Abstract
In 49 patients with known Ebola virus disease outcomes during the ongoing outbreak in Sierra Leone, 13 were coinfected with the immunomodulatory pegivirus GB virus C (GBV-C). Fifty-three percent of these GBV-C+ patients survived; in contrast, only 22% of GBV-C− patients survived. Both survival and GBV-C status were associated with age, with older patients having lower survival rates and intermediate-age patients (21 to 45 years) having the highest rate of GBV-C infection. Understanding the separate and combined effects of GBV-C and age on Ebola virus survival may lead to new treatment and prevention strategies, perhaps through age-related pathways of immune activation.
TEXT
As of this writing, there have been 14,413 confirmed and probable infections and 5,177 deaths in the ongoing and worsening Ebola virus (EBOV) disease outbreak in West Africa (1). Recently, EBOV sequences from Sierra Leone were obtained by unbiased deep sequencing. These patients represented approximately 70% of patients with Ebola virus disease in Sierra Leone from late May to mid-June of 2014 (2).
In the three countries (Sierra Leone, Liberia, and Guinea) where the Ebola virus outbreak is concentrated, GB virus C (GBV-C, also known as human pegivirus) infects between 10 and 28% of individuals (3–6). Although GBV-C causes a prolonged high-titer viremia, GBV-C infection is largely considered to be benign (7, 8). Intriguingly, several epidemiological studies have associated GBV-C infection with lower mortality in HIV-positive people (9–12; see reference 13 for a meta-analysis). Although potential mechanisms explaining this association are still under investigation, a growing body of evidence suggests that GBV-C prevents aberrant immune activation that is a hallmark of HIV pathogenesis and disease progression (i.e., AIDS) (see reference 14 for a review).
We reasoned that the relatively high prevalence of GBV-C in West Africa would result in a significant number of coinfections with EBOV. To examine GBV-C coinfections with EBOV, deep-sequencing data initially published in reference 2 were downloaded from the NCBI Sequence Read Archive (SRA), sequencing run (SRR) files were converted into fastq files using the SRA toolkit, and SRR identifiers (IDs) were correlated with patient sample IDs using information from supplemental Table S2 in reference 2. Further analysis was confined to the 49 patients for whom EBOV infection outcome, age, and gender information were available (see Fig. S2 in the supplemental material in reference 2; also unpublished data). Samples for which two independent library preparations were performed or which were collected from the same individual at multiple time points were merged into single fastq files. fastq files were then imported into CLC Genomics Workbench 7 and short (<90-bp) and low-quality (Phred quality score <Q30) reads were removed. Samples labeled as potential duplicates in supplemental Table S2 of reference 2 were excluded from the analysis. Reads from all patients were aligned with moderate stringency (length fraction, 0.8; similarity fraction, 0.8; mismatch cost, 2; insertion and deletion cost, 3) against a full-genome GBV-C genotype 1 reference, the predominant genotype in West Africa (15) (GenBank accession number HGU36380). Up to 79,619 reads mapped to GBV-C per individual (Table 1).
TABLE 1.
EBOV patients coinfected with GBV-C
| Patient ID | SRR ID(s)a | No. of raw GBV-C reads | No. of unique GBV-C reads | No. of EBOV reads | Patient outcome |
|---|---|---|---|---|---|
| G3670.1 | 1553450 | 191 | 159 | 282,289 | Discharged |
| G3765.2 | 1553501, 1553502 | 2,161 | 1,749 | 2,490 | Discharged |
| G3789.1 | 1553525, 1553526 | 343 | 325 | 5,508 | Discharged |
| G3796 | 1553529, 1553530 | 79,619 | 40,538 | 2,090,829 | Discharged |
| G3819 | 1553559, 1553559 | 601 | 554 | 33,172 | Discharged |
| G3821 | 1553563, 1553564 | 4,529 | 4,047 | 99,766 | Discharged |
| G3850 | 1553595, 1553596 | 797 | 673 | 7,363 | Discharged |
| G3764 | 1553499, 1553500 | 64 | 55 | 5,402,195 | Died |
| G3795 | 1553527, 1553528 | 2,671 | 774 | 1,005,042 | Died |
| G3808 | 1553543, 1553544 | 6,454 | 5,515 | 819,166 | Died |
| G3825 | 1553569, 1553570, 1553571, 1553572 | 5,093 | 4,069 | 2,177,336 | Died |
| G3826 | 1553573, 1553574 | 29,752 | 27,149 | 867,174 | Died |
| G3845 | 1553589, 1553590 | 2,794 | 2,137 | 3,377,501 | Died |
SRR, sequencing run.
