ABSTRACT
Most plant viruses counter the RNA silencing-based antiviral defense by expressing viral suppressors of RNA silencing (VSRs). In this sense, VSRs may be regarded as virulence effectors that can be recognized by the host as avirulence (avr) factors to induce R-mediated resistance. We made use of Agrobacterium-mediated transient coexpression of VSRs in combination with Potato virus X (PVX) to recapitulate in local tissues the systemic necrosis (SN) caused by PVX-potyvirus synergistic infections in Nicotiana benthamiana. The hypersensitive response (HR)-like response was associated with an enhanced accumulation of PVX subgenomic RNAs. We further show that expression of P25, the VSR of PVX, in the presence of VSR from different viruses elicited an HR-like response in Nicotiana spp. Furthermore, the expression of P25 by a Plum pox virus (PPV) vector was sufficient to induce an increase of PPV pathogenicity that led to necrotic mottling. A frameshift mutation in the P25 open reading frame (ORF) of PVX did not lead to necrosis when coexpressed with VSRs. These findings indicate that P25 is the main PVX determinant involved in eliciting a systemic HR-like response in PVX-associated synergisms. Moreover, we show that silencing of SGT1 and RAR1 attenuated cell death in both PVX-potyvirus synergistic infection and the HR-like response elicited by P25. Our study underscores that P25 variants that have impaired ability to suppress RNA silencing cannot act as elicitors when synergized by the presence of other VSRs. These findings highlight the importance of RNA silencing suppression activity in the HR-like response elicited by VSRs in certain hosts.
IMPORTANCE The work presented here describes how the activity of the PVX suppressor P25 elicits an HR-like response in Nicotiana spp. when overexpressed with other VSR proteins. This finding suggests that the SN response caused by PVX-associated synergisms is a delayed immune response triggered by P25, once it reaches a threshold level by the action of other VSRs. Moreover, this work supports the contention that the silencing suppressor activity of PVX P25 protein is a prerequisite for HR elicitation. We propose that unidentified avr determinants could be involved in other cases of viral synergisms in which heterologous “helper” viruses encoding strong VSRs exacerbate the accumulation of the avr-encoding virus.
INTRODUCTION
In host-virus interactions, RNA silencing triggered by viral double-stranded RNA (dsRNA) is a general mechanism involved in immunity against viruses (1). By analogy with the zigzag model that describes plant-microbe interactions, dsRNA and RNA silencing could be regarded as a viral pathogen-associated molecular pattern (PAMP) and PAMP-triggered immunity, respectively (2). Most viruses counter the RNA silencing-based antiviral defense by expressing viral suppressors of RNA silencing (VSRs). Therefore, VSRs may be regarded as virulence effectors that facilitate viral infection in plants. As suggested by the zigzag model, plants may have developed a countermeasure against viral VSRs through the recognition of these viral effectors as avirulence (avr) factors to induce resistance (R) gene-mediated resistance. Indeed, VSRs are recognized by R genes to trigger immune responses. For example, the Cauliflower mosaic virus VSR protein P6 is an avr determinant recognized by R genes in several plants (3, 4). Similarly, the Tomato bushy stunt virus (TBSV) P19 silencing suppressor is also an elicitor of hypersensitive response (HR) in certain Nicotiana species (5).
In many compatible pathosystems, close relationships between defense responses to virus infection and severe necrotic symptoms have been reported (6–8). Several studies have postulated that systemic necrosis (SN) shares HR attributes, as a consequence of the delayed occurrence of biochemical and physiological events that are associated with programmed cell death (PCD) (8–10). Moreover, R proteins have been involved in some cases of SN responses associated with compatible virus interactions (6, 11). In addition, Komatsu et al. (8) determined that SGT1 and RAR1, which are both required for the function of many R proteins in incompatible interactions (12, 13), also mediate SN in compatible virus infections. Thus, SN could be considered an uncontrolled or incomplete HR-associated necrosis response that is triggered in distal tissues when local defense responses fail to limit virus spread.
Multiple infection of plants by unrelated viruses is a frequent phenomenon in nature, and a number of plant diseases are attributed to synergistic interactions (14, 15). Synergy is often manifested by a remarkable increase in both virus accumulation and symptom expression, compared to single infections. The best-studied synergistic interaction involves Potato virus X (PVX) with a number of potyviruses in tobacco (Nicotiana tabacum) plants, in which the level of PVX is enhanced severalfold compared to its level in singly infected plants (16, 17). Potyvirus-associated synergistic diseases have been suggested to result from suppression of the host defense mechanism based on RNA silencing by the potyviral VSR, helper component-proteinase (HC) protein (18). However, the accumulation of PVX genomic RNA (gRNA) did not increase greatly in N. benthamiana when doubly infected with PVX and either Plum pox virus (PPV) or Tobacco etch virus (TEV), despite the extreme enhancement of symptoms in this host that lead to SN, i.e., synergism in pathology (16, 19). PVX-mediated expression of viral genes to determine whether a protein would function as a pathogenicity determinant is a well-established technique (17, 20). The expression of HC by a PVX vector is sufficient to induce the increase of PVX pathogenicity in N. benthamiana, albeit PVX gRNA accumulated at levels similar to those in plants infected with the PVX empty vector (19). Further, exacerbation of pathogenicity, including death in N. benthamiana, produced by recombinant PVX expressing VSRs from other viruses has not been linked to an increase in the accumulation of PVX gRNA (20–23). These conflicting published results on the role of PVX accumulation in inducing SN in Nicotiana spp. prompted us to analyze further the role of PVX proteins in virus pathogenicity. PVX requires three virally encoded proteins, the triple gene block (TGB), for movement between cells. P25 (TGB1) is a multifunctional protein that suppresses RNA silencing and moves from cell to cell through plasmodesmata, while TGB2 and TGB3 are membrane-spanning proteins associated with endoplasmic reticulum (ER)-derived granular vesicles (24, 25). P25 is the elicitor for HR-type resistance to PVX mediated by the Nb gene in potato (Solanum tuberosum) (26). Recent studies showed that transgenic expression of P25 in tobacco caused major alterations in the host transcriptome, with an enhanced resistance against other pathogens (27). TGB3 induces the unfolded protein response during PVX infection, which is important to regulate cellular cytotoxicity that could otherwise lead to cell death if viral proteins reached high levels in the ER (28).
Recently, we have shown that SN was correlated with an enhanced expression of lipoxygenase (LOX) activity in PVX-Potato virus Y (PVY)-infected plants (29). LOX action on polyunsaturated fatty acid substrates is the first step in the jasmonic acid (JA) biosynthesis pathway, a hormone involved in the execution of HR cell death in tobacco (30). In this report, we use transient expression of viral proteins by Agrobacterium tumefaciens (i.e., agroinfiltration) and hybrid viruses to demonstrate that the P25 protein of PVX is the main pathogenicity determinant responsible for the SN response in PVX-associated synergisms. In agreement with previous findings, LOX activity was also stimulated in leaf patches agroinfiltrated with P25/VSR combinations that led to an HR-like response. Moreover, we have shown that SGT1 and RAR1 were involved in the process of cell death in both PVX-potyvirus synergistic infection and the local HR-like response elicited by P25. The elicitor activity of P25 was correlated with the RNA silencing suppressor activity of the protein.
MATERIALS AND METHODS
Binary vector constructs.
The PCR amplification and cloning of PPV HC (HCwt), PPV HCLH (HCL134H) (19), TEV HC (31), TBSV P19 (32), PVY P1 (33), Cucumber mosaic virus (CMV) 2b (34), and Citrus tristeza virus (CTV) P23 genes (35) into A. tumefaciens binary vectors have been described previously. A. tumefaciens carrying pCAMBIA1305.1 containing a gene encoding β-glucuronidase (GUS) was used as negative control.
The complete P25 coding region was amplified by PCR from pP2C2S-402, a cDNA clone of PVX derived from pP2C2S (36). The forward primer P25NcoI, 5′-CATGCCATGGATATTCTCATCAGTAG-3′ (where nucleotides 4486 to 4505 are in italics and the NcoI site is in bold), and the reverse primer P25XmaI, 5′-TCACCCGGGTTATGGCCCTGCGCGGAC-3′ (nucleotides complementary to 5149 to 5166 in italics, XmaI site in bold), were used. The PCR-amplified fragment was cloned into the pRTL2 vector (37) linearized with NcoI and XmaI. The expression cassette from the pRTL2-based construct was PstI excised and inserted into the plant transformation vector pCAMBIA2300 to provide the pCAM-P25 plasmid.
T7-tagged versions of wild-type P25 (P25-T7) or P25 mutants were constructed via PCR, using primers that introduced the T7 epitope at the C terminus of the P25 coding sequence (primers, P25NcoI and P25T7XmaI, [5′-TCACCCGGGCTAACCCATCTGTTGTCCACCCGTCATACTTGCCATTGGCCCTGCGCGGAC-3′]; the T7 epitope is underlined). Single-amino-acid substitutions A104V, T117A, and K124E and a stop codon at position 18, P25stop, were separately introduced in the P25 coding sequence by two consecutive PCRs (38) using the template pCAM-P25, the forward primer P25NcoI, the reverse primer P25T7XmaI, and two specific primers for each mutant, according to the mutant sequences previously reported (39). Each of the PCR products was cloned into NcoI/XmaI-digested pRTL2 and finally transferred to pCAMBIA2300 linearized with PstI. The presence of the nucleotide substitutions and the lack of undesired changes were confirmed by sequencing.
The binary vector pGR107, which contains the infectious cDNA of PVX, was kindly provided by D. C. Baulcombe (University of Cambridge, Cambridge, United Kingdom). To construct PVXΔ25, pGR107 was digested with ApaI (nucleotide 4945 of PVX), end filled with Klenow fragment, and religated.
The infectious cDNA clone of PVX carrying PPV HC (pPVX-HC) sequences under the control of the PVX duplicated coat protein (CP) promoter was described before (19). The PVX HC, PVX P25, and green fluorescent protein (GFP) coding regions were amplified by PCR from pPVX-HC, pCAM-P25, and pAVA393 (19), respectively, using specific primers bearing at their 5′ ends the attB1 and attB2 recombinant sequences. The N. benthamiana luminal binding protein (BiP) coding sequence (accession no. FJ463755.1) was synthesized with SuperScript Reverse Transcriptase II (Invitrogen) from total RNA and amplified by PCR. The primer pairs used to amplify these genes are available upon request. The PCR products were cloned separately into the donor plasmid pDONR207 by BP clonase II recombination (Gateway Technology; Invitrogen). The P25 and GFP sequences from pDONR207 were transferred into the binary destination vector pGWBinPPV-3xHA using LR clonase II (Invitrogen) to render PPV-P25 and PPV-GFP, respectively. pGWBinPPV-3xHA is a derivative of pBinPPV (40), which contains the infectious cDNA of PPV, and was kindly provided by J. A. García (Centro Nacional de Biotecnología, Madrid, Spain). The PVX-HC sequence from pDONR207 was transferred into the binary destination vector pGWB2 (41). The NbBiP sequence from pDONR207 was transferred into the binary destination vector pGWB14. The plasmids obtained were introduced separately into A. tumefaciens GV3101. All PCRs were performed with Phusion DNA polymerase (Finnzymes).
Plant material and agroinfiltration.
