Abstract
Endothelial dysfunction underlies the pathophysiology of vascular disorders such as acute lung injury (ALI) syndromes. Recent work has identified the Abl family kinases (c-Abl and Arg) as important regulators of endothelial cell (EC) barrier function and suggests that their inhibition by currently available pharmaceutical agents such as imatinib may be EC protective. Here we describe novel and differential effects of imatinib in regulating lung pathophysiology in two clinically relevant experimental models of ALI. Imatinib attenuates endotoxin (LPS)-induced vascular leak and lung inflammation in mice but exacerbates these features in a mouse model of ventilator-induced lung injury (VILI). We next explored these discrepant observations in vitro through investigation of the roles for Abl kinases in cultured lung EC. Imatinib attenuates LPS-induced lung EC permeability, restores VE-cadherin junctions, and reduces inflammation by suppressing VCAM-1 expression and inflammatory cytokine (IL-8 and IL-6) secretion. Conversely, in EC exposed to pathological 18% cyclic stretch (CS) (in vitro model of VILI), imatinib decreases VE-cadherin expression, disrupts cell-cell junctions, and increases IL-8 levels. Downregulation of c-Abl expression with siRNA attenuates LPS-induced VCAM-1 expression, whereas specific reduction of Arg reduces VE-cadherin expression in 18% CS-challenged ECs to mimic the imatinib effects. In summary, imatinib exhibits pulmonary barrier-protective and anti-inflammatory effects in LPS-injured mice and lung EC; however, imatinib exacerbates VILI as well as dysfunction in 18% CS-EC. These findings identify the Abl family kinases as important modulators of EC function and potential therapeutic targets in lung injury syndromes.
Keywords: ARDS, VILI, imatinib, c-Abl, Arg, LPS, stretch, endothelium, permeability
acute respiratory distress syndrome (ARDS) is a sudden failure of the respiratory system that occurs in critically ill patients as a consequence of inflammatory conditions including sepsis, pneumonia, gastric acid aspiration, and trauma (37). ARDS is characterized by increased alveolar and vascular permeability, arterial hypoxemia, lung inflammation, and subsequent respiratory failure (37). Despite extensive research on ARDS pathobiology, there are still no effective pharmacological treatments, and supportive care with low tidal volume mechanical ventilation (MV) is the only intervention proven to reduce mortality (58a). However, it is also well established that MV can initiate or aggravate acute lung injury (ALI) syndromes such as ARDS, a process termed ventilator-induced-lung injury (VILI) (50, 56). Inflammatory insults such as endotoxin (LPS) or excessive mechanical stretch (VILI) initiate lung injury through mechanisms that involve endothelial cell (EC) dysfunction as manifested by EC activation, contraction, and leukocyte adhesion (6, 7, 36). Although the mechanisms underlying these processes remain incompletely understood, novel approaches to reverse EC dysfunction may have valuable clinical utility in the treatment of ARDS/VILI.
Recent studies have identified the Abelson (Abl) family of nonreceptor tyrosine kinases, composed of c-Abl and Arg, as novel regulators of endothelial cell (EC) barrier function (12). These kinases are established mediators of multiple cellular processes including adhesion, proliferation, differentiation, and migration (9, 25, 63); however, their role in endothelial barrier regulation has only recently been explored. We previously reported that c-Abl mediates enhancement of the lung EC barrier by the potent agonists S1P or FTY720 (20, 59). In contrast, other reports indicate that c-Abl mediates oxidative stress-induced hyperpermeability (57, 58), whereas Arg mediates EC barrier disruption induced by thrombin or VEGF (2). Additional studies support both similar and distinct roles for c-Abl and Arg kinases in other cellular processes relevant to barrier function including focal adhesion and cytoskeletal dynamics (48, 62). Taken together, these observations support the hypothesis that the Abl family kinases are critical mediators of vascular function and potential targets for ARDS therapeutics.
Imatinib (or STI-571) is a potent inhibitor of c-Abl and Arg that is used to treat Philadelphia chromosome-positive chronic myelogenous leukemia (Ph+CML) and several other malignancies (40, 43, 46). In addition to well-known anticancer effects, imatinib has demonstrated therapeutic potential as an anti-inflammatory and antifibrotic agent in experimental models (51, 61). Furthermore, a recent case report suggests that imatinib has protective effects against vascular edema (1). In contrast to this case report, imatinib treatment frequently results in side effects related to vascular leak, including peripheral, periorbital, and bone edema (18, 19, 38). The mechanisms by which imatinib exerts these differential effects on vascular permeability, as well as its effects on inflammation, remain largely unknown. In the present study, we investigated the effects of imatinib in experimental murine models of acute lung injury (ALI) induced by endotoxin (LPS) or high-tidal-volume MV (HTVMV), both of which are characterized by increased lung vascular permeability and inflammation. We then explored the direct effects of imatinib on human lung EC exposed to ALI/VILI-relevant stimuli [LPS, mechanical cyclic stretch (CS)]. Our data indicate a differential effect of imatinib in LPS vs. CS and suggest that the imatinib targets, c-Abl and Arg, regulate endothelial barrier function in a stimulus-specific manner.
MATERIALS AND METHODS
Murine models of acute lung injury (LPS and VILI).
Male 8- to 10-wk-old wild-type C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used for all experiments. Mice were maintained in autoclaved cages with free access to food and water. All animal experiments were approved by the Animal Care and Use Committees of the University of Illinois at Chicago.
Mice were anesthetized by intraperitoneal injection (IP) of ketamine-xylazine (100 and 5 mg−1·kg−1, respectively) and then treated with imatinib (75 mg/kg) or water (vehicle groups) via IP. For VILI experiments, mice were intubated (20-gauge IV catheter) and placed on MV (Inspira, Harvard Apparatus, Holliston, MA) with room air, using a tidal volume (Vt) of 30 ml/kg, a respiratory rate of 75 breaths/min, and a positive end-expiratory pressure of 0 cmH2O for 4 h. Deep anesthesia was maintained with ketamine-xylazine throughout the experiment. Control mice were intubated and allowed to breathe spontaneously for 4 h (SB groups). For LPS experiments, after intubation, 1 mg/kg of LPS or PBS was instilled intratracheally (IT). Blood, bronchoalveolar lavage (BAL) fluid, and lungs were harvested immediately after LPS (18 h) or VILI (4 h) challenge. BAL was performed by instilling 1 ml of Hanks' balanced salt solution (Invitrogen, Grand Island, NY) through the tracheal cannula into the lungs. Cells were recovered after centrifugation of the lavage fluid (∼0.8 ml) at 500 g for 20 min. In the cell suspension, we measured BAL total numbers of cells using the Bio-Rad TC20 automated cell counter device. A differential cell count (500 cells/sample) was performed on cytospin samples after staining with Kwik Diff Stain (Thermo Scientific, Rockford, IL). The supernatant from BAL fluid was centrifuged again (17,000 g for 10 min) and stored at −80°C for further analysis (protein content, cytokine levels). Lung tissues were snap-frozen in liquid nitrogen or embedded in paraformaldehyde for histology.
Mouse sample analysis.
