Abstract
Subcellular trafficking within host cells plays a critical role in viral life cycles, including influenza A virus (IAV). Thus targeting relevant subcellular compartments holds promise for effective intervention to control the impact of influenza infection. Bafilomycin A1 (Baf-A1), when used at relative high concentrations (≥10 nM), inhibits vacuolar ATPase (V-ATPase) and reduces endosome acidification and lysosome number, thus inhibiting IAV replication but promoting host cell cytotoxicity. We tested the hypothesis that much lower doses of Baf-A1 also have anti-IAV activity, but without toxic effects. Thus we assessed the antiviral activity of Baf-A1 at different concentrations (0.1–100 nM) in human alveolar epithelial cells (A549) infected with IAV strain A/PR/8/34 virus (H1N1). Infected and mock-infected cells pre- and cotreated with Baf-A1 were harvested 0–24 h postinfection and analyzed by immunoblotting, immunofluorescence, and confocal and electron microscopy. We found that Baf-A1 had disparate concentration-dependent effects on subcellular organelles and suppressed affected IAV replication. At concentrations ≥10 nM Baf-A1 inhibited acid lysosome formation, which resulted in greatly reduced IAV replication and release. Notably, at a very low concentration of 0.1 nM that is insufficient to reduce lysosome number, Baf-A1 retained the capacity to significantly impair IAV nuclear accumulation as well as IAV replication and release. In contrast to the effects of high concentrations of Baf-A1, very low concentrations did not exhibit cytotoxic effects or induce apoptotic cell death, based on morphological and FACS analyses. In conclusion, our results reveal that low-concentration Baf-A1 is an effective inhibitor of IAV replication, without impacting host cell viability.
Keywords: influenza A virus, low-dose bafilomycin A1, noncytotoxic, apoptotic cell death, autophagy
influenza viruses are common pathogens of the upper respiratory tract and a substantial disease burden worldwide. It is estimated that seasonal epidemics affect 10–20% of the world's population and the virus is associated with significant morbidity and mortality every year (22, 54). Although immunization is the single most effective way to protect against seasonal influenza infection, the vaccine's effectiveness can be significantly compromised when it does not match the prevalent viral strain(s) in a given year (1, 46). That there are also reports of increasing numbers of drug-resistant flu strains further highlights a need to identify new anti-influenza strategies (19, 20, 53).
Influenza A viruses (IAV) are enveloped, single-stranded RNA viruses that belong to the family Orthomyxoviridae. The genome contains eight negative-sense segments of RNA that encode 10 or more different viral proteins depending on the virus strain (23, 55). The IAV envelope is made up of phospholipids with several embedded proteins including hemagglutinin (HA) and neuraminidase, which are commonly used to classify influenza virus strains. HA is the primary protein responsible for influenza virus binding to receptors on the host cell surface, facilitating cell entry by the virions (26).
Following virus attachment, host cells undergo de novo formation of clathrin-coated pits leading to primary uptake of the virus via endocytosis, although other clathrin- and caveolin-independent pathways also exist (26, 31). The endocytic pathway of internalized viruses is generally thought to lead to formation of acidic late endosomes in which the low pH may trigger HA-catalyzed fusion between the viral and endosomal membranes (28). Furthermore, components inside the virus [i.e., M1 and the viral ribonucleoproteins (vRNPs)] become exposed to the low pH of late endosomes via the M2 ion channel, resulting in disruption of M1-vRNP interactions and uncoating the virus (6). Released vRNPs are subsequently imported to the nucleus for viral gene expression and replication (6). Progeny viral genomes leave the nucleus and assemble into infectious particles at the plasma membrane followed by release by budding from the apical plasma membrane (26, 29). Targeting IAV trafficking at different stages of the infectious cycle could quell the ability of the influenza virus to spread and cause disease in its host.
Bafilomycin A1 (Baf-A1) is a member of the plecomacrolide subclass of macrolide antibiotics isolated from Streptomyces griseus. Baf-A1 has been shown to be a highly specific vacuolar type H+-ATPase (V-ATPase) inhibitor in various cell types, at effective concentrations of ∼0.1–1 μM (100 –1,000 nM) (3, 11). Cellular V-ATPases transport protons across the plasma membrane as well as functioning to acidify intracellular compartments, including endosomes and lysosomes (7). Baf-A1 binds to the cellular V-ATPases and blocks proton translocation, resulting in the inhibition of lysosomal proteases that are activated at low pH (3, 60). These concentrations of Baf-A1 prevent V-ATPase-mediated degradation of sequestered material and block autophagy flux by interfering with late-stage autophagosome-lysosome fusion (12, 34, 58). When used for short duration in vitro at concentrations from 50 to 100 nM, Baf-A1 inhibits growth of influenza A and B viruses and rhinovirus by inhibiting V-ATPase and preventing endosomal acidification (40, 50). Although these findings are of interest conceptually, their impact is tempered by the fact that these Baf-A1 concentrations are also cytotoxic, thus precluding direct in vivo translation (27). In the present study, we demonstrate that much lower Baf-A1 concentrations (below 1 nM) that are not sufficient to inhibit V-ATPase retain capacity to significantly impair the nuclear accumulation of IAV and dramatically attenuate virus replication and release in a human alveolar epithelial cell line.
MATERIALS AND METHODS
Reagents.
Cell culture media, propidium iodide (PI), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), Baf-A1, and type-I agarose were obtained from Sigma (Sigma-Aldrich, Oakville, CA). Nitrocellulose membrane, prestained protein molecular weight markers, and BCA protein assay kit were obtained from Bio-Rad Laboratories (Mississauga, ON, Canada). pHRodo Red Dextran (pRRD) was from Molecular Probes. All other chemicals used were of the highest analytical grade and were purchased from Sigma (Sigma-Aldrich, Oakville, ON, Canada) or Fisher (Fisher Scientific, Ottawa, ON, Canada).
Cells, media, and viruses.
Human alveolar lung A549 cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA) and grown in Dulbecco's Modified Eagle's Medium (DMEM, Invitrogen, Burlington, ON, Canada), supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin/streptomycin (Invitrogen). Cells were maintained as monolayers in 10% CO2 at 37°C and passaged by trypsinization at 80–90% confluence. Human influenza virus strain A/PR/8/34 virus (H1N1) was grown in 10-day-old embryonated hens' eggs from laboratory stocks. The chorioallantoic fluid was harvested, aliquoted, and titered in Madin-Darby canine kidney (MDCK) cells by standard procedures (5).
Antibodies.
Antibodies were used in this study for either Western blotting or immunofluorescence (IF), or both. Primary antibodies were mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit-anti LC3 (Sigma-Aldrich, Oakville, CA), rabbit anti early endosome marker (EEA1, ab2900) (Abcam, Cambridge, MA), and lysosomal-associated membrane protein 3 (LAMP3) (Proteintech Group, Chicago, IL, 12632-1-AP). Alexa Fluor 488 goat anti-rabbit, LysoTracker Red DND-99 (L7528), DAPI, and TO-PRO were obtained from Invitrogen Molecular Probes (Burlington, ON, Canada). Cy3 (goat anti-mouse) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The mouse monoclonal anti-nucleoprotein (NP) 28–73 antibody was a kind gift from Dr. Mingyi Li, National Centre for Foreign Animal Disease, Winnipeg, MB, Canada (59). The mouse monoclonal anti-NS1 3F5 antibody has been previously described (44).