A low level of carryover contamination is common in unbiased deep-sequencing experiments. We were therefore concerned that samples with low numbers of GBV-C reads might represent carryover from other samples with high levels of GBV-C. To more rigorously define samples as GBV-C positive or negative, we determined the GBV-C consensus sequence for each sample. We then remapped the reads from each sample against consensus sequences from all samples with high stringency (length fraction, 0.98; similarity fraction, 0.98; mismatch cost, 2; insertion and deletion cost, 3) and discarded reads that mapped to multiple consensus sequences. Twelve individuals had unambiguous evidence of GBV-C viremia supported by at least 100 uniquely mapped reads covering between 63 and 100% of the genome (Tables 1 and 2). A 13th individual (G3764) was putatively categorized as GBV-C+ on the basis of 55 uniquely mapped reads, resulting in 38% coverage across the genome.
TABLE 2.
EBOV patients not coinfected with GBV-C
| Patient ID | SRR IDsa | No. of raw GBV-C reads | No. of unique GBV-C reads | No. of EBOV reads | Patient outcome |
|---|---|---|---|---|---|
| G3769 | 1553503, 1553504, 1553505, 1553506, 1553507, 1553508, 1553509, 1553510 | 7 | 0 | 4,218,762 | Discharged |
| G3799 | 1553533, 1553534 | 29 | 5 | 1,160,025 | Discharged |
| G3805 | 1553537, 1553538, 1553539, 1553540 | 0 | 0 | 6,054 | Discharged |
| G3809 | 1553545, 1553546 | 6 | 0 | 11,344 | Discharged |
| G3810 | 1553547, 1553548, 1553549, 1553550 | 0 | 0 | 503,396 | Discharged |
| G3817 | 1553555, 1553556 | 1 | 0 | 920,637 | Discharged |
| G3857 | 1553603, 1553604 | 4 | 2 | 9,115 | Discharged |
| NM042 | 1553605, 1553606, 1553607, 1553608, 1553609, 1553610 | 0 | 0 | 574,137 | Discharged |
| EM112 | 1553429, 1553430 | 2 | 0 | 4,495,065 | Died |
| EM121 | 1553439, 1553440 | 0 | 0 | 3,393,644 | Died |
| EM124 | 1553441, 1553442, 1553443, 1553444, 1553445, 1553446, 1553447, 1553448 | 4 | 0 | 2,370,521 | Died |
| G3676 | 1553451, 1553452, 1553453, 1553454 | 0 | 0 | 1,753,365 | Died |
| G3677 | 1553455, 1553456, 1553457, 1553458 | 2 | 0 | 2,195,013 | Died |
| G3707 | 1553471, 1553472 | 4 | 0 | 1,848,917 | Died |
| G3713 | 1553473, 1553474, 1553475, 1553476, 1553477, 1553478 | 4 | 0 | 11,650,343 | Died |
| G3724 | 1553479, 1553480 | 0 | 0 | 5,237,956 | Died |
| G3735 | 1553485, 1553486, 1553487, 1553488 | 0 | 0 | 11,945,730 | Died |
| G3752 | 1553495, 1553496 | 2 | 1 | 1,181,649 | Died |
| G3770 | 1553511, 1553512, 1553513, 1553514 | 15 | 4 | 9,932,933 | Died |
| G3798 | 1553531, 1553532 | 2 | 0 | 1,124,997 | Died |
| G3800 | 1553535, 1553536 | 9 | 0 | 890,300 | Died |
| G3807 | 1553541, 1553542 | 0 | 0 | 822,652 | Died |
