ABSTRACT
A unique HIV-host equilibrium exists in untreated HIV-2-infected individuals. This equilibrium is characterized by low to undetectable levels of viremia throughout the disease course, despite the establishment of disseminated HIV-2 reservoirs at levels comparable to those observed in untreated HIV-1 infection. Although the clinical spectrum is similar in the two infections, HIV-2 infection is associated with a much lower rate of CD4 T-cell decline and has a limited impact on the mortality of infected adults. Here we investigated HIV-2 infection of the human thymus, the primary organ for T-cell production. Human thymic tissue and suspensions of total or purified CD4 single-positive thymocytes were infected with HIV-2 or HIV-1 primary isolates using either CCR5 or CXCR4 coreceptors. We found that HIV-2 infected both thymic organ cultures and thymocyte suspensions, as attested to by the total HIV DNA and cell-associated viral mRNA levels. Nevertheless, thymocytes featured reduced levels of intracellular Gag viral protein, irrespective of HIV-2 coreceptor tropism and cell differentiation stage, in agreement with the low viral load in culture supernatants. Our data show that HIV-2 is able to infect the human thymus, but the HIV-2 replication cycle in thymocytes is impaired, providing a new model to identify therapeutic targets for viral replication control.
IMPORTANCE HIV-1 infects the thymus, leading to a decrease in CD4 T-cell production that contributes to the characteristic CD4 T-cell loss. HIV-2 infection is associated with a very low rate of progression to AIDS and is therefore considered a unique naturally occurring model of attenuated HIV disease. HIV-2-infected individuals feature low to undetectable plasma viral loads, in spite of the numbers of circulating infected T cells being similar to those found in patients infected with HIV-1. We assessed, for the first time, the direct impact of HIV-2 infection on the human thymus. We show that HIV-2 is able to infect the thymus but that the HIV-2 replication cycle in thymocytes is impaired. We propose that this system will be important to devise immunotherapies that target viral production, aiding the design of future therapeutic strategies for HIV control.
INTRODUCTION
The thymus is the primary organ for T-cell production, and despite the age-associated decline, thymic function is maintained until late in life (1, 2). Thymic activity is vital in clinical settings requiring de novo T-cell generation, such as HIV infection (1, 3, 4). Accordingly, impairment of thymic output impacts the rate of HIV-1 disease progression, while the degree of immunological reconstitution achieved after antiretroviral therapy has been shown to rely on thymus recovery (1, 3, 4). Moreover, a functional cure for HIV infection is thought to entail a diverse T-cell repertoire, which can be generated only by the thymus.
HIV-1 targets the thymus in both children and adults, resulting in severe disruption of the thymic microenvironment, as demonstrated by the morphological changes and thymocyte depletion reported in the thymuses of HIV-1-infected individuals (5, 6). Several studies based on HIV-1 infection of the human thymus either in vitro (7–9) or in vivo using the SCID/hu mouse model (10, 11) have indicated that both direct infection of thymic cells and indirect viral effects upon the microenvironment play a role in HIV-1-associated thymic pathology. Furthermore, viral entry, viral replication kinetics, and the cytopathicity of HIV-1 in human thymocytes have been shown to be highly dependent on viral tropism, due to the predominance of CXCR4 (X4) versus CCR5 (R5) expression in the human thymus (9, 12, 13). Thymic disruption has also been described in nonhuman primate models of simian immunodeficiency virus infection (14).
Here we addressed, for the first time, the direct impact of HIV-2 infection on the human thymus. This is particularly relevant because HIV-2-infected individuals feature low rates of CD4 T-cell decline and disease progression (15–17). Moreover, they typically have low to undetectable levels of viremia, with this being observed even in AIDS patients with <200 CD4 T cells/μl (18, 19). The low levels of circulating virus account for the reduced horizontal and vertical transmission observed in HIV-2 infection (20, 21), as well as for its geographical confinement to West Africa and connected countries, such as Portugal. Despite the high prevalence of HIV-2 in several regions of West Africa, such as in Guinea Bissau (8% in adults and up to 20% in people over 40 years of age) (22), there is no significant impact on the mortality of infected adults. HIV-2 thus constitutes a unique naturally occurring model of attenuated HIV disease valuable for the study of HIV pathogenesis.
In spite of the low to undetectable HIV-2 plasma loads, HIV-2- and HIV-1-infected patients at equivalent stages of CD4 T-cell depletion feature comparable levels of cell-associated viral burden (18, 23, 24), indicating the establishment of disseminated HIV-2 reservoirs. They also feature similar levels of T-cell activation (19), suggesting distinct control of viral replication in the presence of cell activation in HIV-2 and HIV-1 infections.
Our previous data support the preservation of thymic function in HIV-2 infection, as estimated by signal joint (sj)/β T cell receptor excision circle (TREC) quantification (3) in circulating T cells of HIV-2-infected patients (25). However, there are no studies on the direct impact of HIV-2 infection on the human thymus either in vivo, due to the difficulty of obtaining thymic tissue from HIV-2-infected patients, or in vitro. We show here that HIV-2 is able to infect the human thymus but that this is associated with limited viral replication, irrespective of viral coreceptor tropism and thymocyte differentiation stage. HIV-2 infection of the human thymus thus offers a novel approach to investigate the mechanisms underlying the establishment of HIV reservoirs and the control of viral replication.
MATERIALS AND METHODS
Ethical statement.
Thymic tissue specimens (from individuals ranging from newborns to children 4 years old) were obtained during routine thymectomy performed during pediatric corrective cardiac surgery at the Hospital de Santa Cruz, Carnaxide, Portugal, after the parents provided written informed consent. Buffy coats from healthy donors were provided by the Instituto Português do Sangue e da Transplantação after they provided written informed consent. The study was approved by the Ethical Boards of the Faculty of Medicine of the University of Lisbon and of the Hospital de Santa Cruz, Carnaxide, Portugal.
HIV stocks.
The viruses used are described in Table 1. Viral stocks were propagated in pools of isolated peripheral blood mononuclear cells (PBMCs) stimulated for 3 days with phytohemagglutinin (PHA; 5 μg/ml; Sigma) and maintained with human recombinant interleukin-2 (IL-2; 10 U/ml; from Maurice Gately, Hoffmann-La Roche Inc., through the NIH AIDS Reagent Program), as described previously (28). Virus in cell-free culture supernatants was quantified by measuring reverse transcriptase (RT) activity using a Lenti-RT activity kit (Cavidi).
TABLE 1.