Influenza virus infection and plaque assay.
A549 cells were infected at 80–90% confluence with influenza A/PR/8/34 virus at a multiplicity of infection (MOI) of 1 plaque-forming unit (PFU)/cell. Cell monolayers were first washed twice with 1× phosphate-buffered saline (PBS; 137 mM NaCl, 0.3 mM KCl, 0.8 mM Na2HPO4, 0.1 mM KH2PO4) followed by infection with viruses diluted in gel saline [137 mM NaCl, 0.2 mM CaCl2, 0.8 mM MgCl2, 19 mM HBO3, 0.1 mM Na2B4O7, 0.3% (wt/vol) gelatin]. Infected and mock-infected cells were harvested 0–24 h postinfection (pi) and analyzed by Western blotting. To quantify the amount of virus released, supernatants were harvested at assigned time points and virus yield was titrated by plaque assay on MDCK cells. Briefly, supernatants were serially diluted in gel saline and adsorbed to confluent monolayers of MDCK cells in six-well plates for 60 min at 37°C. MDCK cell monolayers were then overlaid with 0.6% type-I agarose and incubated at 37°C for 72 h to permit plaque formation (5).
Virus binding assay.
Preparation of cells for binding assay was performed as described previously with slight modifications (8, 49). The cells were washed, fixed, and processed as described below for IF microscopy or fluorescence-activated cell sorting (FACS) with use of anti-nucleoprotein antibodies. Briefly, cells grown in 12-well plates were pretreated with Baf-A1 (0–100 nM, for 24 h) and then incubated with A/PR/8/34 virus at a MOI of 10 PFU/cell on ice for 60 min. After gentle scraping, the cells were harvested by centrifugation at 1,500 g for 5 min, washed once with cold PBS, fixed in 3% paraformaldehyde/PBS for 15 min, permeabilized in 0.1% Triton X-100, and blocked in 10% goat serum/PBS for 60 min. To detect virus binding, cells were incubated with the monoclonal antibody to influenza virus NP for 45 min, followed by Alexa Fluor 488-labeled goat anti-mouse IgG from Invitrogen Molecular Probes for 30 min. Cells were analyzed on a FACSCalibur cytometer by using Cellquest 3.1F software (Becton Dickinson Immunocytometry Systems). Data analysis was performed with Cell Quest Pro Software (BD Biosciences) and FlowJo 4.6 software (Treestar, Ashland, OR). At least 104 cells were analyzed for each sample.
Indirect immunofluorescence microscopy.
For IF staining, A549 cells were seeded on glass coverslips and treated with different doses of Baf-A1 for 24 h, then mock-infected, or infected with A/PR/8/34 virus at MOI of 1–10 PFU/cell. Cells were then fixed for 15 min in 4% paraformaldehyde/120 mM sucrose in PBS, pH 7.4, and permeabilized for 10 min with 0.3% Triton X-100 in PBS. After incubation with 3% BSA blocking solution for 60 min, cells were incubated overnight with the assigned primary antibodies at 4°C. Cells were then incubated with corresponding secondary antibodies diluted in 1% BSA in PBS for 1 h at room temperature. Cell nuclei were stained with DAPI dye or TO-PRO followed by mounting with ProLong Gold antifade reagent from Invitrogen Molecular Probes. The fluorescent signal was examined and analyzed with an Olympus FluoView multilaser confocal microscope. Laser intensity and detector sensitivity settings remained constant for all image acquisitions within a respective experiment. The methods for the quantification of IAV nuclear transportation have been described previously (62). In brief, following IF staining, the cells were analyzed by IF confocal microscopy and total number of infected cells as well as nuclear staining was counted. Data were then presented as average percentages of nuclear staining of IAV nuclear protein (vNP) in infected cells in Baf-A1-treated cells vs. nontreated control cells.
Labeling of lysosomal compartments with LysoTracker.
Lysosomal compartments were labeled by incubating the live IAV-infected A549 cells (pretreated with different doses of Baf-A1 for 24 h) with 200 nM LysoTracker Red DND-99 (L7528, Molecular Probes) in the culture media for 10 min at 37°. After incubation, cells were washed with PBS and immediately fixed for 15 min (4% paraformaldehyde/120 mM sucrose). Fluorescence images were captured by utilizing an Olympus FluoView multilaser confocal microscope. Olympus FluoView software, which measures the intensity of staining through threshold analysis, was used to quantify the amount of LysoTracker fluorescence detectable in the control and Baf-A1 cells (14).
Measurement of lysosome pH.
Lysosomal pH in was measured in A549 epithelial cells by using the pH-sensitive fluorescent indicator pRRD (Molecular Probes). A549 cells were cultured (DMEM/10% FBS) on Nunc Lab-Tek four-well chambered coverglass slides. At confluence, the cultures were treated with Baf-A1 (0, 0.1, 1, and 10 ng/ml) for 24 h. Thereafter, cell nuclei were stained with 10 μg/ml Hoechst 33342 (Hank's balanced salt solution-20 mM HEPES; pH 7.4) for 10 min (37°C). Cells were washed with HBSS than immediately incubated (40 min, 37°C) in HBSS containing pRRD (33 μg/ml). Cells were then washed with HBSS and the cells in each chamber were covered with HBSS containing the appropriate concentration of Baf-A1.
Cellular lysosomal fluorescence resulting from pRRD uptake was quantitated by epifluorescence microscopy by using an Olympus IX70 inverted microscope coupled to a Retiga-SRV fast monochrome charge-coupled device camera and Nikon NIS-Elements imaging software. Each well of a chamber slide was imaged by capturing five images, each from distinct areas (image field dimensions were 2.7 × 105 μm2). Integrated pRRD fluorescence over the entire area of each image field was obtained. Background fluorescence was sampled from three distinct areas and the mean was used to calculate the background fluorescence for each image field. The background-corrected integrated pRRD fluorescence of each image field was normalized for cell number by dividing by total nuclear counts to obtain the total integrated pRRD fluorescence per cell (TIFC). For each chamber well the TIFC values from the five separate image fields were averaged. Averaged TIFC values from triplicate experiments were combined to give a final mean TIFC value (n = 3).
Analysis of cellular morphology.
To assess viability of the cells based on gross cellular appearance (chromatin condensation and cell shrinkage), infected and mock-infected A549 cells treated with different doses of Baf-A1 (0–100 nM) in 12-well plates were examined by phase-contrast microscopy (Nikon TE-2000) and cells were photographed with a Canon-A700 digital camera.
MTT assay.