BAL fluid protein concentration was measured by using the BCA protein assay kit (Thermo Scientific). Lung tissue albumin concentration was measured in lung homogenates by use of a mouse albumin ELISA quantitation kit (Bethyl Labs, Montgomery, TX). Cytokine levels were measured by using the Bio-Rad Bio-Plex Pro Mouse 7-plex Cytokine Assay (Bio-Rad Laboratories, Hercules, CA) according to manufacturer's instructions. For histological evaluation, lung tissue samples fixed in paraformaldehyde were embedded in paraffin, cut into 10-μm sections, and stained with hematoxylin and eosin (H&E). Photomicrographs were taken at ×10 with the Aperio ScanScope. Representative images of histology slides were analyzed by a single individual experienced in lung pathology and blinded to experimental conditions.
Endothelial cell culture.
Human pulmonary artery endothelial cells (HPAEC) were purchased from Lonza (Walkersville, MD) and cultured in the manufacturer's recommended EBM-2 medium containing 10% FBS (Sigma, St. Louis, MO). Cells were grown at 37°C in a 5% CO2 incubator and used at passages 5–7. The Flexcell Strain Unit (FX-5000; Flexcell International, Hillsborough, NC) was used for the cyclic stretch (CS) experiments. HPAEC were plated onto six-well BioFlex plates coated with type I collagen (Flexcell), grown to confluence, and then exposed to pathological (18% elongation) CS for 24 h. Control (no CS) BioFlex plates were kept in static condition in the same incubator. In different experiments, HPAEC were exposed to LPS (E0127:B8, Sigma), 1 μg/ml, for time points as indicated. For all experiments, 10% medium was replaced with 2% medium before imatinib treatment. Imatinib mesylate (PKC Pharmaceuticals/LC Laboratories, Woburn, MA) was dissolved in water and added to cell cultures 1 h prior CS or LPS challenge.
TER measurements.
The integrity of the EC monolayer was evaluated by use of an electrical cell-substrate impedance sensing system (ECIS) (Applied Biophysics, Troy, NY) as we have previously described (22). HPAEC grown on gold microelectrodes were pretreated with imatinib for 1 h and then challenged with LPS (1 μg/ml). Transendothelial electrical resistance (TER) was monitored over time, with TER values normalized to starting resistance. TER values from multiple independent experiments were pooled at discrete time points (maximum LPS effect) by use of custom-designed Epool software and were plotted as means ± SE.
Silencing of c-Abl and Arg.
Reduction of Abl family kinase expression with siRNA was performed by using the Gene silencer transfection reagent (Genlantis, San Diego, CA) according to manufacturer's instructions. Experiments were performed 48 h posttransfection. c-Abl, Arg, and control siRNA were purchased from Santa Cruz Biotechnology (sc-29843, sc-38945, and sc-37003, respectively; Santa Cruz, CA).
Western blotting.
EC were washed with PBS and lysates were prepared in Laemmli's SDS sample buffer (Boston Bioproducts, Ashland, MA). Sample proteins were subjected to SDS-PAGE electrophoresis and then transferred to polyvinylidene difluoride membranes (Bio-Rad). The membranes were incubated overnight (4°C) with primary antibodies including Arg (Novus Biologicals, Littleton, CO), c-Abl (Cell Signaling, Danvers, MA), VCAM-1 and VE-cadherin (Santa Cruz Biotechnology) and β-actin-HRP (Sigma). Secondary antibodies conjugated to horseradish peroxidase (Cell Signaling) were added to membranes for 60 min at room temperature. The Pierce enhanced chemiluminescence detection system (Thermo Scientific) was used to visualize the bands. Band densities were determined by using the Image J software (National Institutes of Health).
Immunofluorescence.
EC were fixed after the indicated experimental conditions in 3.7% formaldehyde (Sigma) for 10 min at room temperature. Cells were then permeabilized with 0.1% Triton X (Sigma) in PBS and stained with VE-cadherin (Santa Cruz) overnight at 4°C. After washing, EC were incubated with secondary antibody (anti-mouse Alexa Fluor 488). For cells grown on Flexcell plates, a piece of the bottom was cut and placed on glass slides. ProLong Gold Antifade reagent (Invitrogen) was used for mounting. Images were taken with a Nikon Eclipse TE2000 microscope.
ELISA.
Following experiments, conditioned medium was collected and centrifuged at 20,000 g for 20 min (4°C). The supernatant was stored at −80°C until use. IL-6 (Biolegend, San Diego, CA) and IL-8 (GenScript, Piscataway, NJ) levels were measured by using commercially available ELISA kits.
Statistical analysis.
Results are expressed as means ± SE. A one-way ANOVA was used for multiple-group comparisons followed by the post hoc Tukey's or Dunnett's test as indicated. Student's t-test was used for the comparison of two groups. Statistical analysis was performed with GraphPad Prism 5 software. P < 0.05 was considered statistically significant.
RESULTS
Effects of imatinib on lung vascular leak in LPS and VILI murine models.
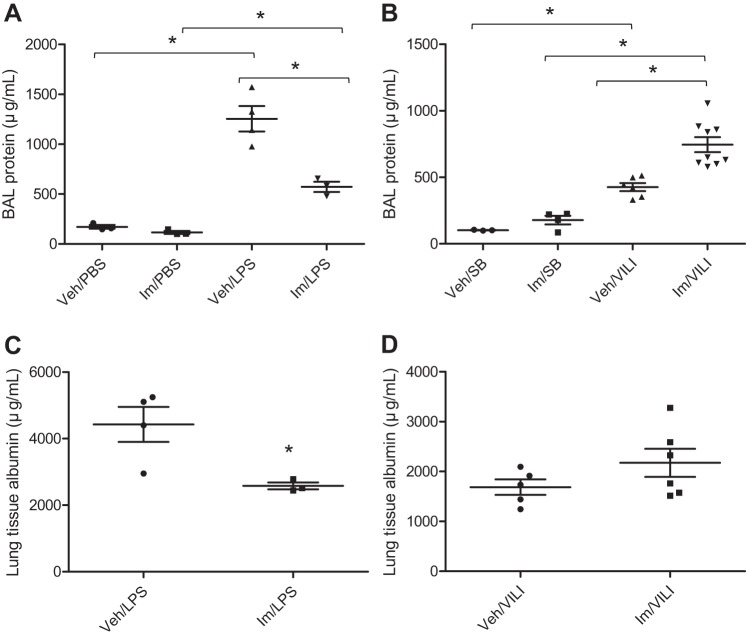
A key event in ALI development is endothelial barrier disruption, which results in vascular leakage and the movement of protein-rich fluid and inflammatory cells into the interstitium and pulmonary air spaces (60). Endothelial disruption can be induced by disease-relevant stimuli including LPS and mechanical CS (6, 24, 39). In this study, we evaluated the effects of imatinib in the well-established murine models of LPS (IT)- and HTVMV (VILI)-induced ALI. As we have previously described, the LPS model is characterized by increased vascular leakage and activation/recruitment of neutrophils to the inflamed lungs (41), whereas the VILI model (Vt: 30 ml/kg) is characterized by more modest increases in pulmonary vascular leak and neutrophil lung recruitment (35). To investigate the effects of imatinib on LPS- or VILI-induced alveolar-capillary permeability, we first measured the concentration of protein in BAL. As demonstrated in Fig. 1, A and B, there is an increase in leakage of proteins into the alveolar space by 7.3-fold and 4.2-fold after challenge with LPS or VILI compared with untreated mice, respectively. Interestingly, imatinib significantly reduced BAL protein levels in LPS-injured mice by 54% (P < 0.01) compared with the LPS-only group (Fig. 1A), but imatinib exhibited the opposite effect in the VILI model and increased BAL protein by 43% compared with VILI alone (P < 0.001) (Fig. 1B). In addition, tissue albumin concentration was determined as another assay for pulmonary endothelial disruption. In this assay, imatinib significantly reduced lung tissue albumin levels by 42% (P < 0.05) in LPS-challenged mice compared with LPS alone (Fig. 1C). In contrast, imatinib had no protective effect in VILI-injured mice but rather caused a 22% increase in lung tissue albumin compared with the untreated VILI group that trended toward significance (P = 0.18) (Fig. 1D). These results demonstrate opposing effects of imatinib on lung permeability in mice depending on the type of ALI insult.
