Abstract
Renalase is a recently identified FAD/NADH-dependent amine oxidase mainly expressed in kidney that is secreted into blood and urine where it was suggested to metabolize catecholamines. The present study evaluated central and peripheral dopaminergic activities in the renalase knockout (KO) mouse model and examined the changes induced by recombinant renalase (RR) administration on plasma and urine catecholamine levels. Compared with wild-type (WT) mice, KO mice presented increased plasma levels of epinephrine (Epi), norepinephrine (NE), and dopamine (DA) that were accompanied by increases in the urinary excretion of Epi, NE, DA. In addition, the KO mice presented an increase in urinary DA-to-l-3,4-dihydroxyphenylalanine (l-DOPA) ratios without changes in renal tubular aromatic-l-amino acid decarboxylase (AADC) activity. By contrast, the in vivo administration of RR (1.5 mg/kg sc) to KO mice was accompanied by significant decreases in plasma levels of Epi, DA, and l-DOPA as well as in urinary excretion of Epi, DA, and DA-to-l-DOPA ratios notwithstanding the accompanied increase in renal AADC activity. In addition, the increase in renal DA output observed in renalase KO mice was accompanied by an increase in the expression of the L-type amino acid transporter like (LAT) 1 that is reversed by the administration of RR in these animals. These results suggest that the overexpression of LAT1 in the renal cortex of the renalase KO mice might contribute to the enhanced l-DOPA availability/uptake and consequently to the activation of the renal dopaminergic system in the presence of renalase deficiency.
Keywords: knockout mouse model; renal dopamine; l-3,4-dihydroxyphenylalanine; epinephrine
the catecholamines epinephrine (Epi), dopamine (DA), and norepinephrine (NE) play a key role in the regulation of blood pressure and sodium homeostasis through their action on central and peripheral adrenergic and dopaminergic receptors. The known pathway for the metabolism of these compounds involves uptake by neuronal and extraneuronal tissues and degradation by the intracellular enzymes monoamine oxidases A and B (MAO-A and -B) and catechol-O-methyltransferase (COMT) (1, 21, 34).
In kidney, the epithelial cells of proximal tubules (PT) are endowed with a high aromatic-l-amino acid decarboxylase (AADC) activity, and filtered or circulating l-3,4-dihydroxyphenylalanine (l-DOPA) can be converted to DA after being taken up into this cellular compartment (14, 20, 31). The candidate transport systems for l-DOPA in renal PT cells include Na+-dependent amino acid transport systems, like the neutral amino acid exchanger ASCT2 (SLC1A5), the broad specific neutral amino acid transporter B0AT1, and Na+-independent system L-type amino acid transporters like L-type amino acid transporter like (LAT) 1 and LAT2 (24–25).
DA of renal origin exerts natriuretic and diuretic effects by activating D1-like receptors located at various regions in the nephron (18). The availability of DA to activate its specific receptors is determined by the interplay between the rate of synthesis and the degree of degradation of the amine (32) by deamination to 3,4-dihydroxyphenylacetic acid (DOPAC), O-methylation to 3-metoxytyramine (3-MT), and deamination plus O-methylation to homovanillic acid (HVA) (2, 9, 11). Previous work from our group demonstrated that both selective and combined inhibition of MAO-A and COMT did not change renal DA excretion, natriuresis, or phosphaturia, notwithstanding the marked decrease in the urinary excretion of the corresponding metabolites (28). This observation suggested that other metabolic pathways besides COMT and MAO might be involved in the regulation of renal DA output.
Renalase is a recently identified FAD/NADH-dependent amine oxidase mainly expressed in kidney that is secreted into blood where it was suggested to metabolize catecholamines with preference for Epi as well as the catecholamine precursor l-DOPA (6, 8, 37, 38). When administered in vivo, renalase acutely lowers blood pressure in a dose-dependent manner, and this was accompanied by decreased cardiac contractility and heart rate without compensatory changes in peripheral vascular tone, thus suggesting that these hemodynamic effects may be accounted for by circulating catecholamine degradation (3, 4).
In addition to the kidney, renalase is also highly expressed in other organs, namely in the heart, intestine, skeletal muscle, liver, as well as in the peripheral and central nervous system (CNS) (15, 16, 38). Thus, one can hypothesize that renalase may be involved not only in circulating catecholamine degradation but also in tissue-specific functions, including the regulation of DA levels in the CNS and peripheral organs (5).
In the present work, we used the renalase knockout (KO) mouse model to gain insight into the influence of renalase deficiency on central and peripheral dopaminergic activities and to evaluate the changes induced by recombinant renalase (RR) administration on plasma and urine catecholamines levels, namely DA and the correspondent metabolites.
MATERIALS AND METHODS
Metabolic studies.
Renalase-deficient (KO) mice were generated as previously described (36).
Male C57BL/6J mice were used as the wild-type (WT) group (Charles River, Barcelona, Spain). All in vivo experiments were performed in accordance to European Directive number 86/609, transposed to the Portuguese Law by DL 129/92 and by Portaria 1005/92, and the rules of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (17). The present study was approved by the scientific committee of the Faculty of Medicine of the University of Porto.
Both KO and WT groups of mice used were 7–8 wk old. All animals were kept under controlled environmental conditions (12:12-h light-dark cycle and room temperature 22 ± 2°C) and were fed ad libitum throughout the study with a standard diet (SAFE A04, by Scientific Animal Food and Engineering). Fluid intake and food consumption were monitored daily throughout the study. The daily sodium intake averaged 25 mmol/kg body wt.
Both groups of animals were placed in metabolic cages (Tecniplast, Buguggiate-VA, Italy) for later determination of biochemical parameters and 24-h quantification of catecholamines and metabolites (29). On the day of death the animals were anaesthetized with pentobarbital sodium (50 mg/kg body wt ip), and blood was collected directly from the heart in tubes containing heparin for later determination of plasma catecholamines and biochemical parameters. Thereafter, the kidneys, heart, gastrocnemius muscle, jejunum, and brain were rapidly removed and weighted. Tibia length was also measured for organ weight normalization. Fragments of renal cortex, jejunum, and brain were stored for later quantification of catecholamines and enzymatic studies.