Cytotoxic effects of Baf-A1 on the A549 cells was determined by MTT assays as previously described (14). Briefly, cells grown in 12-well plates were pretreated with Baf-A1 (0–100 nM, 24 h) and then infected with mock or A/PR/8/34 virus at a MOI of 1 PFU/cell for each experimental time point (10 and 24 h pi). The percentage of cell viability was calculated as (mean OD of treated cells/mean OD of control cells) × 100. For each time point, the treated cells were compared with control cells that had been treated only with medium. For each individual experiment, a vehicle control was performed at the appropriate time point.
Measurement of apoptosis by FACS analysis.
Apoptosis was measured by the Nicoletti method as previously described (37). Briefly, cells grown in 12-well plates were pretreated with Baf-A1 (0–100 nM, 24 h) and then infected with mock or A/PR/8/34 virus at a MOI of 1 PFU/cell for each experimental time point (10 and 24 h pi). After scraping, the cells were harvested by centrifugation at 1,500 g for 5 min, washed once with PBS, and resuspended in hypotonic PI lysis buffer (1% sodium citrate, 0.1% Triton X-100, 0.5 mg/ml RNase A, 40 μg/ml PI). Cell nuclei were incubated for 30 min at 30°C and subsequently analyzed by FACS. Nuclei to the left of the G1 peak containing hypodiploid DNA were considered apoptotic.
Immunoblotting.
Western blotting was used to detect vNP, NS1, LC3β, and GAPDH. Briefly, cell pellets were lysed on ice for 30 min in lysis buffer [20 mM Tris·HCl (pH 7.5), 0.5% Nonidet P-40, 0.5 mM PMSF, 100 μM β-glycerol 3-phosphate, and 0.5% protease inhibitor cocktail]. After a high-speed spin (13,000 g for 10 min), supernatant protein content was determined by Lowry protein assay. Equal amounts of proteins were size fractionated by SDS-PAGE and transferred to nitrocellulose membranes. After membranes were blocked with 5% nonfat dried milk and 0.1% Tween 20, blots were incubated overnight with the primary antibodies at 4°C. Blots were then probed with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature and visualized using an enhanced chemiluminescence detection (Amersham-Pharmacia Biotech).
Transmission electron microscopic analyses.
For transmission electron microscopy (TEM), cells were fixed in 2.5% glutaraldehyde in PBS (pH 7.4) for 1 h at 4°C and postfixed with 1% osmium tetroxide before being embedded in Epon. TEM images were captured via a CM-10 transmission electron microscope (Philips, Netherlands) at 80 kV, on sectioned cells (100 nm on 200 mesh grids) stained with uranyl acetate and counterstained with lead citrate (15).
Statistical analysis.
Data are expressed as means ± SD and statistical differences were evaluated by one-way or two-way ANOVA followed by Tukey's or Bonferroni's post hoc test, with GraphPad Prism 5.0 to determine significant differences at a P value < 0.05. For all experiments data were collected in triplicate unless otherwise indicated.
RESULTS
Baf-A1 reduces IAV replication and release from A549 cells.
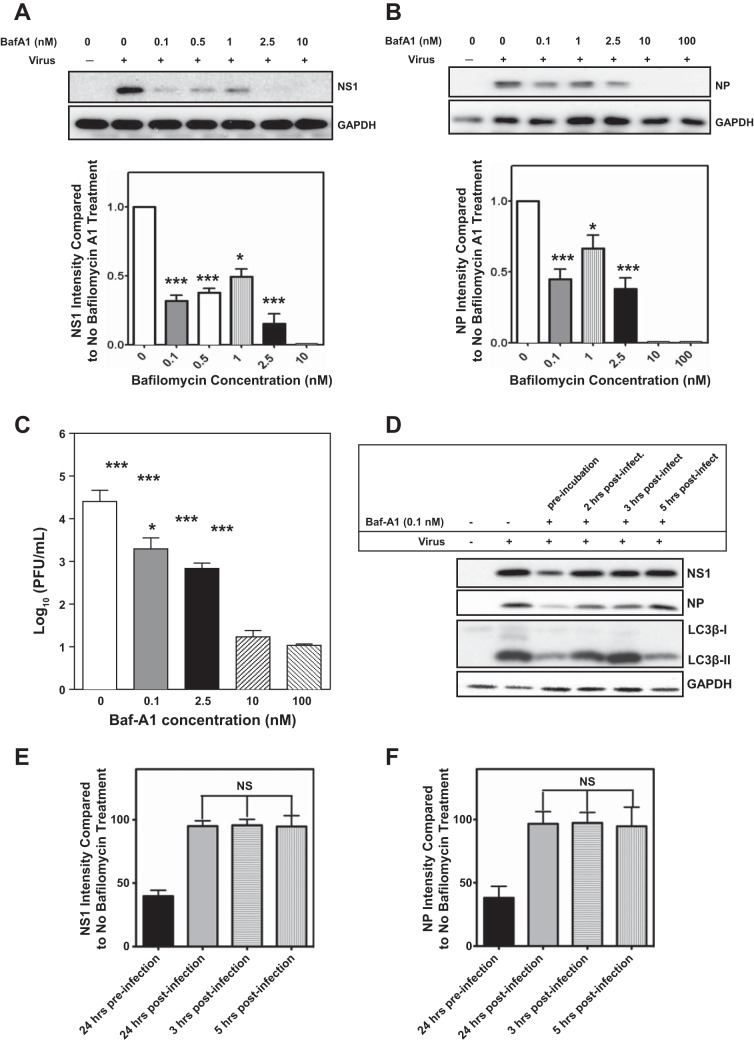
Since 100 nM Baf-A1 is known to inhibit influenza virus replication in MDCK cells (40), we assessed the anti-IAV activity of Baf-A1 at different concentrations in A549 human alveolar epithelial cells. Cells were treated with 0–100 nM Baf-A1 for 24 h and then infected with A/PR/8/34 virus at an MOI of 1 PFU/cell. The highest concentrations of Baf-A1 (10 and 100 nM), which are known to effectively suppress V-ATPases, completely blocked viral replication, confirming previous reports. However, at lower concentrations (0.1, 0.5, and 1 nM), which are well below the Baf-A1 IC50 (13), we also observed significantly diminished viral replication, as evidenced by a reduction in expression of the IAV nonstructural protein 1 (NS1) (P < 0.001, Fig. 1A) and vNP (P < 0.01, Fig. 1B). To examine the effects of Baf-A1 on the production of infectious virus, supernatants from infected A549 cells pretreated with Baf-A1 were collected and assessed using plaque assays (Fig. 1C). Baf-A1 suppressed generation of replicating IAV in a dose-dependent manner, with 0.1 nM Baf-A1 reducing the release of virus by a factor of 1 log10 (P = 0.003). Consistent with prior reports, IAV infectivity was also inhibited by ∼3 log10 (P < 0.001) with 10 and 100 nM Baf-A1. In another experiment we tested whether Baf-A1 treatment was responsible in A/PR/8/34 virus uncoating and affected viral replication. A549 were pretreated with Baf-A1 (0.1 nM, 24 h) and infected with A/PR/8/34 virus (MOI of 1 PFU/cell) or Baf-A1 were added to the infected cells (0.1 nM) 2, 3, and 5 h after A/PR/8/34 virus infection (MOI of 1 PFU/cell). After 24 h of infection cell lysates were prepared and viral proteins (NS1 and NP) protein were investigated by immunoblotting. Baf-A1 suppressed IAV when added to the A549 cells 24 h before infection and did not significantly affect IAV in postinfection treatment (P < 0.001) (Fig. 1, D–F).
