Abstract
Traumatic brain injury (TBI) causes significant mortality, long term disability and psychological symptoms. Gene therapy is a promising approach for treatment of different pathological conditions. Here we tested chitosan and polyethyleneimine (PEI)-coated magnetic micelles (CPmag micelles or CPMMs), a potential MRI contrast agent, to deliver a reporter DNA to the brain after mild TBI (mTBI). CPMM - tomato plasmid (ptd) conjugate expressing a red-fluorescent protein (RFP) was administered intranasally immediately after mTBI or sham surgery in male SD rats. Evans blue extravasation following mTBI suggested CPMM-ptd entry into the brain via the compromised blood-brain barrier. Magnetofection increased the concentration of CPMMs in the brain. RFP expression was observed in the brain (cortex and hippocampus), lung and liver 48 hours after mTBI. CPMM did not evoke any inflammatory response by themselves and were excreted from the body. These results indicate the possibility of using intranasally administered CPMM as a theranostic vehicle for mTBI.
Keywords: TBI, LFPI, Brain, magnetic micelle, bio-distribution, theranostic, DNA delivery
Background
TBI causes significant mortality, disability and psychological symptoms and is a serious health problem in the United States and around the world. Approximately 1.7 million people sustain a TBI annually [1]. Mild to severe TBI can cause intellectual and cognitive deficits, mood and behavioural changes [2–4]. Although the immediate consequences of mTBI are not always obvious, the secondary axonal injury with brain dysfunction may appear months after the damage. The mechanical impact causes a breakdown of the blood-brain barrier (BBB) and induces secondary neurodegeneration. The resident immune cells become activated and the circulating immune cells infiltrate into the brain through the leaky BBB and secrete inflammatory mediators. These pro-inflammatory events lead to continued neurodegeneration and loss of brain function. Recent work from our laboratory showed elevated expression of the chemokine CCL20 (MIP3-alpha) both peripherally and centrally after mTBI [5, 6]. Therefore, pro-inflammatory molecules like CCL20 can be a potential target for TBI therapy.
Gene therapy is a promising treatment strategy in many types of diseases. Viruses are widely used and efficient vectors for gene delivery to target cells but their potential for evoking immune reactions and mutagenesis precludes their use in humans. Nonviral vectors, because of their safety and efficacy, have been gaining popularity as gene delivery vehicles. Among these, the polysaccharide chitosan or chitosan-functionalized nanoparticles (CNPs), e.g., gold-CNPs, carbon nanotubes etc., have been widely used for gene transfer. Chitosan is a biodegradable, nonimmunogenic, cationic polymer but its low transfection efficiency prevents it from being used alone. Modifications, such as by grafting polyethyleneimine (PEI) onto chitosan, or grafting chitosan onto PEI, or by creating a chitosan-PEI composite have been used successfully for gene delivery in vitro and in vivo [7]. Chitosan alone has also been shown to be neuroprotective after traumatic spinal cord injury [8]. The next generation of therapeutics called ‘theranostics’ will combine several functions, such as targeting, drug delivery and imaging, into one particle. For example, superparamagnetic iron oxide nanoparticles (SPIONs) are efficient in cellular imaging applications and can be combined with functionalized chitosan or lipid micelles for targeted drug/gene delivery. These low cost materials are biocompatible and can easily be surfacemodified with a variety of peptides, antibodies or small molecules for targeting and to increase their transfection efficiency. Moreover, it has been shown that ‘magnetofection’ the use of a magnet to draw magnetic nanoparticles to a specific area, can efficiently increase the speed and efficiency of the gene transfection in vitro and site specific targeting in vivo [9, 10].
In a previous report from this laboratory, Wang et al. [11] manufactured chitosan-PEI-modified micelles with cores containing SPIONs, which are efficient theranostic agents that can deliver DNA into cells in vivo and enable MRI monitoring of the tissues at the same time because the SPIONs act as a T2-weighted contrast agent. In the present study we tested the efficacy of the same nanoparticles prepared by Wang et al. [11] in delivering DNA to the brain following mTBI in rats. We also determined the biodistribution of the particles and their transfection efficiency in different tissues.
METHODS
Preparation of chitosan-polyethyleneimine magnetic micelle (CPMM) nanoparticles
CPMM were prepared, conjugated with DNA and characterized as described previously by Wang et al. [11] (see supplement 1 for details and Figure S1 for size).
Cellular uptake of CPMM-cy-5.5
HT22 cells were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin in an atmosphere of 5% CO2. Cells were plated at a density of 20,000 per well in 8 well chamber slides 24 h prior to the experiment. cy-5.5 was conjugated to the CPMM at a ratio of CPMM:cy-5.5 1:10 and dialyzed for 24 h to remove excess cy-5.5. CPMM-cy-5.5 conjugate equivalent to 2.5 µg/ml CPMM was added to the cells and incubated at 37°C for 1h with a magnet of field strength 43.2 mTesla (mT) or without a bar magnet underneath the wells. Magnetic field strength was first optimized in vitro by using magnets of increasing field strengths. 43.2mT was found to be the optimum strength in potentiating the CPMM entry into the cells. An equal amount of free cy-5.5 was used as control. After 1h cells were washed three times with sterile PBS and fixed with 4% paraformaldehyde for 10 minutes, washed with sterile PBS and cover slipped using DAPI containing mounting medium. Cells were observed using a Leica TCS SP2 laser scanning confocal microscope and photos were taken.
