (Fig. 3A and B), indicating that wt RABV is incapable of activating DCs in vitro. However, significantly higher numbers of CD86+, CD80+, or MHC-II+ cells, as well as higher levels of expression of these molecules (measured as mean fluorescent intensity [MFI]), were detected in DCs infected with laboratory-attenuated than with wt RABV (Fig. 3A and B). To determine if RABV G was responsible for DC activation, laboratory-attenuated RABV expressing the glycoprotein from wt RABV as constructed previously (35) was included for comparison. The recombinant RABV (rRABV) expressing P from wt virus (DRV-P) was included as a control. DRV-P induced DC activation as efficiently as CVS-B2c, while rRABV expressing G from wt virus (DRV-G) did not, suggesting that DC activation is G dependent (Fig. 3A and B). UV inactivation of laboratory-attenuated CVS-B2c abolished its ability to activate DCs, as the levels of CD86, CD80, and MHC-II were comparable to those for mock infection (Fig. 3B), which suggests that active RABV infection is required for DC activation.
FIG 3.
wt RABV fails to induce DC activation and maturation in vitro. (A) Flow-cytometric analysis of the number of CD86, CD80, and MHC-II-positive DCs infected with CVS-B2c, DRV-Mexico, B2c(DRV-P), or B2c(DRV-G), treated with LPS, or mock treated. (B) Analysis of CD86, CD80, and MHC-II expression in DCs infected with the indicated RABVs. (C) Analysis of DC activation using various virus doses of the indicated RABVs. (D) Analysis of expression of IFN-β, IP-10, RANTES, IL-6, and TNF-α using qRT-PCR in DCs infected with the indicated RABVs. (E) Quantification of IFN-α and IL-6 by ELISA in the culture supernatants of DCs infected with the indicated RABVs.
In order to study the effects of viral dose on DC activation, DCs were incubated with 1, 10, and 100 gene copies of RABVs per cell. It was observed that the number of CD86+, CD80+, or MHC-II+ DCs did not change in DCs infected with DRV-Mexico or B2c (DRV-G), while the number of CD86+ DCs increased in a gene copy number-dependent manner in DCs infected with CVS-B2c or B2c (DRV-P) (Fig. 3C). These results demonstrate that the inability of wt RABV to activate DCs is not dose dependent, while laboratory-attenuated RABV activates DCs in a dose-dependent manner.
To confirm that wt RABV does not activate DCs, cytokine and chemokine production in DCs or supernatants of DCs infected with different viruses were analyzed by qRT-PCR and ELISA. DRV-Mexico and B2c (DRV-G) stimulated significantly lower levels of IL-6, TNF-α, IFN-β, RANTES, and IFN-γ-induced protein 10 (IP-10) in DCs than CVS-B2c and B2c (DRV-P), as detected by RT-PCR (Fig. 3D) or by ELISA (Fig. 3E). These results confirm the inability of wt RABV (or G from wt RABV) to activate DCs.
wt RABV (or G from wt RABV) does not inhibit LPS-mediated DC activation.
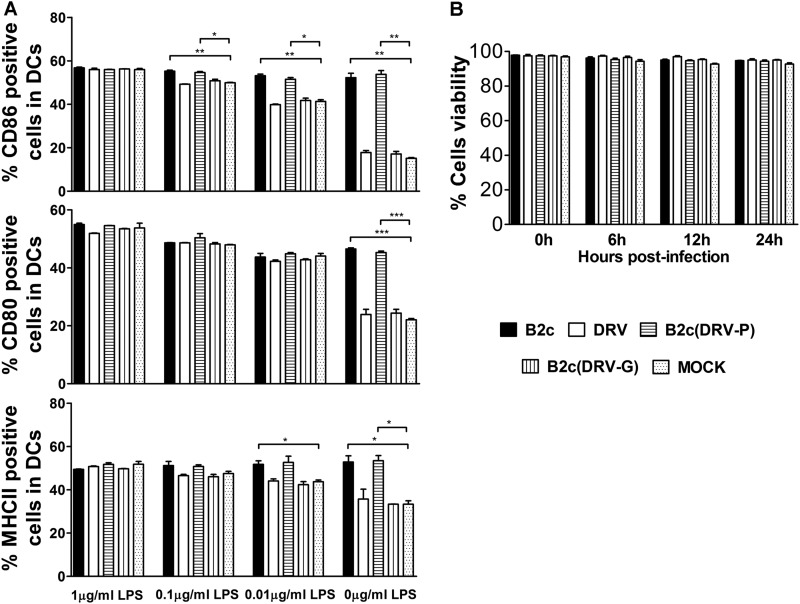
To determine if wt RABV (or G from wt RABV) inhibits DC activation, immature DCs were treated with various concentrations of LPS in the presence or absence of RABV. Mock-treated DCs were included as a control. As expected in the LPS-treated cells, the numbers of CD86+, CD80+, and MHC-II+ DCs were significantly lower in DCs treated with DRV-Mexico or B2c (DRV-G) than in those treated with CVS-B2c or B2c (DRV-P) (Fig. 4A). In DCs treated with 1 μg/ml of LPS, the numbers of CD86+, CD80+, and MHC-II+ DCs remain unchanged irrespective of different RABV infections. For DCs treated with lower concentrations of LPS, only the number of CD86+ DCs was significantly lower in DCs treated with DRV-Mexico or B2c (DRV-G) than in those treated with CVS-B2c or B2c (DRV-P); however, there were no significant differences in the number of CD86+, CD80+, and MHC-II+ DCs treated with DRV-Mexico or B2c (DRV-G) compared to the numbers with LPS treatment alone, indicating that G from DRV-Mexico does not inhibit LPS-mediated DC activation (Fig. 4A). To ensure that these changes were not due to DC viability, a cell viability assay was performed. More than 95% of DCs infected with each virus were found to be viable throughout the observation period, similar to mock-infected DCs (Fig. 4B).
