Abstract
After trauma, obese patients have an increased risk of developing acute kidney injury (AKI). We have demonstrated that obese Zucker (OZ) rats, but not lean Zucker (LZ) rats, develop AKI 24 h after orthopedic trauma. ROS have been implicated in the pathophysiology of AKI in models of critical illness. However, the contribution of ROS to trauma-induced AKI in the setting of obesity has not been determined. We hypothesized that AKI in OZ rats after trauma is mediated by increased oxidative stress. Male LZ and OZ rats were divided into control and trauma groups, with a subset receiving treatment after trauma with the antioxidant apocynin (50 mg/kg ip, 2 mM in drinking water). The day after trauma, glomerular filtration rate, plasma creatinine, urine kidney injury molecule-1, and albumin excretion as well as renal oxidant and antioxidant activity were measured. After trauma, compared with LZ rats, OZ rats exhibited a significant decrease in glomerular filtration rate along with significant increases in plasma creatinine and urine kidney injury molecule-1 and albumin excretion. Additionally, oxidative stress was significantly increased in OZ rats, as evidenced by increased renal NADPH oxidase activity and urine lipid peroxidation products (thiobarbituric acid-reactive substances), and OZ rats also had suppressed renal superoxide dismutase activity. Apocynin treatment significantly decreased oxidative stress and AKI in OZ rats but had minimal effects in LZ rats. These results suggest that ROS play an important role in AKI in OZ rats after traumatic injury and that ROS may be a potential future therapeutic target in the obese after trauma.
Keywords: obesity, trauma, acute kidney injury, reactive oxygen species, inflammation
orthopedic trauma is one of the leading causes of morbidity and mortality in the United States each year, particularly in those under the age of 45 yr (7). Recently, clinical studies have shown that obese patients have an increased incidence of posttrauma remote organ failure, including acute kidney injury (AKI) (5, 13, 28), which is characterized by increased plasma creatinine, decreased glomerular filtration rate (GFR), and, often, renal morphological damage (19). However, the causative mechanisms for this, as well as the disparate pathophysiological responses to trauma in the context of obesity, are not well understood. When AKI does occur in the critically ill, it is associated with increased risk of subsequent multiple organ failure, chronic kidney disease, and mortality (29, 49, 55). Thus, it is important to understand why it occurs more commonly in obese trauma patients so that preventative and therapeutic strategies can be developed for this patient population.
ROS are a byproduct of normal metabolism and have many roles in physiological function within the kidney (3, 17, 40, 41). When ROS are produced in excess and overpower the antioxidant systems, the resulting oxidative stress can have harmful effects (22, 41). ROS and oxidative stress have recently been implicated in the pathogenesis of AKI in various animal models, including that induced by ischemia-reperfusion (39, 54), sepsis (38), severe burns (9), toxins (44), and rhabdomyolysis (51). However, there are few studies that have investigated the role that ROS may play in AKI after orthopedic trauma, and while imbalance between the prooxidant and antioxidant systems has been well recognized to be present in the context of obesity (33), it is not known if ROS play a role in the increased AKI incidence seen in the context of obesity after trauma.
The present study built on our previous work describing a novel model of orthopedic trauma in which obese Zucker (OZ) rats, a commonly used model of metabolic syndrome (8), develop AKI within 24 h after the injury, whereas no AKI occurs in lean Zucker (LZ) rats (35). In the present study, we tested the hypothesis that acute inhibition of ROS production after orthopedic trauma would mitigate the subsequent development of AKI in OZ rats. To investigate the involvement of ROS in the development of posttrauma AKI in the context of obesity, we evaluated renal function and tubular damage markers along with the activities of oxidant and antioxidant systems and the closely linked inflammatory cascades.
METHODS
Animals.
Male LZ and OZ rats were purchased from Harlan Laboratories (Indianapolis, IN). All rats were between 12 and 13 wk of age at the time of experimentation. Rats were fed standard Harlan Teklad rat chow ad libidum, housed at 2–3 rats/cage, and were exposed to a 12:12-h light-dark cycle. All experiments were performed with approval from the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Experimental design and treatment.
The following eight experimental groups were included in the present study (the control animals were those with no trauma and the trauma animals were those that had undergone orthopedic trauma 24 h earlier): 1) LZ control, 2) LZ control with sham apocynin treatment, 3) LZ trauma, 4) LZ trauma with apocynin treatment, 5) OZ control, 6) OZ control with sham apocynin treatment, 7) OZ trauma, and 8) OZ trauma with apocynin treatment. The sham treatment groups (groups 2 and 6) were used only for mean arterial pressure (MAP), GFR, and plasma creatinine measurements because differences in major outcomes were not seen with the sham treatment (see Table 1 and Fig. 1), whereas the other six groups were used for all measurements. We chose the 24-h time point after trauma because clinical studies have shown that AKI commonly occurs within the first 48 h after trauma (6). In the treatment groups, apocynin (Sigma-Aldrich, St. Louis, MO) was administered directly after trauma via intraperitoneal injection (50 mg/kg), and it was also dissolved in drinking water (2 mM) and given to rats for 24 h after trauma, as we have previously described (56). Half of the animals in each group were used for urine collection and subsequent decapitation for blood and tissue collections, whereas the other half were used for systemic and renal hemodynamic measurements. The same animals could not be used for both, due to the infusion of a radioactive substance required for the renal function measurement, as described below.