N. benthamiana plants were grown in a growth chamber with a 16-h light/8-h dark cycle at 25°C. The transgenic N. benthamiana plants expressing the salicylate hydroxylase gene have been described previously (42). For transient expression assays (leaf patch assays), A. tumefaciens cultures were grown to exponential phase in LB medium with antibiotics at 28°C. For infiltration of viral proteins, each bacterial culture was diluted to a final optical density of 0.6 at 600 nm. In the case of viral amplicons, bacterial cultures were diluted to a final optical density of 0.3. Equal volumes of each suspension were then combined in a 1:1 ratio, and infiltration of the mixtures into leaves of N. benthamiana plants was performed as described previously (43). In silencing suppression assays, a free GFP reporter gene expressed from pCAMBIA2300 was coinfiltrated either with the pCAMBIA1305.1 vector (GUS) or with pCAMBIA2300 expressing T7-tagged versions of wild-type P25 (P25wt) or P25 mutants. When possible, all of the treatment types to be compared were infiltrated on a single leaf. To minimize the effects of interleaf variability, all of the leaf disks from different plants corresponding to the same treatment type were pooled.
N. benthamiana plants were sprayed with salicylic acid (SA) (2 mM) (Sigma) or water every day from 1 day before the agroinoculation to 8 days postagroinoculation (dpa).
RNA and protein gel blot analysis.
Total RNA was extracted from infiltrated leaves at 3 and 6 dpa and from upper leaves at 6 and 9 dpa as described previously (10). RNA samples were separated on 1% agarose formaldehyde gels and transferred to Hybond-N membranes (Roche Molecular Biochemicals). Membrane hybridization was carried out overnight at 65°C using digoxigenin-labeled riboprobes corresponding to either PVX CP or GFP sequences (19). Detection of vacuolar processing enzyme (VPE-1) mRNA was performed using a 32P-labeled probe corresponding to a 344-bp fragment from NtVPE-1a (accession no. AB075947.1).
Real-time quantitative reverse transcriptase PCR (qRT-PCR) for virus detection was performed as described by García-Marcos and associates (29). The relative quantification of PCR products was calculated by the comparative cycle threshold (ΔΔCT) method. 18S rRNA was chosen for normalization because of its similar levels of expression across all treatments.
RT-PCR for the analysis of threonine deaminase (TD) and proteinase inhibitor I (PI-I) gene expression was performed as described by García-Marcos et al. (29). The Actin gene transcripts were amplified as an internal control. A PCR using a template derived from RNA without reverse transcription was performed as a negative control. The primer pairs used to amplify all these genes are available upon request.
Viral proteins were analyzed by Western blotting as described by Tena et al. (33). T7-tagged P25 was detected with horseradish peroxidase (HRP)-conjugated anti-T7 antibody (Novagen) (1:5,000 dilution). Hemagglutinin (HA)-tagged P25 or GFP was detected with a rat monoclonal antiserum to HA (Roche Molecular Biochemicals) (1:1,000 dilution). PPV HC was detected with a rabbit polyclonal antiserum (1:300 dilution) (19). PPV CP was detected with a rabbit polyclonal antiserum (1:2,000 dilution) (Instituto Valenciano de Investigaciones Agrarias, Valencia, Spain). An appropriate secondary antibody conjugated with HRP was used in each case. Detection was performed using the ECL system (Amersham Biosciences).
VIGS assays.
For virus-induced gene silencing (VIGS) assays, a 366-bp cDNA fragment corresponding to the 3′ part (nucleotides [nt] 331 to 696) of RAR1 from N. benthamiana (12) was amplified by PCR using oligonucleotides RAR-F1 (5′-CGGGATCCGGTTCACAACCCAGAGAAGT-3′) and RAR-R1 (5′-CCCTCGAGTTAGGACGCTGGGCTGGC-3′), subsequently cut with BamHI and XhoI (sites in bold), and ligated into the binary vector pTRV2 to yield pTRV2:RAR1. pTRV2:SGT1 (13), which contains a 480-bp fragment of NbSGT1, was obtained from the Arabidopsis Biological Resource Center. The pTRV1 vector and the pTRV2 vector and its derivatives were separately transformed into A. tumefaciens strain GV3101. N. benthamiana leaves were infiltrated with A. tumefaciens cultures as described above.
Silencing of NbRAR1 and NbSGT1 mRNA was detected from upper, noninoculated leaf tissue by reverse transcriptase PCR at 14 dpa (29). To ensure that similar amounts of cDNA were used for silenced and nonsilenced plants, amplification of Actin was used as the internal control. Primers (available upon request) that anneal outside the region targeted for silencing were used to ensure that the endogenous gene would be tested.
Cell death and lipoxygenase assays.
Cell damage was assayed by measuring electrolyte leakage. Twenty-four disks of 0.3 cm2 were excised from upper leaf tissue using a core borer. Disks were rinsed briefly with water and floated on 5 ml of double-distilled water for 6 h at room temperature. The conductivity of the water was measured using a Crison conductivity meter. This represented the electrolyte leakage from the leaf disks (Reading 1). Then, samples were boiled for 20 min at 90°C. After the liquid cooled down, the conductivity of the water was measured again. This represented the total ions present in the leaf disks (Reading 2). Electrolyte leakage was represented as the percentage of total ions released [(Reading 1/Reading 2) × 100]. Reported data are means and standard errors of the values obtained in two independent experiments with a minimum of five independent replicates per experiment and pools of 4 plants for each treatment. To clarify the graphics, in each experiment the value of a control sample was set at 1 and other data were calculated relative to this value. Statistical analyses were performed using the statistical software IBM SPSS Statistics v.20 (IBM Corp.).
For 3,3′-diaminobenzidine (DAB) staining to detect H2O2 production, leaves were vacuum infiltrated for 10 min with DAB solution at 1 mg ml−1. Representative phenotypes were photographed with a Leica L2 stereoscope using a Leica DFC 320 camera.
Protein extracts were prepared from agroinfiltrated plants grown at 32°C to maximize LOX activity. LOX activity in soluble fractions was spectrophotometrically measured by monitoring the increase of the conjugated diene hydroperoxide at A234, as described previously (29). The assay was performed in the presence of 0.6 mM KCN to verify that the enzymatic activity was cyanide resistant.
RESULTS
Agroinfiltration of PVX together with VSRs elicits an HR-like necrosis response that correlates with an enhanced accumulation of PVX subgenomic RNAs (sgRNAs).
To determine if expression of PVX together with VSRs recapitulates in local tissues the synergistic interactions of PVX with the viruses that express them, leaf patches of N. benthamiana plants were infiltrated with Agrobacterium mixtures containing binary constructs expressing PVX plus either PPV HCwt, PPV HCLH, TBSV P19, or GUS. HCLH is a variant of PPV HC containing a single point mutation (L134H) and is unable to induce the SN response when expressed from a PVX vector (19). PVX induced a strong necrosis response and H2O2 production within 6 to 8 dpa when coexpressed with HCwt, whereas agroinfiltration of PVX plus P19 resulted in the development of a delayed necrosis (Fig. 1A). PVX in combination with either HCLH or GUS failed to elicit necrosis at any time point. We used an electrolyte leakage assay to quantify the level of necrosis induced by different PVX/VSR combinations at two time points. Consistent with the above results, leaf patches infiltrated with PVX plus HCwt exhibited significantly greater levels of electrolyte leakage than did those with combinations containing PVX plus either P19, HCLH, or GUS at 7 dpa (Fig. 1B). By 12 dpa, a time point at which tissue infiltrated with PVX plus HCwt becomes completely desiccated, there was a significant increase of electrolyte leakage in patches infiltrated with PVX plus P19 compared to controls.
FIG 1.
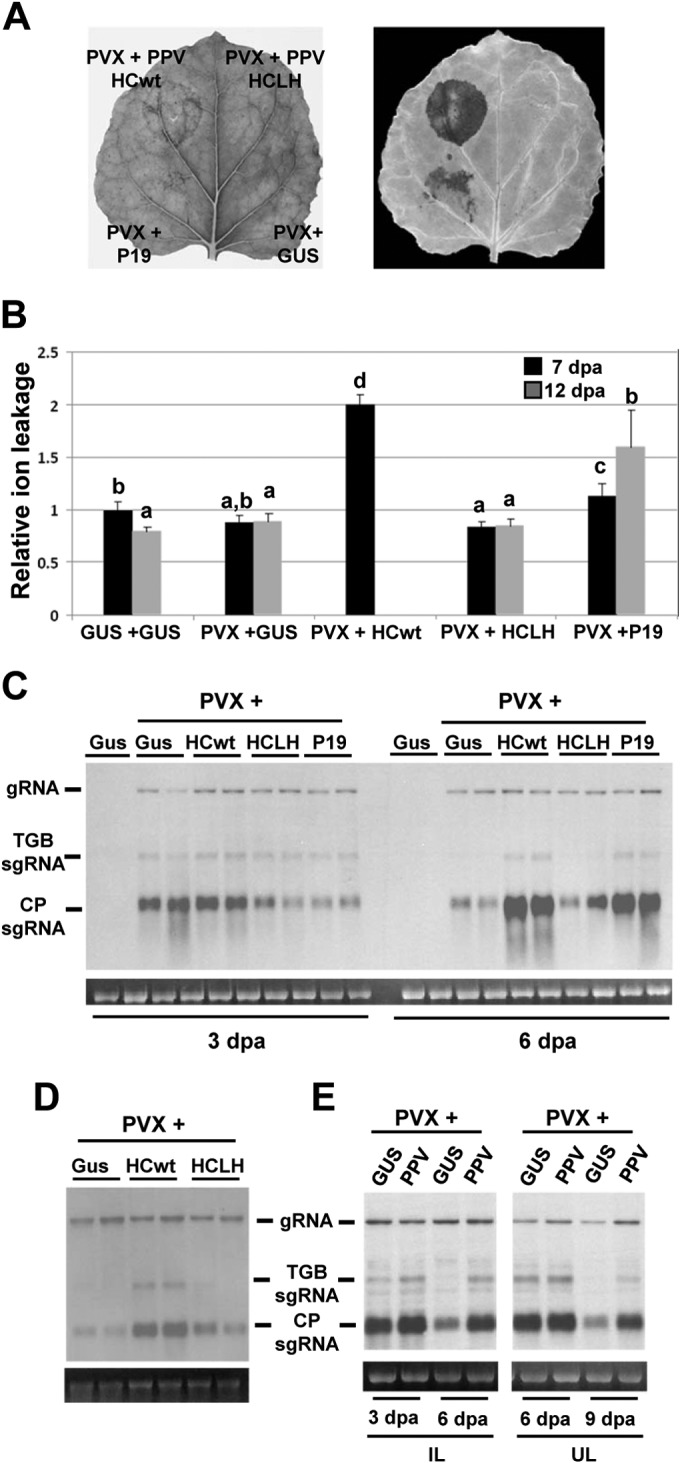
Expression of PVX together with VSRs elicits an HR-like necrosis in Nicotiana benthamiana. (A) Leaves were infiltrated with combinations of Agrobacterium cultures containing binary constructs expressing PVX plus either GUS, PPV HCwt, PPV HCLH, or TBSV P19, as indicated. A leaf was photographed (left panel) and then stained with DAB (right panel) solution at 7 days postagroinoculation (dpa). DAB formed a deep brown polymerization product upon reaction with H2O2. (B) Electrolyte leakage from leaf disks infiltrated with the different combinations at 7 and 12 dpa. Data represent the means ± standard errors for 16 replicates, each consisting of four plants that received the same treatment in two independent experiments. Statistical comparisons between means were made among treatments at 7 or 12 dpa by employing Scheffé's multiple-range test. Different letters indicate significant differences at P values of <0.05. (C) Northern blot analysis of total RNA extracted from agroinfiltrated leaf patches such as those shown in panel A at 3 and 6 dpa. Two independent pooled samples were analyzed for each combination. (D) Northern blot analysis of total RNA extracted from leaf patches of N. tabacum infiltrated with combinations of Agrobacterium cultures containing binary constructs expressing PVX plus either GUS, PPV HCwt, or PPV HCLH at 6 dpa. (E) Northern blot analysis of total RNA extracts of N. benthamiana plants infected with PVX alone (PVX + GUS) or in combination with PPV-GFP (PVX + PPV-GFP). Total RNA extracts were extracted from infiltrated leaf (IL) patches at 3 and 6 dpa or from upper leaves (UL) at 6 and 9 dpa. Total RNA (2 μg) was hybridized with a probe complementary to PVX CP. PVX genomic RNA (gRNA) and the two major subgenomic RNAs (sgRNAs), triple gene block (TGB) sgRNA and CP sgRNA, are indicated. Ethidium bromide staining of 25S rRNA is shown as a loading control.