Fig. 1.
Bafilomycin A1 (Baf-A1) pretreatment inhibits influenza virus NS1 and nucleoprotein (NP) protein and attenuates infectious virus production in lung epithelial cell cultures. A549 cells were treated for 24 h with different concentrations of Baf-A1 prior to infection with influenza A/PR/8/34 virus (H1N1) at multiplicity of infection (MOI) = 1 for 24 h. A and B: immunoblots showing that both high concentrations (10 and 100 nM) and low concentration (0.1 nM) of Baf-A1 markedly reduced expression of viral proteins NS1 (P < 0.001) (Fig. 1A) and NP (P = 0.003) (Fig. 1B). C: amount of virus released at 24 h postinfection (pi) was determined by plaque assays. Data are presented as means ± SE of 3 independent experiments. Statistical significance of Baf-A1 treatment vs. control was determined by a t-test, indicated by ***P value <0.001, *P value <0.05. D: representative immunoblots from A549 cells were infected with A/PR/8/34 virus (MOI = 1, 24 h). Some cultures were pretreated (24 h) with Baf-A1 (0.1 nM) prior to infection, whereas others were treated with Baf-A1 2, 3, or 5 h after A/PR/8/34 virus infection. All cell lysates were prepared 24 h after initial A/PR/8/34 virus infection. Immunoblots show cellular abundance of NS1, NP, and LC3βII. Densitometry analyses of immunoblots from 3 separate experiments, with normalization again a GAPDH loading control, revealed that Baf-A1 treatment after infection had no effect on NS1 or NP, whereas pretreatment significantly reduced vial protein accumulation compared with infected cells that received no Baf-A1 treatment (E and F) (P < 0.001).
IAV binding to A549 cells is not affected by Baf-A1.
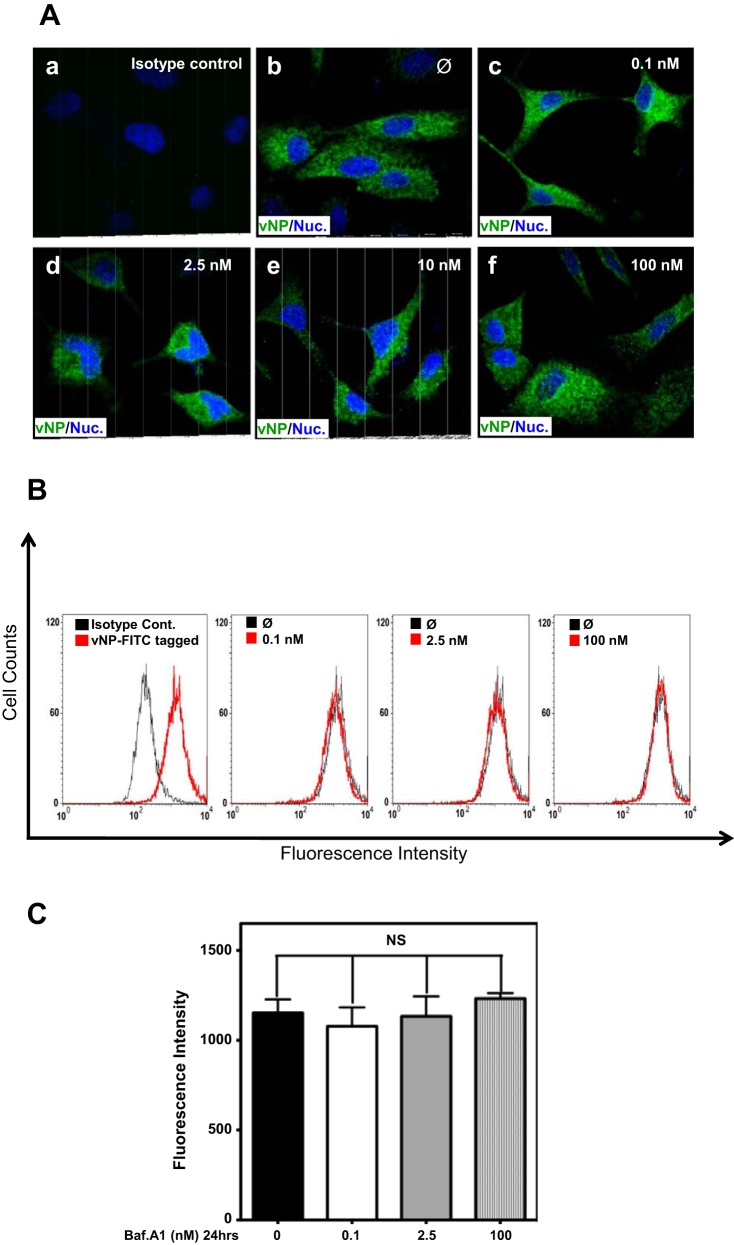
Because influenza virus entry begins with attachment to the surface of the host cell (8, 31, 49), and to gain insight into the mechanism for Baf-A1 inhibition of IAV replication, we next determined the impact of Baf-A1 on virus binding. Influenza virus was allowed to bind for 60 min on ice to A549 cells that had been pretreated with various Baf-A1 concentrations for 24 h. Cells were then washed and fixed without permeabilization. Cell-associated virus particles were determined by IF confocal microscopy and FACS with use of anti-vNP antibodies. IF confocal microscopy did not reveal any clear qualitative differences in virus binding to A549 cells treated with any Baf-A1 concentration (Fig. 2A). Quantitative determination of IAV particles bound to A549 cells by FACS analysis confirmed this observation (Fig. 2, B and C). These data indicate that Baf-A1 does not directly affect IAV binding to A549 cells.
Fig. 2.
Baf-A1 treatment has no effect on influenza A virus (IAV) binding. A549 cells were treated with different concentrations of Baf-A1 for 24 h and then incubated with A/PR/8/34 virus at a MOI of 10 plaque-forming units (PFU)/cell. Cells were exposed to influenza virus on ice for 90 min to allow viral binding, then washed, fixed, and analyzed by immunofluorescence (IF) microscopy and FACS by using anti-vNP antibody. A: typical confocal images of A549 cells stained for IAV particles with a mouse anti-vNP monoclonal antibody. Initial attachment and binding of IAV was not affected by Baf-A1 treatment since both Baf-A1-treated (c, d, e, and f) and Baf-A1-untreated (b) A549 cells showed comparable high levels of virus binding. vNP, IAV nuclear protein. B: representative FACS histograms of Baf-A1 pretreated A549 cells infected with influenza virus, showing number of attached virus particles in Baf-A1-treated cells (in red) compared with nontreated cells (in black). C: quantified data of the FACS histograms displayed in B. Treatment of A549 cells with different concentrations of Baf-A1 did not affect the numbers of viruses attached to the cells. Error bars represent the standard deviation for 3 independent experiments. Ø, No Baf-A1 treatment; Nuc, nucleus; NS, not significant.