| G3814 | 1553551, 1553552 | 0 | 0 | 13,286 | Died |
| G3816 | 1553553, 1553554 | 0 | 0 | 10,101 | Died |
| G3818 | 1553557, 1553558 | 0 | 0 | 2,398,770 | Died |
| G3820 | 1553561, 1553562 | 2 | 0 | 1,890,862 | Died |
| G3822 | 1553565, 1553566 | 0 | 0 | 3,417,852 | Died |
| G3823 | 1553567, 1553568 | 0 | 0 | 4,672,245 | Died |
| G3827 | 1553575, 1553576 | 0 | 0 | 657,986 | Died |
| G3829 | 1553577, 1553578 | 0 | 0 | 1,619,423 | Died |
| G3834 | 1553581, 1553582 | 0 | 0 | 646,694 | Died |
| G3838 | 1553583, 1553584 | 4 | 1 | 2,234,396 | Died |
| G3840 | 1553585, 1553586 | 0 | 0 | 2,978,090 | Died |
| G3846 | 1553591, 1553592 | 6 | 0 | 2,829,761 | Died |
| G3848 | 1553593, 1553594 | 0 | 0 | 3,013,745 | Died |
| G3851 | 1553597, 1553598 | 0 | 0 | 393,873 | Died |
SRR, sequencing run.
The 2014 EBOV sequences from Sierra Leone were on average 99.98% [99.98% to 100%] identical in pairwise comparisons across the genome (data not shown), which is consistent with the recency of this outbreak. In contrast, GBV-C sequences shared on average 91% (86.96 to 98.46%) nucleotide identity (Table 3), suggesting preexisting GBV-C infections rather than cotransmission with EBOV.
TABLE 3.
Pairwise comparison of percentages of nucleotide identity for GBV-C consensus sequences
| Patient ID | % nucleotide identity to GBV-C consensus sequencea: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G3670.1 | G3764 | G3765.2 | G3789.1 | G3795 | G3796 | G3808 | G3819 | G3821 | G3825 | G3826 | G3845 | G3850 | |
| G3670.1 | 100 | ||||||||||||
| G3764 | 90.86 | 100 | |||||||||||
| G3765.2 | 92.46 | 91.36 | 100 | ||||||||||
| G3789.1 | 86.96 | 87.89 | 88.28 | 100 | |||||||||
| G3795 | 91.30 | 90.92 | 92.79 | 88.11 | 100 | ||||||||
| G3796 | 91.36 | 90.97 | 92.79 | 88.11 | 98.46 | 100 | |||||||
| G3808 | 94.22 | 91.52 | 92.68 | 88.61 | 92.18 | 92.13 | 100 | ||||||
| G3819 | 91.36 | 91.41 | 91.30 | 87.34 | 91.19 | 91.03 | 91.03 | 100 | |||||
| G3821 | 92.46 | 92.24 | 92.63 | 88.61 | 92.24 | 92.35 | 92.85 | 91.74 | 100 | ||||
| G3825 | 91.41 | 90.53 | 93.40 | 87.78 | 91.74 | 91.85 | 92.13 | 91.47 | 92.07 | 100 | |||
| G3826 | 92.02 | 91.80 | 91.80 | 88.72 | 91.41 | 91.63 | 91.69 | 91.63 | 92.57 | 92.07 | 100 | ||
| G3845 | 90.97 | 91.14 | 92.63 | 87.89 | 95.60 | 95.82 | 91.85 | 90.75 | 92.18 | 91.52 | 91.30 | 100 | |
| G3850 | 92.18 | 92.29 | 92.29 | 87.40 | 91.69 | 91.96 | 92.68 | 91.36 | 92.24 | 91.69 | 92.13 | 91.85 | 100 |
Pairwise comparisons of GBV-C consensus sequences were generated by aligning sequences with ClustalW. After manual adjustment, the percent nucleotide identity was calculated from the resulting 1,800-bp alignment.