Table 1.
Body weights and mean arterial pressure levels in LZ and OZ rats in control and trauma groups with or without apocynin treatment
| LZ Rats |
OZ Rats |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | Control + apocynin | Trauma | Trauma + apocynin | Control | Control + apocynin | Trauma | Trauma + apocynin | |
| Body weight, g | 321 ± 11 | 325 ± 5 | 312 ± 5 | 312 ± 5 | 470 ± 9* | 470 ± 8* | 472 ± 9* | 498 ± 14* |
| Mean arterial pressure, mmHg | 125 ± 2 | 127 ± 4 | 122 ± 2 | 124 ± 2 | 132 ± 2* | 134 ± 3 | 121 ± 3+ | 122 ± 1+ |
Values are means ± SE; n = 6–8 rats/group. LZ, lean Zucker; OZ, obese Zucker.
P < 0.05 vs. the corresponding LZ groups; †P < 0.05 vs. the control OZ rat group.
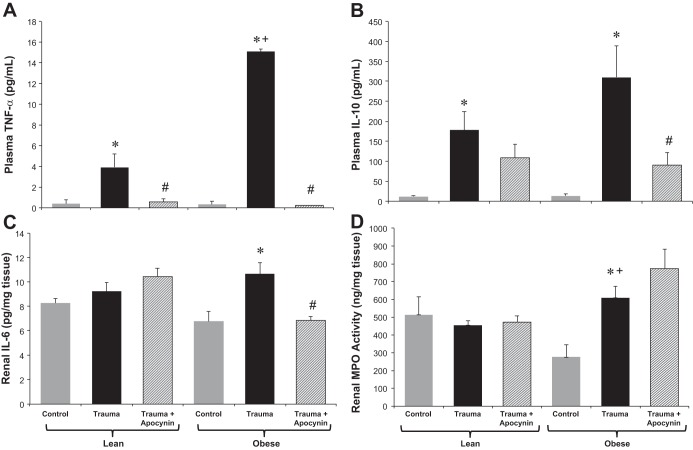
Fig. 1.
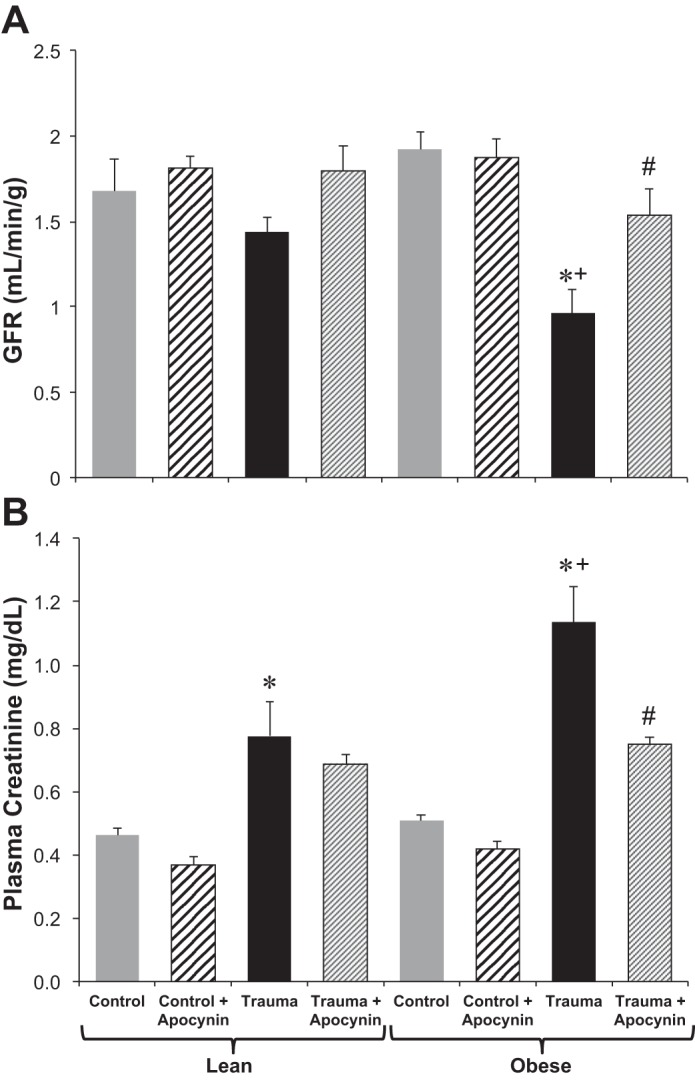
Glomerular filtration rate (GFR; A) and plasma creatinine (B) in lean Zucker (LZ) and obese Zucker (OZ) rats in control and trauma groups with or without apocynin treatment. n = 6–8 rats/group. *P < 0.05 vs. control LZ or OZ rats, respectively; +P < 0.05 vs. LZ rats with trauma; #P < 0.05 vs. OZ rats with trauma.
Orthopedic trauma.