In some experiments, renalase KO mice aged 7–8 wk received RR (1.5 mg/kg) or the vehicle (25 mM BisTris, 10 mM NaCl, 10% glycerol, 0.5 mM dithiothreitol, and 1 mM EDTA, pH 6.5) by a single subcutaneous injection 24 h before death as previously described (6).
Plasma and urine electrolytes and biochemistry.
The quantification of sodium and potassium in plasma and urine samples was performed by ion-selective electrodes. Creatinine was measured by the Jaffé method, whereas urea was measured by an enzymatic test. Total proteins in plasma were quantified by the Biuret reaction. All determinations were performed by a Pentra 200 analyzer (ABX Diagnostics, Geneva, Switzerland).
Assay of catecholamines.
The quantification of catecholamines and its derivatives in urine, plasma samples, renal tissues, brain, and jejunum and in samples from enzymatic studies was performed by high-pressure liquid chromatography with electrochemical detection, as previously described (35). In our laboratory, the lower limit of detection of NE, Epi, l-DOPA, DA, DOPAC, 3-MT, and HVA ranged from 350 to 1,000 fmol.
AADC activity.
Fragments of renal cortex were homogenized at 4°C with a Thomas Teflon homogenizer (Poliscience) in incubation medium containing (in mM): 0.35 NaH2PO4, 0.15 Na2HPO4, 0.11 Na2B4O7, and 0.2 pyridoxal phosphate (pH 7.0). Activity of AADC was determined as previously described by Soares-da-Silva (33) using l-DOPA as substrate (0.1–10 mM; Sigma). The assay of DA was performed by HPLC with electrochemical detection.
MAO-A and -B activities.
Fragments of renal cortex and brain were homogenized in 67 mM phosphate buffer (pH 7.2) at 4°C with a Thomas Teflon homogenizer. MAO activity was determined with 5-[3H]hydroxytryptamine (20–2,000 μM; Sigma) as a preferential substrate for MAO-A and β-[14C]phenylethylamine (0.25–250 μM; Sigma) as a preferential substrate for MAO-B (12). Aliquots of 50 μl of the homogenates were incubated for 10 min with 50 μl of each substrate. At the end of the incubation period the tubes were transferred to ice, and the reaction was stopped by the addition of 50 μl of perchloric acid (2 M). The deaminated products were extracted with ethyl acetate and measured by liquid scintillation counting.
COMT activity.
Fragments of renal cortex and brain were homogenized in 5 mM phosphate buffer (pH 7.8) at 4°C with a Thomas Teflon homogenizer. COMT activity was evaluated by the methylation of Epi (0.01–500 μM; Sigma) to metanephrine (MN), as previously described (23). The assay of MN was performed by HPLC with electrochemical detection.
Immunoblot analysis.
For the determination of LAT1, LAT2, and ASCT2 expression in renal cortex extracts, equal amounts of total protein, determined using the method of Bradford (6), were separated on a 12% SDS-polyacrylamide gel and electrotransferred to a nitrocellulose membrane in Tris-glycine transfer buffer containing 20% methanol. The transblot sheets were blocked in 5% nonfat dry milk in PBS for 60 min and then incubated overnight at 4°C with specific primary antibodies (rabbit anti-LAT1 H-75 antibody, goat anti-LAT2 G-19 antibody, and mouse anti-GAPDH antibody from Santa Cruz Biotechnology and rabbit anti-ASCT2 antibody from USBiological Life Sciences). The immunoblots were subsequently washed and incubated with the respective fluorescently labeled secondary antibody for 1 h at room temperature and protected from light. Membranes were washed and imaged by scanning at 800 nm with the Odyssey Infrared System (LI-COR Biosciences).
Renalase-induced catecholamine oxidation.
The ability of RR to oxidize DA to its corresponding aminochrome was examined by addition of RR (20 μg) (6) in buffer containing 25 mmol/l Tris, 5 mmol/l NaCl, and 250 mmol/l NADH (pH 7.5), to DA (1 mM), during 10 min. Protein precipitation was immediately performed by an acidification with perchloric acid (10%) followed by a rapid neutralization with 0.76 M KHCO3 and centrifuged for 60 s at 16,000 g. Samples were immediately injected into a HPLC system (Waters model 2690) with a photodiode array detector (Waters model 996).
As previously described (27), the chromatograms were analyzed at 279 nm, which corresponds to the maximum absorption wavelength of catecholamine, and at 490 nm, which corresponds to the most specific wavelength of the aminochrome. Control experiments of oxidation of catecholamine to the correspondent aminochrome were carried out using NaIO4 (2 mM).
Statistics.
All data are presented as means ± SE. P < 0.05 was assumed to denote a significant difference. For group comparison, one-way ANOVA followed by a post hoc Tukey test or standard unpaired Student's t-test were used when appropriate. Data were analyzed using Microsoft Excel 2010 and GraphPad Prism 5 software.
RESULTS
Baseline characteristics.
The baseline characteristics of the KO mice, namely renal function and sodium balance, did not differ from those of the corresponding WT controls (Table 1). The KO mice presented increased urine volume without differences in daily urinary excretion of either sodium or potassium (Table 1).
Table 1.
Metabolic balance in WT and renalase KO mice
| WT | KO | |
|---|---|---|
| Plasma | ||
| Creatinine, mg/dl | 0.36 ± 0.01 | 0.38 ± 0.003 |
| Urea, mg/dl | 43.84 ± 3.37 | 41.41 ± 2.52 |
| Protein, g/dl | 37.98 ± 1.43 | 36.87 ± 1.90 |
| Na+, mmol/l | 143.75 ± 1.06 | 143.27 ± 0.66 |
| K+, mmol/l | 6.17 ± 0.28 | 5.67 ± 0.34 |
| Urine | ||
| Volume, μl/24 h | 640.32 ± 95.36 | 1,009.26 ± 105.88* |
| Na+, mmol/24 h | 1.31 ± 0.11 | 1.42 ± 0.20 |
| K+, mmol/24 h | 3.26 ± 0.44 | 3.46 ± 0.24 |
Values represent mean values ± SE; n = 9–11 experiments. WT, wild-type; KO, knockout. Significantly different from corresponding values in WT mice (
P < 0.05).