Cell viability assay
HT22 cell viability was measured using a WST assay kit (Roche Applied Science). Cells at 80% confluence were trypsinized and seeded in a 96-well plate at a density of 3500 cells/ well. At 24 h after plating, the cells were treated with different concentrations of CPMM in a final volume of 100 µL per well and incubated for 1 h, 3 h and 24 h at 37°C with 5% CO2. During incubation the wells were placed on a magnet for the first 1 h. WST reagent was added following the manufacturer’s instructions and after 4 h the plate was read at 540 nm and 630 nm using a Synergy H4 micro plate reader (Biotek). Cell viability was calculated using the formula
Cell Viability (%) = 100 × (ODsample/ODcontrol)
Animals
All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals following a protocol approved by the Institutional Animal Care and Use Committee at the University of South Florida. Male Sprague-Dawley rats (250 – 300g) (Harlan) were housed in a climate-controlled room with 12/12 h day-night cycle, water and laboratory chow available ad libitum. A total of 33 animals were used in this study.
Lateral fluid percussion injury (LFPI)
Brain trauma was delivered to rats as described previously [5](details in supplement).
Conjugation of td-tomato plasmid (ptd) to CPMM and delivery to rats
The pCMV-td Tomato plasmid (Clontech) encoding the tomato-red fluorescent protein DNA (ptd) was extracted and purified using the MegaPrep plasmid purification kit (Qiagen) from a culture of XL1-Blue cells transformed with the plasmid. CPMMs (0.2 µg/µl, 10 kD) and plasmid DNA (0.2 µg/µl) in phosphate-buffered saline (PBS, pH 7.4) were prepared separately. The plasmid DNA solution was added drop wise to CPMM solution and vortexed for 20 minutes. The CPMM-DNA conjugate was instilled into the nostrils of anesthetized rats at 50 µl per nostril immediately after mTBI or sham surgery. The rats were then placed on a 37°C heating pad in their home cage with (8.64 Gauss) or without a magnetic cap on the head for 1 h and allowed to recover (Figure 1).
Figure 1. Schematic representation showing the strategy to administer CPMM nanoparticles to rat after mTBI.
Tissue collection
Rats were deeply anesthetized with ketamine and xylazine (I.P.) and then perfused with 0.9% saline followed by 4% paraformaldehyde (PFA) in phosphate buffer. The brain, lungs, liver, kidneys and spleen were removed, post-fixed in 2% PFA and saturated with increasing sucrose concentrations (20% to 30%) in PBS. Tissues were then frozen in OCT. Brains were sectioned coronally at 30 µm thickness, thaw-mounted onto glass slides and stored at −20°C prior to staining. All other tissues were sectioned at 5µm thickness, thaw-mounted on glass slides and stored at −20°C for future use.
Evans Blue extravasation study
LFPI or sham surgery was performed as described above. Rats were allowed to survive for 1h, 6h or 24 h. 30 min before euthanasia, 2% evans blue (EB) solution was injected through tail vein. At the same time 2.5µg/ml CPMM solution was instilled intra-nasally at a dose of 50 µl/nostril. Rats were deeply anesthetized and 0.9% saline solution was infused transcardially and continued as long as colored solution was coming out of the right atrium. Then rats were perfused with 4% PFA solution and tissues were collected as described above.
Fluoro-Jade histochemistry for neurodegeneration
Fluoro-Jade (FJ) (Histochem) staining was performed to label degenerating neurons [12, 13]. Thaw-mounted sections were placed in 100% ethanol for 3 minutes followed by 70% ethanol and deionized (DI) water for 1 minute each. Sections were then oxidized with 0.06% KMnO4 solution for 15 minutes followed by three rinses in DI water for 1 minute each. Sections were then stained in a 0.001% solution of Fluoro-Jade in 0.1% acetic acid for 30 min. Slides were rinsed, dried at 45°C for 20 min, cleared with xylene, and cover-slipped using DPX mounting medium.
Prussian blue staining for ferric iron
Freshly prepared 5% potassium ferrocyanide, K4Fe(CN)6, solution and 5M HCl were mixed in a ratio of 1:1 (v/v). Slide mounted sections were washed in PBS and incubated in ferrocyanide solution in a Coplin jar for 72 h. The slides were then washed in DI water and counterstained with eosin, dehydrated with graded alcohols, cleared with xylene and mounted with Vectamount mounting medium.
Immunohistochemistry for inflammatory markers and red fluorescent protein
Slide-mounted tissue sections were washed with PBS for 5 min, incubated in 3% hydrogen peroxide for 20 min and washed 3 times in PBS. They were then heated in 1% antigen-unmasking solution (Vector Laboratories) for 20 min at 90°C, incubated for 1 h in permeabilization buffer (10% goat serum, 0.1% Triton X-100 in PBS) and incubated overnight at 4°C with rabbit anti- IL-1β (1:100) or mouse anti - IL-6 (1:100) or mouse anti-TNFα (1:50) primary antibody (Abcam) in antibody solution (5% goat serum, 0.05% Triton X-100 in PBS). Next day, the sections were washed with PBS and incubated 1 h at room temperature with biotinylated goat anti-rabbit, (1:400), or biolinylated goat anti- mouse (1:400) antibodies (Vector Laboratories) in antibody solution. Sections were then washed in PBS, incubated in avidin-biotin complex mixture (ABC,1:100) for 1 h at room temperature, washed again and developed with diaminobenzidine solution (DAB), washed with PBS, dried and cover slipped with vectamount mounting medium. For immunostaining of RFP sections were pre-treated in the same way and incubated with rabbit anti-DsRed (1:1000) primary antibody (Abcam) over night at 4°C, washed with PBS and incubated with biotinylated goat anti-rabbit antibody, 1:400, (Vector Laboratories) for 1 h at room temperature. Sections were washed with PBS, dried and cover slipped with Vectashield aqueous mounting medium with DAPI.