Trauma was performed on LZ and OZ rats, as previously described by our laboratory (35, 56, 57). Briefly, rats were anesthetized with isoflurane (5% via inhalation), and the retrofemoral tissue groups were clamped bilaterally for 30 s with an angled Kelly clamp (19 cm) to induce soft tissue injury. Using a 15-gauge needle, the fibulas were fractured, and 1.5 ml of homogenized bone components (2 g/5 ml PBS) were then injected into the area of the injured tissue bilaterally. The bones for the bone component injection were collected from previously euthanized LZ and OZ rats. To provide analgesia, buprenorphine was injected subcutaneously at a dose of 0.01 mg/kg directly before the trauma and every 8–12 h after trauma at a dose of 0.05 mg/kg. After the brief period of anesthesia, rats regained consciousness and were able to ambulate, had ready access to food and water, and were monitored for evidence of distress.
Systemic hemodynamic and renal function measurements.
In rats used for MAP and GFR measurements, polyethylene catheters (PE-50, BD, Franklin Lakes, NJ) were inserted into the common carotid artery and external jugular vein, and rats were allowed to recover for at least 5 h before the start of the experiments. MAP was measured over a 30-min period (to reach a stable level) in control rats and those who had trauma 24 h earlier using a PowerLab data-acquisition system and LabChart software (AD Instruments, Colorado Springs, CO). GFR (reported as ml·min−1·g kidney wt−1) was measured in control rats and those who had trauma 24 h earlier over a 2.5-h period via the constant infusion method, as previously described (35), using a scintillation counter (LS 6500, Beckman Coulter, Brea, CA) to determine levels of infused tritiated inulin (3H; Perkin-Elmer Health Sciences, Shelton, CT).
Urine measurements.
Rats were placed in metabolic cages for 24 h for urine collection. Urine albumin excretion was measured via ELISA (NR002, Exocell, Philadelphia, PA), and creatinine excretion was measured by colorimetric assay (CR01, Oxford Biomedical Research, Rochester Hills, MI) to acquire the 24-h albumin-to-creatinine ratio. Kidney injury molecule (KIM)-1 excretion was measured by ELISA (RKM100, R&D Systems, Minneapolis, MN) and urine thiobarbituric acid-reactive substances (a nonspecific marker of lipid peroxidation and oxidative stress) were measured by assay (KGE013, R&D Systems). All urine markers were normalized by creatinine to account for differences in urine output between rats.
Tissue and plasma collections.
After urine collection, rats were decapitated for blood and tissue collections. Blood was collected in EDTA-lined tubes in ice and centrifuged at 3,000 rpm in 4°C, and the supernatant was stored in a −80°C freezer for later use. Tissues were harvested immediately, with central hilar slices of the kidneys being placed in formalin for fixation and later histological analysis, whereas the kidney poles were snap frozen in liquid nitrogen. Frozen kidney sections were homogenized via the standard method (45) in RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA) containing protease inhibitors (Genentech, San Francisco, CA) and centrifuged at 12,000 g for 20 min in 4°C, and aliquots of the homogenates were stored in a −80°C freezer for later analysis.
Renal tissue homogenate measurements.
Renal IL-6 was measured via ELISA (R6000B, R&D Systems). Total SOD activity in the renal tissue was measured using an assay kit (catalog no. 706002, Cayman Chemical, Ann Arbor, MI). Superoxide production by NADPH oxidase (NOX) was measured via chemiluminescence detection using a Berthold luminometer after incubation in 4 μM lucigenin, as previously described (11, 58). Myeloperoxidase (MPO) activity was determined using an EnzChek assay kit (E33856, Life Technologies, Grand Island, NY). All renal tissue measurements were normalized by tissue protein concentration (Pierce BCA Protein Assay, Thermo Scientific, Rockford, IL).
Plasma measurements.
Plasma TNF-α (RAB0479, Sigma-Aldrich) and IL-10 (R1000, R&D Systems) were measured via ELISA. Plasma creatinine was measured via colorimetric assay (CR01, Oxford Biomedical Research).
Statistical analyses.
All data are presented as means ± SE. ANOVA was used to make comparisons, and, when differences were observed, Tukey post hoc analyses were performed to evaluate differences between groups. All statistical comparisons were performed using SPSS statistical software (version 22, IBM, New York, NY). Statistically significant differences were deemed to be present when P < 0.05.
RESULTS
Body weights and MAP.
As shown in Table 1, rats in the OZ groups weighed more than the rats in the LZ groups, with no significant differences in weight within LZ or OZ rat groups. MAP levels were not different within any of the LZ rat groups (control vs. 24 h after trauma, with and without apocynin treatment), whereas OZ rats with trauma and those with trauma and apocynin treatment had decreased MAPs compared with control OZ rats with or without treatment with apocynin.
GFR and creatinine.
Figure 1, A and B, shows GFR and plasma creatinine levels, respectively. The day after trauma, OZ rats had a significant decrease in GFR, whereas LZ rats did not. Apocynin treatment mitigated the decrease in GFR in OZ rats with trauma, whereas sham apocynin treatment did not change GFR in either rat group. Plasma creatinine was increased in both LZ and OZ rats after trauma, with the increase being more significant in OZ rats compared with LZ rats. Apocynin significantly decreased plasma creatinine in OZ rats with trauma, whereas it had no effect in LZ rats with trauma or in sham apocynin-treated LZ or OZ animals.
Urine KIM-1 and albumin.