Fig. 1.
Effects of imatinib on lung vascular leak in LPS- and ventilator-induced lung injury (VILI)-treated mice. C57BL/6J mice were injected with imatinib (Im) (75 mg/kg ip) or water [vehicle (Veh)] and then exposed to either LPS (1 mg/kg, 18 h) (vs. PBS) or VILI (30 ml/kg, 4 h) [vs. spontaneously breathing (SB)]. Lung permeability was assessed by measuring the bronchoalveolar lavage (BAL) protein content (A and B) and the albumin concentration in lung homogenates (C and D). Data represent means ± SE (n = 3–8 mice in each group). *P < 0.05, Tukey's post hoc test (A and B); t-test (C and D).
Effects of imatinib on lung histopathology.
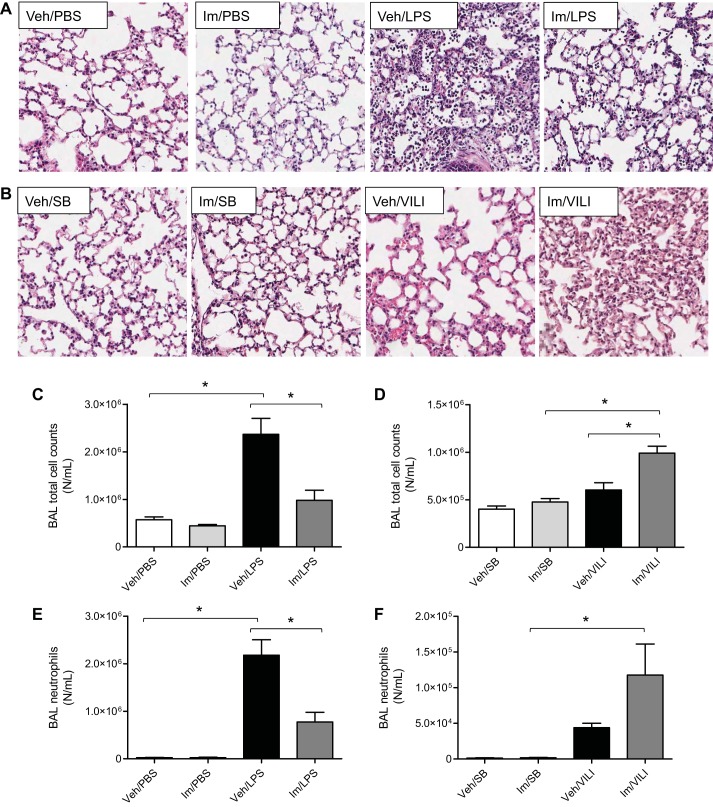
We next examined lung tissue sections stained with H&E to evaluate the effects of imatinib on the histopathological changes induced by LPS (Fig. 2A) or VILI (Fig. 2B). Control groups (no LPS or VILI) injected with vehicle or imatinib displayed normal lung histology without any inflammation. LPS-induced inflammatory cell infiltration, alveolar damage, and interstitial edema compared with the control group, and these histopathological markers of ALI were reduced in imatinib-treated mice (Fig. 2A). VILI induced a milder level of lung injury than LPS, but the addition of imatinib substantially increased the observed inflammatory cell infiltration, alveolar damage, and interstitial edema (Fig. 2B).
Fig. 2.
Effects of imatinib on lung histopathology and inflammation in LPS- and VILI-treated mice. A and B: hematoxylin and eosin staining of lung tissue sections. A: vehicle (Veh) or imatinib (Im) (75 mg/kg) after PBS or LPS (1 mg/kg, 18 h). B: vehicle or imatinib (75 mg/kg) in spontaneously breathing (SB) or VILI (30 ml/kg, 4 h) exposed mice. Images shown are representative lung tissue sections from multiple animals (original magnification, ×100). C and D: BAL total cell counts. E and F: BAL neutrophil cell counts. Data represent means ± SE (n = 3–8 mice in each group). *P < 0.05, Tukey's post hoc test.
Effects of imatinib on lung inflammation.
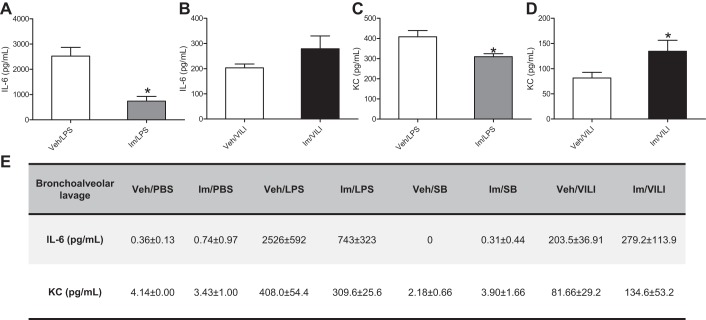
To investigate the effects of imatinib on the infiltration of inflammatory cells into the alveolar space, a characteristic of ALI/ARDS, BAL total and differential cell counts were determined in mice injured by LPS or VILI. Figure 2, C and E, demonstrates that LPS significantly increased BAL total cell counts and neutrophils compared with the untreated group, whereas imatinib significantly reduced this increase in total cells and neutrophils by 58.5% (P < 0.01) and 64.5% (P < 0.01), respectively. In contrast, in the VILI model, imatinib significantly increased BAL total cells by 39% (P < 0.01) and trended toward increased neutrophils (Fig. 2, D and F). In addition, the levels of KC (mouse homolog of IL-8) and IL-6 in BAL were measured in all experimental groups. Imatinib did not affect the basal levels of KC or IL-6 (Fig. 3E), but it significantly reduced the levels of IL-6 by 70% (P = 0.01) and KC by 24% (P = 0.047) in LPS-injured mice (Fig. 3, A and C). In agreement with the previous data, imatinib failed to reduce the levels of inflammatory cytokines in the VILI model but instead increased IL-6 levels by 27.1% (P = 0.16) and KC by 39% (P = 0.044) compared with VILI alone (Fig. 3, B and D).
Fig. 3.
Effects of imatinib on inflammatory cytokine production by LPS or VILI in mice. Mice were injected with imatinib (75 mg/kg ip) and then challenged with LPS (1 mg/kg, 18 h) or VILI (30 ml/kg, 4 h). The levels (pg/ml) of IL-6 (A and B) and KC (C and D) were assayed in the BAL. Data represent means ± SE (n = 3–7 mice per group), *P < 0.05 compared with treated group (LPS or VILI). E: absolute values of cytokines for all experimental groups (means ± SD).