HIV isolates used in the study
| Virus | Major coreceptor(s) used | Source |
|---|---|---|
| Primary isolates | ||
| HIV-192US660 | R5 | NIHa |
| HIV-192HT599 | X4 | NIHa,b |
| HIV-260415K | R5 | NIHa,c |
| HIV-220.04d | X4 | Nuno Taveira |
| Lab-adapted strains | ||
| HIV-1NL4-3 | X4 | MRCe |
| HIV-2ROD10 | R5, X4 | MRCe |
From the Multicenter AIDS Cohort Study, NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH.
Provided by Neal Halsey.
Provided by Feng Gao and Beatrice Hahn.
NIBSC, Centre for AIDS Reagents, Medical Research Council, United Kingdom.
HIV infection of thymocyte suspensions.
Total thymocytes were recovered through tissue dispersion and separation on a Ficoll-Paque Plus (GE Healthcare) density gradient. The CD4 single-positive (CD4SP) population was sorted from total thymocytes as CD3high CD8-negative (CD8neg) cells (purity, >98%), using a FACSAria high-speed cell sorter (BD Biosciences). Viral stocks were ultracentrifuged for 30 min at 50,000 × g and 4°C (Beckman L8 ultracentrifuge), resuspended in complete medium (RPMI 1640 with 10% fetal bovine serum [FBS], 2 mM l-glutamine, 50 U/ml penicillin-streptomycin, and 50 μg/ml gentamicin [all from Gibco/Invitrogen] plus 3 μg/ml Polybrene [Sigma]), and added to the cells at 0.3 ng RT/106 thymocytes. Total and CD4SP thymocytes were cultured for 3 to 4 h at 108 and 3.5 × 107 cells/ml, respectively, in the absence or presence of virus. After infection, the cells were washed and cultured at 107 cells/ml in complete medium supplemented with IL-2 (20 U/ml), IL-4 (20 ng/ml; R&D), and IL-7 (10 ng/ml; R&D) at 37°C. A quarter of the medium was replaced every 3 to 4 days. On day 10, the thymocyte number per well was determined using 10-μm latex beads (Coulter), and the cells were analyzed by flow cytometry or stored as pellets at −80°C. Viral production in supernatants was quantified at day 10 postinfection of CD11cneg CD14neg CD123neg T-cell receptor γδ-negative (TCRγδneg) thymocytes by measuring RT activity using SYBR green product-enhanced RT (SG-PERT), as described previously (29, 30).
HIV infection of TOCs.
Thymic tissue blocks (diameter, 1 to 2 mm) were placed on Millicell organotypic inserts (Millipore) in a 6-well plate containing 1 ml of thymic organ culture (TOC) medium (complete medium with 15% FBS, 10 mM HEPES, 1 mM sodium pyruvate, and 1% minimal essential medium nonessential amino acids [all from Gibco/Invitrogen]). TOCs were placed at 37°C in 5% CO2 overnight, and half of the medium was replaced prior to infection. HIV infection was performed by placing a 5-μl drop containing 3 ng RT of virus on top of each TOC. A third of the medium was replaced every 2 to 3 days. On day 10 or 11 postinfection, TOCs were placed in 4% formaldehyde or mashed. Thymocytes were analyzed by flow cytometry or stored as cell pellets at −80°C. The role of Env proteins was assessed by culturing the TOCs for 7 days on Isopore membranes (Millipore) placed in TOC medium containing 1 μg/ml of purified recombinant glycoproteins: gp105ROD (MRC EVA621), gp120Ba-L (catalog no. 4961; NIH), and gp120IIIB (MRC EVA607) or anti-CD4 monoclonal antibody (MAb; BD Biosciences) as a control.
Flow cytometry.
Surface staining was performed for 20 min at room temperature and always included fixable viability dye (eBioscience) for dead cell exclusion. Thymocytes were fixed, permeabilized, and stained using an intracellular staining kit (eBioscience), as described previously (31). The anti-human MAbs used were (clone designations are given in parentheses) CD3 (UCHT1), CD4 (RPA-T4), CD8α (RPA-T8), CD11c (3.9), CD14 (61D3), CD16 (eBioCB16), CD19 (HIB19), CD123 (6H6), and TCRγδ (B1.1) from eBioscience. The following anti-Gag MAbs were used: KC57 (Beckman Coulter), anti-p24 (MAb Kal-1; Dako), anti-p24 (MAb AG3.0 or 183-H12-5C; NIH), and anti-p27 (MAb ARP396/397; P. Szawlowski, NIBSC, Centre for AIDS Reagents, Medical Research Council [MRC]). Alexa Fluor 488 goat anti-mouse IgG (H+L) antibody (Molecular Probes) was used for secondary detection. Cells were acquired using a LSRFortessa cell analyzer (BD Biosciences), and data were analyzed with FlowJo software (TreeStar).
Immunohistochemistry.
Fixed TOCs were embedded in paraffin and cut into 3-μm sections (Minot microtome Leica RM2145). Epitope retrieval was performed at pH 9 (Leica Biosystems buffer) for 15 min using a microwave (800 W). Samples were stained with the appropriate anti-Gag primary antibodies, incubated with a peroxidase-diaminobenzidine detection system (EnVision; Dako), and counterstained with Harris's hematoxylin (BioOptica). Images were acquired using a Leica DM2500 microscope.
Quantification of total HIV DNA and gag mRNA by real-time PCR.
Total HIV DNA was quantified in cell lysates prepared by treating cell pellets with 100 μg/ml proteinase K in 10 mM Tris-HCl for 1 h at 56°C, followed by 10 min of enzyme inactivation at 95°C. For gag mRNA quantification, 200 ng of total RNA, purified using a mirVana microRNA isolation kit (Ambion), was used to synthesize cDNA using oligo(dT)20 and SuperScript III RT (Invitrogen) according to the manufacturer's instructions. Real-time PCR was performed using Platinum Taq plus carboxy-X-rhodamine (ROX) or TaqMan gene expression master mix (Applied Biosystems) with the primers and probes described in Table S1 in the supplemental material. Standard curves were generated from serial dilutions of cDNA prepared from the mRNA of 3 different thymuses (for GAPDH [glyceraldehyde-3-phosphate dehydrogenase] quantification) or of plasmids containing the amplicons of HIV-1 gag and CD3γ (a kind gift from Rémi Cheynier) (32) or of HIV-2 gag. A plasmid carrying HIV-2 gag was generated by inserting a sequence including the sequences for the long terminal repeat/gag from HIV-2ROD10 into the pGEM-T Easy vector (Promega). Quantification was performed using an Applied Biosystems 7500 Fast real-time PCR system.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism (v5.01) software (GraphPad Software Inc.). Data from two samples were compared using the Wilcoxon matched-pairs test. Data from more than two samples were compared using the Friedman test or the Kruskal-Wallis test with Dunn's multiple-comparison posttest. P values of <0.05 were considered significant.