To examine whether the necrosis phenotype induced by different PVX/VSR combinations was associated with changes in the accumulation of PVX, we measured the amount of viral RNA in infiltrated tissues at 3 and 6 dpa by Northern blotting (Fig. 1C). Remarkably, whereas PVX gRNA accumulated at roughly comparable levels in the different PVX/VSR combinations at 3 and 6 dpa, accumulation of TGB and CP sgRNAs in leaf patches infiltrated with PVX plus either HCwt or P19 was greater than in patches infiltrated with PVX plus either HCLH or GUS at 6 dpa. Similarly, transient expression of PVX plus PPV HCwt in N. tabacum led to a greater accumulation of TGB and CP sgRNAs than that seen in patches infiltrated with PVX plus either HCLH or GUS at 6 dpa (Fig. 1D). However, PVX failed to elicit necrosis in the presence of HCwt, likely due to the fact that transient expression of PVX is less efficient in tobacco than in N. benthamiana (44).
We further evaluated whether the enhanced accumulation of PVX sgRNAs in the presence of VSR could be extrapolated to the PVX/potyvirus synergistic interaction. RNA was extracted from inoculated and upper leaves of N. benthamiana plants infected either with PVX plus PPV-GFP or with PVX alone. As shown in Fig. 1E, not only was PVX sgRNAs accumulation greater in the inoculated leaves but it was greater too in the upper leaves of plants infected with PVX and PPV-GFP than in the plants singly infected with PVX (PVX + GUS) at later stages of infection. Altogether, these results indicate that PVX/VSR combinations capable of inducing a necrosis response in N. benthamiana enhance and/or stabilize PVX sgRNAs.
The P25 protein of PVX triggers an HR-like response in Nicotiana spp.
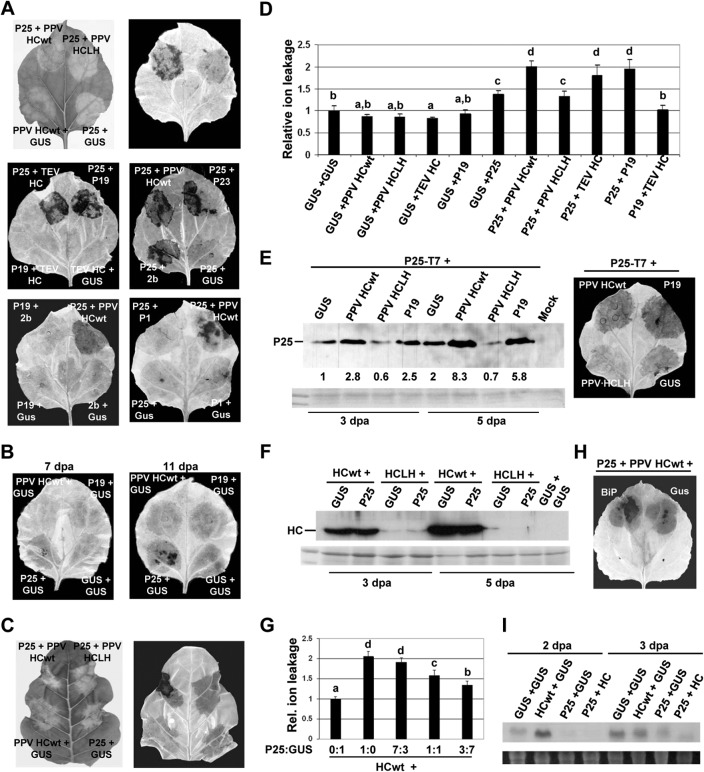
Previous studies had shown that the potexviral P25 protein is a pathogenicity determinant involved in suppression of RNA silencing (25). To determine whether P25 contributes to the necrosis elicited by PVX/VSR combinations, N. benthamiana leaf patches were infiltrated with P25 either alone or in combination with VSRs of viruses belonging to different families (Fig. 2A). The combinations of P25 and HCwt derived from PPV or TEV, P19, closteroviral P23, and cucumoviral 2b, but not the nonfunctional HCLH, resulted in strong production of H2O2 indicative of necrosis at 7 dpa. Expression of P25 alone induced a weak production of H2O2 at this time point, which increased compared to that in controls at 11 dpa (Fig. 2B). The enhancement of P25-induced necrosis by a selection of VSRs was corroborated by electrolyte leakage measurements (Fig. 2D). Expression of the different VSRs alone or the combinations of P19 and TEV HC or CMV 2b did not lead to necrosis or increases in electrolyte leakage compared to controls. Furthermore, expression of P25 in combination with a symptom determinant like the potyviral P1, which has been shown not to be a VSR (33), did not lead to necrosis (Fig. 2A, lower right panel). Thus, the effect of VSRs on P25-induced necrosis seems to depend on their suppression function. Coexpression of P25 and PPV HCwt also triggered an HR-like response in N. tabacum (Fig. 2C).
FIG 2.
Expression of PVX P25 together with VSRs elicits an HR-like necrosis in Nicotiana spp. (A) Leaves of N. benthamiana were infiltrated with combinations of Agrobacterium cultures containing binary constructs expressing PVX P25 plus either GUS, PPV HCwt, PPV HCLH, TEV HC, TBSV P19, CTV P23, CMV 2b, or PVY P1 and expressing P19 plus either TEV HC or 2b, as indicated. Leaves were photographed (upper left panel) and then stained with DAB solution at 7 days postagroinoculation (dpa). (B) Leaves of N. benthamiana were infiltrated with GUS alone or combinations of Agrobacterium cultures containing GUS plus either PVX P25, PPV HCwt, or TBSV P19. Leaves were stained with DAB solution at 7 (left panel) and 11 dpa (right panel). (C) Leaf of N. tabacum was infiltrated with combinations of Agrobacterium cultures containing PVX P25 plus either GUS, PPV HCwt, PPV HCLH, or GUS plus PPV HCwt, as indicated. Leaf was photographed (left panel) and then stained with DAB solution (right panel) at 11 dpa. (D) Electrolyte leakage from leaf disks infiltrated with different combinations of Agrobacterium cultures at 7 dpa. Data represent the means ± standard errors for 12 replicates, each consisting of four plants that received the same treatment in two independent experiments. Statistically significant differences between means were determined by employing Scheffé's multiple-range test. Different letters (a, b, c, d) indicate significant differences at P values of <0.05. (E) Western blot analysis of extracts derived from leaf patches infiltrated with combinations of Agrobacterium cultures containing a T7-tagged version of P25 (P25-T7) plus either GUS, PPV HCwt, PPV HCLH, or TBSV P19 at 3 and 5 dpa, using antibodies against the T7 epitope (left panel). The intensity of each band was quantified by densitometry analyses. The value for the band in leaf patches infiltrated with P25-T7 plus GUS at 3 dpa was set at 1, and other data were calculated relative to this value. The lower panel shows the Ponceau S-stained membrane after blotting, as a loading control. A leaf was stained with DAB solution at 9 dpa (right panel). (F) Western blot analysis of extracts derived from leaf patches infiltrated with combinations of Agrobacterium cultures containing PPV HCwt plus either P25 or GUS and PPV HCLH plus either P25 or GUS at 3 and 5 dpa, using antibodies against HC-Pro. (G) Effects of decreasing the amount of the Agrobacterium culture expressing PVX 25 protein on electrolyte leakage. Leaves were infiltrated with combinations of HCwt plus either GUS or a series of dilutions of P25 as indicated and analyzed at 7 dpa. (H) Leaf of N. benthamiana was infiltrated with a mixture of Agrobacterium expressing P25 and HCwt in combination with either BiP or GUS. Leaf was stained with DAB solution at 7 dpa. (I) Northern blot analysis of total RNA extracted from leaf patches agroinfiltrated with GUS alone or combinations of GUS plus either PVX P25 or PPV HCwt and of PVX P25 plus PPV HCwt at 2 and 3 dpa. Total RNA (15 μg) was hybridized with a 32P-labeled probe complementary to NtVPE-1a.
To determine whether the necrosis induced by the P25/VSR combinations correlated with the level of accumulation of P25, a P25 construct with a carboxy-terminal T7 tag was infiltrated together with HCwt, HCLH, P19, or GUS in N. benthamiana plants. The necrosis induced by the P25-T7 plus HCwt and P25-T7 plus P19 combinations correlated with a greater accumulation of P25 than that induced by the P25-T7 plus HCLH or P25-T7 plus GUS combinations, as assessed by Western blotting (Fig. 2E). The C-terminal tag did not alter the phenotype conferred by the nontagged version of P25, although we did observe a slight delay (1 to 2 days) in HR development. To know whether P25 influences the levels of VSRs, the accumulation of HCwt and HCLH was tested with PPV HC antiserum (Fig. 2F). The presence of the weak suppressor P25 had negligible effects on the accumulation of HCwt and HCLH. The accumulation of HCwt was greater than that of HCLH regardless of the expression of P25, as it has been described that transient expression of mutant variants of HC is posttranscriptionally silenced when the mutation affects its capability to suppress RNA silencing (19). In contrast, the strong suppressor P19 was able to rescue the expression of HCLH mutant at wild-type (wt) levels (45). As coexpression of P25 did not alter significantly the accumulation of HC, HCwt accumulation in leaf patches infiltrated with P25 plus HCwt does not contribute by itself to the HR-like response.
To support that the HR-like response is P25 dose dependent, we conducted a transient expression experiment by coexpressing HCwt with a series of dilutions of P25 (P25/GUS ratios of 7:3, 1:1, and 3:7). The level of electrolyte leakage decreased upon progressive dilution of the Agrobacterium culture expressing P25, with the 3:7 dilution still giving a significantly higher level of electrolyte leakage than that of HCwt plus GUS (Fig. 2G).
It was reported that overexpression of PVX TGB3 protein by Agrobacterium led to ER stress-related cell death in N. benthamiana, which could be alleviated by the ER resident chaperone BiP (28). To determine whether ER stress is involved in P25-mediated necrosis, leaf patches were infiltrated with a mixture of Agrobacterium expressing P25 and HCwt in combination with either BiP or GUS. Overexpression of BiP did not alleviate P25-induced necrosis and resulted in strong production of H2O2 similar to what was observed in controls (Fig. 2H).