Compared with WT mice, the renalase KO mice presented increased renal and cardiac mass and decreased skeletal muscle mass without differences in either body weight or tibia length (Table 2).
Table 2.
Body and organ weights in WT and renalase KO mice
| WT | KO | |
|---|---|---|
| Body weight, g | 20.2 ± 0.3 | 19.6 ± 0.7 |
| Tibia lenght, cm | 1.7 ± 0.02 | 1.6 ± 0.02 |
| Right kidney, mg | 130.5 ± 3.1 | 147.8 ± 4.9* |
| Left kidney, mg | 120.5 ± 4.0 | 141.3 ± 5.1* |
| Heart, mg | 101.0 ± 1.8 | 108.4 ± 1.5* |
| Left ventricle, mg | 69.5 ± 1.2 | 74.8 ± 1.8* |
| Skeletal muscle, mg | 117.6 ± 3.1 | 102.4 ± 6.5* |
| Brain, mg | 305.1 ± 3.1 | 287.0 ± 12.6 |
Values represent mean values ± SE; n = 9–11 experiments. Significantly different from corresponding values in WT mice (
P < 0.05).
Plasma catecholamine levels.
Compared with WT mice, the KO mice presented increased plasma levels of both Epi and NE by ∼254 and 58%, respectively (Fig. 1A). In addition, the plasma levels of DA and the deaminated metabolite DOPAC were significantly increased in KO mice compared with the WT group by ∼87 and 22%, respectively (Fig. 1A). Compared with WT mice, the plasma levels of the DA precursor l-DOPA were also increased in KO mice by ∼41%, although the difference did not reach statistical significance (Fig. 1A).
Fig. 1.
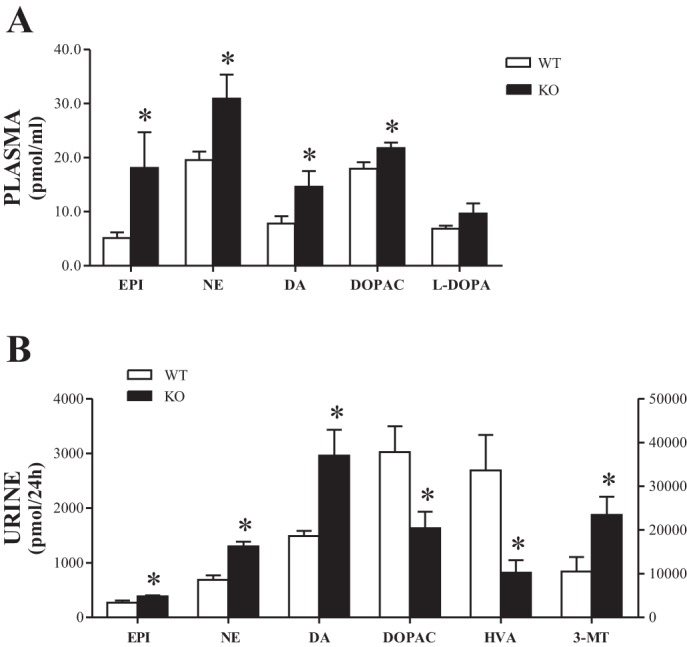
Epinephrine (Epi), norepinephrine (NE), l-3,4-dihydroxyphenylalanine (l-DOPA), dopamine (DA), and DA metabolite levels in plasma (A) and urine (B). Bars represent mean values ± SE. *Knockout (KO) mice significantly different from corresponding values in wild-type (WT) mice (n = 9–11). l-DOPA, DA, and 3,4-dihydroxyphenylacetic acid (DOPAC) correspond to left y-axis; homovanillic acid (HVA) and 3-metoxytyramine (3-MT) correspond to right y-axis.
Renal DA system.
Compared with WT mice, the daily urinary excretions of Epi, NE, and DA were significantly increased in KO mice by ∼42, 89, and 99%, respectively (Fig. 1B). This was accompanied in KO mice by an approximately fourfold increase in the urinary DA-to-l-DOPA ratios (Fig. 3A) compared with WT mice (2.41 ± 0.54 vs. 9.45 ± 2.37, P < 0.01) without changes in renal AADC activity between the two groups (Table 3).
Fig. 3.
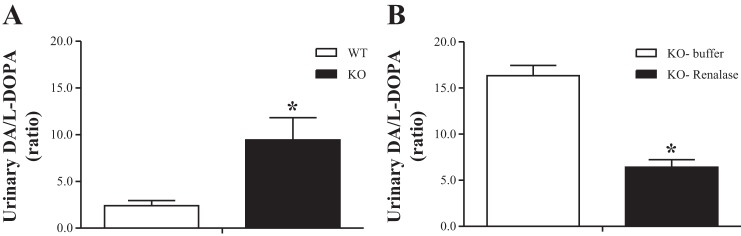
Urinary DA-to-l-DOPA ratios in WT and KO mice at baseline (A) and in KO mice treated with buffer or recombinant renalase (B). Bars represent mean values ± SE. *P < 0.01, KO mice significantly different from corresponding values in WT mice (n = 9–11).
Table 3.