RESULTS
HIV-2 infects the human thymus.
We investigated HIV-2 infection of the human thymus in vitro using both TOCs, which preserve the thymic microenvironment and have been shown to be permissive to HIV-1 infection (7, 8), and thymocyte suspensions. TOCs and thymocyte suspensions were infected with HIV-2 or HIV-1 primary isolates with selective R5 or X4 coreceptor specificity and cultured for 10 days. RT activity was used to normalize the viral input, as HIV-1 and HIV-2 RT enzymes were shown to possess similar specific activities (33).
We found that HIV-2 was able to infect both thymic tissue and total thymocyte suspensions, as indicated by the levels of total HIV DNA, which includes unintegrated and integrated proviral DNA, in HIV-2-infected samples (Fig. 1A). Furthermore, we observed that HIV-2 infection of both thymic tissue and total thymocyte suspensions was less coreceptor dependent than HIV-1 infection.
FIG 1.
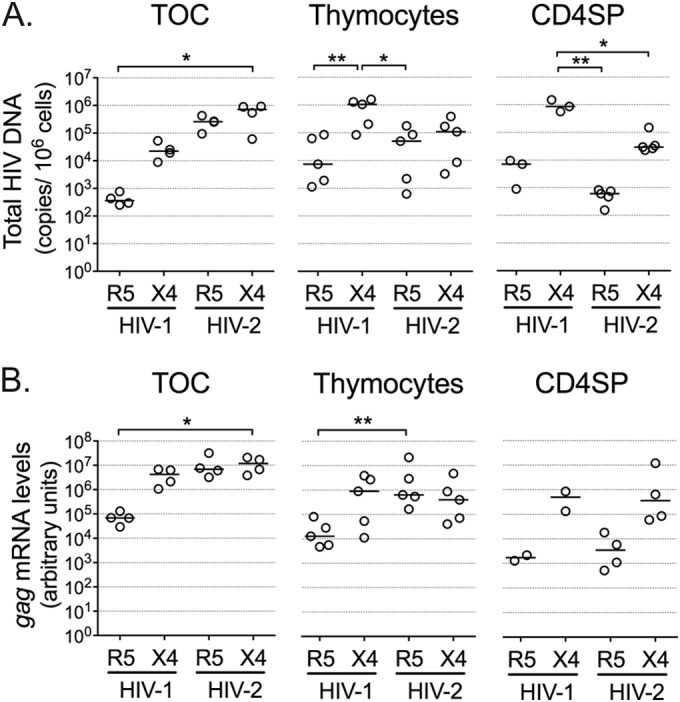
HIV-2 infects human thymic tissue and isolated thymocytes. TOCs were infected with R5- or X4-tropic HIV-2 or HIV-1 primary isolates and cultured for 10 or 11 days on Millipore inserts. Single-cell suspensions of total thymocytes or CD4SP thymocytes, sorted as CD3high CD8neg cells, were also infected with the above-mentioned viruses and cultured for 10 days. Graphs show total HIV DNA (A), as assessed by quantitative real-time PCR, or viral gag mRNA (B), as assessed by real-time RT-PCR (the level of mRNA expression was normalized to the level of GAPDH expression). Each dot represents a single thymus. Lines indicate median values. *, P < 0.05; **, P < 0.01.
Next we sorted purified mature CD4SP thymocytes, which constitute a target of HIV-1 (11), and cultured them for 10 days after infection with the above-mentioned viruses. In contrast to HIV-2 infection of TOCs and thymocyte suspensions, HIV-2 infection of CD4SP thymocytes resulted in coreceptor-dependent levels of total HIV DNA (Fig. 1A). The limited ability of R5-tropic HIV-2 to infect the CD4SP subset was in contrast to our observations in TOCs and total thymocyte suspensions (Fig. 1A), suggesting that R5-tropic HIV-2 targeted other human thymocyte populations.
We next assessed the levels of ongoing viral transcription in HIV-2-infected TOCs and thymocytes by measuring viral gag mRNA. Both R5- and X4-tropic HIV-2 isolates generated gag mRNA levels comparable to those found upon infection with the X4-tropic HIV-1 isolate (Fig. 1B), indicating efficient integration and transcription of HIV-2 in TOCs and thymocyte cultures. Of note, HIV-2 gag mRNA levels showed coreceptor dependency in the case of purified CD4SP thymocytes but not in TOCs or thymocyte suspensions (Fig. 1B), in line with the total HIV DNA levels that were observed in the same samples.
In summary, our results indicated that HIV-2 is able to successfully enter, reverse transcribe, and integrate in human thymocytes in TOCs and in suspension and that viral transcription is ongoing in these systems.
Limited viral production in HIV-2 infection of human thymocytes.
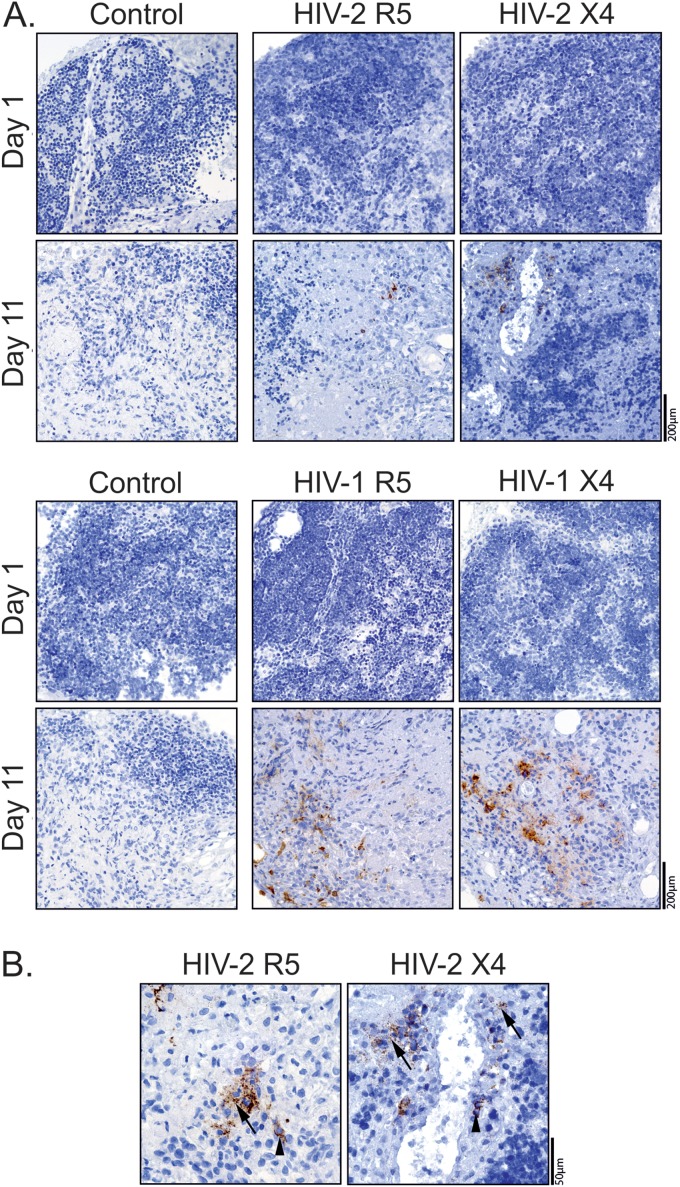
Next we assessed viral production in HIV-2-infected thymic tissue using immunohistochemistry. The Gag protein was detected in R5- and X4-tropic HIV-2-infected TOCs after culture (Fig. 2A), using validated antibodies (see Fig. S1 in the supplemental material) (34). Gag protein expression was both cell associated and extracellular (Fig. 2B), with the latter likely corresponding to virus produced by HIV-infected cells during TOC, as no Gag staining was observed on day 1 (Fig. 2A). Of note, Gag protein levels were often indistinguishable in X4- and R5-tropic HIV-1 TOC infections (Fig. 2A), despite the differences in the levels of the cell-associated viral burden (Fig. 1), indicating a lack of correlation between the amount of virus detected by immunohistochemistry and the levels of thymocyte viral production. Therefore, although our immunohistochemistry data confirmed the ability of HIV-2 to productively infect the human thymus, it was not possible to infer the specific contribution of thymocytes versus that of the thymic stroma to the viral protein detected in the histological analysis.