FIG 4.
wt RABV (or the glycoprotein from wt RABV) does not inhibit LPS-mediated DC activation. (A) Quantitative assessment of CD86, CD80, and MHC-II expression in DCs infected with RABVs left untreated or treated with the indicated concentration of LPS. (B) Analysis of DC viability after infection with RABVs at 10 gene copy numbers/cell using trypan blue.
wt RABV induces low levels of leader RNA synthesis in DCs.
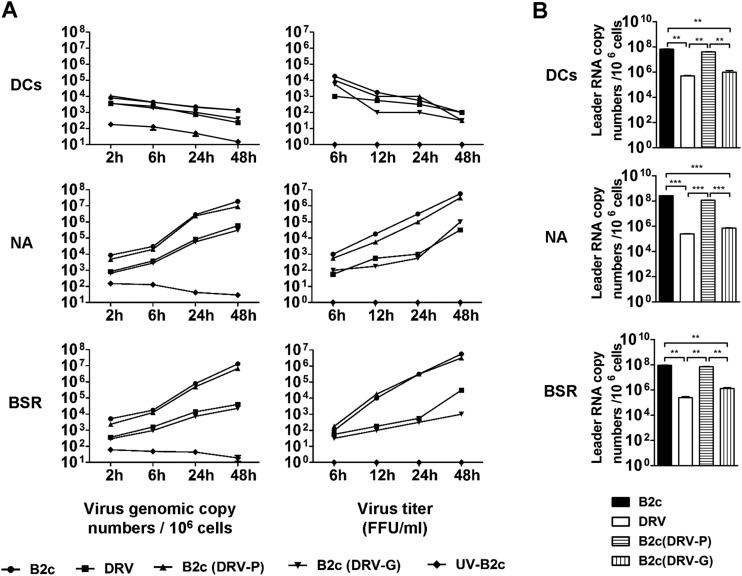
To investigate if these rRABVs can actively replicate in DCs, virus titer and genomic RNA were measured in DCs after infection. As shown in Fig. 5A, virus titers or viral RNA gradually declined in DCs infected with each of the viruses, indicating RABVs do not actively replicate in DCs. All of the viruses replicated in NA and BSR cells, although the rate of replication is much lower in cells infected with DRV-Mexico or B2c (DRV-G) than in those infected with CVS-B2c or B2c (DRV-P) (Fig. 5A).
FIG 5.
wt RABV induces a low level of leader RNA synthesis in DCs. However, RABV replication is suppressed and nonproductive in DCs. (A) DCs as well as NA and BSR cells were infected with the indicated RABVs for viral genome quantification at different time points using qRT-PCR. The quantification of virus production in the cell culture supernatants at different time points is shown. (B) Quantification of viral leader RNA using qRT-PCR in DCs as well as NA and BSR cells infected with indicated viruses.
It has been shown that DC activation is due to the recognition of viral RNA, particularly leader RNA, by RIG-I to induce interferon responses (29). To investigate if the failure of wt RABV to activate DCs is due to its inability to synthesize leader RNA, DCs were infected with CVS-B2c, DRV-Mexico, B2c(DRV-P), or B2c(DRV-G) at an MOI of 10 copy numbers per cell, and viral leader RNA was measured at 24 h postinfection. BSR or NA cells were infected with each virus as positive controls. Using qRT-PCR, the leader RNA levels in DCs and NA and BSR cells were determined. As shown in Fig. 5B, a significantly lower level (2 logs lower) of leader RNA was detected in DCs infected with DRV or B2c (DRV-G) than in those infected with CVS-B2c or B2c (DRV-P). Similar results were obtained with both NA and BSR cells (Fig. 5B). Thus, these results indicate that wt RABV induces significantly less de novo synthesis of leader RNA in DCs than laboratory-attenuated RABV.
Synthetic leader RNA from wt RABV is as capable of inducing DC activation as that from laboratory-attenuated RABV.