The expression of VPE-1 mRNA, a cysteine proteinase with caspase-1-like activity, was transiently increased in Tobacco mosaic virus (TMV)-induced HR in tobacco plants (46). To examine whether expression of P25 affects VPE-1 mRNA accumulation, RNA was extracted from leaf patches infiltrated with GUS, P25, HCwt, or the combination P25 plus HCwt. Northern blot analysis showed that accumulation of VPE-1 mRNA was mostly inhibited in patches infiltrated with P25 plus HCwt and to a lesser extent in patches expressing P25 alone from 2 to 6 dpa (Fig. 2I and data not shown).
P25 is the main pathogenicity determinant involved in eliciting an HR-like response in PVX-associated synergisms.
The expression of the PPV HC protein by a PVX vector was sufficient to induce an increase of PVX pathogenicity that led to SN (19). To determine whether P25 can function as a pathogenicity determinant when expressed from a potyvirus vector, PPV recombinant viruses expressing the HA-tagged versions of either P25 (PPV-P25) or GFP (PPV-GFP) as a control were inoculated onto N. benthamiana plants. Although leaves inoculated with PPV-P25 or PPV-GFP did not exhibit visible alterations, the upper noninoculated leaves displayed mild mosaic symptoms at 7 dpa with either of the two chimeric viruses. Later on, PPV-mediated expression of P25 generated a remarkable worsening of the symptoms, including severe mosaic, curling, and size reduction, that evolved to necrotic mottling in upper leaves at 15 dpa, with no symptom accentuation being observed in PPV-GFP-infected plants (Fig. 3A). Consistent with these results, H2O2 production was more strongly induced in PPV-P25 infected plants than in plants infected with PPV-GFP, as revealed by DAB staining (Fig. 3B). Western blot analysis of proteins extracted from upper leaves showed that P25 accumulated in plants infected with PPV-P25 at 15 dpa (Fig. 3C). Comparative analysis of virus accumulation revealed that the level of PPV CP was similar in PPV-P25-infected plants and in plants infected with PPV-GFP, ruling out that the necrosis phenotype was due to enhanced accumulation of PPV-P25. Therefore, P25, like many other VSRs expressed by a PVX vector, behaves as a pathogenicity determinant in N. benthamiana when expressed from PPV.
FIG 3.
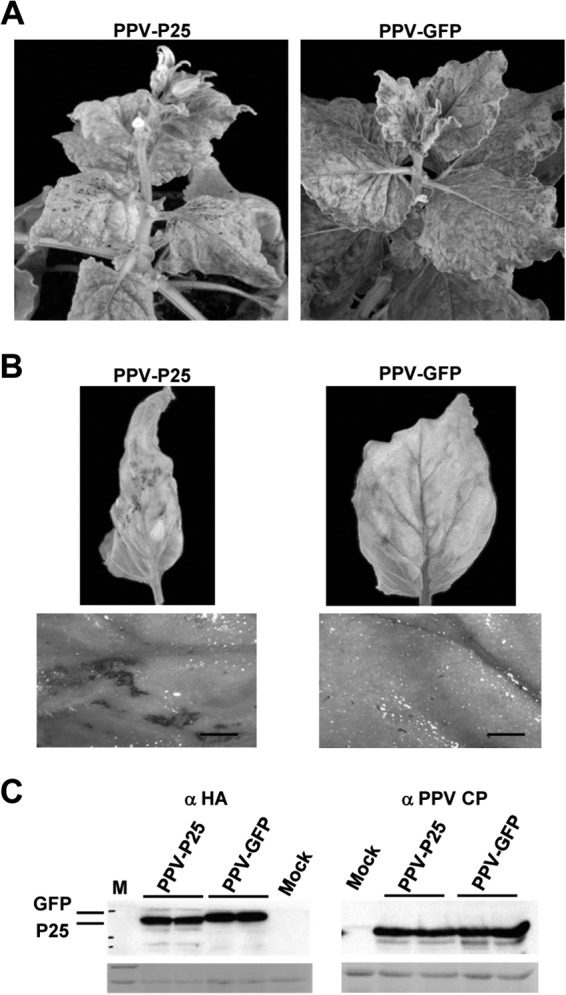
Expression of PVX P25 by a PPV vector increases viral symptoms in Nicotiana benthamiana. (A) Representative disease symptoms induced in N. benthamiana plants by infection with PPV-P25 or PPV-GFP recombinant viruses at 15 days postagroinoculation (dpa). (B) Detached, upper leaves of PPV-P25- and PPV-GFP-infected plants were stained with DAB solution at 15 dpa (upper panels). Representative phenotypes were photographed with a Leica L2 stereoscope (lower panels). Scale bars, 0.5 mm. (C) Western blot analysis of extracts derived from upper leaves of plants infected with PPV-P25 or PPV-GFP at 15 dpa, using antibodies against the HA epitope (left panel) or PPV CP (right panel). The lower panels show the Ponceau S-stained membrane after blotting, as controls of loading.
To determine whether potexviral proteins other than P25 could contribute to the HR-like response elicited by PVX/VSR combinations, we introduced a frameshift mutation in the P25 ORF of PVX to render PVXΔ25. Agrobacterium cultures carrying the PVX or PVXΔ25 constructs were combined with cultures that carry either HCwt or P19 and infiltrated into opposite leaf patches of N. benthamiana plants. It was hypothesized that coexpression of HCwt or P19 and PVXΔ25 would functionally complement the defective virus for the lack of P25 silencing suppression activity (39). This prediction was somewhat confirmed, as shown in Fig. 4A, because accumulation of PVXΔ25 RNA in the presence of HCwt or P19 was similar to that of PVX in combination with either HCwt or P19 at 6 dpa. The lower level of PVX sgRNAs accumulation observed for PVXΔ25 plus P19 was probably due to the fact that the suppressor activity of P19 on PVXΔ25 is weaker than that of HCwt. However, while the combinations of PVX and HCwt or P19 resulted in strong production of H2O2 (Fig. 4B), indicative of necrosis at 7 and 12 dpa, respectively, neither HCwt nor P19 was able to increase the pathogenicity of PVXΔ25. Consistent with these observations, leaf patches infiltrated with PVXΔ25 plus VSRs exhibited significantly lower levels of electrolyte leakage than did plants infiltrated with combinations of PVX and HCwt (at 7 dpa) or P19 (at 12 dpa) (Fig. 4C). Furthermore, the levels of electrolyte leakage in patches infiltrated with PVXΔ25 plus VSRs were comparable to those in patches infiltrated either with PVX plus GUS or with GUS alone. Thus, P25 seems to be the main PVX determinant involved in eliciting an HR-like response in PVX-associated synergisms.
FIG 4.
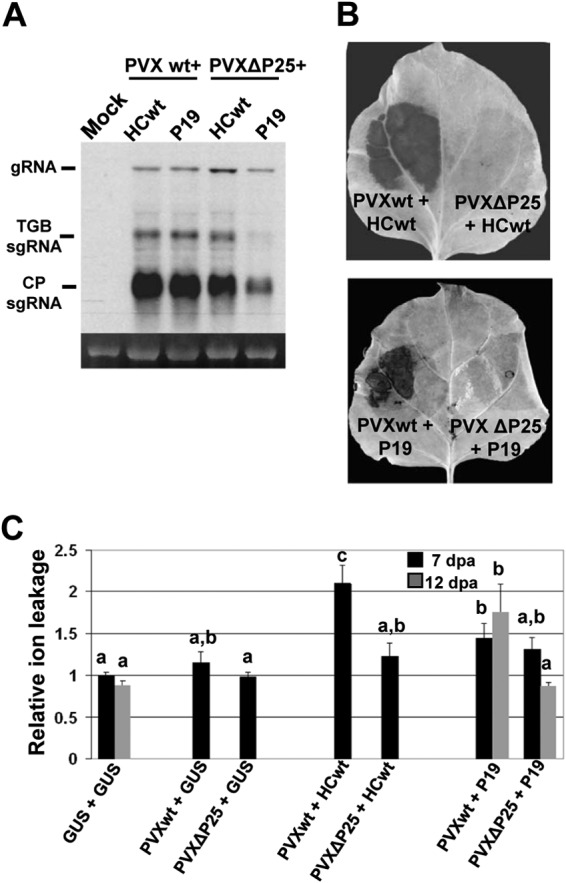
PVXΔ25 in combination with VSRs does not induce the HR-like response. Leaves of N. benthamiana were agroinfiltrated with GUS alone or combinations of PVX plus either GUS, PPV HCwt, or TBSV P19 and of PVXΔ25 plus either GUS, HCwt, or P19. (A) Northern blot analysis of total RNA extracted from leaf patches agroinfiltrated with PVX plus either HCwt or P19 and with PVXΔ25 plus either HCwt or P19, at 6 days postagroinoculation (dpa). Total RNA (2 μg) was hybridized with a probe complementary to PVX CP. PVX gRNA and the two major sgRNAs, triple-gene-block (TGB) sgRNA and CP sgRNA, are indicated. Ethidium bromide staining of 25S rRNA is shown as a loading control. (B) Leaves were stained with DAB solution at 7 (upper panel) and 12 (lower panel) dpa. (C) Electrolyte leakage from leaf disks infiltrated with the different combinations at 7 and 12 dpa. Data represent the means ± standard errors for 10 replicates, each consisting of four plants that received the same treatment in two independent experiments. Statistical comparisons between means were made among treatments at 7 or 12 dpa by employing Scheffé's multiple-range test. Different letters (a, b, c) indicate significant differences at P values of <0.05.
The silencing suppression activity of P25 is necessary for HR elicitation.
To evaluate if suppressor activity of P25 is required for inducing an HR-like response, three single-amino-acid mutants of P25, with mutations A104V, T117A, and K124E, reported previously, were used for analysis (39). In addition, to exclude the possibility that accumulation of P25 RNA is involved in necrosis, we examined the ability of a premature stop codon mutant, P25stop, to induce necrosis. We confirmed previous results showing that mutants T117A and K124E were deficient in suppressing the silencing of the GFP reporter in leaf patch assays whereas A104V was active in this function (Fig. 5A).
FIG 5.