Effects of imatinib on LPS-induced endothelial dysfunction.
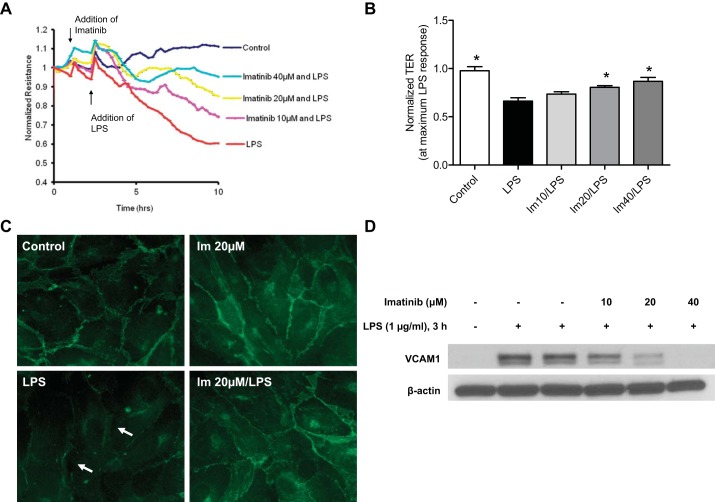
To investigate the effects of imatinib on LPS-induced pathophysiology at the level of the lung endothelium, we next used TER measurements to characterize EC permeability responses to LPS and imatinib. Continuous TER was assayed in confluent HPAEC after treatment with vehicle (water) or imatinib (10, 20, and 40 μM) for 1 h, followed by exposure to LPS (1 μg/ml). LPS significantly decreased EC TER levels over time, indicating increased EC permeability, but imatinib attenuated this response in a dose-dependent manner (Fig. 4, A and B). TER values obtained from multiple independent experiments were pooled at the maximum LPS response (time point at which LPS induces the maximum decrease in TER) to demonstrate that imatinib increased EC TER values compared with LPS alone by 9.8% (P > 0.05), 17.6% (P < 0.05), and 23.6% (P < 0.01) for the 10, 20, and 40 μM concentrations of the inhibitor, respectively (Fig. 4B), indicating protection against LPS-induced EC monolayer disruption.
Fig. 4.
Imatinib prevents LPS-induced endothelial dysfunction. A: representative transendothelial electrical resistance (TER) tracing. Human pulmonary artery endothelial cells (HPAEC) were plated on gold microelectrodes and at the time indicated by arrows were pretreated with imatinib (10, 20, or 40 μM) (Im 10, Im 20, Im 40) followed by stimulation with LPS (1 μg/ml). TER values were normalized to the baseline and then measured over time. Decreased resistance values reflect increases in permeability. B: normalized TER values were pooled from each independent experiment at the time point at which LPS induced the maximum decrease. Data values are means ± SE from 3–5 independent experiments (*P < 0.05 compared with LPS, Tukey's post hoc test). C: immunofluorescence images for VE-cadherin in HPAEC pretreated for 1 h with imatinib (20 μM), followed by LPS challenge (5 h). LPS induced loss of VE-cadherin from the junctions as shown by arrows. Representative images from 3 independent experiments are shown. D: HPAEC were treated with imatinib for 1 h followed by LPS for 3 h. VCAM-1 and β-actin expression were detected by immunoblotting. A representative Western blot is shown demonstrating an upregulation of VCAM-1 expression after LPS challenge that is decreased by imatinib in a dose-dependent manner.
To explore the mechanisms by which imatinib exerts these protective effects on EC permeability, we evaluated its effects on the integrity of cell-cell junctions following LPS challenge. VE-cadherin, a core protein of the adherens junction, is an endothelial-specific cadherin that forms bonds between cells and is required to maintain EC barrier integrity (16). Using immunofluorescence, we observe a strong expression of VE-cadherin in cell-cell contacts in control or imatinib (20 μM, 6 h) treated cells (Fig. 4C). After 5 h of LPS stimulation, there is a significant loss of VE-cadherin from cells junctions with visible formation of intercellular gaps (Fig. 4C, bottom left). Imatinib attenuates these LPS-induced alterations in VE-cadherin distribution and gap formation (Fig. 4C, bottom right). Another pathophysiological characteristic of ALI is increased neutrophil transmigration across the EC monolayer. Upon stimulation by inflammatory agents during ALI, EC are activated, leading to the expression of cellular adhesion molecules (CAMs) which facilitates the adhesion of neutrophils to EC and ultimately infiltration into the lung tissue and alveolar space (36, 53). To assess the effects of imatinib on this process, immunoblotting was performed to analyze in lung EC the levels of VCAM-1, a cell surface receptor that mediates leukocyte adhesion (42). LPS (3 h) induced substantial upregulation of VCAM-1 expression compared with control EC, but imatinib inhibited this increase in a dose-dependent matter (Fig. 4D). Together, these in vitro data indicate that imatinib prevents lung EC barrier disruption and inflammatory activation induced by LPS, suggesting potential mechanisms by which it exerts its protective effects on ALI in LPS-challenged mice (Figs. 1–3).
Effects of imatinib on mechanical CS-induced endothelial dysfunction.
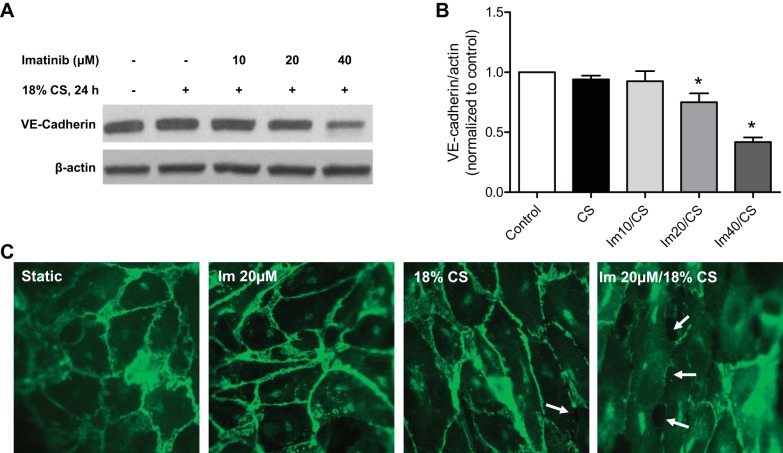
To explore the effects of imatinib on the responses of lung EC to mechanical injury in vitro, a model of pathological 18% CS was used to mimic the injurious forces that are generated during HTVMV and induce vascular barrier dysfunction (6). For these experiments, EC were pretreated with imatinib and then exposed to 18% CS for 24 h. VE-cadherin expression levels in lung EC were decreased in a concentration-dependent manner by imatinib after exposure to 18% CS (Fig. 5, A and B). These results indicate that 20 μM imatinib decreased VE-cadherin expression by 25% (P < 0.05) compared with control whereas 40 μM imatinib produced a 58% decrease in CS-stimulated EC (P < 0.001). To confirm that imatinib induces a loss in VE-cadherin from EC junctions, immunofluorescence staining for VE-cadherin was performed on CS-challenged cells. The combination of 18% CS and imatinib resulted in a substantial loss of this critical junctional protein at the periphery of the cells (Fig. 5C). Prolonged exposure to imatinib alone (20 μM, 24 h) had no effect on VE-cadherin distribution in static EC (Fig. 5C). In addition, imatinib alone (20 μM) did not alter VE-cadherin expression as assessed by Western blotting or EC barrier function as measured by ECIS (data not shown). These data demonstrate that imatinib induces a barrier-disruptive phenotype in lung EC exposed to pathological CS.