Urine KIM-1 and albumin excretion are shown in Fig. 2, A and B, respectively. KIM-1 excretion was increased significantly in OZ rats with trauma, both compared with LZ rats with trauma and control OZ rats. Apocynin treatment significantly decreased KIM-1 excretion in OZ rats with trauma. The urine albumin-to-creatinine ratio was unchanged in LZ rats, whereas it was increased in OZ rats after trauma, with apocynin treatment significantly decreasing it in OZ rats.
Fig. 2.
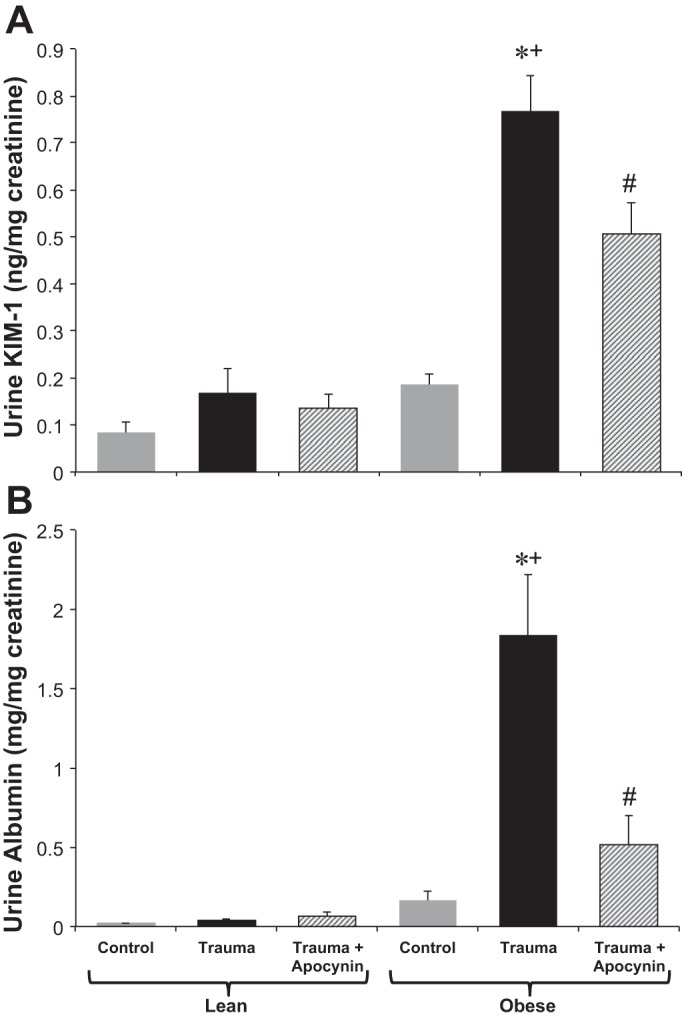
Twenty-four-hour urine kidney injury molecule (KIM)-1 (A) and urine albumin-to-creatinine ratio (B) in LZ and OZ rats in control and trauma groups with or without apocynin treatment. n = 6–8 rats/group. *P < 0.05 vs. control LZ or OZ rats, respectively; +P < 0.05 vs. LZ rats with trauma; #P < 0.05 vs. OZ rats with trauma.
Oxidant/antioxidant activity.
Renal NOX activity was significantly increased after trauma in OZ rats but not in LZ rats, and apocynin treatment decreased its activity (Fig. 3A). Renal total SOD activity was decreased in control OZ rats compared with control LZ rats and in OZ rats with trauma compared with LZ rats with trauma. Neither trauma nor apocynin treatment had an effect on SOD activity in either LZ or OZ rats (Fig. 3B). Urine thiobarbituric acid-reactive substance levels were higher at baseline in OZ rats and increased in OZ rats but not in LZ rats after trauma, whereas apocynin treatment prevented this increase (Fig. 3C).
Fig. 3.
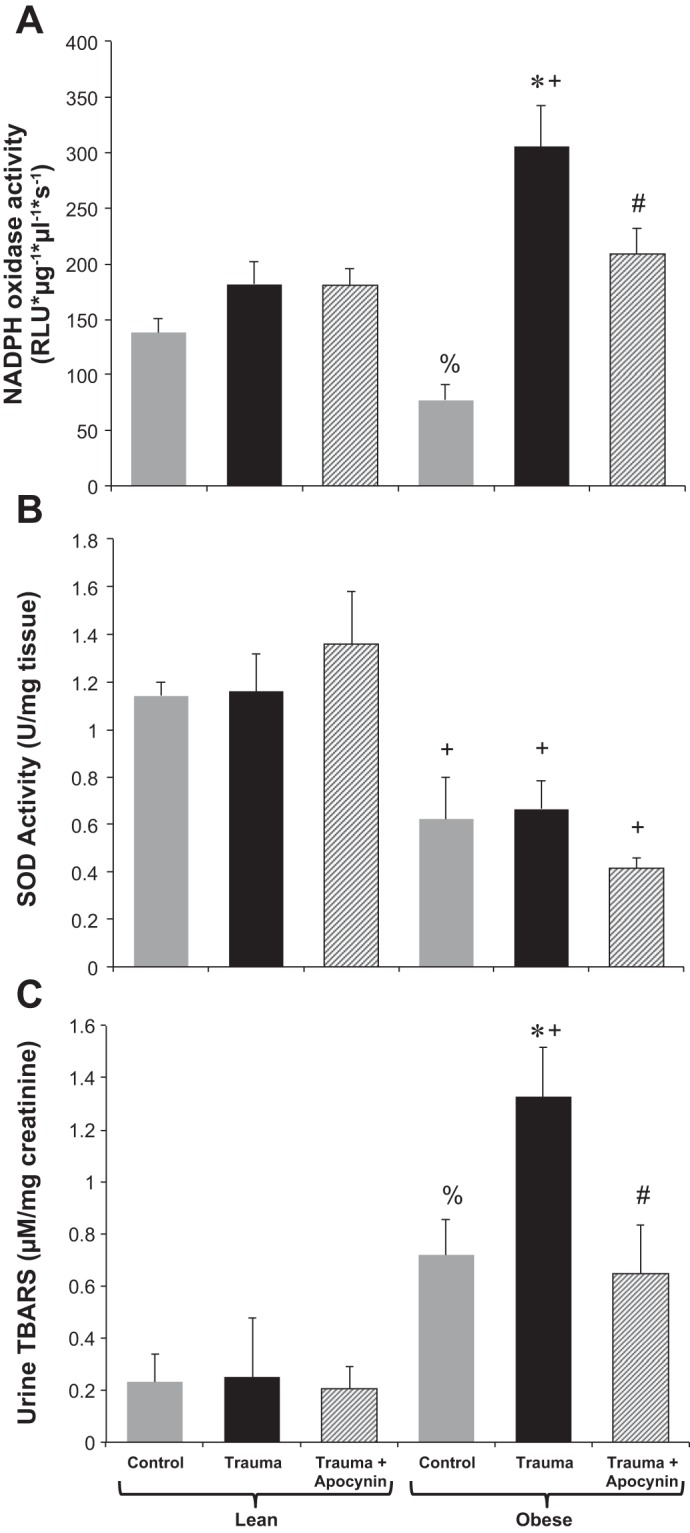
NADPH oxidase activity (A) and total SOD activity (B) in homogenized renal tissue as well as urine thiobarbituric acid-reactive substances (TBARS; C) in LZ and OZ rats in control and trauma groups with or without apocynin treatment. n = 6–8 rats/group. *P < 0.05 vs. control OZ rats; +P < 0.05 vs. the corresponding LZ group; #P < 0.05 vs. OZ rats with trauma; %P < 0.05 vs. control LZ rats.
Systemic and renal inflammatory markers.
In both LZ and OZ rats, plasma TNF-α levels were increased after trauma, with the increase being significantly greater in OZ rats. Apocynin treatment decreased levels of TNF-α in both groups after trauma (Fig. 4A). Plasma IL-10 levels were increased in both LZ and OZ rats the day after trauma, whereas apocynin significantly decreased levels in OZ rats with trauma (Fig. 4B). Renal IL-6 levels were increased in OZ rats after trauma but not in LZ rats, and this increase was blunted by apocynin treatment (Fig. 4C). MPO activity in the kidney was increased in OZ rats after trauma but not in LZ rats, whereas apocynin treatment had no effect (Fig. 4D).
Fig. 4.