Mortality in this cohort of 49 patients with sequence-confirmed EBOV infection was 69% overall, which is comparable to the 65% mortality reported for definitive infections in Sierra Leone before 18 August 2014 (1). Only 6/13 (46%) GBV-C+ individuals died, whereas 28/36 (78%) GBV-C− individuals died. Univariate analyses (Table 4) showed that older age was associated with higher mortality (OR, 1.06; P = 0.0124) and that GBV-C+ status was associated with lower mortality (OR = 0.25; P = 0.0402). However, when these factors were considered together in a multivariate analysis (Table 4), GBV-C status became nonsignificant (OR = 0.25; P = 0.0835), likely reflecting a confounding effect of age. Our finding of a relationship between older age and higher mortality is consistent with a recently published study (16). However, GBV-C infection follows a different pattern, being most common in people aged 21 to 45 years (Fig. 1). Thus, age is associated with both EBOV survival and GBV-C status, but the pattern of association is different in each case.
TABLE 4.
Factors associated with mortality in EBOV+ patientsa
| Variable | Univariate model |
Multivariate model |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age (yr) | 1.06 (1.01–1.11) | 0.0124 | 1.09 (1.02–1.16) | 0.0076 |
| Sex (male) | 0.56 (0.16–2.00) | 0.3735 | 0.30 (0.05–1.91) | 0.2028 |
| GBV-C+ | 0.25 (0.06–0.94) | 0.0402 | 0.25 (0.05–1.20) | 0.0835 |
Analyses are based on 49 patients for whom complete data were available. First-order interaction terms were not statistically significant and are therefore not included. P values are 2-tailed and based on chi-square tests from univariate and multivariate logistic regression, performed using the computer program R (46). Statistically significant P values are shown in bold. CI, confidence interval.
FIG 1.
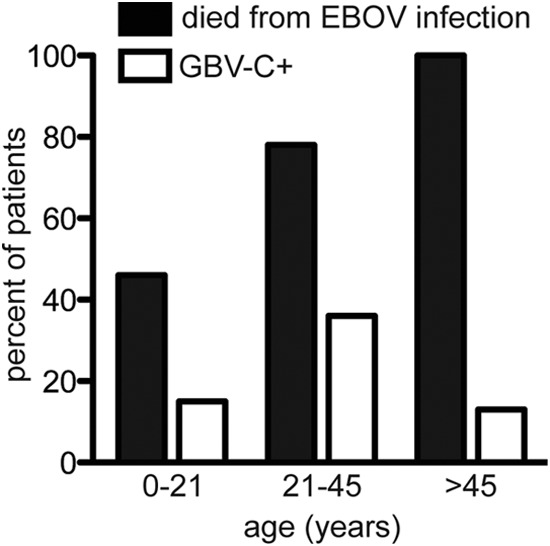
Ebola virus mortality and GBV-C coinfection status by age.
There were both epidemiological and technical aspects of this study that could not be controlled. For example, potentially confounding variables, such as comorbidities, rapidity of diagnosis, and relationships among patients, were not available. Furthermore, the samples were collected opportunistically, possibly introducing selection bias. It is also possible that we were not able to detect low-titer GBV-C viremia in some patients. Because sequencing reads were generated in an “unbiased” fashion, patients with very high EBOV titers may have “swamped” the sample, effectively reducing the number of GBV-C reads. We believe that this is unlikely because (i) we detected GBV-C in patients with EBOV plasma loads of >108 (see supplemental Fig. S2 in reference 2) and (ii) in previous studies, we have detected multiple viruses from a single sample using a similar methodology, even in samples where at least one virus was highly concentrated (17–20). Recovery of unique reads targeting the majority of the viral genome provide unequivocal evidence for GBV-C infection in all but one of the samples; however, in the one sample where less than half of the genome is covered, verification of GBV-C status using an independent assay (e.g., reverse transcription-quantitative PCR [RT-qPCR]) would be ideal but is not currently possible.