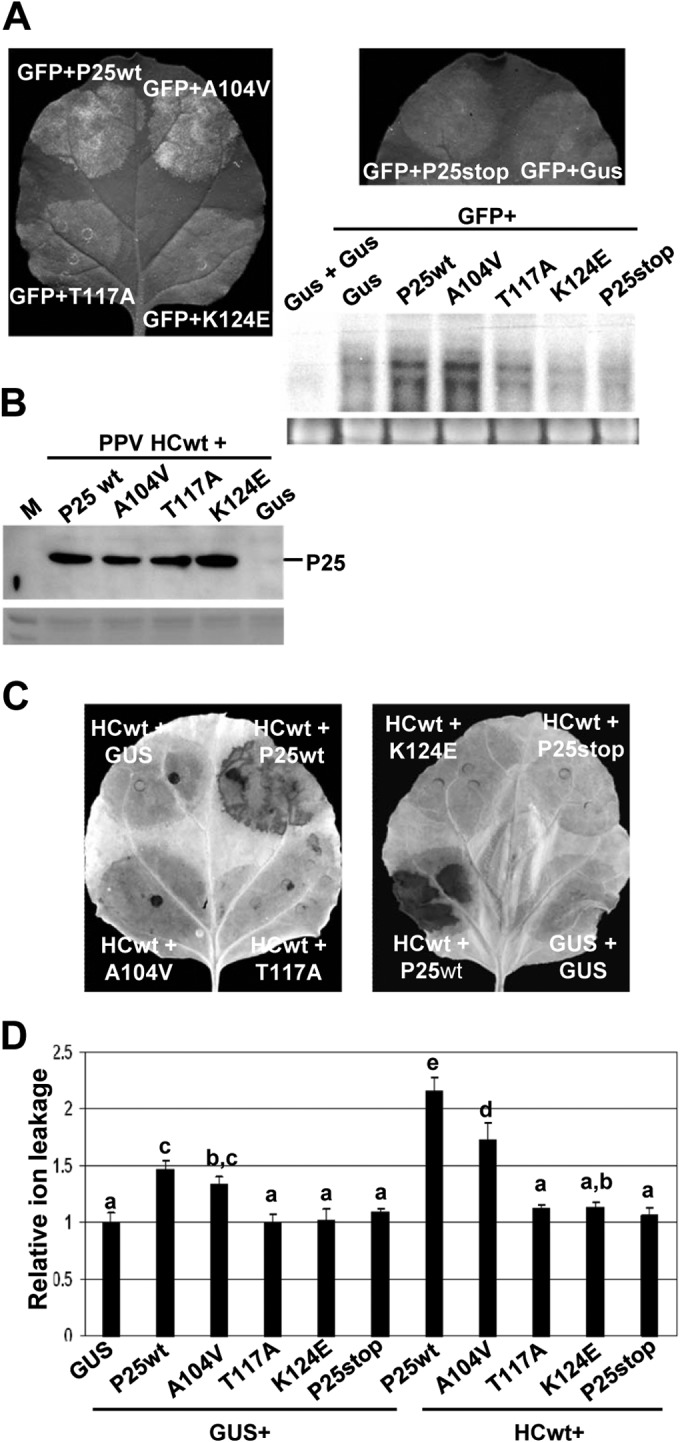
The HR-like response correlates with the silencing suppression activity of P25. (A) Leaves of N. benthamiana were infiltrated with GUS alone or combinations of Agrobacterium cultures containing binary constructs expressing a free GFP reporter gene plus T7-tagged versions of either P25wt or P25 A104V, T117A, K124E mutants or P25stop mutant, as indicated. Photographs were taken with long-wavelength UV light at 3 days postagroinoculation (dpa) (upper panels). Total RNA (10 μg) extracted from infiltrated tissues was hybridized with a probe complementary to GFP (lower panel). Ethidium bromide staining of 25S rRNA is shown as a loading control. (B) Western blot analysis of extracts derived from leaf patches infiltrated with combinations of PPV HCwt plus either P25 wt, A104V, T117A, K124E, P25stop, or GUS at 6 dpa, using antibodies against the T7 epitope. The lower panel shows the Ponceau S-stained membrane after blotting, as a loading control. (C) Leaves were infiltrated with GUS alone or combinations of Agrobacterium cultures containing PPV HCwt plus either T7-tagged versions of P25 wt, A104V, T117A, K124E, P25stop, or GUS. Leaves were stained with DAB solution at 9 dpa. (D) Electrolyte leakage from leaf disks infiltrated with T7-tagged versions of P25 wt, A104V, T117A, K124E, or P25stop, either alone or in combination with PPV HCwt at 9 dpa. GUS alone was infiltrated as a control. Data represent the means ± standard errors for 18 replicates, each consisting of four plants that received the same treatment in three independent experiments. Statistically significant differences between means were determined by employing Scheffé's multiple-range test. Different letters (a, b, c, d, e) indicate significant differences at P values of <0.05.
Leaf patches were infiltrated with T7-tagged versions of P25wt or P25 mutants either alone or in combination with HCwt. The combination of HCwt together with P25 A104V mutant led to slightly reduced necrosis and a low level of electrolyte leakage compared to that of HCwt plus P25wt but still significantly higher than those shown in GUS plus GUS or HCwt plus P25stop combinations (Fig. 5C and D). Remarkably, leaf patches infiltrated with HCwt plus the T117A or the K124E mutant did not undergo necrosis and exhibited levels of electrolyte leakage similar to those of patches infiltrated with controls. T117A or K124E mutants infiltrated alone gave lower levels of electrolyte leakage compared with P25wt or P25 A104V mutant. We confirmed that P25 A104V, T117A, and K124E mutants accumulated in the infiltrated patches at levels comparable to that of P25wt, as assayed by Western blot analysis (Fig. 5B). Altogether, these observations suggest that the suppressor activity of the P25 protein is necessary to trigger the HR-like response in N. benthamiana.
Both RAR1 and SGT1 positively regulate P25-induced HR and virulence of PVX-HC.
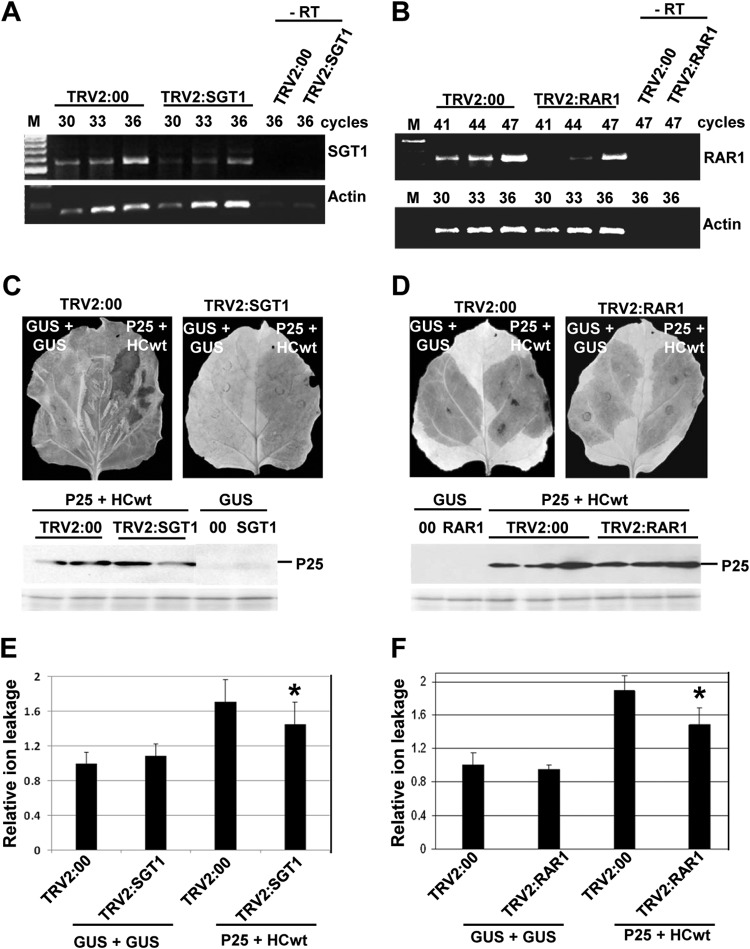
Functioning of R proteins depends on a chaperon complex that includes SGT1 and RAR1 in tobacco (12, 13). To examine the role of SGT1 and RAR1 in the HR-like response elicited by P25/HCwt, fragments of N. benthamiana homologues to SGT1 and RAR1 were cloned separately into a Tobacco rattle virus (TRV)-based VIGS vector. N. benthamiana plants were infiltrated with a recombinant vector or the empty vector (TRV2:00) as a control. At 14 days after infection, RT-PCR analysis using RNA extracted from upper leaves confirmed that SGT1 and RAR1 transcript levels were reduced substantially compared with control plants (Fig. 6A and B). Combinations of P25wt-T7 and either HCwt or GUS (as control) were infiltrated in opposite leaf patches of plants silenced for SGT1 and RAR1 or control plants. The development of the necrosis response and H2O2 production elicited by P25wt-T7/HCwt was attenuated in SGT1- and RAR1-silenced plants compared with control plants (Fig. 6C and D). Consistent with these observations, SGT1- and RAR1-silenced plants infiltrated with P25wt-T7 plus HCwt exhibited significantly lower levels of electrolyte leakage than did control plants (Fig. 6E and F). We confirmed that P25wt-T7 accumulated in SGT1- and RAR1-silenced plants at levels comparable to those of control plants, as assayed by Western blot analysis (Fig. 6C and D, lower panels).
FIG 6.
Silencing of N. benthamiana SGT1 and RAR1 results in attenuated HR-like necrosis induced by PVX P25/PPV HCwt. N. benthamiana plants were infiltrated with Agrobacterium cultures containing binary constructs expressing pTRV2:SGT1, pTRV2:RAR1, or TRV2:00 (vector control). Silencing of SGT1 (A) and RAR1 (B) transcripts was monitored by RT-PCR in uppermost leaves at 14 days after infiltration (dai). The same RT reactions were used to amplify Actin gene transcripts as a control. The numbers of PCR cycles are indicated below the treatments. −RT, control without RT. At 14 dai, leaves of pTRV2:SGT1, pTRV2:RAR1, and control plants were agroinfiltrated with GUS alone or the combination of P25wt-T7 plus HCwt in opposite leaf patches. SGT1-silenced (C) and RAR1-silenced (D) leaves were stained with DAB solution at 9 days postagroinoculation (dpa) (upper panels). Western blot analysis of extracts derived from leaf patches at 6 dpa, using antibodies against the T7 epitope (middle panels). The lower panels below the Western blots show the Ponceau S-stained membranes after blotting, as a loading control. Leaf disks from SGT1-silenced (E) and RAR1-silenced (F) leaves were excised and assayed for electrolyte leakage at 9 dpa. Data represent the means ± standard errors for 12 replicates, each consisting of four plants that received the same treatment in two independent experiments. Asterisks indicate significant differences in SGT1- and RAR1-silenced plants compared with control plants (Mann-Whitney U test, P < 0.05).
To evaluate whether SGT1 and RAR1 are also involved in the SN induced by PVX/potyvirus synergism, PVX-HC was agroinoculated in SGT1- and RAR1-silenced and nonsilenced control plants. By 11 dpa, the number of plants affected by necrosis reached 95 to 100% in both control and silenced plants, although the development of cell death in SGT1- and RAR1-silenced leaves was slower than that in nonsilenced control leaves. Electrolyte leakage measurement revealed that cell death in SGT1- and RAR1-silenced leaves was attenuated compared with nonsilenced control leaves at 11 dpa (Fig. 7A and B). These results were obtained in five biological replicates with 20 plants in each treatment. To examine whether the attenuated cell death phenotype was associated with changes in the accumulation of PVX-HC, we measured the amount of virus in systemically infected leaves from SGT1- and RAR1-silenced and nonsilenced control plants (Fig. 7C and D). qRT-PCR analysis showed that PVX-HC accumulated to slightly lower levels in SGT1- and RAR1-silenced plants than in control plants at 11 dpa.
FIG 7.