Baf-A1 attenuates IAV intracellular trafficking to nuclei and nuclear import.
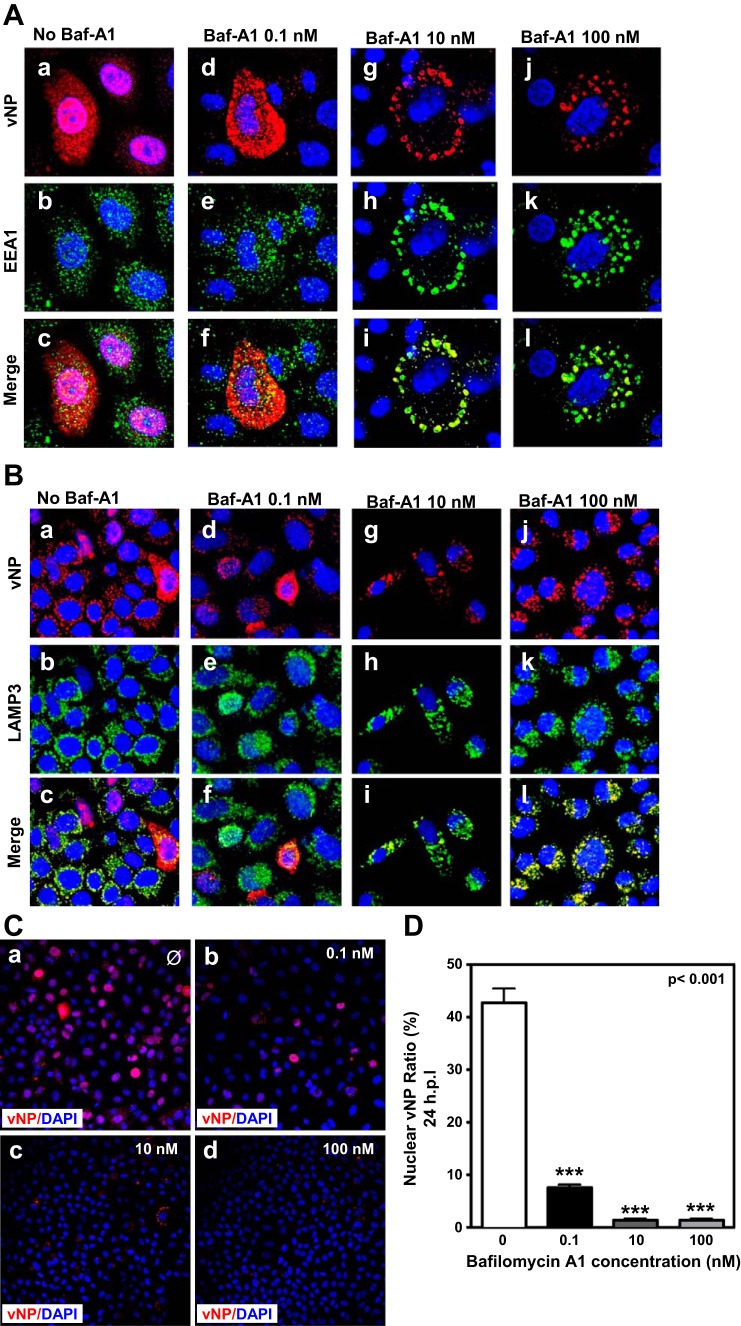
Since viral particles are taken up into coated pits and undergo endocytosis (26, 31), we next determined the effect of different concentrations of Baf-A1 on intracellular virus trafficking in A549 cells by IF confocal microscopy using antibodies for markers of early endosomes (EEA1), late endosomes (lysosome-associated membrane glycoprotein 3; LAMP3), and IAV nucleoprotein (vNP). The distribution of influenza vNP in infected cells in the absence of Baf-A1 treatment was typical for endosomal virus transport, with vNP evident in the nucleus as well as in the cytoplasm, where it appeared to colocalize with LAMP3 and had many areas of overlap with small EEA1-positive punctuate EEA1 (Fig. 3, A and B, a–c). In cells treated with 0.1 nM Baf-A1, reduced labeling of nuclear vNP was evident, but overlapping staining with EEA1 and LAMP3 in the cytoplasm was retained (Fig. 3, A and B, d–f). This effect was enhanced in cells treated with higher, 10 and 100 nM Baf-A1 concentrations (Fig. 3, A and B, g–i and j–l). Consistent with data from prior studies (9), our observations suggest that IAVs are retained in early and late endosomes in cells treated with relatively high concentrations of Baf-A1.
Fig. 3.
Entry of influenza virus ribonucleoproteins (RNPs) into the nucleus is blocked in a dose-dependent manner by Baf-A1. A549 cells grown on glass coverslips and pretreated with different concentrations of Baf-A1 (0–100 nM) for 24 h were infected with A/PR/8/34 virus at MOI of 1 PFU/cell for 24 h. Cells were analyzed by IF confocal microscopy using anti-vNP, anti-EEA1, and anti-LAMP3 antibodies. TO-PRO-3 was used for nuclear staining. A–C: examination of the effect of different concentrations of Baf-A1 (0–100 nM) on subcellular localization of IAV particles by confocal fluorescence microscopy. In the absence of Baf-A1, expression of virus protein NP (red) was observed in the nuclei (blue) of infected cells (Aa and B). The number of vNP within nucleoli was lower in cells treated with 0.1 nM Baf-A1 (Ad). No vNP positive cells (red) were seen in cells treated with high concentrations of Baf-A1 (10 and 100 nM) (A, g and j). The majority of virus particles (red) are trapped in the early endosomes (A, i and l) or late endosomes (B, i and l) of cells treated with 10 and 100 nM Baf-A1. D: quantitative determination of IAV nuclear transportation. Bar graph shows quantitative analysis for ratios of vNP nuclear imported cells/total infected cells (Fig. 3D). About 42% of vNPs were translocated to the cell nuclei in the control cells but this number was reduced to ∼7% in cells treated with 0.1 nM Baf-A1. Translocation of vNP to the cell nuclei was significantly blocked in the cells treated with high concentrations of Baf-A1 (10 and 100 nM). Data show the mean values and SE scored from counting total and infected cells from 5 nonadjacent ×40 high-power fields of view (average 180 cells/field) and presented as average percentages of vNP nuclear staining in infected cells treated with Baf-A1 vs. nontreated control cells. Values are expressed as means ± SD from 3 independent experiments (n = 3), and statistical differences were evaluated by 1-way or 2-way ANOVA followed by Tukey's or Bonferroni's post hoc test, using GraphPad Prism 5.0. P < 0.05 was considered significant. ***P < 0.001.