Kinetic parameters (Vmax and Km) of enzymatic activities of AADC, MAO-A, MAO-B, and COMT in homogenates of renal cortex from WT and renalase KO mice
| Renal Cortex | WT | KO |
|---|---|---|
| AADC | ||
| Vmax, nmol·mg protein−1·h−1 | 106.4 ± 5.2 | 103.5 ± 5.9 |
| Km, mM | 1.4 ± 0.23 | 1.5 ± 0.28 |
| MAO-A | ||
| Vmax, nmol·mg protein−1·h−1 | 12.09 ± 0.87 | 10.45 ± 0.55 |
| Km, μM | 1,001.0 ± 145.0 | 878.3 ± 99.6 |
| MAO-B | ||
| Vmax, nmol·mg protein−1·h−1 | 1.77 ± 0.07 | 1.22 ± 0.05* |
| Km, μM | 48.2 ± 5.3 | 50.60 ± 5.6 |
| COMT | ||
| Vmax, nmol·mg protein−1·h−1 | 3.68 ± 0.07 | 3.99 ± 0.13* |
| Km, μM | 2.5 ± 0.22 | 2.2 ± 0.32 |
Values represent mean values ± SE; n = 9–11 experiments.
Vmax, maximal velocity; Km, Michaelis constant; AADC, aromatic-l-amino acid decarboxylase; MAO-A and -B, monoamine oxidases A and B, respectively; COMT, catechol-O-methyltransferase.
Significantly different from corresponding values in WT mice (
P < 0.05).
Compared with WT mice, the KO mice presented a marked decrease in the daily urinary levels of both DOPAC and HVA, by ∼85 and 229%, respectively (Fig. 1B). This was accompanied in KO mice by a significant decrease in renal MAO-B activity without changes in renal MAO-A activity (Table 3). In addition, the KO mice presented a marked increase in the daily urinary levels of 3-MT by ∼123% (Fig. 1B), which was associated with a significant increase in renal COMT activity (Table 3).
Jejunal DA system.
The jejunal tissue levels of both l-DOPA and DA were similar between KO mice and the WT group (Table 4). The jejunal tissue levels of DOPAC in KO mice were decreased compared with the WT group (Table 4), and this was associated with significant decreases in both MAO-A and MAO-B activities in the jejunum from KO mice (Table 5).
Table 4.
Catecholamines in jejunum from WT and renalase KO mice
| WT | KO | |
|---|---|---|
| l-DOPA | 0.005 ± 0.0004 | 0.005 ± 0.001 |
| DA | 0.03 ± 0.01 | 0.02 ± 0.002 |
| DOPAC | 0.006 ± 0.001 | 0.003 ± 0.0003* |
Values represent mean value ± SE; n = 9–11 experiments. Units are pmol/mg.
l-DOPA, l-3,4-dihydroxyphenylalanine; DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid.
Significantly different from corresponding values in WT mice (
P < 0.05).
Table 5.
Kinetic parameters (Vmax and Km) of enzymatic activities of MAO-A, MAO-B, and COMT in homogenates of brain from WT and renalase KO mice
| Jejunum | WT | KO |
|---|---|---|
| MAO-A | ||
| Vmax, nmol/mg prot/h | 74.76 ± 1.76 | 60.53 ± 3.97* |
| Km, μM | 411.1 ± 26.6 | 355.9 ± 64.3 |
| MAO-B | ||
| Vmax, nmol/mg prot/h | 18.32 ± 1.08 | 13.10 ± 1.30* |
| Km, μM | 61.0 ± 9.1 | 57.9 ± 14.3 |
Values represent mean values ± SE; n = 9–11 experiments. Significantly different from corresponding values in WT mice (
P < 0.05).
Central DA system.
The brain tissue levels of both l-DOPA and DA were similar between KO mice and WT animals (Table 6). The brain tissue levels of DOPAC were significantly increased in KO mice compared with WT mice, and this was associated with a significant increase in brain MAO-A activity (Tables 6 and 7). By contrast, the brain tissue levels of both HVA and 3-MT were significantly decreased in KO mice compared with WT controls, and this was associated with significant decreases in both MAO-B and COMT activities (Tables 6 and 7).
Table 6.
Catecholamines in brain from WT and renalase KO mice
| WT | KO | |
|---|---|---|
| l-DOPA | 0.08 ± 0.01 | 0.07 ± 0.01 |
| DA | 6.27 ± 0.61 | 5.40 ± 0.34 |
| DOPAC | 1.37 ± 0.16 | 1.87 ± 0.12* |
| HVA | 1.73 ± 0.17 | 1.23 ± 0.11* |
| 3-MT | 1.17 ± 0.13 | 0.72 ± 0.08* |
Values represent mean value ± SE; n = 9–11 experiments. Units are pmol/mg.
HVA, homovanillic acid; 3-MT, 3-metoxytyramine.
Significantly different from corresponding values in WT mice (
P < 0.05).
Table 7.
Kinetic parameters (Vmax and Km) of enzymatic activities of MAO-A, MAO-B, and COMT in homogenates of brain from WT and renalase KO mice
| Brain | WT | KO |
|---|---|---|
| MAO-A | ||
| Vmax, nmol/mg prot/h | 9.88 ± 0.53 | 13.01 ± 0.69* |
| Km, μM | 630.1 ± 78.76 | 952.9 ± 115.6 |
| MAO-B | ||
| Vmax, nmol/mg prot/h | 8.61 ± 0.31 | 7.58 ± 0.31* |
| Km, μM | 27.9 ± 3.4 | 27.3 ± 3.9 |
| COMT | ||
| Vmax, nmol/mg prot/h | 1.47 ± 0.05 | 0.94 ± 0.04* |
| Km, μM | 7.1 ± 0.8 | 7.5 ± 1.1 |
Values represent mean values ± SE; n = 9–11 experiments. Significantly different from corresponding values in WT mice (
P < 0.05).
Effects of RR on plasma and urine catecholamine levels.
The administration of RR did not change the baseline characteristics of KO mice, namely renal function, sodium balance, as well as body and organ weights.