Image analysis and quantitation
All quantitation was performed using the NIH Image J software. For immunohistochemical analysis, photomicrographs captured at 200× magnification with an Olympus DP70 camera were used for quantitation. Images were taken at the same exposure and digital gain settings for a given magnification to minimize differential background intensity or false-positive immunoreactivity across sections. The channels of the RGB images were converted to gray-scale and then adjusted for brightness and contrast to exclude noise pixels. The images were also adjusted for the threshold to highlight all the positive cells to be counted and a binary version of the image was created with pixel intensities between 0 and 255. Integrated density (IntDen) was calculated and background correction was performed for each image. The corrected integrated density of immunoreactivity of the sections from 1.5, 2.5 and 3.5 mm caudal to the bregma were averaged to represent the IntDen of immunoreactivity from each brain and expressed as mean IntDen ± S.E.M.
Statistical analysis
All data are presented as mean ± S.E.M. Statistical significance was evaluated by one-way ANOVA with Bonferroni’s post-hoc test. A p value of less than 0.05 was considered statistically significant for all comparisons.
Results
Cellular uptake of CPMM and its effect on cell viability
HT22 cells were treated with free cy-5.5 dye or cy-5.5 conjugated CPMM with or without an applied magnet for 1 h. Confocal microscopic images show cy-5.5 fluorescence after 1 h in all three groups but free cy-5.5 showed least fluorescence. On the other hand cells incubated with CPMM nanoparticles with the magnet showed significantly higher fluorescence compared to the cy-5.5-CPMM group without the magnet (Figure 2A). Integrated density calculation corrected for the background showed highest fluorescence intensity in the cells incubated with cy-5.5 – CPMM conjugate with magnet (Figure 2B). The cytotoxicity of the CPMM nanoparticles was also tested on HT22 cells. Results from WST assays (Figure. 2C) show that these particles at concentrations of 0.5 – 10 µg/ml and incubated up to 24h do not compromise viability of HT22 cells. At these concentrations cells show healthy growth and normal cellular architecture even after 24 h of treatment.
Figure 2. Uptake and Viability of HT22 cells treated with CPMM.
A, B, cy-5.5-CPMM was readily up-taken by HT22 cells within 1 hour of incubation under the magnetic field. HT22 cells incubated with the same concentration of cy-5.5-CPMM without a magnetic field or with free cy-5.5 dye showed significantly less uptake. A, confocal images showing uptake. B, fluorescence intensity (mean ± SEM) of cy-5.5 inside the cells measured as integrated density by image J. * p< 0.01, ** p< 0.001. C, HT22 cell viability after incubation with different concentrations of CPMMs for various times periods.
Effect of the magnetic field on the concentration of CPMM in the brain after mTBI
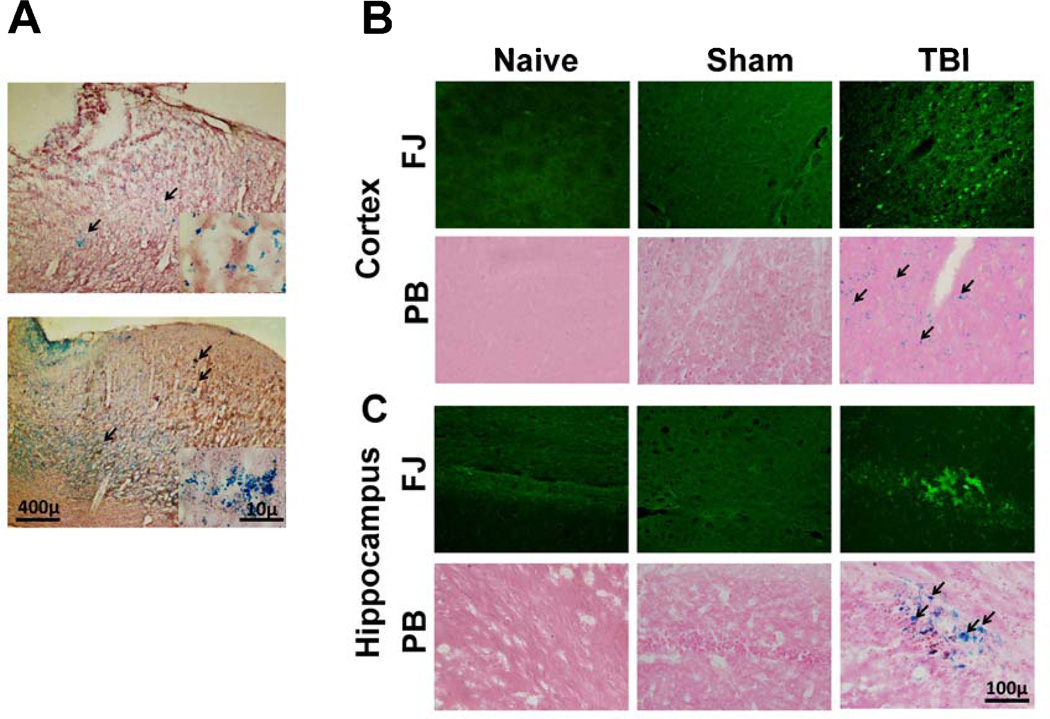
To optimize the magnetic field strength to be used, rats were subjected to increasing magnetic field strengths for equal duration (1 hour) and CPMM entry into the brain was observed. 86.4 mT was found to be the optimum field strength and used in all subsequent experiments. To observe whether magnetofection causes any differences in concentrating CPMM nanoparticles in the brain or not, rats were subjected to mTBI to the cerebral cortex or sham operated and immediately thereafter ptd-complexed CPMMs were administered intranasally (i.n.). One group of rats comprised of 6 rats including 3 sham and 3 mTBI animals were subjected to a magnetic field for 1 h. while the other group, also including 3 sham and 3 mTBI animals, were not. The degree of PB staining was compared between the two groups. PB staining reveals that more CPMMs were concentrated in the cortex of the rats subjected to a magnetic field (Figure 3A lower image) compared to the other group recovered without the magnet (Figure 3A upper image). All subsequent experiments were performed by subjecting the rats to the same magnetic field following sham or mTBI and i.n. CPMM administration.