Fig. 5.
Effects of imatinib on 18% cyclic stretch (CS)-induced endothelial dysfunction. HPAEC were treated with imatinib as indicated for 1 h and then subjected to cyclic stretch (18% CS, 24 h). A: representative Western blot for VE-cadherin. B: densitometry of VE-cadherin normalized to β-actin. Data values are means ± SE from 3 independent experiments. (*P < 0.05 compared with control, Dunnett's post hoc test). C: immunofluorescence staining for VE-cadherin. 18% CS in combination with imatinib induced a loss of VE-cadherin from the junctions as indicated by arrows. Representative images from multiple independent experiments are shown.
Effects of imatinib on cytokine production in EC after stimulation with LPS or 18% CS.
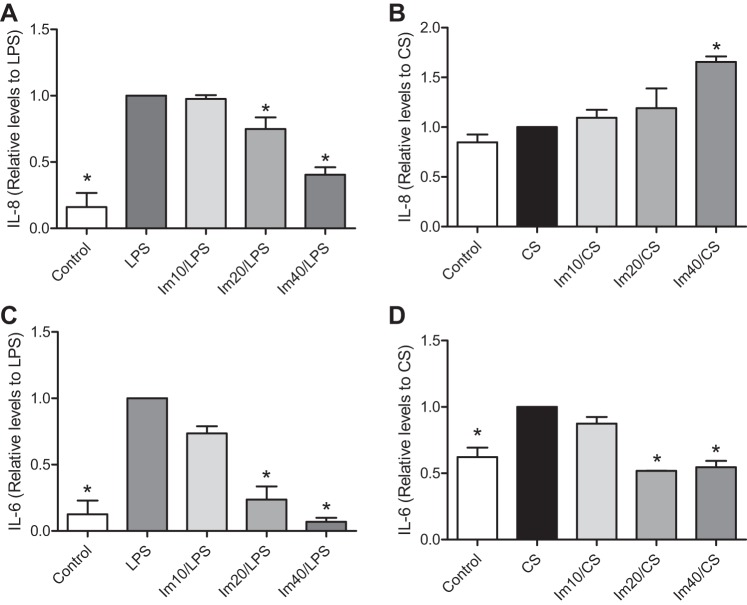
The release of proinflammatory cytokines after cell activation can further potentiate lung injury. Given the effects of imatinib on BAL cytokine levels in mouse lungs (Fig. 3), we next determined the regulation of cytokine release by imatinib in stimulated lung EC. HPAEC were pretreated with imatinib and exposed to LPS (1 μg/ml, 3 h) or 18% CS (24 h), and then IL-8 and IL-6 levels were determined by ELISA in the conditioned medium. LPS increased IL-8 levels by a factor of 6.2 (P < 0.001) compared with control, whereas imatinib attenuated this increase by 25.0% (P > 0.05) at a concentration of 20 μM and by 59.7% (P < 0.001) at 40 μM (Fig. 6A). In contrast, imatinib increased IL-8 levels in 18% CS-challenged EC by 9.5% (P > 0.05), 19% (P > 0.05) and 65% (P < 0.01) at doses of 10, 20, and 40 μM, respectively, compared with 18% CS alone (Fig. 6B). This effect was not due to prolonged exposure to imatinib alone since IL-8 levels did not increase in static EC exposed to these concentrations of imatinib for 24 h (data not shown). Interestingly, this discrepant pattern was not replicated when IL-6 secretion was examined. IL-6 levels were significantly higher than control in EC stimulated with LPS (1 μg/ml, 3 h) or 18% CS (24 h) by a factor of 7.9 (P < 0.001) and 1.6 (P < 0.001), respectively (Fig. 6, C and D). However, imatinib inhibited IL-6 levels after both types of stimuli. In LPS-stimulated EC, imatinib significantly decreased IL-6 release by 26.5% (P > 0.05), 76.3% (P < 0.001), and 93.0% (P < 0.001) at doses of 10, 20, and 40 μM, respectively (Fig. 6C). In EC subjected to 18% CS, imatinib inhibited IL-6 levels by 48.2% (P < 0.001) and 45.4% (P < 0.001) at doses of 20 and 40 μM, respectively (Fig. 6D).
Fig. 6.
Effects of imatinib on inflammatory cytokine production by LPS or 18% CS in HPAEC. HPAEC were treated with imatinib as indicated for 1 h followed by challenge with LPS (1 μg/ml, 3 h) or 18% CS (24 h). Cell supernatants were then collected and assayed for IL-8 (A and B) and IL-6 (C and D) by ELISA. Data represent means ± SE from 3 or more independent experiments. *P < 0.05 compared with LPS or 18% CS group, Dunnett's post hoc test.
Roles of c-Abl and Arg in mediating endothelial dysfunction.
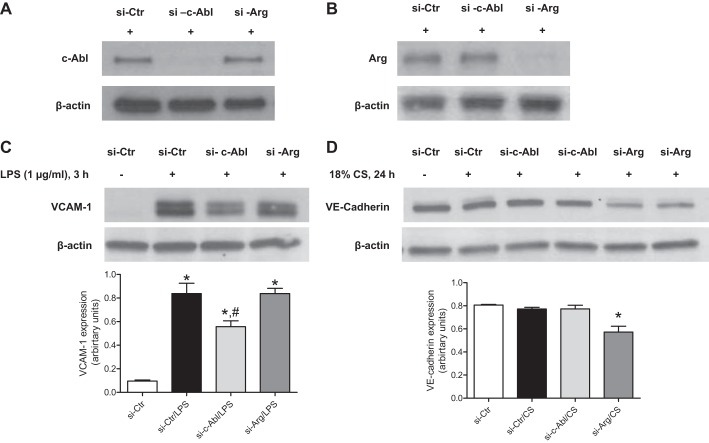
Previous studies have suggested differential roles for the Abl family kinases, c-Abl and Arg, in mediating EC barrier function (2, 11). Because imatinib inhibits both these kinases, siRNA technology was employed to individually downregulate the expression of c-Abl or Arg in HPAEC to investigate the specific functions of c-Abl and Arg in our models of EC dysfunction. The specificity of these siRNA approaches for each kinase is depicted in Fig. 7, A and B. Following siRNA transfection, EC were stimulated with LPS (1 μg/ml, 3 h) or 18% CS (24 h), and measures of EC activation or dysfunction were determined. LPS induction of VCAM-1 expression was significantly decreased after c-Abl siRNA (by 33%, P < 0.05), but not by Arg knockdown (Fig. 7C). When lung EC were exposed to 18% CS, a significant loss of VE-cadherin expression was observed (by 26% compared with 18% CS, P < 0.01) in Arg siRNA-treated cells, whereas c-Abl siRNA had no significant effect (Fig. 7D). These data suggest differential roles for each Abl kinase in regulating pulmonary EC function depending on the stimuli.
Fig. 7.