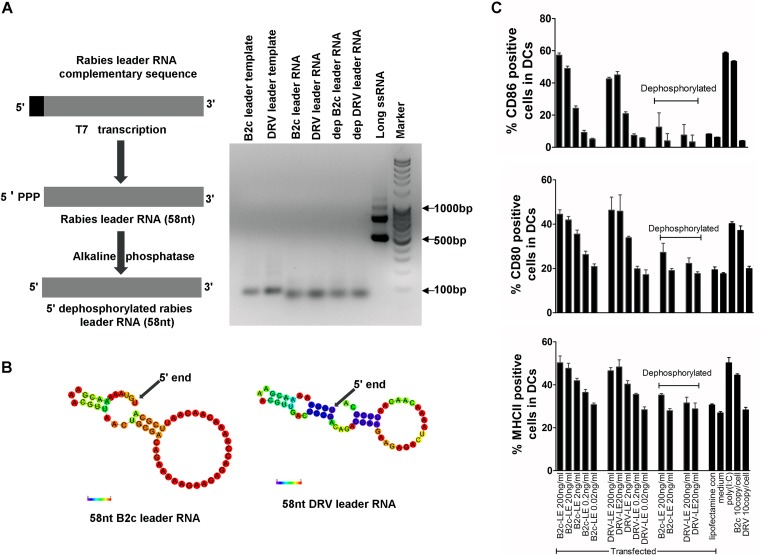
To determine if the leader RNA from wt RABV is able to activate DCs, the leader RNAs from CVS-B2c and DRV-Mexico were synthesized using a T7 RiboMAX in vitro transcription system. The synthesized leader RNAs were verified by gel electrophoresis (Fig. 6A). Analysis of the minimum free energy secondary structure calculation using the RNAfold web server program suggests that the leader RNA from both viruses have similar hairpin structures (Fig. 6B). Purified leader RNAs were used to transfect DCs using Lipofectamine 2000 at various concentrations; 5′ dephosphorylated leader RNAs were included as a control. The number of CD11c cells that were CD86+, CD80+, and MHC-II+ were measured to assess the activation status using flow cytometry. As shown in Fig. 6C, the number of CD86+, CD80+, and MHC-II+ DCs increased in a dose-dependent manner when transfected with the leader RNA from both viruses, indicating that leader RNAs from both viruses are capable of activating DCs (Fig. 6C). However, the dephosphorylation of leader RNA abolishes DC activation. These data further demonstrate that it is the level of leader RNA, not the leader RNA per se, from wt RABV that determines the status of DC activation.
FIG 6.
Transient transfection of leader RNA from wt or laboratory-attenuated RABV can induce activation of DCs in a dose-dependent manner. (A) Schematic representation of enzymatic synthesis of rabies leader ssRNA. (B) In silico calculations of minimum free energy secondary structures of CVS-B2c and DRV leader RNA. The structures are colored according to base-pairing probability, ranging from 0 (least likely; red) to 1 (most likely; violet). The 5′ end is indicated by the black arrow. (C) Indicated concentration of enzymatically synthesized leader RNA was used to transfect DCs using Lipofectamine 2000 (lipofectamine con). The number of CD86+, CD80+, and MHC-II+ DCs was measured using flow cytometry.
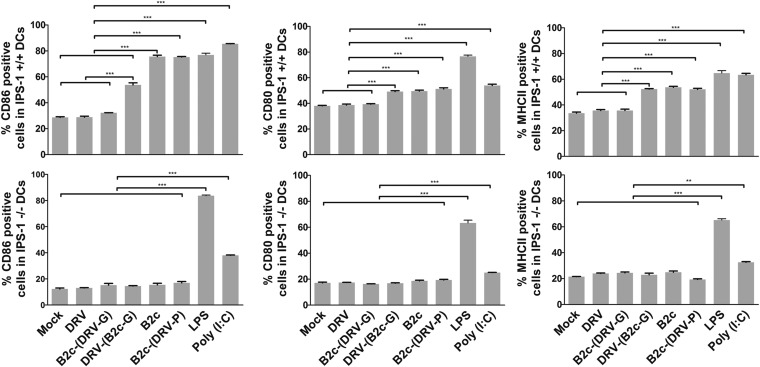
Laboratory-adapted RABV and wt RABV trans-complemented with G from laboratory-attenuated virus activated DCs in an IPS-1-dependent manner.
In order to determine whether RABVs induce DC activation through an RNA-dependent pathway, BMDCs from IPS-1 knockout (IPS-1−/−) or wild-type IPS-1 (IPS-1+/+) mice were cocultured with each RABV. DCs treated with LPS or poly(I·C) were included as positive controls. The activation status of DCs was measured for the expression of CD86 and MHC-II molecules on CD11c+ cells by flow cytometry. Compared to mock-infected DCs, no increase in the number of CD86-, CD80-, or MHC-II-positive cells was detected in IPS-1−/− or IPS-1+/+ DCs infected with B2c (DRV-G) or DRV-Mexico. On the other hand, infection of IPS-1+/+ DCs with CVS-B2c or B2c (DRV-P) induced significantly higher numbers of CD86-, CD80-, or MHC-II-positive DCs. However, the level of CD86, CD80, or MHC-II expression in IPS-1−/− DCs infected with these viruses was similar to that in mock-infected DCs (Fig. 7). Since the wt RABV or the laboratory-attenuated RABV expressing G from wt RABV is unable to activate either IPS-1−/− or IPS-1+/+ DCs, wt RABV (DRV-Mexico) was trans-complemented with G from laboratory-attenuated virus [DRV(B2c-G)] and included for comparison. As shown in Fig. 7, treatment of IPS-1+/+ DCs with [DRV(B2c-G)] induced significantly higher numbers of CD86-, CD80-, or MHC-II-positive cells than the mock treatment; however, the treatment of IPS-1−/− DCs with this virus did not increase the number of CD86-, CD80-, or MHC-II-positive cells compared to mock infection. Thus, these results suggest that DC activation by RABVs involves the IPS-1 pathway and is G dependent.
FIG 7.