A and B: plasma TNF-α (A) and IL-10 (B) in LZ and OZ rats in control and trauma groups with or without apocynin treatment. C and D: IL-6 (C) and myeloperoxidase (MPO) activity (D) in homogenized renal tissue of LZ and OZ rats in control and trauma groups with or without apocynin treatment. n = 6–8 rats/group. *P < 0.05 vs. control LZ or OZ rats, respectively; +P < 0.05 vs. LZ rats with trauma; #P < 0.05 vs. OZ rats with trauma.
DISCUSSION
Based on previous studies showing that ROS are involved in the pathophysiology of AKI in various animal models, the goal of the present study was to determine if ROS contribute to the development of AKI after orthopedic trauma in obesity (5, 13). In agreement with our previous work, we found that the day after trauma, OZ rats had decreased GFR and increased systemic inflammation compared with LZ rats (35). New findings from this study include the following: 1) OZ rats have increased tubular injury compared with LZ rats after trauma, 2) OZ rats have increased renal ROS production after trauma compared with LZ rats as well as impaired baseline and posttrauma antioxidant activity, and 3) apocynin treatment attenuates AKI in OZ rats and reduces systemic and renal inflammation.
Increased renal oxidative stress in OZ rats after trauma.
Studies have shown that ROS are acutely upregulated in the setting of critical illness and can have adverse effects on a number of organs (1, 18, 31, 52). However, little work has been done comparing the obese and lean acute oxidative stress responses to critical illness. We demonstrated that OZ rats, compared with LZ rats, have elevated oxidative stress after trauma, as evidenced by increased lipid peroxidation products in the urine after trauma. In addition, we found increased renal NOX activity along with suppressed SOD activity. This suggests that, after trauma, renal oxidative stress is exacerbated in OZ rats due at least in part to an imbalance between superoxide generation and scavenging. Our previous studies have demonstrated that OZ rats exhibit early posttrauma hyperglycemia, which contributes to increased lung NOX activity and acute lung injury (56, 57). Recent studies by others have also demonstrated that acute bouts of hyperglycemia can lead to increases in ROS levels (32, 48). The contributions of posttrauma hyperglycemia to renal NOX activity and AKI development after trauma in OZ rats are unclear and will be elucidated in future studies. In addition, the present study showed decreased renal SOD activity in control OZ rats compared with control LZ rats, consistent with previous findings in obese humans and animals (12, 23). Although the mechanisms have not been thoroughly determined, these results may reveal the importance of antioxidant treatment in the obese after trauma. Indeed, we have found that apocynin treatment effectively prevents the development of AKI (present study) and acute lung injury (56) in OZ rats after trauma.