The administration of RR to KO mice significantly reduced the plasma levels of Epi by ∼61%, and also decreased the plasma levels of NE by ∼39%, although the difference did not reach statistical significance (Fig. 2A). In addition, the administration of RR to KO mice significantly reduced the plasma levels of both DA and l-DOPA by ∼38 and 57%, respectively (Fig. 2A). Moreover, the administration of RR to KO mice decreased the plasma levels of DOPAC by ∼27%, although the difference did not reach statistical significance (Fig. 2A).
Fig. 2.
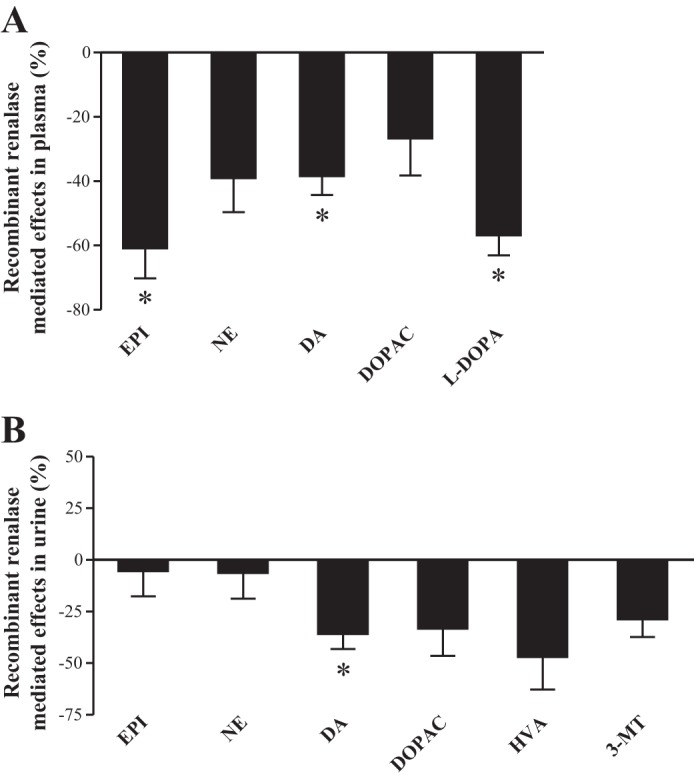
Recombinant renalase-mediated effects in Epi, NE, l-DOPA, DA, DOPAC, HVA, and 3-MT levels in plasma (A) and urine (B). Bars represent mean values ± SE, expressed as %control. *KO mice treated with recombinant renalase significantly different from corresponding values in KO mice treated with vehicle (n = 4–8).
The administration of RR to KO mice did not change the daily urinary excretion of either Epi or NE (Fig. 2B) but significantly decreased the daily urinary excretion of DA by ∼36% (Fig. 2B). This was accompanied in KO mice receiving the RR by a 60% reduction in the urinary DA-to-l-DOPA ratios (16.34 ± 1.12 vs. 6.43 ± 0.79, P < 0.001) (Fig. 3B). By contrast, the KO mice receiving the RR presented a marked increase in renal AADC activity (maximal velocity = 106.6 ± 11.3 vs. 66.1 ± 7.7 nmol/mg protein/h, P < 0.05). In addition, the administration of RR to KO mice was accompanied by decreases in the daily urinary excretion of DOPAC, HVA, or 3-MT by ∼33, 47, and 29%, respectively, although the differences did not reach statistical significance (Fig. 2B).
Immunoblot analysis.
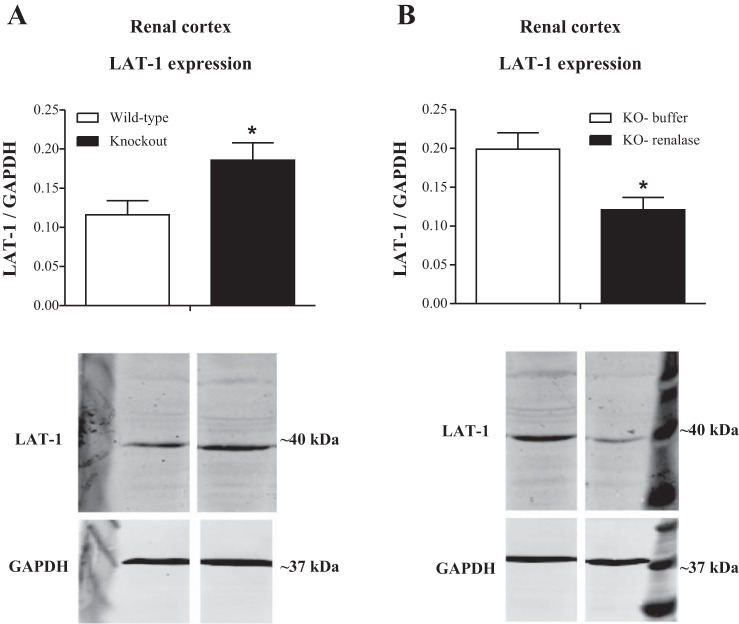
The changes in the amino acid transporters that are potentially involved in the uptake of l-DOPA were evaluated in the renal cortex of both WT and KO animals. As depicted in Fig. 4A, LAT1 expression was significantly increased in KO compared with WT animals (P < 0.04). On the other hand, the administration of RR induced a significant reduction in the expression of LAT1 (P < 0.04) in the renal cortex of the KO animals (Fig. 4B).
Fig. 4.
Semiquantitative analysis and representative immunoblots of the expression of L-type amino acid transporter like (LAT) 1 in the renal cortex from WT and renalase KO mice (A) as well as in KO mice injected with buffer or recombinant renalase (B). Bars represent mean values ± SE. *P < 0.05, KO mice treated with recombinant renalase significantly different from corresponding values in KO mice treated with vehicle (n = 4–8).
LAT2 and ASCT2 expression did not change in the renal cortex of either WT or KO animals. The administration of RR did not change the expression in either LAT2 or ASCT2 (data not shown).