Laboratory-attenuated RABV and wt RABV trans-complemented with G from laboratory-attenuated virus can activate DCs in an IPS-1-dependent manner. (A) Flow-cytometric analysis of the number of CD86-, CD80-, and MHC-II-positive DCs derived from IPS-1 knockout or wild-type mice that were mock treated, infected with CVS-B2c, DRV-Mexico, B2c(DRV-P), B2c(DRV-G), or DRV(B2cG), or treated with LPS or poly(I·C).
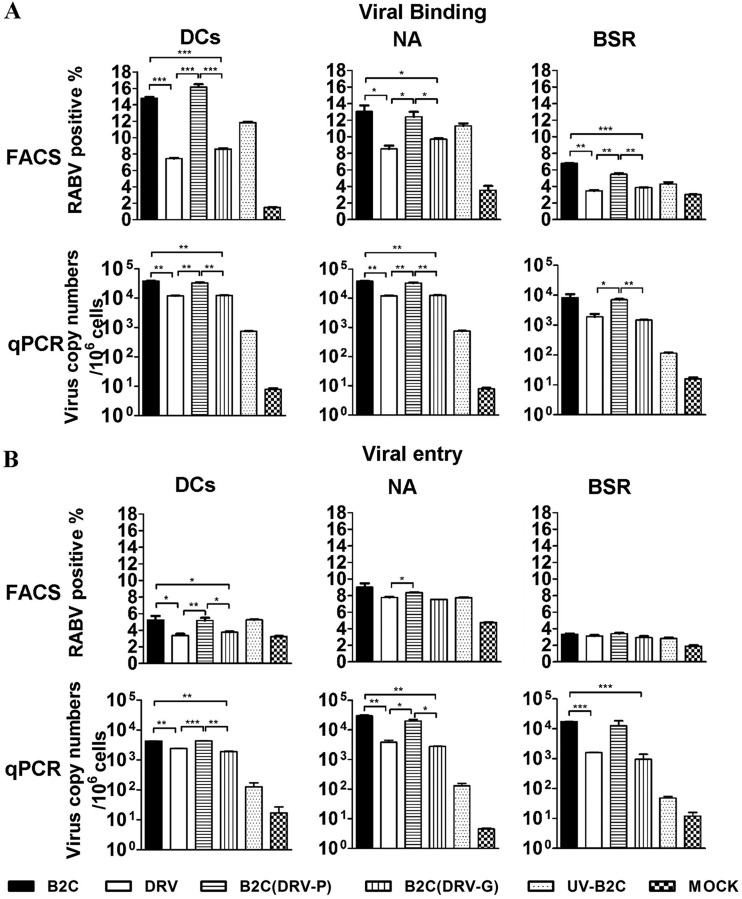
wt RABV (G) is inefficient in binding and entry into DCs.
To determine the mechanism(s) by which wt RABV induces low levels of leader RNA synthesis in DCs, virus binding and entry into DCs were investigated after infection with each RABV. As RABVs are known to replicate in NA and BSR cells, these cells were included as positive controls. For virus binding, DCs and NA and BSR cells were incubated with 10 gene copies of different RABVs at 4°C for 2 h. The cells then were washed three times to remove unbound viruses. Cell surface-bound viruses were detected by FACS using FITC-labeled anti-RABV N monoclonal antibodies. Alternatively, viral RNA inside the bound viruses was measured by qRT-PCR using N gene-specific primers. As shown in Fig. 8A, significantly fewer RABV-positive cells (<8%) were detected in DCs treated with DRV-Mexico or B2c (DRV-G) than in DCs treated with CVS-B2c or B2c (DRV-P) (>15%) (Fig. 8A, top left). The flow-cytometric data were further confirmed by qRT-PCR for viral N gene (Fig. 8A, bottom left), and significantly less RABV N RNA was detected in DCs treated with DRV-Mexico (1.1 × 104) or B2c (DRV-G) (1.2 × 104) than in DCs treated with CVS-B2c (3.8 × 104) or B2c (DRV-P) (3.3 × 104) (Fig. 8A, bottom left). A similar binding phenomenon was observed in NA (Fig. 8A, middle) and, to a lesser degree, in BSR (Fig. 8A, right) cells.
FIG 8.
wt RABV (G) is inefficient in binding and entry into DCs. (A) NA and BSR cells as well as DCs were infected with the indicated viruses for the analysis of binding using FACS and qRT-PCR. (B) NA and BSR cells as well as DCs were infected with the indicated viruses for the analysis of entry using FACS and qRT-PCR (see Materials and Methods for details).
In order to assess virus entry, cells were incubated with 10 gene copies of different RABVs at 37°C for 4 h, and then the cells were treated with trypsin (10 min for DCs, 4 min for NA and BSR cells) to remove the surface-bound viruses. As shown in Fig. 8B, significantly fewer RABV-positive cells (<3.5%) were detected in DCs treated with DRV-Mexico or B2c (DRV-G) than in DCs treated with CVS-B2c or B2c (DRV-P) (>5%) by flow cytometry. Further, the flow-cytometric data were confirmed by qRT-PCR. These data indicate that significantly less DRV or B2c (DRV-G) entered into DCs than CVS-B2c or B2c (DRV-P) (Fig. 8B, bottom left). A similar trend also was observed in NA and BSR cells, particularly by qRT-PCR (Fig. 8B, right).