Figure 3.
A, Magnetofection increases CPMM concentration in the brain after TBI. Bright field images showing the localization of iron oxide nanoparticles (blue stained particles stained with acidic potassium ferrocyanide, black arrows) in the brain after mTBI followed by i.n. administration of CPMM without (upper image) or with (lower image) application of magnetic field. Counterstained with nuclear fast red. B,C, TBI induced neurodegeneration in specific areas of the rat brain and entry of CPMM nanoparticles to the sites of injury. (B) cortex; (C) hippocampus, B & C, upper panels, mTBI or sham-operated rats immediately given i.n. CPMMs and the head was subjected to a magnetic field for 1 h. Naïve rats did not receive any CPMMs or any surgical manipulations or magnetic field. After 48 h, the brains were removed and cryosections were stained with Fluoro-Jade B (FJ, green) to identify damaged neurons. B & C, lower panels, serial sections matched for area and level of those in the FJ staining were stained with acidic potassium ferrocyanide to identify iron oxide particles (blue staining; black arrows). Counterstained with eosin
Following mTBI the pattern and extent of neurodegeneration observed in these experiments were in accordance with the those reported previously [9]. mTBI causes neurodegeneration and the majority of FJ-positive cells were found within the cerebral cortex (Figure 3B), hippocampus (Figure 3C) and thalamus (data not shown). Degenerating cortical neurons were observed around the epicentre of the trauma and on the lateral cortex. Hippocampal neurodegeneration was localized to the pyramidal cell layers with some diffuse labelling throughout the general structure. PB staining of matching sections shows the presence of iron oxide nanoparticles in the cortex and hippocampus even 48h after mTBI followed by i.n. administration of CPMM (Figure 3B, C, lower panels). Also, Most of the particles were found on side of the brain ipsilateral to the injury. No PB staining was observed in the thalamus. Both FJ and PB staining was absent in sham animals.
ptd protein expressed in the brain and other tissues 48h after mTBI
Wang et al. (2012) have clearly shown using the gel retardation assay that the DNA binds completely and stably to CPMM nanoparticles. The optimum DNA binding was observed at a chitosan to DNA ratio of 10 to 1. It has also been shown that DNA was completely protected when conjugated with CPMM particles [11]. Plasmid ptd was conjugated to CPMMs and the particles were instilled into the nose of sham-operated rats or rats subjected to mTBI. Naïve rats received no nanoparticles or surgery and no RFP expression was observed in their brain tissues. A few ptd-positive cells were found in the cortex of sham animals. On the other hand, animals with mTBI showed substantial RFP expression in the cortex and hippocampus (Figure 4A). Most expression in the cortex was observed in the tissues around the trauma epicentre and in the lateral cortex where most of the FJ-positive cells were observed. Although, other areas of the cortex also had cells expressing RFP. In the hippocampus the ptd expression was observed in the pyramidal cell layer and also in the general structure of the hippocampus.