Nonetheless, these results demonstrate that approximately 27% of EBOV patients in this cohort are coinfected with GBV-C, an immunomodulatory virus that attenuates the pathogenesis of HIV. The association between GBV-C status and Ebola virus disease survival is intriguing, although confounded by age. We speculate that GBV-C may interact with the host immune system in ways that modulate the overexuberant immune response characteristic of EBOV-related pathogenesis (21–27). However, our analyses are also consistent with a primary effect of age on both Ebola virus disease-related survival and GBV-C infection. Resolving the direction of causality would require additional data on the time course of infection and coinfection, as well as direct measures of immunity.
EBOV and GBV-C appear to infect different types of immune cells. EBOV infects primarily myeloid-lineage cells (28–31), while GBV-C appears to target lymphoid-lineage cells (32, 33). The interaction of immune cell populations—both locally in lymphoid tissues and systemically via secreted factors—provides a biologically plausible mechanism for an interaction between GBV-C and EBOV. If GBV-C infection attenuates EBOV pathogenesis, it is possible that this occurs through modulation of the host immune response. In the context of HIV infection, GBV-C has been associated with a reduced production of proinflammatory cytokines and a reduction in T-cell activation in vivo and in vitro (34–44). Conversely, robust production of proinflammatory cytokines and lymphocyte activation followed by massive T-cell death are thought to play a major role in EBOV pathogenesis and have been associated with poor clinical outcome in retrospective studies (21–27).
Although our data are preliminary and potentially influenced by confounding variables, the results that we present here indicate that further study of GBV-C/EBOV coinfection may be warranted. Such investigations should endeavor to follow patients of different ages longitudinally and to collect immunological data, with the goal of establishing the temporal sequence of events that leads to EBOV-related survival and mortality, with and without coinfecting GBV-C.
Nucleotide sequence accession number.
We have made consensus GBV-C sequences available in GenBank (accession numbers KM670096 to KM670110).
ACKNOWLEDGMENTS
Five coauthors of the study that provided the original data used in the manuscript lost their lives to Ebola (45). This paper would not have been possible without their courageous efforts. We thank all the authors of the original paper (2) for making their source data publicly available for reanalysis. We thank Erin Bailey, Thomas Friedrich, and Esper Kallas for helpful discussion.
This work was funded by the NIH (grants R01 AI077376-01 and R01 AI077376). This publication was made possible in part by a grant (P51 RR000167) from the Office of Research Infrastructure Programs (ORIP), a component of the National Institutes of Health (NIH), to the Wisconsin National Primate Research Center (WNPRC), University of Wisconsin—Madison. This research was conducted in part at a facility constructed with support from the Research Facilities Improvement Program (grants RR15459-01 and RR020141-01). A.L.B. performed this work with support from the University of Wisconsin's Medical Scientist Training Program (MSTP) (grant T32 GM008692) and a National Research Service Award (NRSA) through the Microbes in Health and Disease (MHD) training program at the University of Wisconsin (T32 AI055397). We thank the University of Wisconsin Department of Pathology and Laboratory Medicine and the WNPRC for funding and the use of its facilities and services. The funders of this research had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.World Health Organization. 14November2014. Ebola response roadmap—situation report update. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/disease/ebola/situation-reports/en/. [Google Scholar]