DISCUSSION
It has long been known that rabies patients rarely develop VNA (41). Likewise, laboratory animals infected with wt RABV do not develop VNA (9). In the present study, we present evidence that RABV evades host immune responses by failure to activate DCs both in vitro and in vivo. Adoptive transfer of DCs primed with wt RABV did not activate DCs and, as a result, did not induce the production of VNA or provide protection against challenge. On the other hand, adoptive transfer of DCs primed with laboratory-attenuated RABV activated DCs, induced the production of VNA, and provided protection against challenge. Furthermore, our studies revealed that wt RABV binding to DCs is severely blocked, and de novo synthesis of its leader RNA is limited (two logs lower than that in DCs infected with laboratory-attenuated RABV). Thus, our studies indicate that wt RABV evades DC-mediated immune activation by inefficient binding of the virus to DCs and the subsequent low level of leader RNA transcription in DCs.
DCs are antigen-presenting cells (APCs) specially equipped with a highly efficient mechanism that allows them to detect pathogens, to capture, process, and present antigens, and to initiate and regulate immune responses (25). DCs possess properties and abilities enabling them to act as unique immune live adjuvants (42). However, viruses have developed ways to evade the host immune system. One of the common escape mechanisms is to interfere with DC activation, and this has been observed in infection with viruses such as vaccinia virus, herpes simplex virus (HSV-1), measles virus, and human cytomegalovirus (HCMV) (43–45). Salio et al. have shown that DCs infected with HSV-1 fail to upregulate costimulatory molecules, produce cytokines, or acquire responsiveness to chemokines required for migration to secondary lymphoid organs (45). Similarly, our findings indicate that wt RABV evades the host immune responses by failure to induce DC activation. After infection with wt RABV, costimulatory molecules (CD86 and CD80) and MHC-II were not upregulated in DCs cultivated in vitro. Likewise, adoptive transfer of DCs primed with wt RABV failed to activate DCs or to produce VNA in the recipients. On the other hand, laboratory-attenuated RABV stimulated DC activation both in vitro and in vivo. These results somewhat contradict previous studies. Li et al. (28) reported that wt RABV induced a level of DC activation similar to that of laboratory-attenuated RABV when the surface markers were measured. These discrepancies may be due to different wt viruses used, and these viruses may have different passage histories in experimental animals or cell culture. Nevertheless, the upregulation of NF-κB signaling pathway-related genes in DCs is less robust, and the level of IFN-α mRNA is lower in monocytes and DCs infected with DOG4 (wt) than in those infected with SPBNGAS-GAS (laboratory attenuated) (28).
To determine the mechanism by which wt RABV evades DC activation, rRABVs with the exchange of G as described previously (35) were used to infect DCs. Recombinant RABV expressing G from wt RABV failed to stimulate DC activation, while those expressing G from laboratory-attenuated RABV activated DCs. Therefore, DC activation is G dependent. It has been shown that HCMV and vaccinia virus inhibit DC maturation to evade the host innate immune response by failing to upregulate MHC-II and costimulatory molecules or by inducing DC death, respectively (43). We next sought to determine whether wt RABV could inhibit the LPS-induced DC activation as their immune evasive strategy. Our data indicate that wt RABV or rRABV expressing G from wt RABV does not suppress the expression of CD86, CD80, or MHC-II molecules, indicating that wt RABV or G from wt RABV does not inhibit LPS-mediated DC activation. Previous studies have shown that rRABV expressing G from wt RABV possesses the phenotype of wt RABV in pathogenicity, which correlates with the failure to induce apoptosis and innate immune responses (35, 46, 47). Therefore, it is likely that G from wt RABV contributes to rabies pathogenicity through evasion of the innate and adaptive immune responses.
Further, we sought to determine the role of viral RNA on DC activation. It is known that double-stranded RNA and 5′-triphosphate leader RNA from negative-stranded RNA viruses can be recognized in the cytoplasm by RLRs (RIG-I-like receptors), namely, RIG-I and Mda-5 (48–51). RIG-I recognizes 5′-triphosphated RNA, including the leader RNA from negative-stranded RNA viruses (30, 52). It has been shown that 5′-triphosphated leader RNA from measles virus acts as an activator of the RIG-I-mediated immune response (50). Forsbach et al. (53) reported that the conserved U- and G-rich nucleotides of the 3′-terminal sequence of negative-strand RNA viruses, such as vesicular stomatitis virus, Sendai virus, and influenza virus, can stimulate cytokine responses via TLR7 and TLR8 (53). Once these pathogen-associated molecular patterns (PAMPS) encounter pathogens, they will induce the activation of immune cells to produce antiviral molecules, such as IFNs (54, 55). Laboratory-attenuated RABV has been reported to activate DCs by RIG-1 to induce IFN production through recognition of viral RNA, particularly leader RNA, which is IPS-1 dependent (29, 30). To counter these host innate immune responses, viruses completing transcription in the cytosol have developed various strategies, such as an efficient capping process, by the polymerases (56, 57) or shielding nascent genome and anti-genome in the nucleocapsid (58, 59). It also has been shown that the processing of 5′ termini of the genome by negative-strand RNA viruses is one of the strategies to avoid RIG-I-dependent interferon induction (60). Here, we describe that one of the mechanisms to evade the immune responses by wt RABV is limited de novo synthesis of leader RNA. Our analysis indicates that the leader RNA from wt RABV is as capable of activating DCs as that from laboratory-attenuated virus. Thus, the level of leader RNA plays a crucial role in DC activation after infection with RABV. One of the major differences between wt and laboratory-attenuated RABV is the amount of leader RNA synthesized de novo in DCs. wt RABVs or rRABV expressing G from wt virus induces 2-log lower de novo synthesis of leader RNA than laboratory-attenuated RABV. These results led us to believe that the failure of wt RABV to activate DCs is due to the small amount of leader RNA synthesized de novo. To ensure that it is the viral leader RNA that activates the DCs, BMDCs isolated from IPS-1 knockout or wild-type mice were used for infection with each of the viruses. Our results indicate that laboratory-attenuated RABV or wt RABVs trans-complemented with G from laboratory-attenuated virus can activate DCs in an IPS-1-dependent manner. However, wt RABV or the laboratory-attenuated RABV expressing G from wt virus fails to do so. Together these results indicate that it is RABV leader RNA that activates DCs.