Effects of c-Abl or Arg siRNA on LPS and 18% CS-induced endothelial dysfunction. HPAEC were transfected with control, c-Abl-, or Arg-specific siRNA for 48 h and then stimulated with LPS (1 μg/ml, 3 h) or 18% CS (24 h). Cell lysates were then collected and analyzed by immunoblotting. Representative Western blots of at least 3 experiments depict the specific downregulation of c-Abl (A) or Arg (B) protein expression by siRNA, VCAM-1 expression, and the corresponding densitometry (P < 0.05, *compared with si-Ctr and #compared with si-Ctr/LPS and si-c-Abl/LPS), Tukey's post hoc test (C) and VE-cadherin expression and the corresponding densitometry (D). Data represent means ± SE from 3 or more independent experiments. *P < 0.05, compared with si-Ctr, si-Ctr/CS, and si-c-Abl/CS, Tukey's post hoc test.
DISCUSSION
ARDS is a devastating process marked by severe vascular leak and sustained inflammation that can result from multiple insults including bacterial infection and mechanical injury (60). Recent studies have demonstrated that the chemotherapeutic drug imatinib, an inhibitor of the Abl family of tyrosine kinases as well as several other enzymes, has both vascular barrier-protective and anti-inflammatory properties (2, 61). These intriguing observations suggest that imatinib may be a promising therapeutic strategy for ALI syndromes. However, it has been reported that more than 50% of patients who receive imatinib develop edema-related side effects (21, 26, 47). Given these seemingly contradictory effects on vascular leak, additional studies are needed to explore the potential efficacy of imatinib in targeting lung injury syndromes and to better understand its mechanism of action in regulating permeability. The present study advances this discussion by characterizing the effects of imatinib in two models of lung injury induced by clinically relevant stimuli. The major findings of the present work are the following: 1) imatinib attenuates LPS-induced lung injury in mice and endothelial dysfunction in human lung EC; 2) imatinib exacerbates VILI in mice and CS-induced endothelial dysfunction in human lung EC; and 3) Abl kinases differentially regulate EC responses induced by LPS and CS.
Our data demonstrate that imatinib attenuates lung permeability (BAL protein, lung tissue albumin) (Fig. 1) and inflammation (histology, cell counts, and cytokine levels) (Figs. 2 and 3) in a well-established mouse model of LPS-induced ALI. The mechanistic basis for these protective effects of imatinib in the LPS model was explored in vitro with human lung EC. Imatinib protects EC monolayer integrity and suppresses the inflammatory responses that occur after exposure to LPS (Figs. 4 and 6). Specifically, imatinib prevents the loss of VE-cadherin from cell junctions, an endothelial-specific cell-cell adhesion molecule that plays an essential role in the formation and the stability of EC monolayers (23). Barrier-disruptive agents like LPS induce dissociation of VE-cadherin and decrease its expression, resulting in EC monolayer disruption and gap formation (10). The ability of imatinib to maintain VE-cadherin at cell-cell junctions after LPS (Fig. 4C) indicates a barrier-protective effect in this response. In addition, our novel data indicate that imatinib exerts anti-inflammatory effects in lung EC by suppressing the LPS-induced upregulation of VCAM-1 and the release of IL-6 and IL-8 (Figs. 4D and 6).
These data are consistent with recent studies demonstrating inhibitory effects of imatinib on inflammation and permeability. For example, imatinib potently suppressed TNF-α production and NF-κB signaling in macrophages/monocytes stimulated with LPS (61). At the whole animal level, imatinib inhibited the production of cytokines such as IL-6 and attenuated the development of pulmonary edema in a neutropenic model of LPS-induced lung injury and in bleomycin-induced ALI (32, 51). The anti-inflammatory effects of imatinib also were evident in a murine model of asthma (5) and in a Bcr-Abl-transfected hematopoietic cell line (4). Moreover, Ph+CML patients treated with imatinib displayed decreased levels of NF-κB, IL-6, and IL-8 in lymphomonocytes (13). Previous studies also have revealed barrier-protective effects of imatinib. Imatinib attenuated endothelial barrier disruption induced by thrombin or histamine in vitro and VEGF or murine sepsis (2). Another study demonstrated the capability of imatinib to inhibit endothelial permeability induced by the inflammatory agents VEGF, thrombin, and histamine in human microvascular EC (11). Imatinib also protected against H2O2-induced increased permeability in an in vitro model of oxidative stress in mouse lung EC (57). Finally, a recent case report of a patient with systemic capillary leak syndrome described impressive reversal of vascular leak after imatinib treatment, suggesting the clinical potential of this approach (1).
Despite this growing body of evidence from our group and others about the protective effects of imatinib in several models of vascular dysfunction, in this study we provide evidence that imatinib exacerbates lung injury in experimental models of HTVMV. Mechanical ventilation is the primary supportive therapy for critically ill patients with ARDS and the only known intervention proven to lower mortality when a low-tidal-volume strategy is pursued (58a). However, it is well established that MV also can induce or worsen lung injury (i.e., VILI) (56). To explore the effects of imatinib on VILI, we employed a well-characterized murine model of HTVMV (30 ml/kg) that produces moderate vascular leakage and inflammation (35). In contrast to its protective effects on LPS-induced ALI, imatinib exacerbates vascular leakage (BAL protein, lung tissue albumin) (Fig. 1) and inflammation (cell counts, histology, cytokine release) (Figs. 2 and 3) in VILI-exposed mice. In agreement with these in vivo observations, imatinib decreases expression of VE-cadherin and induces junctional disruption and intercellular gap formation in human lung EC exposed to pathological cyclic stretch (18% CS) (Fig. 5), a stimulus that mimics injurious mechanical forces that can be generated during mechanical ventilation. Moreover, in lung EC exposed to 18% CS, imatinib increases IL-8 secretion in contrast to its anti-inflammatory effects in EC after LPS (Fig. 6). To our knowledge, these intriguing results are the first to describe exacerbating effects of imatinib on lung injury in vivo and EC dysfunction in vitro.
To better understand the disparate effects of imatinib on LPS-induced lung injury and VILI, we investigated the potential differential roles of the Abl family kinases, c-Abl and Arg, which are both inhibited by imatinib. These two kinases have very similar NH2-terminal regions comprised of sequential SH3, SH2, and kinase domains; however, their COOH-terminal structures differ significantly and include cytoskeletal binding domains that contribute to their intracellular localization and the regulation of cell structure (14, 55). These kinases are involved in a variety of cytoskeleton processes, regulate cell-cell adhesion (9, 29, 49, 63), and recently have emerged as important mediators of endothelial function in a stimulus-dependent manner. Specifically, we previously reported that c-Abl mediates the EC cytoskeletal rearrangements that are critical for enhanced barrier integrity after S1P, a barrier-protective sphingolipid, and the pharmaceutical agent FTY720 (20, 59) In contrast to this barrier-enhancing role, c-Abl also mediates H2O2-induced disruption of rat lung microvascular endothelium (58), but not thrombin-induced human EC permeability, which is specifically dependent on Arg expression (2). From these studies, it is clear that c-Abl and Arg regulate EC barrier function in a stimulus-specific manner despite their structural and functional similarities. Our present results support differential roles for c-Abl and Arg. Using siRNA to specifically decrease expression of either c-Abl or Arg, we examined human lung EC responses to LPS and CS. The inhibitory effect of imatinib on LPS-induced VCAM-1 expression is mimicked by c-Abl downregulation, whereas the loss of VE-cadherin expression that occurs after imatinib in EC exposed to 18% CS is mimicked by Arg siRNA (Fig. 7). These data are consistent with the developing paradigm that c-Abl and Arg have multiple nonredundant roles in cellular responses to various stimuli and may partially explain the differential effects of imatinib in our LPS-induced and VILI models of ALI.