FIG 2.
Viral production in HIV-2-infected TOCs. TOCs infected with R5- or X4-tropic HIV-2 or HIV-1 primary isolates were analyzed for viral production by immunohistochemistry using anti-Gag antibodies (brown) and hematoxylin counterstain (blue). (A) Viral production at days 1 and 11 after infection with HIV-2 (top) or HIV-1 (bottom), as assessed using an anti-p27 MAb (ARP396/397 from MRC) or an anti-p24 MAb (Kal-1 from Dako), respectively. (B) Extracellular (arrow) and cytoplasmic (arrowhead) Gag expression in R5- or X4-tropic HIV-2-infected TOCs at day 11 (determined with the ARP396/397 anti-p27 antibody).
We thus evaluated viral production at the single-cell level by flow cytometry. Notably, we found very low levels of intracellular Gag protein in cells isolated from HIV-2-infected TOCs or in thymocyte suspensions, irrespective of the virus used (Fig. 3) and the time point analyzed (see Fig. S2 in the supplemental material). In contrast, Gag protein expression was clearly detected in cells infected with HIV-1, particularly with the X4-tropic isolate (Fig. 3), excluding the possibility that the low level of Gag detection in HIV-2 infections was due to sample processing. Of note, intracellular Gag protein was also barely detected upon thymocyte infection with the lab-adapted strain HIV-2ROD (see Fig. S2 in the supplemental material), despite the high levels of total HIV DNA detected (181,499 copies/106 cells in the example presented). Low Gag levels upon HIV-2 infection were particularly evident in CD4SP thymocytes, which featured extremely low levels of intracellular Gag after infection with HIV-2 primary isolates or HIV-2ROD (Fig. 3; see also Fig. S2 in the supplemental material).
FIG 3.
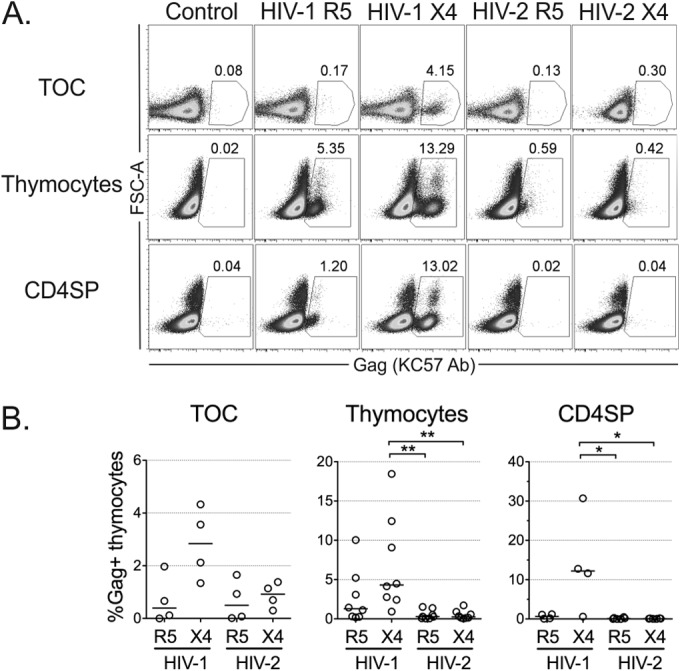
Limited replication of HIV-2 in human thymocytes. Viral production at the single-cell level was determined by flow cytometry using the anti-Gag antibody (Ab) KC57 at 10 days postinfection of TOCs, total thymocytes, or CD4SP thymocytes with R5- or X4-tropic HIV-1 and HIV-2 primary isolates. Representative dot plots of intracellular Gag expression (numbers show the proportion of cells inside the gate) (A) and frequency of productively infected KC57-positive thymocytes (B) under each condition are shown. Flow cytometric analysis was performed after exclusion of dead cells and aggregates. Each dot represents a single thymus. Lines indicate median values. *, P < 0.05; **, P < 0.01. FSC, forward scatter.
The antibody used (KC57) has previously been shown to be specific for both HIV-2 Gag and HIV-1 Gag (35). We further confirmed the ability of the antibody to efficiently detect HIV-2 Gag by flow cytometry in activated PBMCs infected with the HIV-2 strains used in the current study (see Fig. S3 in the supplemental material). Moreover, the level of KC57 staining was comparable to that obtained with other available anti-Gag antibodies in HIV-2-infected PBMCs or thymocytes (see Fig. S2 and S3 in the supplemental material), confirming the low level of viral production in HIV-2-infected thymocytes.
The low levels of intracellular Gag protein translated into reduced viral production in HIV-2 infections, as indicated by the lower levels of virus in the supernatant of HIV-2-infected thymocyte cultures than in the supernatant of their HIV-1-infected counterparts (RT activities, 5.97 ± 3.23 pg/ml for R5-tropic HIV-1, 1,318 ± 583.5 pg/ml for X4-tropic HIV-1, 0.31 ± 0.15 pg/ml for R5-tropic HIV-2, and 8.54 ± 3.40 pg/ml for X4-tropic HIV-2; n = 3).