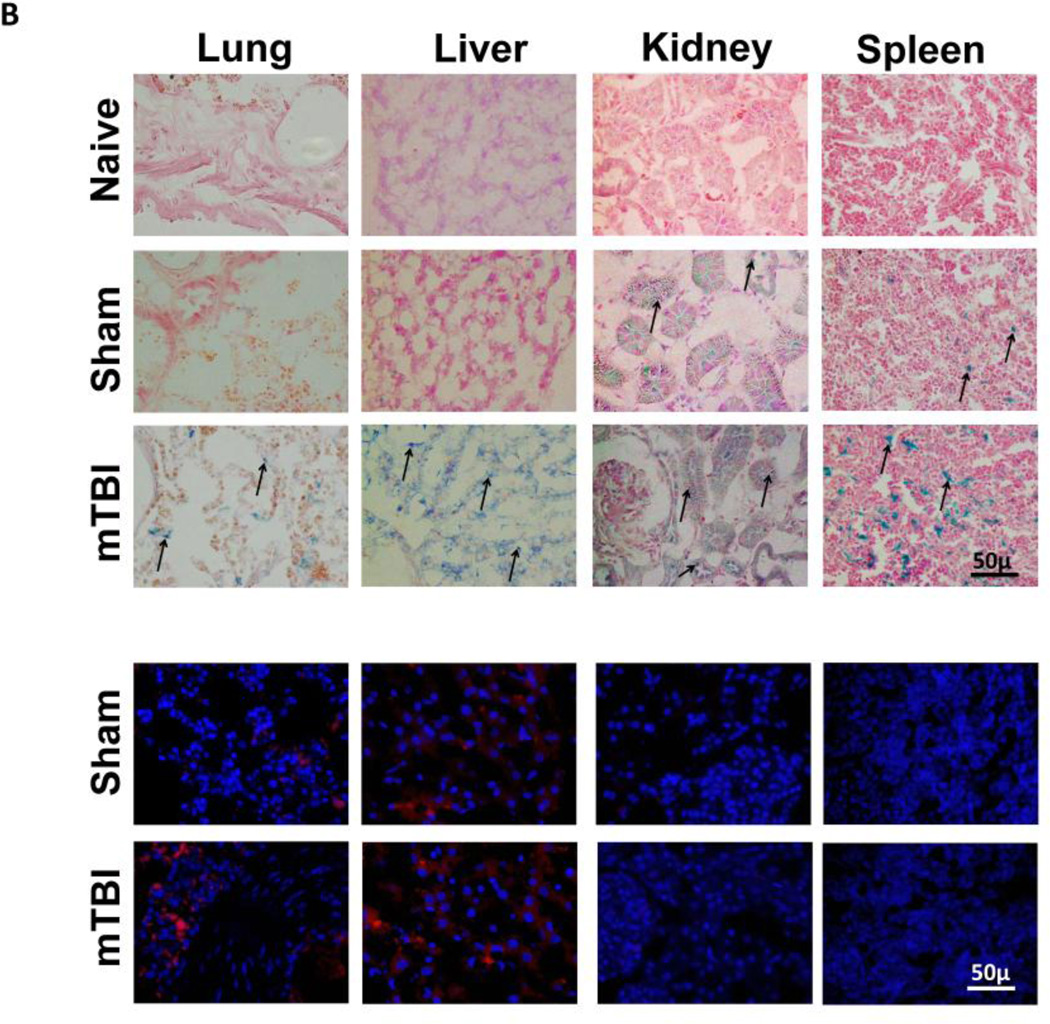
Figure 4. Biodistribution of CPMM and expression of RFP in different organs following mTBI.
A, ptd, delivered with CPMM is expressed in cortex and hippocampus 48h after mTBI. Left panel showing representative low magnification fluorescence photomicrographs of ptd expression (red) in the cortex; insets, high magnification. Right panel, high magnification fluorescence images of ptd expression (red) in the hippocampus. B, Distribution of CPMM and RFP expression in different organs 48h after i.n. administration in naïve, sham or mTBI rats. Upper panel, Prussian blue staining (blue) shows the presence of iron oxide NPs in different tissues. Naïve rats did not receive any CPMMs or any surgical manipulations. Sham or mTBI rats received same amount of CPMM-ptd. Counterstained with eosin. CPMMs significantly increased in these tissues 48h after mTBI. Lower panel, Fluorescence immunostaining shows RFP expression (red) in different organs. Blue shows DAPI positive nuclei. C, Histograms showing the mean intensity of ptd immunofluorescence (integrated density) using Image J software in the rat brain (cortex, hippocampus), lung, liver, kidney and spleen 48 hours post mTBI. ** p< 0.001 compared to sham.
Biodistribution of CPMM and RFP expression following mTBI
PB staining was performed on lung, liver, kidney and spleen sections to determine if CPMM nanoparticles were present in those organs (Figure 4B, upper panels). PB staining was undetectable in the naïve animals that received no CPMMs. In sham-operated animals insignificant labelling was observed in lung and liver after 48 hours of administration. The spleens of these animals showed some PB-positive particles but most of the particles were observed in the kidneys indicating their excretion from the body. In the animals given mTBI, large amounts of PB-positive particles were seen in the lung, liver, kidney and spleen 48 hours after administration. The lungs contained the least amount of CPMM particles while liver and kidney had higher concentrations. These findings suggest that CPMM nanoparticles were excreted from the body and that healthy rats excrete these nanoparticles from the body faster than those with mTBI. Also, it was observed that among these tissues only lung and liver showed RFP expression in both sham and mTBI animals. Although spleen and kidney showed the presence of substantial amounts of iron-oxide particles, no RFP expression was observed in these organs (Figure 4B, lower panels). A comparative analysis of the RFP expression in brain (cortex and hippocampus), lung, liver, spleen and kidney showed the highest expression in the cortex. RFP expressions in the cortex and hippocampus were significantly higher than in the corresponding areas of the sham animals. On the other hand, although lung and liver showed RFP expression, there was not much difference between sham and mTBI animals (Figure 4C).
mTBI causes BBB disruption and CPMM entry into the brain
Evans blue extravasation study showed EB entry in the brain within 1 h of mTBI. EB entry was also observed at 24 h of mTBI although maximum intensity and penetration into the tissue, as seen in the coronal sections was observed at 6 h post TBI. On the other hand the sham animals did not show any EB entry in the brain (Figure 5 A, C). Confocal microscopic images of cerebral microvessels immunostained with antibody against smooth muscle actin (SMA) showed disrupted microvasculature in mTBI rats (Figure 5B). These observations clearly indicated disruption of the BBB following mTBI. Simultaneous EB extravasation and i.n. CPMM administration experiments revealed the localization of CPMM nanoparticles (PB staining) in the same areas of the cortex and hippocampus where EB fluorescence was also observed (Figure 5C). Since, EB and PB staining were absent in the sham animals and it is known that EB enters the brain parenchyma via ruptured blood vessels, these findings clearly suggest that CPMM nanoparticles entered the brain parenchyma via the compromised cerebral microvasculature.