We also quantified abundance of the vNP during IAV infection of A549 cells (24 h pi) pretreated with different concentrations of Baf-A1. Quantitative analysis of vNP abundance by IF microscopy showed that at least 42% of vNP in infected cells accumulated in nuclei of Baf-A1 naive control cells (Fig. 3, Ca and D). Nuclear vNP was significantly decreased to ∼7% in cells pretreated with 0.1 nM Baf-A1 (Fig. 3, Cb and D). Nuclear vNP was virtually absent in cells treated with 10 or 100 nM Baf-A1 (Fig. 3, Cc, Cd, and D). Collectively, these data show that, even at low concentration, Baf-A1 attenuates nuclear accumulation of IAV NP protein, suggesting that endosomal and vesicular trafficking are significantly impacted.
Low concentrations of Baf-A1 have no effect on lysosome number.
In addition to the suspected requirement of acidified endosomes for uncoating and fusion of viral and endosomal membranes (6), the formation of vacuoles with low intracellular pH is required for autophagosome-lysosome fusion during autophagy, a process that has been shown to be induced in association with IAV replication (33, 61). Because our data indicate that high concentrations of Baf-A1 greatly diminish nuclear staining for vNP (Fig. 3), we next investigated whether this observation is associated with incomplete virus uncoating and reduced formation of acid lysosomes.
In cells treated with LysoTracker Red DND-99, the dye normally accumulates in acidic vacuoles, in particular lysosomes. Thus this compound can reveal abnormalities in formation of vacuoles with low pH that are capable of fusion with autophagosomes (16, 21, 57). We pretreated A549 cells with different concentrations of Baf-A1 for 24 h, infected the cells with IAV at MOI = 1 for another 24 h, and then labeled the cells with LysoTracker Red DND-99 for 10 min. Thereafter cells were fixed and then analyzed by fluorescence microscopy. Treatment with 0.1 nM Baf-A1 did not appear to alter the pattern of lysosome staining compared with IAV-infected cells that were not treated with Baf-A1 (Fig. 4A, top). This suggests that this very low Baf-A1 concentration had no effect on V-ATPase activity, formation of vacuoles with low pH, or the number of lysosomes. Consistent with prior reports (60), higher concentrations of Baf-A1 diminished LysoTracker Red DND-99 staining (Fig. 4A, bottom). Indeed, no detectable labeling appeared in cells treated with 100 nM Baf-A1 (Fig. 4A, bottom right). To quantify these responses we used Olympus FluoView software to determine the average pixel intensity in confocal images. This analysis revealed a significant reduction in the number of stained lysosomes in cells treated with 10 and 100 nM Baf-A1, compared with IAV-infected A549 cells treated with either no or with low-concentration (0.1 nM) Baf-A1 (P < 0.001). Collectively, the altered pattern and reduction in the number of stained lysosomes suggests that high, but not low, concentrations of Baf-A1 block vATPase activity to diminish the abundance of acidic lysosomes. We also confirmed the effect of different concentrations of Baf-A1 on lysosomal pH using a newly developed pH-sensitive dye, pRRD. We used 0.1, 1, and 10 nM concentration of Baf-A1. Our result showed that low concentration of Baf-A1 (0.1, 1 nM) did not significantly affect lysosomal pH whereas higher concentration of Baf-A1 (10 nM) significantly diminished lysosomal pH (P < 0.001) (Fig. 4, C and D).
Fig. 4.
Baf-A1 at high concentration inhibits lysosome acidification and autophagosome flux in A549 cells. A: confocal imaging of lysosomes. Confocal micrographs showing LysoTracker Red DND-99 staining of A549 cells treated for 24 h with different concentrations of Baf-A1 (0.1, 10, or 100 nM), prior to infection with A/PR/8/34 virus. Images were obtained 24 h after initiation of infection. Control cultures were not pretreated with Baf-A1. B: histogram showing quantification of integrated fluorescence of LysoTracker Red DND-99 in A549 cells under conditions shown in A. By use of Olympus FluoView software, LysoTracker fluorescence was determined from 6 non-adjacent ×60 high-power fields with an average of 65 cells/field. Values are expressed as means ± SD compared with nontreated control cells of 2 independent experiments. ***P < 0.001 vs. bib-treated cells. C: epifluorescence micrographs showing labeling with pHRodoRed (red) in A549 cells treated with different concentrations of Baf-A1 as indicated, included untreated (control) cultures. Cell nuclei were stained with Hoechst 333442 (blue). D: histogram showing integrated pHRodoRed Dextran (pRRD) fluorescence per cell in A549 cultures under conditions described for images in C. Fluorescence was determined from individual image fields by using Nikon NIS-Elements imaging software as described in materials and methods. Five distinct image areas were captured from individual wells. Mean background fluorescence was determined from 3 distinct areas devoid of cells and subtracted from pRRD fluorescence of each image field. The corrected fluorescence was normalized for cell number by dividing by total nuclear counts to obtain the total integrated pRRD fluorescence per cell (y-axis). For each well, mean corrected values per image field were averaged and used to determine mean ± SD from 3 independent experiments. ***P < 0.001 compared with untreated control cultures. E: immunoblot showing effects of Baf-A1 on infection-induced LC3-II accumulation. A549 cells were pretreated with different concentrations of Baf-A1 for 24 h prior to being infected with influenza A/PR/8/34 virus. The blot shown is typical of 3 independent experiments. As a control, infection burden was monitored by assessing NP accumulation. As a loading control GAPDH abundance was determined for each sample. F: densitometric analysis of LC3-II in A549 cells from immunoblots under conditions described for E. LC3-II levels were normalized to GAPDH as a loading control. ***P < 0.001 for uninfected control cells (C) vs. A/PR/8/34 virus-only infected cells (0). *P < 0.05 compared with A/PR/8/34 virus-only infected cells (0). #P < 0.01 compared to A/PR/8/34 virus-only infected cells (0). Results represent mean ± SD from 3 independent experiments.
Baf-A1 effects on autophagy and autophagosome formation in A549 cells.
Recent studies indicate that autophagy pathways are induced during various pathogenic infections (10, 52), although the precise role of autophagy during influenza virus infection is not yet elucidated (61). Autophagy is a multistep homeostatic lysosome-dependent degradation pathway that can be greatly increased by stress stimuli, such as viral infection. Autophagy involves formation of a double-membrane phagophore that elongates to form autophagosome vesicles. These structures undergo maturation and ultimately form autolysosomes, either through direct fusion with a lysosome or by first forming an amphisome (fusion with a late endosome) that associates with a lysosome (10). Baf-A1 treatment blocks autophagy flux as inhibition of V-ATPase interferes with fusion of autophagosomes and lysosomes, preventing autophagosome-linked degradation (24, 58).