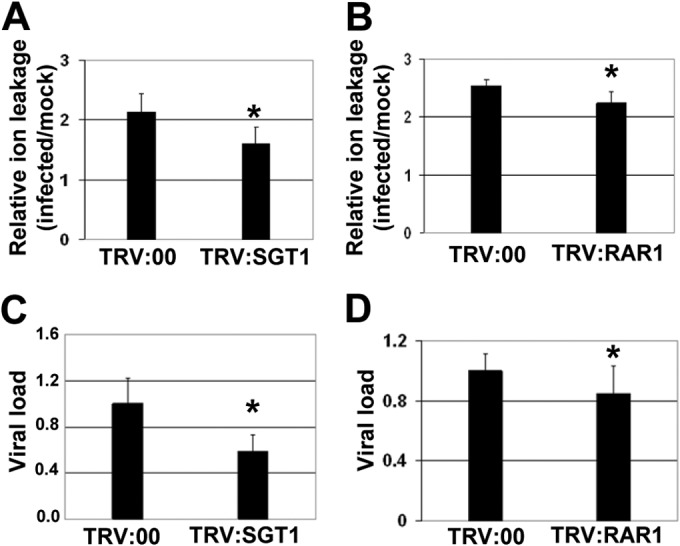
Silencing of N. benthamiana SGT1 and RAR1 results in attenuated systemic necrosis induced by PVX-HC. N. benthamiana plants were infiltrated with Agrobacterium cultures containing binary constructs expressing pTRV2:SGT1, pTRV2:RAR1, or TRV2:00 (vector control). Agroinfiltrated plants were mock inoculated or inoculated with PVX-HC at 14 days after infiltration (dai). Leaf disks from SGT1-silenced (A) and RAR1-silenced (B) leaves were excised and assayed for electrolyte leakage at 11 days postagroinoculation (dpa). Relative electrolyte leakage is expressed as the ratio of levels in inoculated plants over the mock-inoculated plants. qRT-PCR was used to analyze the accumulation of PVX-HC in SGT1-silenced (C) and RAR1-silenced (D) plants compared with controls at 11 dpa. Data represent the means ± standard errors for five replicates, each consisting of four plants that received the same treatment. Asterisks indicate significant differences in SGT1- and RAR1-silenced plants compared with control plants (Mann-Whitney U test, P < 0.05).
Coexpression of P25 and VSRs enhances LOX activity.
SN was correlated with an increase in LOX activity in PVX-potyvirus infected plants, which suggested the involvement of JA signaling in symptom expression (29). We analyzed LOX activity in leaf patches infiltrated with P25 in combination with HCwt, HCLH, P19, or GUS at 7 dpa (Fig. 8A). The level of enzymatic activity was higher in combinations that led to an HR-like response, e.g., P25 plus either HCwt or P19, than in combinations of P25 and HCLH or GUS. We further tested the effect of treatment with SA on the P25/VSR-induced HR because SA functions as a positive regulator in several defense responses. Treatment with SA induced a delayed HR and a statistically significant reduction in electrolyte leakage in leaf patches infiltrated with P25 in combination with either HCwt or P19 compared with that in control-treated plants (Fig. 8B). We confirmed that P25 accumulated in P25/VSR-infiltrated patches of SA-treated plants at levels comparable to those of control-treated plants (Fig. 8B, lower panel). The activation of SA signaling has been shown to result in the inhibition of JA biosynthesis or signaling (47). To determine whether the delayed HR triggered by P25/VSR combinations in SA-treated plants was based on the inhibition of JA-mediated responses, expression of two JA-responsive genes, TD and PI-I, was assayed in nonagroinfiltrated leaf tissue by RT-PCR. As expected, SA treatment repressed the expression of TD and PI-I compared to control plants (Fig. 8C). We therefore used transgenic N. benthamiana plants expressing the SA-degrading enzyme salicylate hydroxylase (NahG plants) to evaluate the SA contribution, if any, in the P25/VSR-induced HR. There was no significant difference in electrolyte leakage between the wt and NahG plants, suggesting that the effect of SA treatment in HR elicited by P25/VSR depends on the antagonistic relationship between the JA and SA signaling pathways (Fig. 8D).
FIG 8.
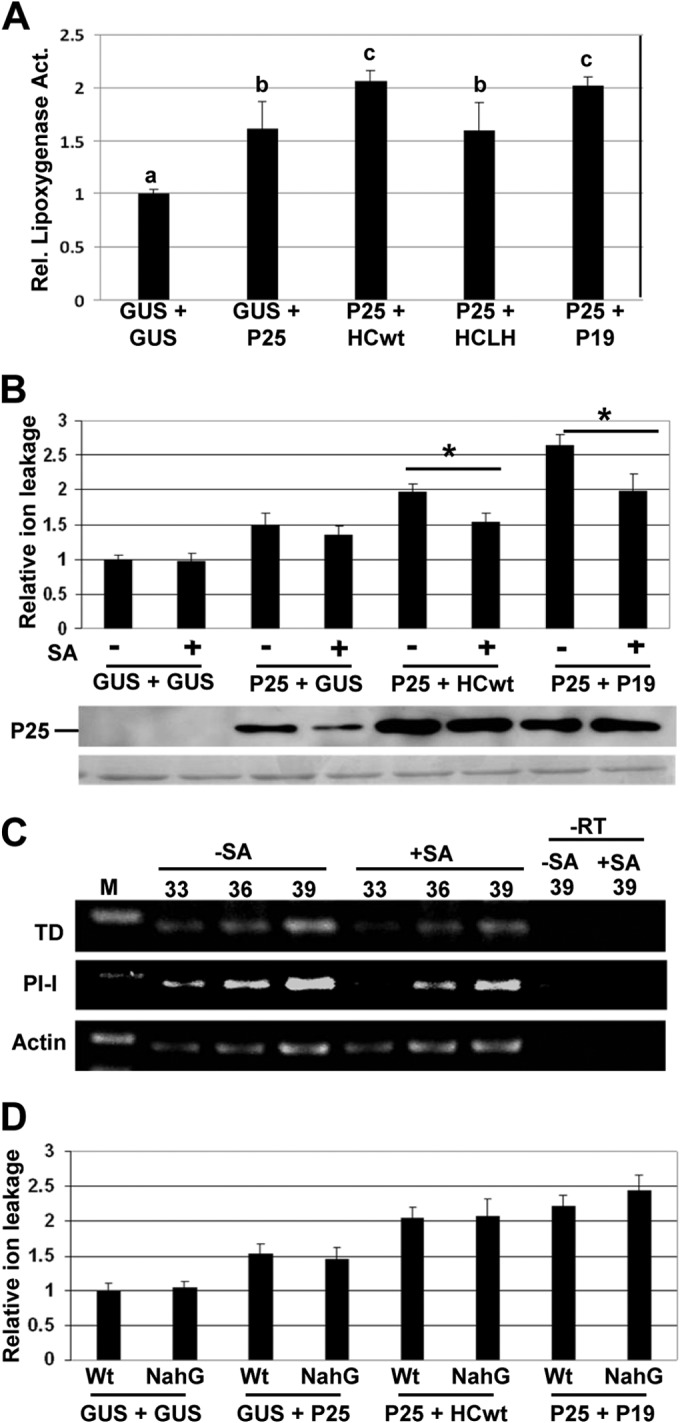
Enhanced LOX activity in N. benthamiana agroinfiltrated with PVX P25/VSR. (A) Leaves were infiltrated with combinations of Agrobacterium cultures containing binary constructs expressing PVX P25 plus either GUS, PPV HCwt, PPV HCLH, or TBSV P19. LOX activity was assayed in leaf extracts at 7 days postagroinoculation (dpa) using linoleic acid (0.1 mM) as a substrate. Data represent the means ± standard errors for 6 replicates, each consisting of four plants that received the same treatment. Statistically significant differences between means were determined by employing Scheffé's multiple-range test. Different letters (a, b, c) indicate significant differences at P values of <0.05. (B) Salicylic acid (SA) or water solution as a control was applied to leaves that were agroinfiltrated with GUS alone or combinations of cultures containing P25wt-T7 plus either GUS, PPV HCwt, or TBSV P19. Leaf disks were excised and assayed for electrolyte leakage at 8 dpa (upper panel). Western blot analysis of extracts derived from leaf patches at 6 dpa, using antibodies against the T7 epitope (middle panel). The lower panel below the Western blot shows the Ponceau S-stained membrane after blotting, as loading control. (C) RT-PCR analysis of expression of the threonine deaminase (TD) and proteinase inhibitor I (PI-I) genes in SA-treated and control-treated plants. The same RT reactions were used to amplify Actin gene transcripts as a control. The numbers of PCR cycles are indicated below the treatments. −RT, control without RT. (D) SA or mock solution as a control was applied to wild-type (Wt) plants and plants expressing the salicylate hydroxylase gene (NahG). Leaf disks were excised and assayed for electrolyte leakage at 8 dpa. Data represent the means ± standard errors for 12 replicates, each consisting of four plants that received the same treatment in two independent experiments. Asterisks indicate significant differences in SA-treated plants compared with control-treated plants (Student's t test, P < 0.05).
DISCUSSION
We have used an agroinfiltration assay to recreate in local tissues the SN response induced by PVX recombinant viruses expressing VSR proteins in N. benthamiana (19, 20). We demonstrated that the accumulation of PVX sgRNAs, a product of virus replication, but not gRNA increased in plants when PPV HCwt or TBSV P19 was coexpressed with PVX and when PVX was coinoculated with PPV. Thus, the PVX-potyvirus interaction in N. benthamiana can be defined as synergism in a broad sense, where both symptom severity and viral products are increased. The extent of PVX RNA accumulation seems to reflect the differential accessibility of viral target RNAs to degradation by RNA silencing; both HCwt and P19 prolonged the expression of the sgRNAs but did not have any obvious effect on accumulation of gRNA. This suggests that subcellular compartmentalization of PVX gRNA in active viral replication complexes and in specific membrane compartments (48) plays an important role in target accessibility to the machinery of RNA silencing. Alternatively, encapsidation might protect the PVX genome from silencing.
We further show that expression of the TGB sgRNA-encoded P25 protein in the presence of VSR from different viruses resulted in an HR-like response in Nicotiana spp. Moreover, P25 protein behaves as a pathogenicity determinant that induces necrotic symptoms when expressed by a PPV vector. This finding suggests that SN during PVX-associated synergisms is a delayed immune response triggered by P25, once it reaches a threshold level by the action of VSRs. This model indicates that SN is caused when recognition of virus multiplication at early stages of infection is weak or late and not sufficiently strong to restrict virus propagation. Later on, when the levels of the elicitor (P25) arise due to the activity of VSRs, a systemic HR-like response is mounted and the plant dies. Our findings on the role of elicitor dose dependency for SN were not completely unprecedented. Komatsu and associates (49) reported that necrotic symptoms induced by Plantago asiatica mosaic virus (PlAMV) in N. benthamiana depended on the level of accumulation of the helicase domain (HEL) of RNA-dependent RNA polymerase (RdRp), whose expression was indirectly regulated by the polymerase domain (POL) of RdRp. Thus, coinfections of PVX with other viruses expressing strong VSRs could mimic PlAMV POL action on HEL, allowing PVX P25 to accumulate beyond a threshold level and induce an HR-like defense response. Expression of P25 from the PVX genome is tightly regulated at low levels via TGB sgRNA (24). The control of P25 expression might represent a strategy of the virus to avoid detrimental effects in the host, with small changes in TGB sgRNA level having a significant impact on protein accumulation. This is evidenced when we compare the effect of expressing P25 at various levels in the presence or absence of VSR on electrolyte leakage, an indicator of cell membrane injury.
It was reported that overexpression of the PVX TGB3 protein by Agrobacterium led to necrotic lesions in N. benthamiana (28). Our results with the frameshift mutant PVXΔ25, in which TGB3 ORF was unaltered, indicate that P25 was the major determinant of PVX involved in SN induced by PVX-associated synergisms. Moreover, in contrast to what was observed for TGB3, the elicitor activity of P25 in N. benthamiana could not be ameliorated by coexpression of BiP, suggesting that ER stress was not involved in HR elicited by P25. We suspect that the elicitor function of P25 might also have contributed to the exacerbation of symptoms observed in other studies by recombinant PVX viruses expressing viral proteins with RNA silencing suppressor activity (20–23). Thus, caution should be taken when evaluating viral proteins as pathogenicity determinants in PVX chimeric viruses. Another implication drawn from our results is that unidentified pathogenicity determinants could be involved in other cases of viral synergisms whereby “helper” viruses encoding strong VSRs such as potyviruses exacerbate the accumulation of the avr-encoding virus (15).