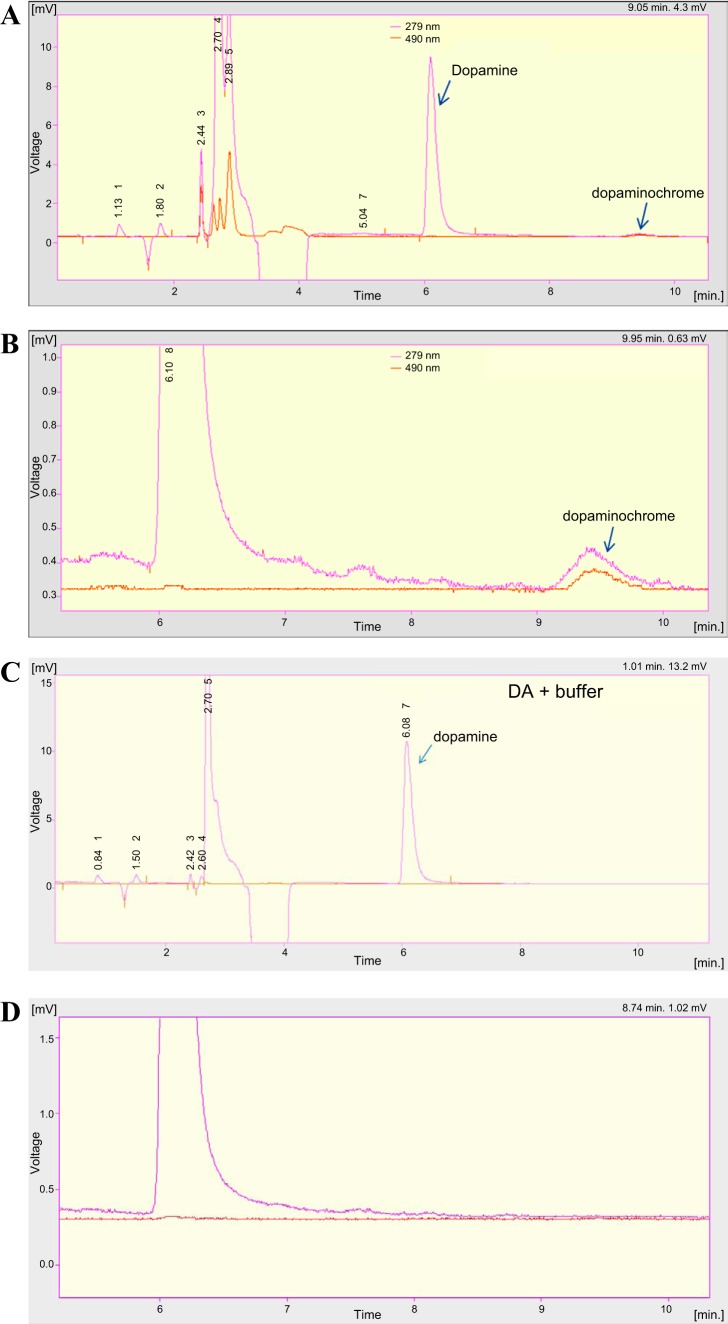
Oxidation of catecholamines by RR.
Figure 5 illustrates the chromatogram showing the oxidation profile of DA obtained in the presence of RR. As can be observed, DA was slightly oxidized to the correspondent aminochrome, dopaminochrome (Fig. 5, A and B). No oxidation of DA was observed in the absence of the RR (negative control in Fig. 5, C and D). This catecholamine was also completely oxidized with NaIO4 to the respective aminochrome (data not shown).
Fig. 5.
Renalase-induced DA oxidation assessed by HPLC-diode array detector (DAD) at normal range (A) and amplified (B). Negative control in the absence of renalase (RR, C) at normal range (A) and amplified (D). The pink line represents the channel at 279 nm, which corresponds to the maximum absorption wavelength of catecholamine, and the orange line represents the channel at 490 nm, which corresponds to the most specific wavelength of the respective aminochrome.
DISCUSSION
The results of the present study show that the increase in sympathetic activity in renalase KO mice is accompanied by enhanced renal DA activity, as was previously reported by Sizova et al. (30). The increased renal DA activity in KO mice was observed notwithstanding an enhanced O-methylation of renal DA and can be explained on the basis of increased renal DA synthesis going along with decreased deamination of the newly formed amine. The finding that the administration of RR to KO mice was accompanied by significant decreases in both plasma l-DOPA levels and in urine DA-to-l-DOPA ratios without changes in the urinary excretion of either DOPAC or 3-MT provides evidence favoring the view that upregulation of the renal DA synthesis may be the main mechanism underlying the increased renal DA output observed in renalase KO mice.
As was previously reported (36), we found significant increases in circulating levels of Epi, NE, and DA in renalase KO mice, thus providing evidence for increased sympathetic activity in the renalase deficiency status. Because Epi was found to be the preferred substrate for renalase (6), it was suggested that the mechanism underlying the increased sympathetic activity in KO mice might be related to Epi-induced release of NE in sympathetic neurons (13, 26). The same mechanism may also account, at least in part, for the increase in circulating DA levels in KO mice because enhanced sympathetic nervous system activity has been shown to increase the secretion of NE and DA (19). The finding that the administration of RR to KO mice induced significant decreases in circulating levels of both Epi and DA is also in agreement with this suggestion.
The increased urine DA output in KO mice was observed in the presence of significant changes in the renal activities of DA-metabolizing enzymes, namely an increase in renal COMT activity and a decrease in renal MAO-B activity, without changes in renal MAO-A activity. Because the urinary excretions of DOPAC and 3-MT are used as good parameters for the assessment of deamination and O-methylation of renal DA, respectively (10–11), the observation that KO mice presented a significant decrease in urinary excretion of DOPAC going along with a significant increase in urinary excretion of 3-MT further reinforces the view that renal DA is less deaminated and more O-methylated in KO mice compared with WT controls. On the other hand, the finding that the urinary excretion of both 3-MT and DOPAC did not increase after RR administration to KO mice suggests that increases in neither deamination nor O-methylation of renal DA can account for the RR-induced decrease in urine DA output. However, one cannot exclude that RR administration may have contributed to decreased renal DA output through oxidation of DA in the corresponding aminochrome as was evidenced in vitro.