Western blot analyses of microtubule-associated protein 1 light chain, LC3, is considered a marker for autophagosome formation because during autophagosome maturation it is cleaved and becomes conjugated with phosphatidylethanolamine to form the LC3-II variant (33). We used immunoblotting and densitometry to measure cellular levels of LC3-II in A549 cells pretreated with different concentrations of Baf-A1 for 24 h then subjected to IAV infection. Infection alone was sufficient to significantly induce an increase in LC3-II, but pretreatment with 0.1 and 1 nM Baf-A1 suppressed infection-induced LC3-II accumulation (Fig. 4, E and F). This effect of Baf-A1 could be due to either a decrease in formation or an increase in degradation of autophagic vacuoles. As expected, increasing Baf-A1 concentrations that are known to inhibit autophagy flux augmented infection-induced LC3-II accumulation (Fig. 4, E and F).
Baf-A1 effects on ultrastructural features of IAV-infected A549 cells.
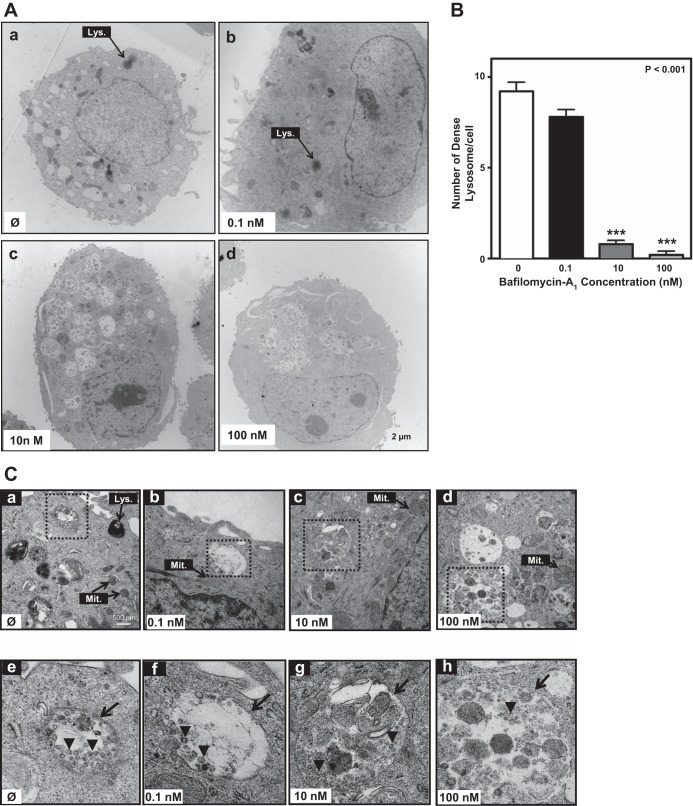
To fully characterize cellular responses to treatment with different Baf-A1 concentrations, we examined A549 cells 24 h after infection by TEM. In infected A549 cells without Baf-A1 exposure, or exposed to low (0.1 nM) Baf-A1 concentration, we observed organelles, nuclei, and chromatin with typical morphology. Numerous electron-dense lysosomes that appear as dark vesicles, indicating lysosomal activation, were evident scattered throughout the cytoplasm (Fig. 5A, a and b). In contrast, but consistent with our observations using LysoTracker labeling (Fig. 4), cells treated with 10 or 100 nM Baf-A1 possessed markedly fewer dark lysosomes but accumulated both double-membrane vacuole structures resembling autophagosomes and vesicles that contained what appeared to be undegraded material (Fig. 5A, c and d). To more fully appreciate these TEM-detected features, we performed semiquantitative analysis for the number of lysosomes, identifying dark-stained lysosomes in the cytoplasm. This confirmed there was a significant reduction in the number of active electron-dense lysosomes in infected cells treated with 10 or 100 nM Baf-A1 compared with counterparts that either were unexposed to Baf-A1 or were treated with 0.1 nM Baf-A1 (Fig. 5B) (P < 0.001 vs. control group).
Fig. 5.
Ultrastructural changes in A549-infected cells pretreated with Baf-A1. A549 cells were treated with Baf-A1 (0–100 nM, 24 h), then infected with A/PR/8/34 virus (MOI 5 PFU/cell), and transmission electron microscopy (TEM) analyses were performed 24 h thereafter. A: TEM photomicrographs showing typical ultrastructural features (organelles, nucleus), including darkly stained lysosomes (Lys; arrow) in control (Ø) cultures (no infection, no Baf-A1) (Aa). Lysosomes are readily seen in infected cells cotreated with 0.1 nM Baf-A1 (Ab, arrow), but are absent in cells treated with 10 nM (Ac) or 100 nM Baf-A1 (Ad). Scale bar for all images is shown at bottom right of Ad. B: bar graph shows quantitative analysis of the number of darkly stained lysosomes (described in materials and methods) in A549 cells under the conditions noted for images in A above. ***P < 0.0001 vs. control conditions (0) (no infection, no Baf-A1 treatment). Results are expressed as means ± SD of total numbers of lysosomes in images captured from 4 different cells. C: high-power TEM micrographs of A549 cells showing mitochondria (Mit.) and lysosomes under the same conditions described for A. Scale bar for Ca–Cd is shown in the lower right corner of Ca. With increasing Baf-A1 concentration there is loss of lysosomal structures, mitochondrial swelling, and increased vacuolation. Boxed areas (hatched lines) identify regions that are enlarged in the corresponding panels (Ce–Ch) in the lower row of images. Arrowheads in each panel point to viral particles within autophagosomes. Arrows in Ce and Cf identify double-membrane autophagosomes. In Cg and Ch the arrows identify disorganized-membrane autophagosomes.
Using high magnification TEM, we also examined the presence and contents of the double-membrane autophagosomes. These structures, containing virus particles, were readily seen in IAV-infected cells (Fig. 5C, a and e). In cells treated with 0.1 nM Baf-A1 prior to IAV infection, autophagic vacuoles with intact membrane containing virus particles were visible (Fig. 5C, b and f). However, in cells pretreated with 10 or 100 nM Baf-A1 we observed accumulation of incomplete autophagic vacuoles that appeared to envelop both viral particles and undigested material (Fig. 5C, c, d, g, and h). Collectively, these data suggest that, although both low and high concentrations of Baf-A1 reduced IAV replication in A549 cells, the mechanisms of action, with respect to autophagy flux, are different. Cells treated with Baf-A1 at higher concentrations (10 and 100 nM) displayed lysosomal acidification inhibition, attenuated fusion between autophagosomes and lysosomes, blockage of the autophagy pathway, and accumulation of IAV within vesicles that lead to an impaired virus life cycle. However, lysosomal pH and fusion between autophagosomes and lysosomes were unaffected in cells treated with 0.1 nM Baf-A1.
Baf-A1 induces cell death in a concentration-dependent manner.