DC activation by RABV is also G dependent. The laboratory-attenuated RABV expressing G from wt virus failed to activate DCs like the wt virus, and the wt RABV trans-complemented with G from laboratory-attenuated virus activated DCs like the laboratory-attenuated virus in an IPS-1-dependent manner as reported previously (29). It is known that RABV glycoprotein is the only protein capable of delivering viral RNA into the host cells by facilitating binding and entry. In this study, we further determined the ability of RABV to bind and to enter DCs. Our results indicate that the binding to and entry into DCs by wt RABV or recombinant RABV expressing G from wt RABV were severely blocked compared to those by laboratory-attenuated RABV or recombinant RABV expressing P from wt RABV. Indeed, previous studies have shown the differential level of G expression between wt and laboratory-attenuated RABVs (19, 61). It also has been shown that CVS-B2c expresses a higher level of G than DRV-Mexico (35), and these two viruses were used in this study. Although it has been reported that the level of G expression alone may not necessarily contribute to viral pathogenicity (62), it is possible the low level of G expression by wt RABV results in a low level of G incorporation into budding virions, consequently affecting virus binding and entry. Thus, further studies are warranted to investigate these possibilities.
In summary, we found that the adoptive transfer of DCs primed with wt RABV did not activate DCs, stimulate VNA, or protect mice against lethal challenge. RABV-mediated activation of DCs involves the IPS-1 pathway and is G dependent. We further show that wt RABV evades DC-mediated immune activation by inefficient binding/entry into DCs, resulting in a reduced level of de novo-synthesized leader RNA.
ACKNOWLEDGMENTS
This work was supported by grants from the Natural Science Foundation of China (31330078), Chinese Department of Sciences and Technology (863 program; 2011AA10A212), Chinese Department of Agriculture (special fund for Agroscientific Research in the Public Interest; 201303042), and Public Health Service grants AI-051560 and AI-093369 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.WHO. 2005. WHO expert consultation on rabies. World Health Organ Tech Rep Series 931:1–88. [PubMed] [Google Scholar]
- 2.Wunner WH, Larson JK, Dietzschold B, Smith CL. 1988. The molecular-biology of rabies viruses. Rev Infect Dis 10:S771–S784. doi: 10.1093/clinids/10.Supplement_4.S771. [DOI] [PubMed] [Google Scholar]
- 3.Cox JH, Dietzschold B, Schneider LG. 1977. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun 16:754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lentz TL, Burrage TG, Smith AL, Tignor GH. 1983. The acetylcholine-receptor as a cellular receptor for rabies virus. Yale J Biol Med 56:315–322. [PMC free article] [PubMed] [Google Scholar]
- 5.Thoulouze MI, Lafage M, Schachner M, Hartmann U, Cremer H, Lafon M. 1998. The neural cell adhesion molecule is a receptor for rabies virus. J Virol 72:7181–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benmansour A, Leblois H, Coulon P, Tuffereau C, Gaudin Y, Flamand A, Lafay F. 1991. Antigenicity of rabies virus glycoprotein. J Virol 65:4198–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lafon M, Wiktor TJ, Macfarlan RI. 1983. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J Gen Virol 64(Part 4):843–851. [DOI] [PubMed] [Google Scholar]
- 8.Dietzschold B, Wunner WH, Wiktor TJ, Lopes AD, Lafon M, Smith CL, Koprowski H. 1983. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A 80:70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemachudha T. 1994. Human rabies: clinical aspects, pathogenesis, and potential therapy. Curr Topics Microbiol Immunol 187:121–143. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Zhang G, Wen Y, Yang S, Xia X, Fu ZF. 2011. Intracerebral administration of recombinant rabies virus expressing GM-CSF prevents the development of rabies after infection with street virus. PLoS One 6:e25414. doi: 10.1371/journal.pone.0025414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnanadurai CW, Zhou M, He W, Leyson CM, Huang CT, Salyards G, Harvey SB, Chen Z, He B, Yang Y, Hooper DC, Dietzchold B, Fu ZF. 2013. Presence of virus neutralizing antibodies in cerebral spinal fluid correlates with non-lethal rabies in dogs. PLoS Negl Trop Dis 7:e2375. doi: 10.1371/journal.pntd.0002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tolson ND, Charlton KM, Lawson KF, Campbell JB, Stewart RB. 1988. Studies of ERA/BHK-21 rabies vaccine in skunks and mice. Can J Vet Res 52:58–62. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M, Zhang GQ, Ren GP, Gnanadurai CW, Li ZG, Chai QQ, Yang Y, Leyson CM, Wu WX, Cui M, Fu ZF. 2013. Recombinant rabies viruses expressing GM-CSF or flagellin are effective vaccines for both intramuscular and oral immunizations. PLoS One 8:e63384. doi: 10.1371/journal.pone.0063384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Toriumi H, Wang HL, Kuang Y, Guo XF, Morimoto K, Fu ZF. 2010. Expression of MIP-1 alpha (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J Virol 84:9642–9648. doi: 10.1128/JVI.