In addition to the kidney, renalase is also highly expressed in other organs, namely in the intestine and CNS where it was suggested to contribute to tissue-specific modulation of catecholamine levels (15–16). In the present study, renalase deficiency in KO mice was associated with significant changes in brain and jejunal activities of catecholamine-metabolizing enzymes, namely MAO-A and -B and COMT, which were accompanied by the expected changes in the tissue levels of the corresponding metabolites. These findings, when viewed collectively with the observation that both jejunal and brain tissue levels of DA did not differ between renalase KO mice and WT controls, suggest that in the presence of renalase deficiency both central and peripheral monoaminergic systems may undergo adjustments involving changes in the local activities of enzymes that ultimately regulate local catecholamine levels.
In the present study, renalase deficiency in KO mice was accompanied by a twofold increase in urinary DA levels. The regulation of renal DA output is well recognized to depend mainly on the renal availability/uptake of l-DOPA by renal PT cells, its fast decarboxylation to DA by AADC, as well as on both deamination and methylation of the newly formed amine (2, 9, 11, 22). Because urinary DA-to-l-DOPA ratios are a rough measure of renal uptake/decarboxylation of l-DOPA in PT cells, the finding that renalase KO mice presented a fourfold increase in the urinary DA-to-l-DOPA ratios compared with WT controls provides evidence for enhanced renal DA synthesis in KO mice. Furthermore, the finding that renal AADC activity was similar between KO and WT mice suggests that increased availability/uptake of l-DOPA in renal PT cells might be the main mechanism underlying the enhanced renal DA synthesis in KO mice. The effects of RR administration to KO mice further reinforce this view. Actually, the administration of RR to KO mice significantly decreased both urine DA output and urinary DA-to-l-DOPA ratios notwithstanding the accompanied increase in renal AADC activity. Interestingly, this was accompanied by a RR-induced decrease in plasma levels of l-DOPA, which was recently described as a good substrate for the enzyme (6).
The semiquantitative evaluation of the renal cortical expression of the Na+-independent amino acid transporter LAT1, involved in renal tubular uptake of l-DOPA, also reinforces the view that the increased renal DA activity in renalase KO mice appears to result mainly from an enhanced availability/uptake of l-DOPA in renal PT cells.
Although LAT1 has been previously described to have a very limited tissue distribution in the kidney, in the present study we found that the increase in renal DA output observed in renalase KO mice was accompanied by an increase in LAT1 expression that is reversed by the administration of RR in these animals. These results suggest that the overexpression of LAT1 in the renal cortex of the renalase KO mice might contribute to the enhanced l-DOPA uptake and consequently to the activation of the renal dopaminergic system in the presence of renalase deficiency. This conclusion is supported further by the findings that RR administration induced a marked decrease of LAT1 expression in the renal cortex of renalase KO mice, which would account for decreased l-DOPA uptake, and diminished renal DA output.
In summary, the upregulation of renal DA activity in renalase KO mice appears to result mainly from an enhanced availability/uptake of l-DOPA in renal PT cells that is reverted by the administration of RR.
GRANTS
This study was supported by Grant PIC/IC/83029/2007 from Fundação para a Ciência e a Tecnologia/FEDER. J. Quelhas-Santos is a PhD student supported by the fellowship SFRH/BD/39066/2007 from Fundação para a Ciência e Tecnologia/COMPETE/FEDER. This work was supported in part by VA Connecticut (G. V. Desir), and National Institute of Diabetes and Digestive and Kidney Diseases Grants RC1-DK-086465, RC1-DK-086402, and R01-DK-081037 (G. V. Desir).
DISCLOSURES
G. V. Desir is a named inventor on issued patents for the discovery and use of renalase.
AUTHOR CONTRIBUTIONS
Author contributions: J.Q.-S., B.S.-M., and M.P. conception and design of research; J.Q.-S., M.P.S., I.S.-S., C.F.-C., L.S.-S., M.J.P., and F.R. performed experiments; J.Q.-S., M.P.S., I.S.-S., C.F.-C., L.S.-S., M.J.P., F.R., B.S.-M., G.V.D., and M.P. analyzed data; J.Q.-S., M.P.S., I.S.-S., F.R., B.S.-M., G.V.D., and M.P. interpreted results of experiments; J.Q.-S. prepared figures; J.Q.-S., G.V.D., and M.P. drafted manuscript; J.Q.-S., B.S.-M., and G.V.D. edited and revised manuscript; J.Q.-S., M.P.S., I.S.-S., C.F.-C., L.S.-S., M.J.P., F.R., B.S.-M., G.V.D., and M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Department of Biochemistry and the Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Porto for the facilities. We also thank Dr. Luisa Guardão for veterinarian assistance and Liliana Leite for technical support.