- 2.Gire SK, Goba A, Andersen KG, Sealfon RS, Park DJ, Kanneh L, Jalloh S, Momoh M, Fullah M, Dudas G, Wohl S, Moses LM, Yozwiak NL, Winnicki S, Matranga CB, Malboeuf CM, Qu J, Gladden AD, Schaffner SF, Yang X, Jiang PP, Nekoui M, Colubri A, Coomber MR, Fonnie M, Moigboi A, Gbakie M, Kamara FK, Tucker V, Konuwa E, Saffa S, Sellu J, Jalloh AA, Kovoma A, Koninga J, Mustapha I, Kargbo K, Foday M, Yillah M, Kanneh F, Robert W, Massally JL, Chapman SB, Bochicchio J, Murphy C, Nusbaum C, Young S, Birren BW, Grant DS, Scheiffelin JS, Lander ES, Happi C, Gevao SM, Gnirke A, Rambaut A, Garry RF, Khan SH, Sabeti PC. 2014. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 345:1369–1372. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C, Collini P, Danso K, Owusu-Ofori S, Dompreh A, Candotti D, Opare-Sem O, Allain JP. 2006. GB virus C and HIV-1 RNA load in single virus and co-infected West African individuals. AIDS 20:379–386. doi: 10.1097/01.aids.0000200536.79360.03. [DOI] [PubMed] [Google Scholar]
- 4.Compston LI, Li C, Sarkodie F, Owusu-Ofori S, Opare-Sem O, Allain JP. 2009. Prevalence of persistent and latent viruses in untreated patients infected with HIV-1 from Ghana, West Africa. J Med Virol 81:1860–1868. doi: 10.1002/jmv.21614. [DOI] [PubMed] [Google Scholar]
- 5.Sathar M, Soni P, York D. 2000. GB virus C/hepatitis G virus (GBV-C/HGV): still looking for a disease. Int J Exp Pathol 81:305–322. doi: 10.1046/j.1365-2613.2000.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohr EL, Stapleton JT. 2009. GB virus type C interactions with HIV: the role of envelope glycoproteins. J Viral Hepat 16:757–768. doi: 10.1111/j.1365-2893.2009.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stapleton JT, Foung S, Muerhoff AS, Bukh J, Simmonds P. 2011. The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the family Flaviviridae. J Gen Virol 92:233–246. doi: 10.1099/vir.0.027490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alter HJ. 1997. G-pers creepers, where'd you get those papers? A reassessment of the literature on the hepatitis G virus. Transfusion 37:569–572. [DOI] [PubMed] [Google Scholar]
- 9.Williams CF, Klinzman D, Yamashita TE, Xiang J, Polgreen PM, Rinaldo C, Liu C, Phair J, Margolick JB, Zdunek D, Hess G, Stapleton JT. 2004. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med 350:981–990. doi: 10.1056/NEJMoa030107. [DOI] [PubMed] [Google Scholar]
- 10.Xiang J, Wünschmann S, Diekema DJ, Klinzman D, Patrick KD, George SL, Stapleton JT. 2001. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med 345:707–714. doi: 10.1056/NEJMoa003364. [DOI] [PubMed] [Google Scholar]
- 11.Vahidnia F, Petersen M, Stapleton JT, Rutherford GW, Busch M, Custer B. 2012. Acquisition of GB virus type C and lower mortality in patients with advanced HIV disease. Clin Infect Dis 55:1012–1019. doi: 10.1093/cid/cis589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tillmann HL, Heiken H, Knapik-Botor A, Heringlake S, Ockenga J, Wilber JC, Goergen B, Detmer J, McMorrow M, Stoll M, Schmidt RE, Manns MP. 2001. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med 345:715–724. doi: 10.1056/NEJMoa010398. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Chaloner K, Tillmann HL, Williams CF, Stapleton JT. 2006. Effect of early and late GB virus C viraemia on survival of HIV-infected individuals: a meta-analysis. HIV Med 7:173–180. doi: 10.1111/j.1468-1293.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhattarai N, Stapleton JT. 2012. GB virus C: the good boy virus? Trends Microbiol 20:124–130. doi: 10.1016/j.tim.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker TJ, Smuts HE. 2000. GBV-C/HGV genotypes: proposed nomenclature for genotypes 1–5. J Med Virol 62:82–83. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A, Sealfon RS, Kanneh L, Moigboi A, Momoh M, Fullah M, Moses LM, Brown BL, Andersen KG, Winnicki S, Schaffner SF, Park DJ, Yozwiak NL, Jiang PP, Kargbo D, Jalloh S, Fonnie M, Sinnah V, French I, Kovoma A, Kamara FK, Tucker V, Konuwa E, Sellu J, Mustapha I, Foday M, Yillah M, Kanneh F, Saffa S, Massally JL, Boisen ML, Branco LM, Vandi MA, Grant DS, Happi C, Gevao SM, Fletcher TE, Fowler RA, Bausch DG, Sabeti PC, Khan SH, Garry RF. 2014. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 371:2092–2100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sibley SD, Lauck M, Bailey AL, Hyeroba D, Tumukunde A, Weny G, Chapman CA, O'Connor DH, Goldberg TL, Friedrich TC. 2014. Discovery and characterization of distinct simian pegiviruses in three wild African Old World monkey species. PLoS One 9:e98569. doi: 10.1371/journal.pone.0098569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauck M, Sibley SD, Hyeroba D, Tumukunde A, Weny G, Chapman CA, Ting N, Switzer WM, Kuhn JH, Friedrich TC, O'Connor DH, Goldberg TL. 2013. Exceptional simian hemorrhagic fever virus diversity in a Wild African primate community. J Virol 87:688–691. doi: 10.1128/JVI.02433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey AL, Lauck M, Weiler A, Sibley SD, Dinis JM, Bergman Z, Nelson CW, Correll M, Gleicher M, Hyeroba D, Tumukunde A, Weny G, Chapman C, Kuhn JH, Hughes AL, Friedrich TC, Goldberg TL, O'Connor DH. 2014. High genetic diversity and adaptive potential of two simian hemorrhagic fever viruses in a wild primate population. PLoS One 9:e90714. doi: 10.1371/journal.pone.0090714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey AL, Lauck M, Sibley SD, Pecotte J, Rice K, Weny G, Tumukunde A, Hyeroba D, Greene J, Correll M, Gleicher M, Friedrich TC, Jahrling PB, Kuhn JH, Goldberg TL, Rogers J, O'Connor DH. 3September2014. Two novel simian arteriviruses in captive and wild baboons (Papio spp.). J Virol doi: 10.1128/JVI.02203-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villinger F, Rollin PE, Brar SS, Chikkala NF, Winter J, Sundstrom JB, Zaki SR, Swanepoel R, Ansari AA, Peters CJ. 1999. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis 179(Suppl 1):S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 22.Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB. 2000. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Invest 80:171–186. doi: 10.1038/labinvest.3780021. [DOI] [PubMed] [Google Scholar]
- 23.Baize S, Leroy EM, Georges AJ, Georges-Courbot MC, Capron M, Bedjabaga I, Lansoud-Soukate J, Mavoungou E. 2002. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol 128:163–168. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hensley LE, Young HA, Jahrling PB, Geisbert TW. 2002. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett 80:169–179. doi: 10.1016/S0165-2478(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez A, Lukwiya M, Bausch D, Mahanty S, Sanchez AJ, Wagoner KD, Rollin PE. 2004. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol 78:10370–10377. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch SP, McCormick JB, Georges AJ. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med 5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 27.Ignatiev GM, Dadaeva AA, Luchko SV, Chepurnov AA. 2000. Immune and pathophysiological processes in baboons experimentally infected with Ebola virus adapted to guinea pigs. Immunol Lett 71:131–140. doi: 10.1016/S0165-2478(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 28.Ryabchikova EI, Kolesnikova LV, Luchko SV. 1999. An analysis of features of pathogenesis in two animal models of Ebola virus infection. J Infect Dis 179:S199–S202. doi: 10.1086/514293. [DOI] [PubMed] [Google Scholar]
- 29.Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Scott DP, Kagan E, Jahrling PB, Davis KJ. 2003. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol 163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, Kagan E, Hensley LE. 2003. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol 163:2371–2382. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldmann H, Bugany H, Mahner F, Klenk HD, Drenckhahn D, Schnittler HJ. 1996. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J Virol 70:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chivero ET, Bhattarai N, Rydze RT, Winters MA, Holodniy M, Stapleton J. 2014. Human Pegivirus (HPgV or GB virus C) RNA is found in multiple blood mononuclear cells in vivo and serum-derived viral RNA containing particles are infectious in vitro. J Gen Virol 95:1307–1319. doi: 10.1099/vir.0.063016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George SL, Varmaz D, Stapleton JT. 2006. GB virus C replicates in primary T and B lymphocytes. J Infect Dis 193:451–454. doi: 10.1086/499435. [DOI] [PubMed] [Google Scholar]