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen Y, Wang H, Wu H, Yang F, Tripp RA, Hogan RJ, Fu ZF. 2011. Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination. J Virol 85:1634–1644. doi: 10.1128/JVI.01552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rupprecht CE, Hanlon CA, Blanton J, Manangan J, Morrill P, Murphy S, Niezgoda M, Orciari LA, Schumacher CL, Dietzschold B. 2005. Oral vaccination of dogs with recombinant rabies virus vaccines. Virus Res 111:101–105. doi: 10.1016/j.virusres.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Li JW, Ertel A, Portocarrero C, Barkhouse DA, Dietzschold B, Hooper DC, Faber M. 2012. Postexposure treatment with the live-attenuated rabies virus (RV) vaccine TriGAS triggers the clearance of wild-type RV from the central nervous system (CNS) through the rapid induction of genes relevant to adaptive immunity in CNS tissues. J Virol 86:3200–3210. doi: 10.1128/JVI.06699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuang Y, Lackay SN, Zhao L, Fu ZF. 2009. Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection. Virus Res 144:18–26. doi: 10.1016/j.virusres.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZW, Sarmento L, Wang Y, Li XQ, Dhingra V, Tseggai T, Jiang B, Fu ZF. 2005. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J Virol 79:12554–12565. doi: 10.1128/JVI.79.19.12554-12565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson N, Cunningham AF, Fooks AR. 2010. The immune response to rabies virus infection and vaccination. Vaccine 28:3896–3901. doi: 10.1016/j.vaccine.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Prehaud C, Megret F, Lafage M, Lafon M. 2005. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol 79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Toriumi H, Kuang Y, Chen HC, Fu ZF. 2009. The roles of chemokines in rabies virus infection: overexpression may not always be beneficial. J Virol 83:11808–11818. doi: 10.1128/JVI.01346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker Y. 2003. Immunological and regulatory functions of uninfected and virus infected immature and mature subtypes of dendritic cells–a review. Virus Genes 26:119–130. doi: 10.1023/A:1023427228024. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. 2000. Immunobiology of dendritic cells. Annu Rev Immunol 18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 26.Reis e Sousa C, Stahl PD, Austyn JM. 1993. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med 178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heath WR, Belz GT, Behrens GMN, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. 2004. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev 199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 28.Li J, McGettigan JP, Faber M, Schnell MJ, Dietzschold B. 2008. Infection of monocytes or immature dendritic cells (DCs) with an attenuated rabies virus results in DC maturation and a strong activation of the NFkappaB signaling pathway. Vaccine 26:419–426. doi: 10.1016/j.vaccine.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faul EJ, Wanjalla CN, Suthar MS, Gale M, Wirblich C, Schnell MJ. 2010. Rabies virus infection induces type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog 6:e1001016. doi: 10.1371/journal.ppat.1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 31.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed.National Academies Press, Washington, DC. [Google Scholar]
- 32.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morimoto K, Hooper DC, Spitsin S, Koprowski H, Dietzschold B. 1999. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J Virol 73:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietzschold B, Morimoto K, Hooper DC, Smith JS, Rupprecht CE, Koprowski H. 2000. Genotypic and phenotypic diversity of rabies virus variants involved in human rabies: implications for postexposure prophylaxis. J Hum Virol 3:50–57. [PubMed] [Google Scholar]
- 35.Zhang GQ, Wang HL, Mahmood F, Fu ZF. 2013. Rabies virus glycoprotein is an important determinant for the induction of innate immune responses and the pathogenic mechanisms. Vet Microbiol 162:601–613. doi: 10.1016/j.vetmic.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu X, Tang L, Tseggai T, Guo Y, Fu ZF. 2013. Wild-type rabies virus phosphoprotein is associated with viral sensitivity to type I interferon treatment. Arch Virol 158:2297–2305. doi: 10.1007/s00705-013-1743-2. [DOI] [PubMed] [Google Scholar]
- 37.Sarmento L, Li ZQ, Howerth E, Jackson AC, Fu ZF. 2005. Glycoprotein-mediated induction of apoptosis limits the spread of attenuated rabies viruses in the central nervous system of mice. J Neurovirol 11:571–581. doi: 10.1080/13550280500385310. [DOI] [PubMed] [Google Scholar]
- 38.Finke S, Conzelmann KK. 1997. Ambisense gene expression from recombinant rabies virus: random packaging of positive- and negative-strand ribonucleoprotein complexes into rabies virions. J Virol 71:7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pawan JL. 1959. Rabies in the vampire bat of Trinidad, with special reference to the clinical course and the latency of infection. Caribb Med J 21:137–156. [PubMed] [Google Scholar]