While apocynin has historically been used as an inhibitor of NOX, it has been shown to have various nonselective antioxidant effects (15, 46), making it difficult to deduce the exact source of ROS production in our model. An important area of future research will be to better understand the mechanisms and sources responsible for the exacerbated ROS levels seen in the context of obesity after trauma. However, apocynin was ideal for use in this study because it is a highly efficacious therapy, even when used acutely (56).
Oxidative stress-mediated renal damage in OZ rats after trauma.
We observed a decrease in GFR and an increase in plasma creatinine the day after trauma in OZ rats compared with LZ rats. By the latest clinical criteria [set forth by the Kidney Disease: Improving Global Outcomes study (19)], the renal dysfunction exhibited by our OZ rats falls well within the range of an AKI diagnosis. Apocynin treatment partially prevented this decrease in GFR and increase in plasma creatinine, suggesting that ROS are important in mediating posttrauma AKI in OZ rats. While ROS can cause direct damage to renal tubular epithelial cells (9, 39) as well as endothelial cells (16, 43), they may also have an effect on renal hemodynamics. For example, superoxide has been shown to enhance tubuloglomerular feedback-mediated afferent arteriolar vasoconstriction (10, 27). We previously reported a large decrease in renal plasma flow the day after trauma in OZ rats but not in LZ rats (35). However, the contribution of ROS on hemodynamics would be difficult to determine in an injured kidney. This is because the interactions among renal hemodynamics, function, and injury are extremely complex, and the changes in perfusion and filtration can either be a cause or a consequence of renal cellular injury (4). Thus, a hemodynamic change after apocynin treatment, if present, would not prove a direct effect of ROS on renal hemodynamics.
Changes in systemic blood pressure can affect renal perfusion. We did observe a significant decrease (∼11 mmHg) in MAP in OZ rats after trauma, whereas in LZ rats MAP was unchanged. However, it is unlikely that the decrease in GFR is caused by this decrease in MAP. Blood pressure levels are still within the autoregulatory range of the kidney, even of OZ rats, which may have an altered baseline renal autoregulatory response (30, 47). Additionally, OZ rats with trauma that were treated with apocynin exhibited a similar decrease in MAP and had significantly higher GFR levels than untreated OZ rats with trauma. The increased GFR is not likely to be due to nonspecific vasodilator effects of apocynin, as the increase in GFR was only observed in OZ rats with trauma treated with apocynin and not LZ rats with trauma or sham groups treated with it.
In experimental models of AKI, ROS have been shown to contribute to renal damage (9, 38, 39, 54) by eliciting renal tubular cell damage and apoptosis (9, 20, 39) as well as renal endothelial cell dysfunction (16, 43). In our study, we observed an increase in urine KIM-1 and albumin excretion after trauma in OZ rats but not in LZ rats. KIM-1 is excreted in the urine after damage to renal tubular cells, particularly those in the proximal tubule (14, 50), and has shown promise as a clinical biomarker for early AKI (42). Albumin is well known to be excreted in the urine in the setting of chronic kidney disease and has also been recently shown to be a biomarker for AKI (53). Tubular injury can perpetuate AKI by leading to renal vasoconstriction, coagulopathy, and microvascular obstruction (2). Nevertheless, the elevated urine excretion of KIM-1 and albumin after trauma in OZ rats was mitigated by apocynin, suggesting that ROS are involved in the development of the intrinsic AKI. An important consideration is whether the ROS are inducing necroptosis in the kidneys of OZ rats (26), and this will be determined in future studies.
Oxidative stress and inflammation.
After trauma, we observed a greater increase in plasma TNF-α in OZ rats than in LZ rats, whereas apocynin treatment significantly decreased levels of the cytokine in both groups. We previously reported an increase in plasma IL-6 levels in OZ and LZ rats after trauma (56). These findings suggest that although OZ and LZ rats had the same injury, OZ rats may have an exaggerated innate immune response after trauma. This is important because studies have shown that markers of inflammation have a strong, direct correlation with outcomes after traumatic injury. High levels of TNF-α have the potential to induce necroptosis in renal cells (25), and our data suggest that the increases in plasma TNF-α could be a downstream mechanism by which ROS could be inducing renal injury. Plasma IL-10 levels were also increased in both OZ and LZ rats, whereas apocynin treatment reduced its levels only in OZ rats. This suggests that 24 h after trauma is a period where both the pro- and anti-inflammatory systems are upregulated. Renal IL-6 levels were increased in OZ rats but not in LZ rats after trauma, which is in agreement with our previous data showing increased macrophage infiltration after trauma in the kidneys of OZ rats (35). Interestingly, renal MPO activity, a marker of neutrophil activity, was increased in OZ rats compared with LZ rats after trauma. However, apocynin did not decrease MPO levels, suggesting that the increased MPO activity does not mediate AKI, which is different from what we observed with the development of posttrauma acute lung injury, where MPO does appear to play a major role (56).