Overall, we found that HIV-2 infection of human thymocytes occurred without significant viral production per cell, despite evidence of cell-associated viral burden and active ongoing viral transcription.
Impact of HIV-2 infection on thymocyte populations.
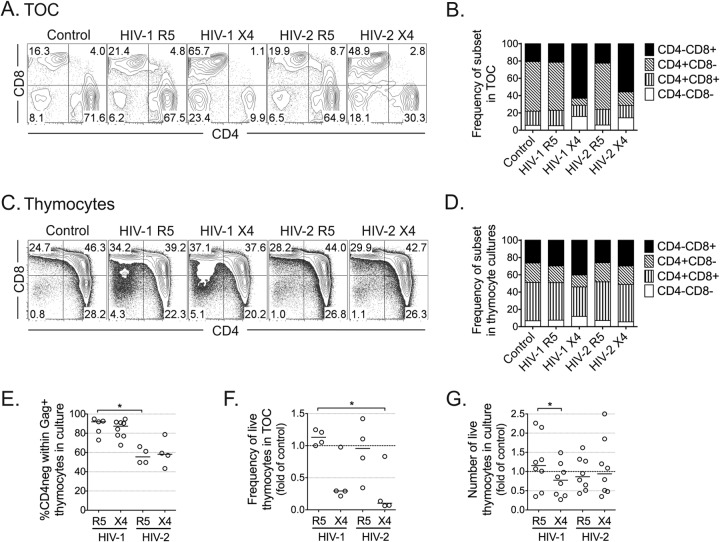
Finally, we assessed the impact of HIV-2 on the distribution of thymocyte populations in infected TOCs and thymocyte suspensions. TOCs infected with X4-tropic viruses, whether they were HIV-2 or HIV-1, featured a dramatic decrease in CD4+ thymocytes compared to the amounts in TOCs infected with R5-tropic viruses (Fig. 4A and B). This was also observed in thymocyte suspensions infected with X4-tropic HIV-1 but not with X4-tropic HIV-2, infection with which did not result in significant alterations of the thymocyte phenotype in culture (Fig. 4C and D). One possible explanation for these results is that downregulation of the CD4 coreceptor is distinctly induced by HIV-2 and HIV-1. Accordingly, our analysis of productively infected cells indicated that HIV-2 was less efficient than HIV-1 at downregulating CD4 (Fig. 4E).
FIG 4.
Distinct cytopathic impact of HIV-2 infection on TOCs and thymocytes. HIV-infected TOCs (A and B) or thymocyte suspensions (C and D) were cultured for 10 days. Representative dot plots of CD4 and CD8 expression in cells in TOCs (A) and in total thymocyte suspensions (C), with the graphs showing the mean frequency of thymocyte subsets in all thymuses analyzed (n = 4 for panel B and n = 8 for panel D). TOC analysis was performed in CD14neg CD16neg CD19neg CD123neg cells. Dead cells and aggregates were excluded from the flow cytometric analysis. Numbers in dot plots represent the proportion of cells inside quadrants. (E) Frequency of CD4neg cells within Gag-positive (KC57 antibody-positive) thymocytes in HIV-infected thymocyte suspensions. (F) Fold change in the frequency of live cells in TOCs relative to that in the uninfected controls, as assessed by flow cytometry. (G) Fold change in the number of live thymocytes in culture relative to that in the uninfected control. Lines indicate median values. *, P < 0.05.
The effects on the subset distribution were mirrored by the cytopathic effect of the respective virus: both X4-tropic HIV-2 and X4-tropic HIV-1 but not R5-tropic viruses were cytopathic in TOCs (Fig. 4F), while X4-tropic HIV-1 had a small effect on cell viability in thymocyte suspensions (Fig. 4G). The low level of cell recovery in X4-tropic HIV-2-infected TOCs, together with the imbalance in cell subsets and the apparent low impact of HIV-2 infection on CD4 downregulation, suggested that X4-tropic HIV-2 infection of TOCs directly or indirectly led to the death of CD4+ thymocytes. This occurred despite the lack of impact of X4-tropic HIV-2 infection on total thymic cell suspensions, supporting the contribution of other cell populations present in the whole tissue.
Given our previous data supporting the immunosuppressive properties of the HIV-2 envelope proteins in peripheral blood lymphocytes mediated, at least in part, by monocytes (36–38), we also evaluated whether the alterations observed in X4-tropic HIV-2-infected TOCs were induced by the direct action of viral envelope proteins. We cultured TOCs in the presence of recombinant HIV-2 and HIV-1 Env proteins binding to either X4 (HIV-2 gp105ROD and HIV-1 gp120IIIB) or R5 (HIV-1 gp120Ba-L). In our system, Env proteins did not induce CD4+ thymocyte depletion or subset imbalances (Fig. 5), indicating that the effects observed may require viral entry and/or postentry steps.
FIG 5.
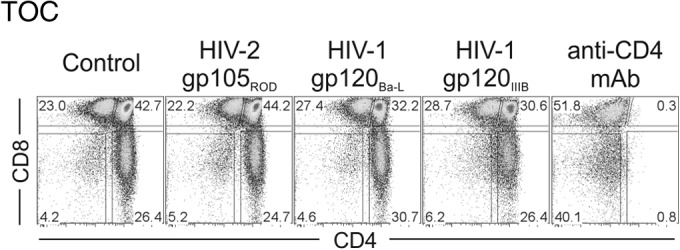
The X4-tropic HIV-2 envelope per se does not impact the thymocyte distribution in TOCs. TOCs were cultured for 7 days with medium only (control) or in the presence of the recombinant envelope protein HIV-2 gp105ROD, HIV-1 gp120Ba-L, or HIV-1 gp120IIIB (all at 1 μg/ml) or anti-CD4 MAb. The dot plots show a representative example (from one of three independent experiments with different thymuses) of the frequency of subsets in TOCs, as determined by CD4 and CD8 expression. Numbers correspond to the proportion of cells inside gates.
Altogether, our data support a cytopathic effect of X4-tropic HIV-2 on the human thymus that was not observed in thymocyte suspensions, suggesting a dependency on the stromal compartment. Importantly, R5-tropic HIV-2 had no significant cytopathic effect on either TOCs or thymocyte suspensions.
DISCUSSION
We addressed here, for the first time, the ability of HIV-2 to infect the human thymus. We showed that HIV-2 is able to infect thymic tissue and thymocyte suspensions in the absence of exogenous stimulation, although with a very low level of viral production per thymocyte, supporting the existence of posttranscriptional control in viral replication.