Figure 5. Blood-brain barrier disruption after mTBI.
A, Evans Blue extravasation in sham rats and at different time points after mTBI. Maximum extravasation is observed 6h after mTBI. Upper panel, the dorsal surface of the whole brain showing the spread of the EB dye. Lower panels, 4 mm thick coronal sections from the same brains showing the internal spread of EB. B, confocal images of sections immunostained with anti SMA antibody showing an intact microvessel in the sham animal and a ruptured micro-vessel in an area close to the trauma epicenter after mTBI. C, EB florescence and PB staining in cortex and hippocampus after mTBI. Sequential 30 µm coronal sections showing the EB fluorescence and PB staining in the similar regions.
CPMM do not evoke an inflammatory response in rats
Naïve or CPMM treated sham or mTBI animals euthanized 24 h after mTBI were used to study the inflammatory response evoked by CPMM particles, if any. Brain sections were stained with antibodies against early inflammatory markers interleukine-6 (IL-6), interleukine-1β or tumour necrosis factor-α (TNF- α). The levels of cytokines expression did not change in either naïve or sham animals, although changes were observed in mTBI animals (Figure 6A). Measurement of the integrated density of the immune-reactivity by image J showed no changes in IL-6, IL-1β or TNF-α expression in naïve or sham animals and significant increase in mTBI animals irrespective of whether they were treated with CPMM or not (Figure 6B). These observations clearly indicate that the CPMM nanoparticles are non-inflammatory.
Figure 6. CPMM nanoparticles do not evoke inflammatory response in rats.
Brain sections from naïve, sham or mTBI rats were immunostained for IL-6, IL-1b or TNF-a. A, Representative bright field photomicrographs showing the immunoreactivities. B, Histogram showing the integrated density of immunoreactivity measured by image J.* p<0.05, **p,0.001.
Discussion
The use of nanotechnology in medicine has opened up a new frontier for the development of drug delivery systems. A wide variety of nanoparticles has been tested experimentally in vitro and in animal models as carriers for nucleic acids, peptides and drugs [14–17]. Magnetic nanoparticles combined with viral or non-viral vectors has been shown to be effective for gene transfection and can be focused to the target site (in vivo) or cells via magnets [18]. SPIONs, are widely used as T2 MRI contrast agents and can be functionalized for in vivo gene delivery while allowing real-time tracking [19]. Also, incorporation of nanoparticles into micelles increases their half-life and biocompatibility in vivo [20]. Wang et al[11] prepared and characterized the CPMM nanoparticles (ζ = +17.89mV) and demonstrated that these nanoparticles can simultaneously deliver genes in vitro and in vivo in mice and serve as an MRI contrast agent. In this study we used CPMMs to deliver a reporter gene to the rat brain after mild traumatic brain injury (mTBI). First, we observed that CPMM nanoparticles were not toxic to the neuronal cell line HT22 even after 24 hours. Neurons are difficult to transfect, although, these nanoparticles are readily taken up by HT22 cells, similar to previous observations from this laboratory on HEK293 and PC3 cell lines, [11] and uptake efficiency increased in the magnetic field.
One of the major drawbacks of non-viral gene vectors is their low transfection efficiency compared to viral vectors [21, 22]. Using CPMM nanoparticles, however, we observed moderate transfection in cortex and hippocampus after mTBI as measured by the integrated density of the ptd immunofluorescence. Iron oxide from the particles as well as redfluorescent protein was observed in different areas of the cortex and the hippocampus in close proximity to degenerating neurons. This is important because specific expression of the reporter protein in brain cells of mTBI rats demonstrates that the CPMM particles could be used to carry therapeutic DNA to injured areas of the brain. Expression of ptd was observed 48 h after mTBI and i.n. administration of CPMM-ptd complex. It indicates that the ptd DNA was released at a slow rate and is of special interest that the time of reporter gene expression coincides with the time of pro-inflammatory gene expression as observed by us previously in similar brain regions and under similar experimental conditions [9]. This leads to the possibility for gene therapy using CPMM nanoparticles to minimize proinflammatory chemokine production in the brain after mTBI and thereby reduce neurodegeneration
The main challenge of gene delivery to the brain is to pass the BBB. Some relatively small nanoparticles (about 35 nm) and highly lipophilic molecules can pass through the BBB and reach the CNS [23]. But loading our nanoparticles with DNA increased the size to around 290 nm[11] which hindered their delivery across the intact BBB, as observed in the naïve and sham animals in this study. Following mTBI the BBB is transiently compromised, and during that period molecules of different sizes can enter the brain tissues. We utilized this time window for intranasal administration of CPMM-ptd complexes in order to evaluate the efficacy of these particles to deliver the reporter gene into cells in the damaged tissues. Also, applying the magnetic field helped in concentrating the particles in the brain. We observed that in this model of mTBI the BBB remains open at least 24 h after injury. This suggests a therapeutic time window for gene/drug delivery after mTBI. We used biocompatible CPMM-ptd conjugates given to sham or mTBI rats and showed that they are an excellent DNA carrier that releases DNA slowly over a period of 48 h in the cells. These nanoparticles are non- immunogenic as observed in this study and previously [11]. Also, the particles do not accumulate in the system longer than 7 days as observed by Wang et al [11] which is in line of our observation. The presence of iron oxide particles in the liver, kidney and spleen, and at the same time no RFP expression in kidney and spleen, are indicative of the enzymatic degradation and excretion of these particles within 48 h of application. Moreover, careful observation indicates most of the particles in the kidney were present in the renal tubules indicating their excretion from the system. Although, there is no direct evidence for whether or not the DNA is unconjugated in the systemic circulation, the circumstantial evidences show that is not the case. We have seen expression of RFP in the brain, lung and liver. Unconjugation of DNA from the CPMM particles in the systemic circulation would not allow RFP expression in those organs.