Although it is not known how PVX P25 induces SN once it reaches a threshold level by the action of VSRs, our findings predict that Nicotiana spp. carry a matching R gene that can specifically recognize P25 and mount an HR. In support of this hypothesis, silencing of SGT1 and RAR1, which are known to play essential roles in many R-gene-triggered resistance responses against viruses (8, 12), ameliorated both P25-mediated HR and SN induced by PVX-HC. Moreover, typical hallmarks of HR-type PCD (30, 48), e.g., accumulation of reactive oxygen species (H2O2) and enhanced expression of LOX activity, have been documented in both plants infected with the synergistic pair PVX-PVY (29) and plants expressing P25/VSR combinations (this study). Several studies have shown that HR in plants is regulated by different caspase-like activities (46, 50). However, whereas VPE-1 was rapidly and transiently increased at an early stage of TMV-induced HR, accumulation of VPE-1 mRNA was downregulated by P25, suggesting that VPE-1 does not play a role as executer in P25-mediated cell death. Plant immunity is regulated by a complex network of cross-communicating signaling pathways. We investigated if the HR-like response induced by P25 was SA dependent. Electrolyte leakage was as extensive in leaves of NahG plants as it was in nontransgenic plants, indicating that the HR triggered by P25 was not dependent on SA signaling. The effect of SA treatment on P25-mediated HR was attributable to the antagonistic effect of SA on JA signaling (47). The participation of oxylipin metabolism in the signaling cascade leading to SN in PVX/potyviruses-associated synergism has been previously documented (29).
It was reported previously that the PVX 25 protein is the elicitor of Nb-mediated HR in potato (26). Since Nicotiana spp. and Solanum tuberosum are members of the family Solanaceae, many of which serve as PVX hosts, the possibility exists that Nb and the putative R gene in Nicotiana are not only functionally related but structurally homologous as well. Evidence from cloned R genes supports this possibility in that orthologues of known R genes have been identified in diverse species of Solanaceae (51). Alternatively, PVX P25 was recently shown to be necessary and sufficient to remodel host actin and endomembranes (ER and Golgi) and to recruit potexviral TGB2 and TGB3 proteins to viral replication and movement complexes (48, 52). This suggests that subcellular rearrangements required for PVX replication and/or movement might be involved in the induction of the necrosis response elicited by overexpression of P25. However, we consider this explanation unlikely for several reasons. First, specific point mutations, e.g., T117A and K124E, but not A104V, abolished the capacity of P25 protein to elicit the HR-like necrosis despite accumulating protein to approximately wild-type levels. Moreover, typical structures resembling PVX movement complexes were observed when GFP-tagged T117A but not A104V was expressed in N. benthamiana (52), which supports the view that redistribution of plant cytoskeleton and elicitation of the necrosis response are not interlinked phenomena triggered by P25.
Several studies have shown that some viral suppressors of RNA silencing can also act as avr gene products, and there have been conflicting findings as to whether or not this suppressor function might be directly related to R-gene-mediated resistance. The suppressor function of both the CP of Turnip crinkle virus and the NSs protein of Tomato spotted wilt virus appears to be independent of its role in elicitation of the resistance response mediated by HRT and Tsw R genes, respectively (53, 54). However, other studies support the idea that triggering of the corresponding R genes requires VSR activity of the avr determinant. The Tobacco aspermy virus (TAV) 2b protein elicited a strong resistance in tobacco when expressed from a recombinant TMV vector. Moreover, two separate studies have shown the importance of the same Arg residue at position 28 from TAV 2b for both VSR activity (55) and resistance elicitation (56). Sansregret et al. (57) reported that the TBSV P19 protein acts as an elicitor of an extreme resistance response in N. tabacum and that small RNA binding by P19, and thus VSR activity, is necessary to trigger the onset of defense in tobacco. Our study underscores that potexviral P25 variants T117A and K124E mutants, which have impaired ability to suppress RNA silencing, cannot act as avr determinants when synergized by the presence of PPV HC and adds further strength to the importance of RNA silencing suppression activity in the HR-like response elicited by VSR, at least in some cases.
P25 of potexviruses has been reported to target multiple components in the antiviral RNA silencing pathway. PVX P25 interacts with Argonaute1 (AGO1) and leads to its degradation via a proteasome-dependent pathway (58). Recently, PlAMV P25 has also been shown to interact with RNA-dependent RNA polymerase6 (RDR6) and Suppressor of Gene Silencing3 (SGS3) and to inhibit dsRNA and secondary small interfering RNA synthesis (59). Although the mode of action of dominant R genes is a matter of debate, one of the most commonly accepted models is the guard hypothesis (48). In this model of indirect detection, the pathogen avr protein targets a host protein under surveillance of the R protein rather than binding directly to the R protein. Whether AGO1, RDR6, or SGS3 represents a candidate protein for the guardee in P25-mediated HR would be a hypothesis to be tested in the future. Recently, it was found that different VSRs, like HC and P19, are responsible for overaccumulation of microRNA168, which resulted in downregulation of AGO1 protein level (60). In addition, VSRs are potent inhibitors of RDR6-dependent mechanisms that normally restrict PVX accumulation in tobacco (61) and Arabidopsis (62). Whether multiple VSRs contribute together with P25 to depletion of AGO1/RDR6 activities and the subsequent increase of PVX sgRNAs in PVX-associated synergisms and how these events are related to P25-mediated HR are key points that need to be further investigated.
ACKNOWLEDGMENTS
E.A. was the recipient of an FPU fellowship from the Spanish Ministry of Education, Culture and Sport. R.P. was the recipient of a contract from the Spanish Council for Scientific Research (CSIC).
We thank J. A. García (Centro Nacional de Biotecnología, Spain) for providing pGWBinPPV-3xHA, L. Peña (Instituto Valenciano de Investigaciones Agrarias, Spain) for providing the binary vector expressing the CTV P23 gene, F. Aparicio (Instituto de Biología Molecular y Celular de Plantas, Spain) for providing the NtVPE-1a clone, and M. Castellano (Centro de Biotecnología y Genómica de Plantas, Spain) for providing pGWB2.
This work was funded by a grant from the Rural Development Administration (RDA) of the Republic of Korea and by grant BIO2013-47940-R from the Spanish Ministry of Economy and Competitiveness.
We declare that we have no conflicts of interest.
REFERENCES
- 1.Ruiz-Ferrer V, Voinnet O. 2009. Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 60:485–510. doi: 10.1146/annurev.arplant.043008.092111. [DOI] [PubMed] [Google Scholar]
- 2.Mandadi KK, Scholthof KB. 2013. Plant immune responses against viruses: how does a virus cause disease? Plant Cell 25:1489–1505. doi: 10.1105/tpc.113.111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Király L, Cole AB, Bourque JE, Schoelz JE. 1999. Systemic cell death is elicited by the interaction of a single gene in Nicotiana clevelandii and gene VI of Cauliflower mosaic virus. Mol Plant Microbe Interact 12:919–925. doi: 10.1094/MPMI.1999.12.10.919. [DOI] [Google Scholar]
- 4.Love AJ, Laird J, Holt J, Hamilton AJ, Sadanandom A, Milner JJ. 2007. Cauliflower mosaic virus protein P6 is a suppressor of RNA silencing. J Gen Virol 88:3439–3444. doi: 10.1099/vir.0.83090-0. [DOI] [PubMed] [Google Scholar]
- 5.Angel CA, Schoelz JE. 2013. A survey of resistance to Tomato bushy stunt virus in the genus Nicotiana reveals that the hypersensitive response is triggered by one of three different viral proteins. Mol Plant Microbe Interact 26:240–248. doi: 10.1094/MPMI-06-12-0157-R. [DOI] [PubMed] [Google Scholar]
- 6.Atsumi G, Kagaya U, Kitazawa H, Nakahara KS, Uyeda I. 2009. Activation of the salicylic acid signaling pathway enhances Clover yellow vein virus virulence in susceptible pea cultivars. Mol Plant Microbe Interact 22:166–175. doi: 10.1094/MPMI-22-2-0166. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Marcos A, Pacheco R, Martiáñez J, González-Jara P, Díaz-Ruíz JR, Tenllado F. 2009. Transcriptional changes and oxidative stress associated with the synergistic interaction between Potato virus X and Potato virus Y and their relationship with symptom expression. Mol Plant Microbe Interact 22:1431–1444. doi: 10.1094/MPMI-22-11-1431. [DOI] [PubMed] [Google Scholar]
- 8.Komatsu K, Hashimoto M, Ozeki J, Yamaji Y, Maejima K, Senshu H, Himeno M, Okano Y, Kagiwada S, Namba S. 2010. Viral-induced systemic necrosis in plants involves both programmed cell death and the inhibition of viral multiplication, which are regulated by independent pathways. Mol Plant Microbe Interact 23:283–293. doi: 10.1094/MPMI-23-3-0283. [DOI] [PubMed] [Google Scholar]
- 9.Xu P, Roossinck MJ. 2000. Cucumber mosaic virus D satellite RNA-induced programmed cell death in tomato. Plant Cell 12:1079–1092. doi: 10.1105/tpc.12.7.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacheco R, García-Marcos A, Manzano A, García de Lacoba M, Camañes G, García-Agustín P, Díaz-Ruíz JR, Tenllado F. 2012. Comparative analysis of transcriptomic and hormonal responses to compatible and incompatible plant-virus interactions that lead to cell death. Mol Plant Microbe Interact 25:709–723. doi: 10.1094/MPMI-11-11-0305. [DOI] [PubMed] [Google Scholar]
- 11.Seo YS, Rojas MR, Lee JY, Lee SW, Jeon JS, Ronald P, Lucas WJ, Gilbertson RL. 2006. A viral resistance gene from common bean functions across plant families and is up-regulated in a non-virus-specific manner. Proc Natl Acad Sci U S A 103:11856–11861. doi: 10.1073/pnas.0604815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP. 2002. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30:415–429. doi: 10.1046/j.1365-313X.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Schiff M, Serino G, Deng XW, Dinesh-Kumar SP. 2002. Role of SCF ubiquitin-ligase and the COP9 signalosome in the N gene-mediated resistance response to Tobacco mosaic virus. Plant Cell 14:1483–1496. doi: 10.1105/tpc.002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latham JR, Wilson AK. 2008. Transcomplementation and synergism in plants: implications for viral transgenes? Mol Plant Pathol 9:85–103. doi: 10.1111/j.1364-3703.2007.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syller J. 2011. Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol Plant Pathol 13:204–216. doi: 10.1111/j.1364-3703.2011.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González-Jara P, Tenllado F, Martínez-García B, Atencio FA, Barajas D, Vargas M, Díaz-Ruiz J, Díaz-Ruiz JR. 2004. Host-dependent differences during synergistic infection by Potyviruses with potato virus X. Mol Plant Pathol 5:29–35. doi: 10.1111/j.1364-3703.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 17.Vance VB, Berger PH, Carrington JC, Hunt AG, Shi BM. 1995. 5′ proximal potyviral sequences mediate potato virus X/potyviral synergistic disease in transgenic tobacco. Virology 206:583–590. doi: 10.1016/S0042-6822(95)80075-1. [DOI] [PubMed] [Google Scholar]