- 34.Bhattarai N, McLinden JH, Xiang J, Landay AL, Chivero ET, Stapleton JT. 2013. GB virus C particles inhibit T cell activation via envelope E2 protein-mediated inhibition of TCR signaling. J Immunol 190:6351–6359. doi: 10.4049/jimmunol.1300589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattarai N, McLinden JH, Xiang J, Kaufman TM, Stapleton JT. 2012. GB virus C envelope protein E2 inhibits TCR-induced IL-2 production and alters IL-2-signaling pathways. J Immunol 189:2211–2216. doi: 10.4049/jimmunol.1201324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattarai N, Rydze RT, Chivero ET, Stapleton JT. 2012. GB virus C viremia is associated with higher levels of double-negative T cells and lower T-cell activation in HIV-infected individuals receiving antiretroviral therapy. J Infect Dis 206:1469–1472. doi: 10.1093/infdis/jis515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maidana-Giret MT, Silva TM, Sauer MM, Tomiyama H, Levi JE, Bassichetto KC, Nishiya A, Diaz RS, Sabino EC, Palacios R, Kallas EG. 2009. GB virus type C infection modulates T-cell activation independently of HIV-1 viral load. AIDS 23:2277–2287. doi: 10.1097/QAD.0b013e32832d7a11. [DOI] [PubMed] [Google Scholar]
- 38.Moenkemeyer M, Schmidt RE, Wedemeyer H, Tillmann HL, Heiken H. 2008. GBV-C coinfection is negatively correlated to Fas expression and Fas-mediated apoptosis in HIV-1 infected patients. J Med Virol 80:1933–1940. doi: 10.1002/jmv.21305. [DOI] [PubMed] [Google Scholar]
- 39.Nattermann J, Nischalke H-D, Kupfer B, Rockstroh J, Hess L, Sauerbruch T, Spengler U. 2003. Regulation of CC chemokine receptor 5 in hepatitis G virus infection. AIDS 17:1457–1462. doi: 10.1097/00002030-200307040-00006. [DOI] [PubMed] [Google Scholar]
- 40.Rydze RT, Xiang J, McLinden JH, Stapleton JT. 2012. GB virus type C infection polarizes T-cell cytokine gene expression toward a Th1 cytokine profile via NS5A protein expression. J Infect Dis 206:69–72. doi: 10.1093/infdis/jis312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarze-Zander C, Neibecker M, Othman S, Tural C, Clotet B, Blackard JT, Kupfer B, Luechters G, Chung RT, Rockstroh JK, Spengler U. 2010. GB virus C coinfection in advanced HIV type-1 disease is associated with low CCR5 and CXCR4 surface expression on CD4(+) T-cells. Antivir Ther 15:745–752. doi: 10.3851/IMP1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stapleton JT, Chaloner K, Martenson JA, Zhang J, Klinzman D, Xiang J, Sauter W, Desai SN, Landay A. 2012. GB virus C infection is associated with altered lymphocyte subset distribution and reduced T cell activation and proliferation in HIV-infected individuals. PLoS One 7:e50563–e50563. doi: 10.1371/journal.pone.0050563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiang J, George SL, Wünschmann S, Chang Q, Klinzman D, Stapleton JT. 2004. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta, and SDF-1. Lancet 363:2040–2046. doi: 10.1016/S0140-6736(04)16453-2. [DOI] [PubMed] [Google Scholar]
- 44.Stapleton JT, Martinson JA, Klinzman D, Xiang J, Desai SN, Landay A. 2013. GB virus C infection and B-cell, natural killer cell, and monocyte activation markers in HIV-infected individuals. AIDS 27:1829–1832. doi: 10.1097/QAD.0b013e328363089f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel G. 28August2014. Ebola's heavy toll on study authors. ScienceInsider http://news.sciencemag.org/health/2014/08/ebolas-heavy-toll-study-authors. [Google Scholar]
- 46.Developmental Core Team R. 2014. R: a language and environment for statistical computing. R Development Core Team, Vienna, Austria. [Google Scholar]