Additionally, SPIONs incorporated into the CPMMs would allow real-time monitoring of the gene delivery into the brain. The ability to be cleared relatively rapidly, the nontoxic nature of these particles and their MRI contrast property make them an ideal candidate for gene therapy in cases of mTBI. Moreover, the SPIO core allows the nanoparticles to be condensed in the brain under magnetic field and thereby make the delivery of larger payload of DNA to the tissue possible. In absence of a magnetic field these particles show less aggregation which prevents their accumulation in the blood vessels [10]. Since its discovery, the technique of magnetofection has been primarily used to increase the gene transfection of the in vitro cell cultures using the superparamagnetic nanoparticles [15, 24, 25]. The gradient of the magnetic field enhances the efficiency of magnetofection compared to that without magnetic field [10, 26]. Gersting et al. have shown that magnetofection accelerated the enrichment of non-viral vector – DNA conjugate on the epithelial surface and enhanced the transfection efficiency ex vivo [14]. Use of this method to enhance site specific drug/gene delivery have also been reported by different authors [27–29]. Magnetic drug targeting (MDT) or magnetic gene targeting (MGT) combined with magnetofection has opened up the possibilities to develop methods for more efficient and less invasive drug/gene therapy strategies for major pathologies like cancer, neurodegeneration and myocardial infarction [24]. In the present study, the CPMM nanoparticles were found to be non-toxic both in vitro and in vivo systems and could be manipulated by inducing a magnetic field which offers the opportunity to develop an efficient MGT vector, in which the gene coding for a therapeutic molecule can be delivered to a therapeutic target area in the body. We used combination of simple bar magnets to create a static magnetic field of 86.4 mT which is within the safety limit of rodent or human tolerance [30–32] to prevent any possible physiological disturbances. Being able to deliver nanoparticles to the brain through the intranasal route helped us keeping the amount of the CPMM particle low and avoided over accumulation of iron in the brain and other organs.
In conclusion, we developed an efficient method for delivering therapeutic DNA to rat brain cells after mTBI. The chitosan-based nanoparticles containing SPION in the core are efficient MGT agent, which can deliver DNA to the brain and potentially can be imaged by MRI, and therefore, can be utilized as a vehicle for therapeutic DNA delivery in injured brain tissues.
Supplementary Material
Acknowledgement
We would like to acknowledge Transgenex NanoBioTech, Inc. Tampa for the kind donation of chitosan, Dr. Bruce Citron, Bay Pines V.A. for his kind donation of the HT22cell lines and Dr. Gary Hellermann for his critical editing of the manuscript.
Sources of support for research: The Office of Naval Research grant (N000140810914) to SSM and VA reintegration grant to SM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that they have no conflict of interests.