- 18.Pruss G, Ge X, Shi XM, Carrington JC, Vance VB. 1997. Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 9:859–868. doi: 10.1105/tpc.9.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González-Jara P, Atencio FA, Martínez-García B, Barajas D, Tenllado F, Díaz-Ruíz JR. 2005. A single amino acid mutation in the Plum pox virus helper component-proteinase gene abolishes both synergistic and RNA silencing suppression activities. Phytopathology 95:894–901. doi: 10.1094/PHYTO-95-0894. [DOI] [PubMed] [Google Scholar]
- 20.Brigneti G, Voinnet O, Li WX, Ji LH, Ding SW, Baulcombe DC. 1998. Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J 17:6739–6746. doi: 10.1093/emboj/17.22.6739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Pfeffer S, Dunoyer P, Heim F, Richards KE, Jonard G, Ziegler-Graff V. 2002. P0 of Beet western yellows virus is a suppressor of posttranscriptional gene silencing. J Virol 76:6815–6824. doi: 10.1128/JVI.76.13.6815-6824.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Luna AP, Morilla G, Voinnet O, Bejarano ER. 2012. Functional analysis of gene-silencing suppressors from tomato yellow leaf curl disease viruses. Mol Plant Microbe Interact 25:1294–1306. doi: 10.1094/MPMI-04-12-0094-R. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Dong J, Xu Y, Wu J. 2012. V2 protein encoded by Tomato yellow leaf curl China virus is an RNA silencing suppressor. Virus Res 163:51–58. doi: 10.1016/j.virusres.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Verchot-Lubicz J, Ye CM, Bamunusinghe D. 2007. Molecular biology of potexviruses: recent advances. J Gen Virol 88:1643–1655. doi: 10.1099/vir.0.82667-0. [DOI] [PubMed] [Google Scholar]
- 25.Voinnet O, Lederer C, Baulcombe DC. 2000. A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell 103:157–167. doi: 10.1016/S0092-8674(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 26.Malcuit I, Marano MR, Kavanagh TA, De Jong W, Forsyth A, Baulcombe DC. 1999. The 25-kDa movement protein of PVX elicits Nb-mediated hypersensitive cell death in potato. Mol Plant Microbe Interact 12:536–543. doi: 10.1094/MPMI.1999.12.6.536. [DOI] [Google Scholar]
- 27.Jada B, Soitamo AJ, Lehto K. 2013. Organ-specific alterations in tobacco transcriptome caused by the PVX-derived P25 silencing suppressor transgene. BMC Plant Biol 13:8. doi: 10.1186/1471-2229-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye C, Dickman MB, Whitham SA, Payton M, Verchot J. 2011. The unfolded protein response is triggered by a plant viral movement protein. Plant Physiol 156:741–755. doi: 10.1104/pp.111.174110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Marcos A, Pacheco R, Manzano A, Aguilar E, Tenllado F. 2013. Oxylipin biosynthesis genes positively regulate PCD during compatible infections by the synergistic pair Potato virus X-Potato virus Y and by Tomato spotted wilt virus. J Virol 87:5769–5783. doi: 10.1128/JVI.03573-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montillet JL, Chamnongpol S, Rusterucci C, Dat J, van de Cotte B, Agnel JP, Battesti C, Inze D, Van Breusegem F, Triantaphylides C. 2005. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol 138:1516–1526. doi: 10.1104/pp.105.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goytia E, Fernández-Calvino L, Martínez-García B, López-Abella D, López-Moya JJ. 2006. Production of plum pox virus HC-Pro functionally active for aphid transmission in a transient-expression system. J Gen Virol 87:3413–3423. doi: 10.1099/vir.0.82301-0. [DOI] [PubMed] [Google Scholar]
- 32.Uhrig JF, Canto T, Marshall D, MacFarlane SA. 2004. Relocalization of nuclear ALY proteins in the cytoplasm by the Tomato bushy stunt virus P19 pathogenicity protein. Plant Physiol 135:2411–2423. doi: 10.1104/pp.104.046086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tena F, González I, Doblas P, Rodríguez C, Sahana N, Kaur H, Tenllado F, Praveen S, Canto T. 2013. The influence of cis-acting P1 protein and translational elements on the expression of Potato virus Y HCPro in heterologous systems and its suppression of silencing activity. Mol Plant Pathol 14:530–541. doi: 10.1111/mpp.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canto T, Cillo F, Palukaitis P. 2002. Generation of siRNAs by TDNA sequences does not require active transcription or homology to sequences in the plant. Mol Plant Microbe Interact 15:1137–1146. doi: 10.1094/MPMI.2002.15.11.1137. [DOI] [PubMed] [Google Scholar]
- 35.Fagoaga C, López C, Hermoso de Mendoza A, Moreno P, Navarro L, Flores R, Peña L. 2006. Post-transcriptional gene silencing of the p23 silencing suppressor of Citrus tristeza virus confers resistance to the virus in transgenic Mexican lime. Plant Mol Biol 60:153–165. doi: 10.1007/s11103-005-3129-7. [DOI] [PubMed] [Google Scholar]
- 36.Baulcombe DC, Chapman S, Santa-Cruz S. 1995. Jellyfish green fluorescent protein as a reporter for virus infections. Plant J 7:1045–1053. doi: 10.1046/j.1365-313X.1995.07061045.x. [DOI] [PubMed] [Google Scholar]
- 37.Restrepo MA, Freed DD, Carrington JC. 1990. Nuclear transport of plant potyviral proteins. Plant Cell 2:987–998. doi: 10.1105/tpc.2.10.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higuchi R, Krummel B, Saiki R. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res 16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayne EH, Rakitina DV, Morozov SY, Baulcombe DC. 2005. Cell-to-cell movement of potato potexvirus X is dependent on suppression of RNA silencing. Plant J 44:471–482. doi: 10.1111/j.1365-313X.2005.02539.x. [DOI] [PubMed] [Google Scholar]
- 40.Alamillo JM, Saenz P, García JA. 2006. Salicylic acid-mediated and RNA-silencing defense mechanisms cooperate in the restriction of systemic spread of Plum pox virus in tobacco. Plant J 48:217–227. doi: 10.1111/j.1365-313X.2006.02861.x. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. 2007. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- 42.Ying XB, Dong L, Zhu H, Duan CG, Du QS, Lv DQ, Fang YY, Garcia JA, Fang RX, Guo HS. 2010. RNA-dependent RNA polymerase 1 from Nicotiana tabacum suppresses RNA silencing and enhances viral infection in Nicotiana benthamiana. Plant Cell 22:1358–1372. doi: 10.1105/tpc.109.072058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenllado F, Díaz-Ruíz JR. 2001. Double-stranded RNA-mediated interference with plant virus infection. J Virol 75:12288–12297. doi: 10.1128/JVI.75.24.12288-12297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nausch H, Mikschofsky H, Koslowski R, Meyer U, Broer I, Huckauf J. 2012. High-level transient expression of ER-targeted human interleukin 6 in Nicotiana benthamiana. PLoS One 7:e48938. doi: 10.1371/journal.pone.0048938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valli A, Gallo A, Calvo M, de Jesús Pérez J, García JA. 2014. A novel role of the potyviral helper component proteinase contributes to enhance the yield of viral particles. J Virol 88:9808–9818. doi: 10.1128/JVI.01010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I. 2004. A plant vacuolar protease, VPE-1, mediates virus-induced hypersensitive cell death. Science 305:855–858. doi: 10.1126/science.1099859. [DOI] [PubMed] [Google Scholar]
- 47.Thaler JS, Humphery PT, Whiteman NK. 2012. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17:260–270. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Tilsner J, Linnik O, Wright KM, Bell K, Roberts AG, Lacomme C, Santa Cruz S, Oparka KJ. 2012. The TGB1 movement protein of Potato virus X reorganizes actin and endomembranes into the X-body, a viral replication factory. Plant Physiol 158:1359–1370. doi: 10.1104/pp.111.189605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komatsu K, Hashimoto M, Maejima K, Shiraishi T, Neriya Y, Miura C, Minato N, Okano Y, Sugawara K, Yamaji Y, Namba S. 2011. A necrosis-inducing elicitor domain encoded by both symptomatic and asymptomatic Plantago asiatica mosaic virus isolates, whose expression is modulated by virus replication. Mol Plant Microbe Interact 24:408–420. doi: 10.1094/MPMI-12-10-0279. [DOI] [PubMed] [Google Scholar]
- 50.Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 51.Grube RC, Radwanski ER, Jahn M. 2000. Comparative genetics of disease resistance within the solanaceae. Genetics 155:873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan F, Lu Y, Lin L, Zheng H, Chen J. 2012. The ability of PVX p25 to form RL structures in plant cells is necessary for its function in movement, but not for its suppression of RNA silencing. PLoS One 7:e43242. doi: 10.1371/journal.pone.0043242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi CW, Qu F, Ren T, Ye X, Morris TJ. 2004. RNA silencing-suppressor function of Turnip crinkle virus coat protein cannot be attributed to its interaction with the Arabidopsis protein TIP. J Gen Virol 85:3415–3420. doi: 10.1099/vir.0.80326-0. [DOI] [PubMed] [Google Scholar]
- 54.de Ronde D, Pasquier A, Ying S, Butterbach P, Lohuis D, Kormelink R. 2014. Analysis of Tomato spotted wilt virus NSs protein indicates the importance of the N-terminal domain for avirulence and RNA silencing suppression. Mol Plant Pathol 15:185–195. doi: 10.1111/mpp.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen HY, Yang J, Lin C, Yuan YA. 2008. Structural basis for RNA-silencing suppression by Tomato aspermy virus protein 2b. EMBO Rep 9:754–760. doi: 10.1038/embor.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li HW, Lucy AP, Guo HS, Li WX, Ji LH, Wong SM, Ding SW. 1999. Strong host resistance targeted against a viral suppressor of the plant gene silencing defence mechanism. EMBO J 18:2683–2691. doi: 10.1093/emboj/18.10.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sansregret R, Dufour V, Langlois M, Daayf F, Dunoyer P, Voinnet O, Bouarab K. 2013. Extreme resistance as a host counter-counter defense against viral suppression of RNA silencing. PLoS Pathog 9:e1003435. doi: 10.1371/journal.ppat.1003435. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Chiu MH, Chen IH, Baulcombe DC, Tsai CH. 2010. The silencing suppressor P25 of Potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol Plant Pathol 11:641–649. doi: 10.1111/j.1364-3703.2010.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okano Y, Senshu H, Hashimoto M, Neriya Y, Netsu O, Minato N, Yoshida T, Maejima K, Oshima K, Komatsu K, Yamaji Y, Namba S. 2014. In planta recognition of a double-stranded RNA synthesis protein complex by a potexviral RNA silencing suppressor. Plant Cell 26:2168–2183. doi: 10.1105/tpc.113.120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varallyay E, Havelda Z. 2013. Unrelated viral suppressors of RNA silencing mediate the control of ARGONAUTE1 level. Mol Plant Pathol 14:567–575. doi: 10.1111/mpp.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallory AC, Parks G, Endres MW, Baulcombe D, Bowman LH, Pruss GJ, Vance VB. 2002. The amplicon-plus system for high-level expression of transgenes in plants. Nat Biotechnol 20:622–625. doi: 10.1038/nbt0602-622. [DOI] [PubMed] [Google Scholar]
- 62.Moissiard G, Parizotto EA, Himber C, Voinnet O. 2007. Transitivity in Arabidopsis can be primed, requires the redundant action of the antiviral Dicer-like 4 and Dicer-like 2, and is compromised by viral-encoded suppressor proteins. RNA 13:1268–1278. doi: 10.1261/rna.541307. [DOI] [PMC free article] [PubMed] [Google Scholar]