Although LPS- and ventilator-induced lung injury share some characteristics in terms of their effects on cytoskeletal structure [e.g., Rho kinase activation, myosin light chain (MLC) phosphorylation, disruption of VE-cadherin] and inflammation (e.g., NF-κB activation), their initiation by different stimuli results in important signaling differences potentially relevant to the present study. Lung EC sense LPS and mechanical forces (i.e., CS) via distinct mechanisms. LPS is a cell wall component of gram-negative bacteria that ligates TLR4 on EC and other pulmonary cells (39). In contrast, mechanical forces like CS are translated into intracellular biochemical signaling through more complex and less well-defined mechanisms involving stimulation of mechanoreceptors, stretch-activated ion channels and extracellular matrix-cytoskeleton interactions (17, 34). These differential upstream events in lung EC after LPS and CS provide alternative mechanisms for triggering common downstream pathways that result in barrier disruption and inflammation, such as calcium release, Rho activation, MLC phosphorylation, expression of adhesion molecules, NF-κB translocation, and cytokine production (6, 8, 10, 24, 31, 36). Interestingly, although both LPS and VILI can induce NF-κB activation, in VILI this can be mediated independently of TLR4 (28). Moreover, CS induces NF-κB activation via stretch-activated ion channels and reactive oxygen species production in human lung fibroblasts (3, 30). In addition, mechanical stretch induces release of cytokines such as IL-6 and IL-8 (27) by both NF-κB-dependent (15, 33, 45) and independent mechanisms (31). As a result, we hypothesize that imatinib may attenuate release of these cytokines in the LPS model through NF-κB inhibition, while augmenting IL-6 and IL-8 production in mechanical injury via its effects on an alternative pathway. Future studies will focus on elucidating the differential effects of imatinib downstream of LPS and CS on important signaling events such as Abl kinases activity, MLC phosphorylation, NF-κB activation, and others to provide additional mechanistic insights into these clinically relevant injury processes in lung EC.
It is important to note several limitations of our study. First, imatinib inhibits other kinases in addition to the Abl family, including platelet-derived growth factor receptor (PDGFR) and c-kit (52) that may contribute to modulation of lung injury by imatinib described in this study. Secondly, technical issues limit the duration of MV exposure to 4 h in the mice, so the effects of imatinib on VILI during more prolonged ventilation remain unclear. Third, mouse models of inflammatory injury, such as ALI and sepsis, may not accurately predict responses in humans (54), and therefore translation of these results to potential therapeutic efficacy of imatinib in patients with ARDS requires further validation.
In summary, our present results indicate that the Abl family kinase inhibitor imatinib attenuates LPS-induced ALI but exacerbates VILI. To identify potential mechanisms underlying these effects, in vitro studies of the direct effects of this drug on lung EC function also were performed. In LPS-stimulated cells, imatinib exerts anti-inflammatory and barrier-protective effects; however, in pathological CS-challenged cells, imatinib exacerbates endothelial dysfunction. The effects of imatinib were mimicked by downregulation of c-Abl in LPS-stimulated EC and by downregulation of Arg in pathological CS-challenged cells, suggesting differential roles for these kinases in mediating the effects of imatinib in these models. Although the precise mechanisms by which imatinib exerts its effects on EC function and ALI remain to be elucidated, these data support nonredundant roles for c-Abl and Arg as key mediators of EC function. Given the distinct functions of c-Abl and Arg, the development and characterization of specific inhibitors for each kinase may be worthwhile for targeting vascular dysfunction in multiple disease processes. Finally, imatinib and related kinase inhibitors that currently are in extensive clinical use represent promising potential therapies for inflammatory vascular leak syndromes and are worthy of additional study.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL058064 (J. G. N. Garcia) and American Heart Association Grant 14PRE18860021 (A. N. Rizzo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.L., J.G.G., and S.M.D. conception and design of research; E.L., A.N.R., S.S., and P.N. performed experiments; E.L. and A.N.R. analyzed data; E.L., S.S., and S.M.D. interpreted results of experiments; E.L. prepared figures; E.L. drafted manuscript; E.L., A.N.R., J.R.J., J.G.G., and S.M.D. edited and revised manuscript; E.L., A.N.R., S.S., P.N., J.R.J., J.G.G., and S.M.D. approved final version of manuscript.
REFERENCES
- 1.Aman J, Peters MJ, Weenink C, van Nieuw Amerongen GP, Vonk Noordegraaf A. Reversal of vascular leak with imatinib. Am J Respir Crit Care Med 188: 1171–1173, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Aman J, van Bezu J, Damanafshan A, Huveneers S, Eringa EC, Vogel SM, Groeneveld AB, Vonk Noordegraaf A, van Hinsbergh VW, van Nieuw Amerongen GP. Effective treatment of edema and endothelial barrier dysfunction with imatinib. Circulation 126: 2728–2738, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Amma H, Naruse K, Ishiguro N, Sokabe M. Involvement of reactive oxygen species in cyclic stretch-induced NF-κB activation in human fibroblast cells. Br J Pharmacol 145: 364–373, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron F, Turhan AG, Giron-Michel J, Azzarone B, Bentires-Alj M, Bours V, Bourhis JH, Chouaib S, Caignard A. Leukemic target susceptibility to natural killer cytotoxicity: relationship with BCR-ABL expression. Blood 99: 2107–2113, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Berlin AA, Lukacs NW. Treatment of cockroach allergen asthma model with imatinib attenuates airway responses. Am J Respir Crit Care Med 171: 35–39, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Birukova AA, Tian Y, Meliton A, Leff A, Wu T, Birukov KG. Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6. Am J Physiol Lung Cell Mol Physiol 302: L965–L975, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birukova AA, Wu T, Tian Y, Meliton A, Sarich N, Tian X, Leff A, Birukov KG. Iloprost improves endothelial barrier function in lipopolysaccharide-induced lung injury. Eur Respir J 41: 165–176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci 122: 3441–3454, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee A, Snead C, Yetik-Anacak G, Antonova G, Zeng J, Catravas JD. Heat shock protein 90 inhibitors attenuate LPS-induced endothelial hyperpermeability. Am J Physiol Lung Cell Mol Physiol 294: L755–L763, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Chislock EM, Pendergast AM. Abl family kinases regulate endothelial barrier function in vitro and in mice. PLoS One 8: e85231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chislock EM, Ring C, Pendergast AM. Abl kinases are required for vascular function, Tie2 expression, and angiopoietin-1-mediated survival. Proc Natl Acad Sci USA 110: 12432–12437, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciarcia R, Vitiello MT, Galdiero M, Pacilio C, Iovane V, d'Angelo D, Pagnini D, Caparrotti G, Conti D, Tomei V, Florio S, Giordano A. Imatinib treatment inhibit IL-6, IL-8, NF-KB and AP-1 production and modulate intracellular calcium in CML patients. J Cell Physiol 227: 2798–2803, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Colicelli J. ABL tyrosine kinases: evolution of function, regulation, and specificity. Sci Signal 3: re6, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras M, Ansari B, Curley G, Higgins BD, Hassett P, O'Toole D, Laffey JG. Hypercapnic acidosis attenuates ventilation-induced lung injury by a nuclear factor-κB-dependent mechanism. Crit Care Med 40: 2622–2630, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci 126: 2545–2549, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol 89: 1645–1655, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Dos Santos LV, Lima JP, Abdalla KC, Bragagnoli AC, Santos FA, Dos Anjos Jacome A, Porto FE. Imatinib-induced bone edema: case report and review of literature. J Natl Compr Canc Netw 11: 1187–1191, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344: 1031–1037, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Dudek SM, Chiang ET, Camp SM, Guo Y, Zhao J, Brown ME, Singleton PA, Wang L, Desai A, Arce FT, Lal R, Van Eyk JE, Imam SZ, Garcia JG. Abl tyrosine kinase phosphorylates nonmuscle Myosin light chain kinase to regulate endothelial barrier function. Mol Biol Cell 21: 4042–4056, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esmaeli B, Diba R, Ahmadi MA, Saadati HG, Faustina MM, Shepler TR, Talpaz M, Fraunfelder R, Rios MB, Kantarjian H. Periorbital oedema and epiphora as ocular side effects of imatinib mesylate (Gleevec). Eye 18: 760–762, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavard J. Breaking the VE-cadherin bonds. FEBS Lett 583: 1–6, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Gawlak G, Tian Y, O'Donnell JJ 3rd, Tian X, Birukova AA, Birukov KG. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex. FASEB J 28: 3249–3260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer 13: 559–571, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guilhot F. Indications for imatinib mesylate therapy and clinical management. Oncologist 9: 271–281, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Halbertsma FJ, Vaneker M, Scheffer GJ, van der Hoeven JG. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med 63: 382–392, 2005. [PubMed] [Google Scholar]
- 28.Held HD, Boettcher S, Hamann L, Uhlig S. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-κB and is blocked by steroids. Am J Respir Crit Care Med 163: 711–716, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Hernández SE, Krishnaswami M, Miller AL, Koleske AJ. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol 14: 36–44, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Inoh H, Ishiguro N, Sawazaki S, Amma H, Miyazu M, Iwata H, Sokabe M, Naruse K. Uni-axial cyclic stretch induces the activation of transcription factor nuclear factor κB in human fibroblast cells. FASEB J 16: 405–407, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Iwaki M, Ito S, Morioka M, Iwata S, Numaguchi Y, Ishii M, Kondo M, Kume H, Naruse K, Sokabe M, Hasegawa Y. Mechanical stretch enhances IL-8 production in pulmonary microvascular endothelial cells. Biochem Biophys Res Commun 389: 531–536, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim IK, Rhee CK, Yeo CD, Kang HH, Lee DG, Lee SH, Kim JW. Effect of tyrosine kinase inhibitors, imatinib and nilotinib, in murine lipopolysaccharide-induced acute lung injury during neutropenia recovery. Crit Care 17: R114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko YA, Yang MC, Huang HT, Hsu CM, Chen LW. NF-κB activation in myeloid cells mediates ventilator-induced lung injury. Respir Res 14: 69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Tanswell AK, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol Lung Cell Mol Physiol 277: L667–L683, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Ma SF, Xie L, Pino-Yanes M, Sammani S, Wade MS, Letsiou E, Siegler J, Wang T, Infusino G, Kittles RA, Flores C, Zhou T, Prabhakar BS, Moreno-Vinasco L, Villar J, Jacobson JR, Dudek SM, Garcia JG. Type 2 deiodinase and host responses of sepsis and acute lung injury. Am J Respir Cell Mol Biol 45: 1203–1211, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maniatis NA, Kotanidou A, Catravas JD, Orfanos SE. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol 49: 119–133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClelland CM, Harocopos GJ, Custer PL. Periorbital edema secondary to imatinib mesylate. Clin Ophthalmol 4: 427–431, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Moen MD, McKeage K, Plosker GL, Siddiqui MA. Imatinib: a review of its use in chronic myeloid leukaemia. Drugs 67: 299–320, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Moreno-Vinasco L, Quijada H, Sammani S, Siegler J, Letsiou E, Deaton R, Saadat L, Zaidi RS, Messana J, Gann PH, Machado RF, Ma W, Camp SM, Wang T, Garcia JG. Nicotinamide phosphoribosyltransferase inhibitor is a novel therapeutic candidate in murine models of inflammatory lung injury. Am J Respir Cell Mol Biol 51: 223–228, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res 105: 223–230, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagar B. c-Abl tyrosine kinase and inhibition by the cancer drug imatinib (Gleevec/STI-571). J Nutr 137: 1518S–1523S; discussion 1548S, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Ning Q, Wang X. Role of Rel A and IκB of nuclear factor κB in the release of interleukin-8 by cyclic mechanical strain in human alveolar type II epithelial cells A549. Respirology 12: 792–798, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Okuda K, Weisberg E, Gilliland DG, Griffin JD. ARG tyrosine kinase activity is inhibited by STI571. Blood 97: 2440–2448, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Ostro D, Lipton J. Unusual fluid retention with imatinib therapy for chronic myeloid leukemia. Leuk Lymphoma 48: 195–196, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Peacock JG, Couch BA, Koleske AJ. The Abl and Arg non-receptor tyrosine kinases regulate different zones of stress fiber, focal adhesion, and contractile network localization in spreading fibroblasts. Cytoskeleton (Hoboken) 67: 666–675, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res 85: 51–100, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Plataki M, Hubmayr RD. The physical basis of ventilator-induced lung injury. Expert Rev Respir Med 4: 373–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhee CK, Lee SH, Yoon HK, Kim SC, Lee SY, Kwon SS, Kim YK, Kim KH, Kim TJ, Kim JW. Effect of nilotinib on bleomycin-induced acute lung injury and pulmonary fibrosis in mice. Respiration 82: 273–287, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Köcher T, Superti-Furga G. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 110: 4055–4063, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol 83: 536–545, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sirvent A, Benistant C, Roche S. Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and cancer cells. Biol Cell 100: 617–631, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 369: 2126–2136, 2013. [DOI] [PubMed] [Google Scholar]
- 57.Stephens RS, Servinsky LE, Rentsendorj O, Kolb TM, Pfeifer A, Pearse DB. Protein kinase G increases antioxidant function in lung microvascular endothelial cells by inhibiting the c-Abl tyrosine kinase. Am J Physiol Cell Physiol 306: C559–C569, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y, Hu G, Zhang X, Minshall RD. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res 105: 676–685, 615 p. following 685, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58a.Ventilation with lower tidal volumes compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342: 1301–1308, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Chiang ET, Simmons JT, Garcia JG, Dudek SM. FTY720-induced human pulmonary endothelial barrier enhancement is mediated by c-Abl. Eur Respir J 38: 78–88, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Wolf AM, Wolf D, Rumpold H, Ludwiczek S, Enrich B, Gastl G, Weiss G, Tilg H. The kinase inhibitor imatinib mesylate inhibits TNFα production in vitro and prevents TNF-dependent acute hepatic inflammation. Proc Natl Acad Sci USA 102: 13622–13627, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodring PJ, Hunter T, Wang JY. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J Cell Sci 116: 2613–2626, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci USA 104: 17686–17691, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]