HIV-2 infection of the human thymus was confirmed by the levels of cell-associated HIV DNA and gag mRNA measured following HIV-2 infection of either TOCs or thymocyte suspensions. The high levels of total HIV DNA observed were consistent with those from previous studies where unintegrated plus integrated proviral DNA were quantified (39, 40). HIV-1 infection of human thymocytes was shown to be coreceptor dependent (9, 12), and our data recapitulated these results. Conversely, we showed that HIV-2 infection of TOCs and thymocyte suspensions was much less dependent on coreceptor tropism than HIV-1 infection in terms of both cell-associated HIV DNA and viral RNA, which could be related to the broader coreceptor usage reported for HIV-2 than for HIV-1 (41).
On the other hand, HIV-2 cytopathicity in human thymic tissue was coreceptor dependent, with X4-tropic virus inducing significant T-cell death in TOCs, which was not observed for R5-tropic HIV-2. This was in line with the findings of a previous study of HIV-2 infection of lymphoid tissue, where lymphocyte depletion also occurred with X4- but not R5-tropic isolates (42). Importantly, we showed that X4-tropic HIV-2 cytopathicity in TOCs was not due to the direct action of HIV-2 Env, indicating a requirement for viral entry and/or postentry steps for CD4+ thymocyte depletion in the human thymus.
The observed cytopathicity caused by X4-tropic HIV-2 in tissue but not in thymocyte suspensions could be related to the infection of components of the thymic stroma that are present in TOCs. Thymic stromal cells, including dendritic cells, macrophages, and even thymic epithelial cells, have been reported to be permissive to HIV-1 infection (43–45). It is not known whether HIV-2 infects the thymic stroma, and due to alterations in tissue morphology that occurred during the culture process, we were not able to directly infer the type of infected cells from our immunohistochemistry data. However, in contrast to the HIV-1 genome, the HIV-2 genome encodes the lentiviral accessory protein Vpx, which targets for degradation the restriction factor sterile alpha motif and histidine/aspartic acid domain-containing protein 1 (SAMHD1), a factor that has been shown to limit the productive infection of HIV-1 in myeloid cells (46). Moreover, additional cell targets may be considered, given the broader coreceptor usage reported for HIV-2 (41). We are currently addressing the possibility that infection of thymic stroma components might have distinct consequences in HIV-1 and HIV-2 infections.
We had previously reported that thymic function, estimated via sj/βTREC measurement, was better preserved in HIV-2-infected than HIV-1-infected patients (25). It is likely that most of these individuals were infected with R5-tropic HIV-2 (25). Importantly, we showed here that R5-tropic HIV-2 infection did not significantly impact TOCs or thymocyte suspensions in terms of cytopathicity or the subset distribution or induce CD4 downregulation in the latter. Nevertheless, the elevated levels of total HIV DNA and viral mRNA support the potential establishment of HIV-2 reservoirs in human thymocytes. It would thus be important to investigate whether, as reported for HIV-1 (32), CD4+ recent thymic emigrant T cells from HIV-2-infected patients may constitute viral reservoirs.
Our data suggest that, in our system, distinct regulation of the replicative cycle of HIV-2 and HIV-1 occurs at the posttranscriptional/translational level. The discrepant levels of Gag protein in the two infections markedly contrasted with the similarly high levels of total HIV DNA and viral mRNA documented. HIV-2 and HIV-1 have been reported to differ significantly in their mechanisms of translation initiation (47). For instance, HIV-2 was described to have a lower translational efficiency than HIV-1 due to differences in the 5′ untranslated region (5′ UTR) of viral genomic RNA (48, 49). In agreement with our observations, in the presence of equivalent T-cell-associated viral gag mRNA levels, the levels of Gag protein production were reported to be lower in T-cell lines and macrophages infected with HIV-2 than those infected with HIV-1 (49). Importantly, and in relation to our data, this was not due to the lower stability of the Gag protein or to the higher rate of viral particle release (49). Interestingly, the authors raised the possibility that host factors may differentially regulate the initiation of Gag translation in HIV-2 and HIV-1 (49). HIV-2 infection of the human thymus provides, to our knowledge, the first model of HIV-2 posttranscriptional regulation in ex vivo T cells using primary isolates, providing a unique opportunity to study the molecular factors and mechanisms involved in the regulation of HIV translation.
Human thymocytes represent an ideal system for the study of HIV latency due to their ability to become infected in the absence of external activation (50). Models using peripheral CD4+ T cells require cellular activation followed by quiescence induction (51), thus inducing molecular modifications that may impact the processes under study. The system described here, utilizing HIV-2 infection of the human thymus in the absence of exogenous stimulation, thus provides an important cellular model for the study of latency and reservoir generation in HIV pathogenesis.
Our results regarding HIV-2 infection also highlight the potential importance of posttranscriptional control of viral production, possibly through the regulation of translation, in viral pathogenesis. Further studies using this model will enable the discovery of potential molecular targets to be used as the basis for new immunotherapies aimed at achieving a functional cure for HIV infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Fundação para a Ciência e a Tecnologia (FCT) and by the Programa Operacional Ciência e Inovação 2010 (POCI2010), grant PTDC/SAU-MII/66248/2006 to A.E.S. H.N.-C., R.B.F., R.T., and R.S.S. received scholarships from FCT cofinanced by POCI2010.
We thank Miguel Abecasis and Rui Anjos for human thymus sample collection; Rémi Cheynier, Andreia Amaral, and Íris Caramalho for discussion; Francisca Matos and nurses and staff at the Hospital de Santa Cruz for technical assistance; Nuno Taveira, Rémi Cheynier, and Nicolas Manel for reagents; the patients; and the families.
We declare no conflicting financial interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03047-14.