REFERENCES
- 1.Aperia A, Eklöf AC, Holtbäck U, Nowicki S, Sundelöf M, Greengard P. The renal dopamine system. Adv Pharmacol 42: 870–873, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol 62: 621–647, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Desir GV. Regulation of blood pressure and cardiovascular function by renalase. Kidney Int 76: 366–370, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Desir GV. Renalase is a novel renal hormone that regulates cardiovascular function. J Am Soc Hypertens 1: 99–103, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Desir GV. Role of renalase in the regulation of blood pressure and the renal dopamine system. Curr Opin Nephrol Hypertens 20: 31–36, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Desir GV, Tang L, Wang P, Li G, Sampaio-Maia B, Quelhas-Santos J, Pestana M, Velazquez H. Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J Am Heart Assoc 1: e002634, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farzaneh-Far R, Desir GV, Na B, Schiller NB, Whooley MA. A functional polymorphism in renalase (Glu37Asp) is associated with cardiac hypertrophy, dysfunction, and ischemia: data from the heart and soul study. PLoS One 5: e13496, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes MH, Soares-da-Silva P. Sequential involvement of monoamine oxidase and catechol-O -methyltransferase in the metabolism of newly-formed dopamine in rat renal tissues. In: Cardiovascular Renal Actions of Dopamine. London, UK: Pergamon, 1993, p. 21–30. [Google Scholar]
- 10.Fernandes MH, Soares-da-Silva P. Role of monoamine oxidase A and B in the deamination of newly-formed dopamine in the rat kidney. J Neural Transm Suppl 32: 155–159, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes MH, Soares-da-Silva P. Role of monoamine oxidase and catechol-O -methyltransferase in the metabolism of renal dopamine. J Neural Transm Suppl 41: 101–105, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes MH, Soares-da-Silva P. Type A and B monoamine oxidase activities in the human and rat kidney. Acta Physiol Scand 145: 363–367, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Floras JS, Aylward PE, Victor RG, Mark AL, Abboud FM. Epinephrine facilitates neurogenic vasoconstriction in humans. J Clin Invest 81: 1265–1274, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi M, Yamaji Y, Kitajima W, Saruta T. Aromatic l-amino acid decarboxylase activity along the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 258: F28–F33, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Hennebry SC, Eikelis N, Socratous F, Desir G, Lambert G, Schlaich M. Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol Psychiatry 15: 234–236, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Hennebry SC, Eikelis N, Socratous F, Desir G, Lambert GW, Straznicky N, Schlaich MP. Central and peripheral distribution of renalase, a novel soluble monoamine oxidase, in human tissue. J Hypertens 27: S33–S34, 2009. [Google Scholar]
- 17.Institute of Laboratory Animal Research, Commission on Life Sciences, and National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press, 1996. [Google Scholar]
- 18.Jose PA, Raymond JR, Bates MD, Aperia A, Felder RA, Carey RM. The renal dopamine receptors. J Am Soc Nephrol 2: 1265–1278, 1992. [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc J, Ducharme MB. Plasma dopamine and noradrenaline variations in response to stress. Physiol Behav 91: 208–211, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Lee MR. Dopamine and the kidney: ten years on. Clin Sci (Lond) 84: 357–375, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Pestana M, Soares-da-Silva P. Effect of type A and B monoamine oxidase selective inhibition by Ro 41-1049 and Ro 19-6327 on dopamine outflow in rat kidney slices. Br J Pharmacol 113: 1269–1274, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pestana M, Soares-da-Silva P. The renal handling of dopamine originating from l-dopa and gamma-glutamyl-l-dopa. Br J Pharmacol 112: 417–422, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pestana M, Vieira-Coelho MA, Pinto-do OP, Fernandes MH, Soares-da-Silva P. Assessment of renal dopaminergic system activity during cyclosporine A administration in the rat. Br J Pharmacol 115: 1349–1358, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinho MJ, Serrao MP, Soares-da-Silva P. High-salt intake and the renal expression of amino acid transporters in spontaneously hypertensive rats. Am J Physiol Renal Physiol 292: F1452–F1463, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Pinto V, Pinho MJ, Soares-da-Silva P. Renal amino acid transport systems and essential hypertension. FASEB J 27: 2927–2938, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Quinn P, Borkowski KR, Collis MG. Epinephrine enhances neurogenic vasoconstriction in the rat perfused kidney. Hypertension 7: 47–52, 1985. [DOI] [PubMed] [Google Scholar]
- 27.Remiao F, Milhazes N, Borges F, Carvalho F, Bastos ML, Lemos-Amado F, Domingues P, Ferrer-Correia A. Synthesis and analysis of aminochromes by HPLC-photodiode array Adrenochrome evaluation in rat blood. Biomed Chromatogr 17: 6–13, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Sampaio-Maia B, Moreira-Rodrigues M, Pestana M. Role of chronic inhibition of dopamine-metabolizing enzymes in the regulation of renal sodium and phosphate excretion in the rat remnant kidney. Nephron Physiol 103: 14–24, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Sampaio-Maia B, Serrão P, Guimarães JT, Vieira-Coelho MA, Pestana M. Renal dopaminergic system activity in the rat remnant kidney. Nephron Exp Nephrol 99: e46–e55, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Sizova D, Velazquez H, Sampaio-Maia B, Quelhas-Santos J, Pestana M, Desir GV. Renalase regulates renal dopamine and phosphate metabolism. Am J Physiol Renal Physiol 305: F839–F844, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soares-da-Silva P, Fernandes MH. Regulation of dopamine synthesis in the rat kidney. J Auton Pharmacol 10, Suppl 1: s25–s30, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Soares-da-Silva P, Pestana M, Fernandes MH. Involvement of tubular sodium in the formation of dopamine in the human renal cortex. J Am Soc Nephrol 3: 1591–1599, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Soares-Da-Silva P, Serrao MP, Vieira-Coelho MA. Apical and basolateral uptake and intracellular fate of dopamine precursor l-dopa in LLC-PK1 cells Am J Physiol Renal Physiol 274: F243–F251, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Soares-da-Silva P, Vieira-Coelho MA. Nonneuronal dopamine. Adv Pharmacol 42: 866–869, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Vieira-Coelho MA, Hussain T, Kansra V, Serrão MP, Guimarães JT, Pestana M, Soares-Da-Silva P, Lokhandwala MF. Aging, high salt intake, and renal dopaminergic activity in Fischer 344 rats. Hypertension 34: 666–672, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Xu J, Velazquez H, Wang P, Li G, Liu D, Sampaio-Maia B, Quelhas-Santos J, Russell K, Russell R, Flavell RA, Pestana M, Giordano F, Desir GV. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int 79: 853–860, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Xu J, Desir GV. Renalase, a new renal hormone: its role in health and disease. Curr Opin Nephrol Hypertens 16: 373–378, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, Wu Y, Peixoto A, Crowley S, Desir GV. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest 115: 1275–1280, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]