- 40.Fekadu M. 1988. Pathogenesis of rabies virus infection in dogs. Rev Infect Dis 10(Suppl 4):S678–S683. doi: 10.1093/clinids/10.Supplement_4.S678. [DOI] [PubMed] [Google Scholar]
- 41.Hemachudha T, Phanuphak P, Sriwanthana B, Manutsathit S, Phanthumchinda K, Siriprasomsup W, Ukachoke C, Rasameechan S, Kaoroptham S. 1988. Immunologic study of human encephalitic and paralytic rabies. Preliminary report of 16 patients. Am J Med 84:673–677. [DOI] [PubMed] [Google Scholar]
- 42.Steinman RM. 2007. Dendritic cells: understanding immunogenicity. Eur J Immunol 37(Suppl 1):S53–S60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 43.Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox WI, Steinman RM, Bhardwaj N. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol 163:6762–6768. [PubMed] [Google Scholar]
- 44.Moutaftsi M, Mehl AM, Borysiewicz LK, Tabi Z. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913–2921. doi: 10.1182/blood.V99.8.2913. [DOI] [PubMed] [Google Scholar]
- 45.Salio M, Cella M, Suter M, Lanzavecchia A. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol 29:3245–3253. [DOI] [PubMed] [Google Scholar]
- 46.Faber M, Pulmanausahakul R, Hodawadekar SS, Spitsin S, McGettigan JP, Schnell MJ, Dietzschold B. 2002. Overexpression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune response. J Virol 76:3374–3381. doi: 10.1128/JVI.76.7.3374-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarmento L, Tseggai T, Dhingra V, Fu ZF. 2006. Rabies virus-induced apoptosis involves caspase-dependent and caspase-independent pathways. Virus Res 121:144–151. doi: 10.1016/j.virusres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 49.Lawrence TM, Hudacek AW, de Zoete MR, Flavell RA, Schnell MJ. 2013. Rabies virus is recognized by the NLRP3 inflammasome and activates interleukin-1beta release in murine dendritic cells. J Virol 87:5848–5857. doi: 10.1128/JVI.00203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plumet S, Herschke F, Bourhis JM, Valentin H, Longhi S, Gerlier D. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS One 2:e279. doi: 10.1371/journal.pone.0000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cui S, Eisenacher K, Kirchhofer A, Brzozka K, Lammens A, Lammens K, Fujita T, Conzelmann KK, Krug A, Hopfner KP. 2008. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol Cell 29:169–179. doi: 10.1016/j.molcel.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 52.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 53.Forsbach A, Nemorin JG, Volp K, Samulowitz U, Montino C, Muller C, Tluk S, Hamm S, Bauer S, Lipford GB, Vollmer J. 2007. Characterization of conserved viral leader RNA sequences that stimulate innate immunity through TLRs. Oligonucleotides 17:405–417. doi: 10.1089/oli.2007.0098. [DOI] [PubMed] [Google Scholar]
- 54.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 55.Helin E, Vainionpaa R, Hyypia T, Julkunen I, Matikainen S. 2001. Measles virus activates NF-kappa B and STAT transcription factors and production of IFN-alpha/beta and IL-6 in the human lung epithelial cell line A549. Virology 290:1–10. doi: 10.1006/viro.2001.1174. [DOI] [PubMed] [Google Scholar]
- 56.Ogino T, Yadav SP, Banerjee AK. 2010. Histidine-mediated RNA transfer to GDP for unique mRNA capping by vesicular stomatitis virus RNA polymerase. Proc Natl Acad Sci U S A 107:3463–3468. doi: 10.1073/pnas.0913083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogino T, Banerjee AK. 2010. The HR motif in the RNA-dependent RNA polymerase L protein of Chandipura virus is required for unconventional mRNA-capping activity. J Gen Virol 91:1311–1314. doi: 10.1099/vir.0.019307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moyer SA, Abraham G, Adler R, Banerjee AK. 1975. Methylated and blocked 5′ termini in vesicular stomatitis virus in vivo mRNAs. Cell 5:59–67. doi: 10.1016/0092-8674(75)90092-6. [DOI] [PubMed] [Google Scholar]
- 59.Gerlier D, Lyles DS. 2011. Interplay between innate immunity and negative-strand RNA viruses: towards a rational model. Microbiol Mol Biol Rev 75:468–490. doi: 10.1128/MMBR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Habjan M, Andersson I, Klingstrom J, Schumann M, Martin A, Zimmermann P, Wagner V, Pichlmair A, Schneider U, Muhlberger E, Mirazimi A, Weber F. 2008. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS One 3:e2032. doi: 10.1371/journal.pone.0002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan X, Prosniak M, Curtis MT, Weiss ML, Faber M, Dietzschold B, Fu ZF. 2001. Silver-haired bat rabies virus variant does not induce apoptosis in the brain of experimentally infected mice. J Neurovirol 7:518–527. doi: 10.1080/135502801753248105. [DOI] [PubMed] [Google Scholar]
- 62.Wirblich C, Schnell MJ. 2011. Rabies virus (RV) glycoprotein expression levels are not critical for pathogenicity of RV. J Virol 85:697–704. doi: 10.1128/JVI.01309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]