REFERENCES
- 1.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 2.Nunes-Cabaço H, Sousa AE. 2013. Repairing thymic function. Curr Opin Organ Transplant 18:363–368. doi: 10.1097/MOT.0b013e3283615df9. [DOI] [PubMed] [Google Scholar]
- 3.Dion ML, Poulin JF, Bordi R, Sylvestre M, Corsini R, Kettaf N, Dalloul A, Boulassel MR, Debre P, Routy JP, Grossman Z, Sekaly RP, Cheynier R. 2004. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity 21:757–768. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Li T, Wu N, Dai Y, Qiu Z, Han Y, Xie J, Zhu T, Li Y. 2011. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis 53:944–951. doi: 10.1093/cid/cir552. [DOI] [PubMed] [Google Scholar]
- 5.Haynes BF, Hale LP, Weinhold KJ, Patel DD, Liao H-X, Bressler PB, Jones DM, Demarest JF, Gebhard-Mitchell K, Haase AT, Bartlett JA. 1999. Analysis of the adult thymus in reconstitution of T lymphocytes in HIV-1 infection. J Clin Invest 103:453–460. doi: 10.1172/JCI5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi VV, Oleske JM, Saad S, Gadol C, Connor E, Bobila R, Minnefor AB. 1986. Thymus biopsy in children with acquired immunodeficiency syndrome. Arch Pathol Lab Med 110:837–842. [PubMed] [Google Scholar]
- 7.Bonyhadi ML, Su L, Auten J, McCune JM, Kaneshima H. 1995. Development of a human thymic organ culture model for the study of HIV pathogenesis. AIDS Res Hum Retroviruses 11:1073–1080. doi: 10.1089/aid.1995.11.1073. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary SK, Choudhary NR, Kimbrell KC, Colasanti J, Ziogas A, Kwa D, Schuitemaker H, Camerini D. 2005. R5 human immunodeficiency virus type 1 infection of fetal thymic organ culture induces cytokine and CCR5 expression. J Virol 79:458–471. doi: 10.1128/JVI.79.1.458-471.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurney KB, Uittenbogaart CH. 2006. Human immunodeficiency virus persistence and production in T-cell development. Clin Vaccine Immunol 13:1237–1245. doi: 10.1128/CVI.00184-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonyhadi ML, Rabin L, Salimi S, Brown DA, Kosek J, McCune JM, Kaneshima H. 1993. HIV induces thymus depletion in vivo. Nature 363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 11.Stanley SK, McCune JM, Kaneshima H, Justement JS, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J. 1993. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med 178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedroza-Martins L, Gurney KB, Torbett BE, Uittenbogaart CH. 1998. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J Virol 72:9441–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkowitz RD, Alexander S, Bare C, Linquist-Stepps V, Bogan M, Moreno ME, Gibson L, Wieder ED, Kosek J, Stoddart CA, McCune JM. 1998. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J Virol 72:10108–10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutrieux J, Fabre-Mersseman V, Charmeteau-De Muylder B, Rancez M, Ponte R, Rozlan S, Figueiredo-Morgado S, Bernard A, Beq S, Couëdel-Courteille A, Cheynier R. 2014. Modified interferon-α subtypes production and chemokine networks in the thymus during acute simian immunodeficiency virus infection, impact on thymopoiesis. AIDS 28:1101–1113. doi: 10.1097/QAD.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 15.Marlink R, Kanki P, Thior I, Travers K, Eisen G, Siby T, Traore I, Hsieh CC, Dia MC, Gueye EH. 1994. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 265:1587–1590. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 16.Poulsen AG, Aaby P, Larsen O, Jensen H, Nauclér A, Lisse IM, Christiansen CB, Dias F, Melbye M. 1997. 9-year HIV-2-associated mortality in an urban community in Bissau, West Africa. Lancet 349:911–914. doi: 10.1016/S0140-6736(96)04402-9. [DOI] [PubMed] [Google Scholar]
- 17.Clavel F, Mansinho K, Chamaret S, Guetard D, Favier V, Nina J, Santos-Ferreira MO, Champalimaud JL, Montagnier L. 1987. Human immunodeficiency virus type 2 infection associated with AIDS in West Africa. N Engl J Med 316:1180–1185. doi: 10.1056/NEJM198705073161903. [DOI] [PubMed] [Google Scholar]
- 18.Popper SJ, Sarr AD, Gueye-NDiaye A, Mboup S, Essex ME, Kanki PJ. 2000. Low plasma human immunodeficiency virus type 2 viral load is independent of proviral load: low virus production in vivo. J Virol 74:1554–1557. doi: 10.1128/JVI.74.3.1554-1557.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino R. 2002. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol 169:3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 20.Kanki PJ, Travers KU, Mboup S, Hsieh CC, Marlink RG, Gueye-NDiaye A, Siby T, Thior I, Hernandez-Avila M, Sankalé JL. 1994. Slower heterosexual spread of HIV-2 than HIV-1. Lancet 343:943–946. doi: 10.1016/S0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 21.O'Donovan D, Ariyoshi K, Milligan P, Ota M, Yamuah L, Sarge-Njie R, Whittle H. 2000. Maternal plasma viral RNA levels determine marked differences in mother-to-child transmission rates of HIV-1 and HIV-2 in the Gambia. MRC/Gambia Government/University College London Medical School Working Group on Mother-Child Transmission of HIV. AIDS 14:441–448. [DOI] [PubMed] [Google Scholar]
- 22.Poulsen AG, Aaby P, Gottschau A, Kvinesdal BB, Dias F, Mølbak K, Lauritzen E. 1993. HIV-2 infection in Bissau, West Africa, 1987-1989: incidence, prevalences, and routes of transmission. J Acquir Immune Defic Syndr 6:941–948. [PubMed] [Google Scholar]
- 23.Soares R, Foxall R, Albuquerque A, Cortesao C, Garcia M, Victorino RMM, Sousa AE. 2006. Increased frequency of circulating CCR5+ CD4+ T cells in human immunodeficiency virus type 2 infection. J Virol 80:12425–12429. doi: 10.1128/JVI.01557-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares RS, Tendeiro R, Foxall RB, Baptista AP, Cavaleiro R, Gomes P, Camacho R, Valadas E, Doroana M, Lucas M, Antunes F, Victorino RMM, Sousa AE. 2011. Cell-associated viral burden provides evidence of ongoing viral replication in aviremic HIV-2-infected patients. J Virol 85:2429–2438. doi: 10.1128/JVI.01921-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautier D, Beq S, Cortesao CS, Sousa AE, Cheynier R. 2007. Efficient thymopoiesis contributes to the maintenance of peripheral CD4 T cells during chronic human immunodeficiency virus type 2 infection. J Virol 81:12685–12688. doi: 10.1128/JVI.01131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borrego P, Marcelino J, Rocha C, Doroana M, Antunes F, Maltez F, Gomes P, Novo C, Barroso H, Taveira N. 2008. The role of the humoral immune response in the molecular evolution of the envelope C2, V3 and C3 regions in chronically HIV-2 infected patients. Retrovirology 5:78. doi: 10.1186/1742-4690-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcelino JM, Borrego P, Nilsson C, Família C, Barroso H, Maltez F, Doroana M, Antunes F, Quintas A, Taveira N. 2012. Resistance to antibody neutralization in HIV-2 infection occurs in late stage disease and is associated with X4 tropism. AIDS 26:2275–2284. doi: 10.1097/QAD.0b013e328359a89d. [DOI] [PubMed] [Google Scholar]