The relationship between ROS and inflammation has been well documented; each has been shown to be able to stimulate the other, leading to a potentially damaging positive feedback cycle (34, 37). Our data suggest that after trauma, oxidative stress is upstream from inflammation, as shown by apocynin treatment effectively reducing inflammatory cell concentrations in both the plasma and kidney. Other investigators have shown similar anti-inflammatory effects of apocynin (21, 46). However, inhibition of inflammatory pathways has also been shown to reduce oxidative stress and associated organ damage (24, 36). Further studies are needed to better understand the relationship of these variables in the posttrauma physiological milieu as well as the contribution of each to remote organ injury.
Perspectives and significance.
The present study shows that ROS are involved in the pathophysiology of posttrauma AKI, particularly in the context of obesity. Future studies should further elucidate the timeline and mechanisms of ROS-induced kidney injury and clarify the cellular sources and causes of increased ROS upregulation in the obese after trauma. Given that ROS have many roles in normal physiological function, it is critical to understand the appropriate balance between oxidant and antioxidant activity after trauma in lean and obese patients to improve patient outcomes.
GRANTS
This work was supported by National Institutes of Health Grants HL-89581 and HL-51971 (to R. L. Hester) and P20-GM-104357 (to L. Xiang), American Heart Association Grant 12SDG12050525 (to L. Xiang), and an Orthopaedic Trauma Association Basic Science Research Grant (to R. L. Hester). P. N. Mittwede received Predoctoral Fellowship 14PRE17810005 from the American Heart Association Greater Southeast Affiliate.
DISCLAIMER
This content is solely the responsibility of the authors, and does not necessarily represent the official views of the funding organizations.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.N.M., L.X., J.S.C., and R.L.H. conception and design of research; P.N.M., L.X., and S.L. performed experiments; P.N.M. analyzed data; P.N.M., L.X., S.L., and R.L.H. interpreted results of experiments; P.N.M. prepared figures; P.N.M. drafted manuscript; P.N.M., L.X., S.L., J.S.C., and R.L.H. edited and revised manuscript; P.N.M., L.X., S.L., J.S.C., and R.L.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Huimin Zhang for technical assistance with this project.
P. N. Mittwede presented part of this work at the Experimental Biology meeting in 2014 in San Diego, CA.
REFERENCES
- 1.Abilés J, de la Cruz AP, Castaño J, Rodríguez-Elvira M, Aguayo E, Moreno-Torres R, Llopis J, Aranda P, Argüelles S, Ayala A, de la Quintana AM, Planells EM. Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: a cohort study. Crit Care 10: R146, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 380: 756–766, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Belmont PJ Jr, Garcia EJ, Romano D, Bader JO, Nelson KJ, Schoenfeld AJ. Risk factors for complications and in-hospital mortality following hip fractures: a study using the National Trauma Data Bank. Arch Orthop Trauma Surg 134: 597–604, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Bihorac A, Delano MJ, Schold JD, Lopez MC, Nathens AB, Maier RV, Layon AJ, Baker HV, Moldawer LL. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg 252: 158–165, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention, National Vital Statistics Reports. Deaths: Preliminary Data for 2011 (online). www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06. pdf [23 Jul 2014]. [Google Scholar]
- 8.Fellmann L, Nascimento AR, Tibiriça E, Bousquet P. Murine models for pharmacological studies of the metabolic syndrome. Pharmacol Ther 137: 331–340, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Feng Y, Liu Y, Wang L, Cai X, Wang D, Wu K, Chen H, Li J, Lei W. Sustained oxidative stress causes late acute renal failure via duplex regulation on p38 MAPK and Akt phosphorylation in severely burned rats. PLoS One 8: e54593, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Y, Lu Y, Liu EY, Zhu X, Mahajan GJ, Lu D, Roman RJ, Liu R. Testosterone enhances tubuloglomerular feedback by increasing superoxide production in the macula densa. Am J Physiol Regul Integr Comp Physiol 304: R726–R733, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Y, Zhang R, Lu D, Liu H, Chandrashekar K, Juncos LA, Liu R. NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa. Am J Physiol Regul Integr Comp Physiol 298: R707–R712, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glance LG, Li Y, Osler TM, Mukamel DB, Dick AW. Impact of obesity on mortality and complications in trauma patients. Ann Surg 259: 576–581, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int 82: 867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev 63: 218–242, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Kan WH, Hsieh CH, Schwacha MG, Choudhry MA, Raju R, Bland KI, Chaudry IH. Flutamide protects against trauma-hemorrhage-induced liver injury via attenuation of the inflammatory response, oxidative stress, and apoptosis. J Appl Physiol 105: 595–602, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care 17: 204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JI, Kim J, Jang HS, Noh MR, Lipschutz JH, Park KM. Reduction of oxidative stress during recovery accelerates normalization of primary cilia length that is altered after ischemic injury in murine kidneys. Am J Physiol Renal Physiol 304: F1283–F1294, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, Cho YS, Chun E, Lee KY. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol 90: 441–448, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Kleikers PW, Wingler K, Hermans JJ, Diebold I, Altenhöfer S, Radermacher KA, Janssen B, Görlach A, Schmidt HH. NADPH oxidases as a source of oxidative stress and molecular target in ischemia/reperfusion injury. J Mol Med (Berl) 90: 1391–1406, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Krautbauer S, Eisinger K, Lupke M, Wanninger J, Ruemmele P, Hader Y, Weiss TS, Buechler C. Manganese superoxide dismutase is reduced in the liver of male but not female humans and rodents with non-alcoholic fatty liver disease. Exp Mol Pathol 95: 330–335, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Zhang H, Huang W, Qian H, Li Y. TNF-α inhibitors with anti-oxidative stress activity from natural products. Curr Top Med Chem 13: 1408–1421, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Linkermann A, Green DR. Necroptosis. N Engl J Med 30:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Liu T, Chen JJ, Bai XJ, Zheng GS, Gao W. The effect of obesity on outcomes in trauma patients. Injury 44: 1145–1152, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manzanares W, Dhaliwal R, Jiang X, Murch L, Heyland DK. Antioxidant micronutrients in the critically ill: a systematic review and meta-analysis. Crit Care 16: R66, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariappan MM, DeSilva K, Sorice GP, Muscogiuru G, Jimenez F, Ahuja S, Barnes JL, Choudhury GG, Musi N, DeFronzo R, Kasinath BS. Combined acute hyperglycemic and hyperinsulinemic clamp induced profibrotic and proinflammatory responses in the kidney. Am J Physiol Cell Physiol 306: C202–C211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 7: e330–e341, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20: 1126–1167, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mittwede PN, Xiang L, Lu S, Clemmer JS, Hester RL. A novel experimental model of orthopedic trauma with acute kidney injury in obese Zucker rats. Physiol Rep 1: e00097, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo TNF-α inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol 287: H1813–H1820, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 1: 103–115, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair AR, Masson GS, Ebenezer PJ, Del Piero F, Francis J. Role of TLR4 in lipopolysaccharide-induced acute kidney injury: protection by blueberry. Free Radic Biol Med 71: 16–25, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Qiao X, Li RS, Li H, Zhu GZ, Huang XG, Shao S, Bai B. Intermedin protects against renal ischemia-reperfusion injury by inhibition of oxidative stress. Am J Physiol Renal Physiol 304: F112–F119, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedeek M, Nasrallah R, Touyz RM, Hébert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol 24: 1512–1518, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao X, Tian L, Xu W, Zhang Z, Wang C, Qi C, Ni Z, Mou S. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One 9: e84131, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh A, Ramnath RD, Foster RR, Wylie EC, Fridén V, Dasgubta I, Haraldsson B, Welsh GI, Mathieson PW, Satchell SC. Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS One 8: e55852, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin HS, Yu M, Kim M, Choi HS, Kang DH. Renoprotective effect of red ginseng in gentamicin-induced acute kidney injury. Lab Invest 94: 1147–1160, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Soljancic A, Ruiz AL, Chandrashekar K, Maranon R, Liu R, Reckelhoff JF, Juncos LA. Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. Am J Physiol Regul Integr Comp Physiol 304: R951–R958, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm 2008: 106507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stepp DW, Boesen El Sullivan JC, Mintz JD, Hair CD, Pollock DM. Obesity augments vasoconstrictor reactivity to angiotensin II in the renal circulation of the Zucker rat. Am J Physiol Heart Circ Physiol 293: H2537–H2542, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Su H, Ji L, Xing W, Zhang W, Zhou H, Qian X, Wang X, Gao F, Sun X, Zhang H. Acute hyperglycaemia enhances oxidative stress and aggravates myocardial ischaemia/reperfusion injury: role of thioredoxin-interacting protein. J Cell Mol Med 17: 181–191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290: F517–F529, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Shah SV, Liu H, Baliga R. Inhibition of cytochrome P450 2E1 and activation of transcription factor Nrf2 are Renoprotective in myoglobinuric acute kidney injury. Kidney Int 86: 338–349, 2014. [DOI] [PubMed] [Google Scholar]
- 52.Ware LB, Fessel JP, May AK, Roberts LJ 2nd. Plasma biomarkers of oxidative stress and development of organ failure in severe sepsis. Shock 36: 12–17, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ware LB, Johnson AC, Zager RA. Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury. Am J Physiol Renal Physiol 300: F628–F638, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winterberg PD, Wang Y, Lin KM, Hartono JR, Nagami GT, Zhou XJ, Shelton JM, Richardson JA, Lu CY. Reactive oxygen species and IRF1 stimulate IFNα production by proximal tubules during ischemic AKI. Am J Physiol Renal Physiol 305: F164–F172, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wohlauer MV, Sauaia A, Moore EE, Burlew CC, Banerjee A, Johnson J. Acute kidney injury and posttrauma multiple organ failure: the canary in the coal mine. J Trauma Acute Care Surg 72: 373–378, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Xiang L, Lu S, Mittwede PN, Clemmer JS, Hester RL. Inhibition of NADPH oxidase prevents acute lung injury in obese rats following severe trauma. Am J Physiol Heart Circ Physiol 306: H684–H689, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiang L, Lu S, Mittwede PN, Clemmer JS, Husband GW, Hester RL. β2-Adrenoreceptor blockade improves early post-trauma hyperglycemia and pulmonary injury in obese rats. Am J Physiol Heart Circ Physiol 307: H621–H627, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Chandrashekar K, Lu Y, Duan Y, Qu P, Wei J, Juncos LA, Liu R. Enhanced expression and activity of Nox2 and Nox4 in the macula densa of ANG II-induced hypertensive mice. Am J Physiol Renal Physiol 306: F344–F350, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]