References
- 1.Faul M, Wald XL, Coronado MMVG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 2.Ettenhofer ML, Abeles N. The significance of mild traumatic brain injury to cognition and self-reported symptoms in long-term recovery from injury. J Clin Exp Neuropsychol. 2009;31(3):363–372. doi: 10.1080/13803390802175270. [DOI] [PubMed] [Google Scholar]
- 3.Halldorsson JG, et al. The scope of early traumatic brain injury as a long-term health concern in two nationwide samples: prevalence and prognostic factors. Brain Inj. 2012;26(1):1–13. doi: 10.3109/02699052.2011.635359. [DOI] [PubMed] [Google Scholar]
- 4.Ozen LJ, Fernandes MA. Slowing down after a mild traumatic brain injury: a strategy to improve cognitive task performance? Arch Clin Neuropsychol. 2012;27(1):85–100. doi: 10.1093/arclin/acr087. [DOI] [PubMed] [Google Scholar]
- 5.Das M, et al. Lateral fluid percussion injury of the brain induces CCL20 inflammatory chemokine expression in rats. J Neuroinflammation. 2011;8:148. doi: 10.1186/1742-2094-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das M, Mohapatra S, Mohapatra SS. New perspectives on central and peripheral immune responses to acute traumatic brain injury. J Neuroinflammation. 2012;9:236. doi: 10.1186/1742-2094-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lou YL, et al. Poly(ethylene imine)-g-chitosan using EX-810 as a spacer for nonviral gene delivery vectors. J Biomed Mater Res A. 2009;88(4):1058–1068. doi: 10.1002/jbm.a.31961. [DOI] [PubMed] [Google Scholar]
- 8.Cho Y, Shi R, Borgens RB. Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J Exp Biol. 2010;213(Pt 9):1513–1520. doi: 10.1242/jeb.035162. [DOI] [PubMed] [Google Scholar]
- 9.Al-Deen FN, Selomulya C, Williams T. On designing stable magnetic vectors as carriers for malaria DNA vaccine. Colloids Surf B Biointerfaces. 2013;102C:492–503. doi: 10.1016/j.colsurfb.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Dobson J. Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Ther. 2006;13(4):283–287. doi: 10.1038/sj.gt.3302720. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, et al. Dual-purpose magnetic micelles for MRI and gene delivery. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duckworth EA, et al. Temporary focal ischemia in the mouse: technical aspects and patterns of Fluoro-Jade evident neurodegeneration. Brain Res. 2005;1042(1):29–36. doi: 10.1016/j.brainres.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751(1):37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- 14.Gersting SW, et al. Gene delivery to respiratory epithelial cells by magnetofection. J Gene Med. 2004;6(8):913–922. doi: 10.1002/jgm.569. [DOI] [PubMed] [Google Scholar]
- 15.Castillo B, et al. Intracellular Delivery of siRNA by Polycationic Superparamagnetic Nanoparticles. J Drug Deliv. 2012;2012:218940. doi: 10.1155/2012/218940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marra M, et al. New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative study. Biotechnol Adv. 2012;30(1):302–309. doi: 10.1016/j.biotechadv.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Caraglia M, et al. Stealth liposomes encapsulating zoledronic acid: a new opportunity to treat neuropathic pain. Mol Pharm. 2013;10(3):1111–1118. doi: 10.1021/mp3006215. [DOI] [PubMed] [Google Scholar]
- 18.McBain SC, et al. Magnetic nanoparticles as gene delivery agents: enhanced transfection in the presence of oscillating magnet arrays. Nanotechnology. 2008;19(40):405102. doi: 10.1088/0957-4484/19/40/405102. [DOI] [PubMed] [Google Scholar]
- 19.Yun J, et al. A novel adenoviral vector labeled with superparamagnetic iron oxide nanoparticles for real-time tracking of viral delivery. J Clin Neurosci. 2012;19(6):875–880. doi: 10.1016/j.jocn.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasongkla N, et al. Multifunctional polymeric micelles as cancer-targeted, MRIultrasensitive drug delivery systems. Nano Lett. 2006;6(11):2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 21.Dalby B, et al. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33(2):95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Guerra-Crespo M, et al. Polyethylenimine improves the transfection efficiency of primary cultures of post-mitotic rat fetal hypothalamic neurons. J Neurosci Methods. 2003;127(2):179–192. doi: 10.1016/s0165-0270(03)00125-0. [DOI] [PubMed] [Google Scholar]
- 23.Caraglia M, et al. Nanotech revolution for the anti-cancer drug delivery through blood-brain barrier. Curr Cancer Drug Targets. 12(3):186–196. doi: 10.2174/156800912799277421. [DOI] [PubMed] [Google Scholar]
- 24.Schwerdt JI, et al. Magnetic field-assisted gene delivery: achievements and therapeutic potential. Curr Gene Ther. 2012;12(2):116–126. doi: 10.2174/156652312800099616. [DOI] [PubMed] [Google Scholar]
- 25.Vainauska D, et al. A novel approach for nucleic acid delivery into cancer cells. Medicina (Kaunas) 2012;48(6):324–329. [PubMed] [Google Scholar]
- 26.Yang SY, et al. Ex vivo magnetofection with magnetic nanoparticles: a novel platform for nonviral tissue engineering. Artif Organs. 2008;32(3):195–204. doi: 10.1111/j.1525-1594.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 27.Plank C, et al. Enhancing and targeting nucleic acid delivery by magnetic force. Expert Opin Biol Ther. 2003;3(5):745–758. doi: 10.1517/14712598.3.5.745. [DOI] [PubMed] [Google Scholar]
- 28.Plank C, et al. The magnetofection method: using magnetic force to enhance gene delivery. Biol Chem. 2003;384(5):737–747. doi: 10.1515/BC.2003.082. [DOI] [PubMed] [Google Scholar]
- 29.Plank C, et al. MagnetofectionTM platform: from magnetic nanoparticles to novel nucleic acid therapeutics. Ther Deliv. 2011;2(6):717–726. doi: 10.4155/tde.11.37. [DOI] [PubMed] [Google Scholar]
- 30.Brix G, et al. Static magnetic fields affect capillary flow of red blood cells in striated skin muscle. Microcirculation. 2008;15(1):15–26. doi: 10.1080/10739680701410850. [DOI] [PubMed] [Google Scholar]
- 31.Repacholi MH. Electromagnetic fileds and cancer: How ICNIRP has dealt with the issue. International Commission of Non Ionizing Radiation Protection. 1996 [Google Scholar]
- 32.Leavey JA. Magnetic field safety guide. Cornell University Environmental Health and Safety Standard Operating Procedure. 2010;MFS 1 ver 4:1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.