- 28.Soares RS, Matoso P, Calado M, Sousa AE. 2011. Strategies to quantify unspliced and multiply spliced mRNA expression in HIV-2 infection. J Virol Methods 175:38–45. doi: 10.1016/j.jviromet.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Pizzato M, Erlwein O, Bonsall D, Kaye S, Muir D, McClure MO. 2009. A one-step SYBR green I-based product-enhanced reverse transcriptase assay for the quantitation of retroviruses in cell culture supernatants. J Virol Methods 156:1–7. doi: 10.1016/j.jviromet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Vermeire J, Naessens E, Vanderstraeten H, Landi A, Iannucci V, Van Nuffel A, Taghon T, Pizzato M, Verhasselt B. 2012. Quantification of reverse transcriptase activity by real-time PCR as a fast and accurate method for titration of HIV, lenti- and retroviral vectors. PLoS One 7:e50859. doi: 10.1371/journal.pone.0050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunes-Cabaço H, Caramalho Í Sepúlveda N, Sousa AE. 2011. Differentiation of human thymic regulatory T cells at the double positive stage. Eur J Immunol 41:3604–3614. doi: 10.1002/eji.201141614. [DOI] [PubMed] [Google Scholar]
- 32.Fabre-Mersseman V, Dutrieux J, Louise A, Rozlan S, Lamine A, Parker R, Rancez M, Nunes-Cabaço H, Sousa AE, Lambotte O, Cheynier R. 2011. CD4+ recent thymic emigrants are infected by HIV in vivo, implication for pathogenesis. AIDS 25:1153–1162. doi: 10.1097/QAD.0b013e3283471e89. [DOI] [PubMed] [Google Scholar]
- 33.Hizi A, Tal R, Shaharabany M, Loya S. 1991. Catalytic properties of the reverse transcriptases of human immunodeficiency viruses type 1 and type 2. J Biol Chem 266:6230–6239. [PubMed] [Google Scholar]
- 34.Fernandes SM, Pires AR, Ferreira C, Tendeiro R, Correia L, Paulo SE, Victorino RMM, Sousa AE. 2014. Gut disruption in HIV-2 infection despite reduced viremia. AIDS 28:290–292. doi: 10.1097/QAD.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 35.Duvall MG, Lore K, Blaak H, Ambrozak DA, Adams WC, Santos K, Geldmacher C, Mascola JR, McMichael AJ, Jaye A, Whittle HC, Rowland-Jones SL, Koup RA. 2007. Dendritic cells are less susceptible to human immunodeficiency virus type 2 (HIV-2) infection than to HIV-1 infection. J Virol 81:13486–13498. doi: 10.1128/JVI.00976-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavaleiro R, Sousa AE, Loureiro A, Victorino RMM. 2000. Marked immunosuppressive effects of the HIV-2 envelope protein in spite of the lower HIV-2 pathogenicity. AIDS 14:2679–2686. doi: 10.1097/00002030-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 37.Cavaleiro R, Brunn GJ, Albuquerque AS, Victorino RMM, Platt JL, Sousa AE. 2007. Monocyte-mediated T cell suppression by HIV-2 envelope proteins. Eur J Immunol 37:3435–3444. doi: 10.1002/eji.200737511. [DOI] [PubMed] [Google Scholar]
- 38.Cavaleiro R, Baptista AP, Foxall RB, Victorino RMM, Sousa AE. 2009. Dendritic cell differentiation and maturation in the presence of HIV type 2 envelope. AIDS Res Hum Retroviruses 25:425–431. doi: 10.1089/aid.2008.0247. [DOI] [PubMed] [Google Scholar]
- 39.MacNeil A, Sarr AD, Sankalé JL, Meloni ST, Mboup S, Kanki P. 2007. Direct evidence of lower viral replication rates in vivo in human immunodeficiency virus type 2 (HIV-2) infection than in HIV-1 infection. J Virol 81:5325–5330. doi: 10.1128/JVI.02625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suspene R, Meyerhans A. 2012. Quantification of unintegrated HIV-1 DNA at the single cell level in vivo. PLoS One 7:e36246. doi: 10.1371/journal.pone.0036246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mörner A, Björndal Å, Albert J, KewalRamani VN, Littman DR, Inoue R, Thorstensson R, Fenyö EM, Björling E. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol 73:2343–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schramm B, Penn ML, Palacios EH, Grant RM, Kirchhoff F, Goldsmith MA. 2000. Cytopathicity of human immunodeficiency virus type 2 (HIV-2) in human lymphoid tissue is coreceptor dependent and comparable to that of HIV-1. J Virol 74:9594–9600. doi: 10.1128/JVI.74.20.9594-9600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitt N, Nugeyre M-T, Scott-Algara D, Cumont M-C, Barré-Sinoussi F, Pancino G, Israël N. 2006. Differential susceptibility of human thymic dendritic cell subsets to X4 and R5 HIV-1 infection. AIDS 20:533–542. doi: 10.1097/01.aids.0000210607.63138.bc. [DOI] [PubMed] [Google Scholar]
- 44.Rozmyslowicz T, Murphy SL, Conover DO, Gaulton GN. 2010. HIV-1 infection inhibits cytokine production in human thymic macrophages. Exp Hematol 38:1157–1166. doi: 10.1016/j.exphem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun J, Valentin H, Nugeyre M-T, Ohayon H, Gounon P, Barré-Sinoussi F. 1996. Productive and persistent infection of human thymic epithelial cells in vitro with HIV-1. Virology 225:413–418. doi: 10.1006/viro.1996.0617. [DOI] [PubMed] [Google Scholar]
- 46.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Ségéral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricci EP, Soto-Rifo R, Herbreteau CH, Decimo D, Ohlmann T. 2008. Lentiviral RNAs can use different mechanisms for translation initiation. Biochem Soc Trans 36:690. doi: 10.1042/BST0360690. [DOI] [PubMed] [Google Scholar]
- 48.Strong CL, Lanchy J-M, Dieng Sarr A, Kanki PJ, Lodmell JS. 2009. A 5′UTR-spliced mRNA isoform is specialized for enhanced HIV-2 gag translation. J Mol Biol 391:426–437. doi: 10.1016/j.jmb.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soto-Rifo R, Limousin T, Rubilar PS, Ricci EP, Decimo D, Moncorge O, Trabaud MA, Andre P, Cimarelli A, Ohlmann T. 2012. Different effects of the TAR structure on HIV-1 and HIV-2 genomic RNA translation. Nucleic Acids Res 40:2653–2667. doi: 10.1093/nar/gkr1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks DG, Kitchen SG, Kitchen C, Scripture-Adams DD, Zack JA. 2001. Generation of HIV latency during thymopoiesis. Nat Med 7:459–464. doi: 10.1038/86531. [DOI] [PubMed] [Google Scholar]
- 51.Marini A, Harper JM, Romerio F. 2008. An in vitro system to model the establishment and reactivation of HIV-1 latency. J Immunol 181:7713–7720. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.