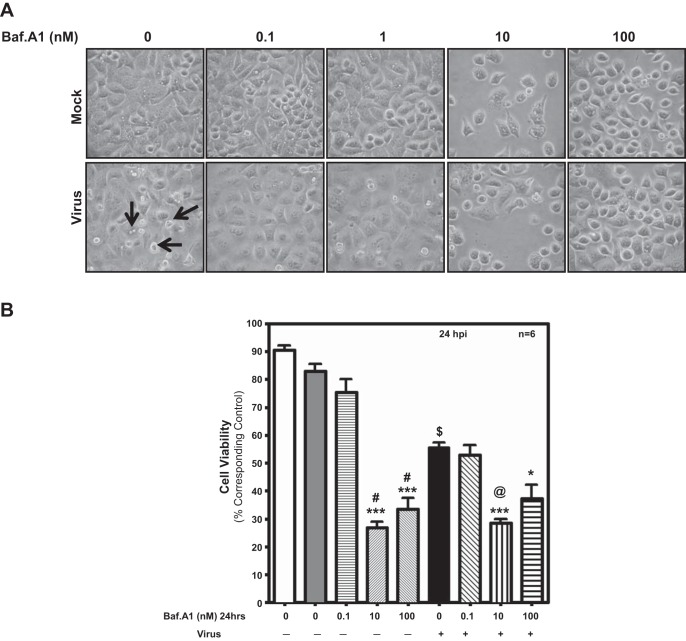
Previous studies have shown that higher Baf-A1 concentrations have cytotoxic effects in vitro (27, 56). To study cytotoxicity of Baf-A1 in our system, we examined cellular morphology of mock- and virus-infected A549 cells pretreated with different doses of Baf-A1, focusing on chromatin condensation and cell shrinkage. Virus-infected cells exhibit characteristic morphological features of cytopathic effects and cell death, such as cell shrinkage, cell disassembly, and rounding and membrane blebbing (Fig. 6A, arrows). Cells treated with 0.1 nM Baf-A1 show relatively fewer signs of death and appear more normal in both infected and noninfected cells compared with virus-infected cells without Baf-A1 pretreatment (Fig. 6A). In contrast, treatment of cells with 10 or 100 nM Baf-A1 resulted in cells with morphological changes in shape and appearance as well as reduced the number of adherent cells, revealing cytotoxic effects of Baf-A1 at these concentrations (Fig. 6A).
Fig. 6.
Analysis of cellular morphology. Phase-contrast microscopy images to capture gross cellular appearance (chromatin condensation and cell shrinkage) at different doses of Baf-A1 in the presence and absence of virus in A549 cells. A: reduced density and poor morphological appearance of cells treated with high concentrations of Baf-A1 (10 and 100 nM), indicating cytotoxicity at high concentrations of Baf-A1. At low concentration of Baf-A1 (0.1 nM), both infected and noninfected cells appeared healthy compared with virus-infected cells without pretreatment with Baf-A1. B: quantification of cytotoxic effects of Baf-A1 on A549 cells. Cells grown in 12-well plates were pretreated with Baf-A1 for 24 h, and either mock-infected or infected with virus and examined by MTT assay. Low-dose Baf-A1 (0.1 nM) was not toxic to A549 cells. Treatment with Baf-A1 at 10 and 100 nM decreases cell viability. Baf-A1 (0.1 nM) also protected virus-infected cells against cell death. Results represent means ± SD from 6 independent experiments. ***P < 0.001, *P < 0.05 vs. matched control; $P < 0.001, virus only vs. Mock; @P < 0.001, virus + 0.1 nM Baf-A1 vs. virus + 10 nM Baf-A1; #P < 0.001, 0.1 nM Baf-A1 vs. 10 and 100 nM Baf-A1.
The concentration-dependent effects of Baf-A1 exposure on mock- and virus-infected A549 cell viability was also examined using the MTT assay (38). Infection of A549 cells with IAV significantly reduced cell viability compared with mock-infected cells (P < 0.001). In IAV-infected as well as uninfected A549 cells, we observed reduced cell viability, with higher doses of 10 and 100 nM Baf-A1 inducing significant levels of cell death after 48 h (P < 0.001) (Fig. 6B). These data confirm that the low concentration of Baf-A1 used in our study has limited, if any, cytotoxic effect on uninfected and IAV-infected human lung epithelial cells.
To better understand the mechanism underpinning reduced cell viability in cells treated with high concentrations of Baf-A1, we performed FACS analysis to identify cells with sub-G1 DNA content (i.e., apoptotic) by labeling with PI (45). Treatment of uninfected cells with 10 and 100 nM Baf-A1 for 24 h also significantly increased the number of cells in sub-G1 phase (P < 0.001) (Fig. 7, A and B). Conversely, 0.1 nM Baf-A1 alone had no statistically significant effect on the number of cells with sub-G1 DNA content (P > 0.05) (Fig. 7, A and B). Infection without Baf-A1 pretreatment was sufficient to increase the fraction of sub-G1-phase A549 cells (Fig. 7, A and B), and this effect was increased when cells were pretreated with 10 or 100 nM Baf-A1 (P < 0.001) (Fig. 7, A and B). Notably, pretreatment of cells with 0.1 nM Baf-A1 did not increase infection-induced apoptosis, confirming that at this low-concentration Baf-A1 has very limited, if any, cytotoxic effect and, compared with the nontreated cells, is protective.
Fig. 7.
Apoptotic cell death induced by different concentrations of Baf-A1 (Baf). Representative FACS histograms for A549 cells treated with different concentrations of Baf-A1 (24 h) (indicated by text in relevant panels), and infected with influenza A/PR/8/34 virus (MOI 1 PFU/cell). For controls, cultures either underwent Mock infection or were not treated at all (labeled as Control). Cells were lifted, and nuclei were stained with propidium iodide, then subjected to FACS analysis to identify apoptotic cells with a reduced DNA content, called the sub-G1 subgroup (delineated as M1). B: bar graph showing mean data for fraction of cells in sub-G1 subgroup) from FACS analyses displayed in A. White bar represents the Control condition, whereas dark gray bar (2nd from left) is for mock-infected cultures. Results represent means ± SD from 6 independent experiments. ***P < 0.001 compared with Control. *P < 0.01 compared with Mock; **P < 0.001 compared with Mock.
DISCUSSION
The antagonistic effect of Baf-A1 on the replication of viruses, including influenza A and B, has been reported by other groups (30, 32, 36, 40, 50). The antiviral activity of Baf-A1 at concentrations ≥10 nM has been attributed to its ability to inhibit V-ATPase, an enzyme that acidifies intracellular compartments, including lysosomes (60). Although this demonstrates that preventing the genesis and/or maintenance of acid lysosomes impacts viral uncoating to prevent replication, the toxicity of such high concentrations of Baf-A1 limits its antiviral therapeutic potential in vivo (27).
Lysosomes are cellular acidic organelles that rely on V-ATPases for the maintenance of their intraluminal acidity. Thus, to explore the effect of different concentrations of Baf-A1 on acid lysosomes, LysoTracker Red was employed. This fluorescent acidotropic probe consists of a fluorophore linked to a weak base that is only partially protonated at neutral pH. LysoTracker Red is freely permeant to cell membranes and typically concentrates in acidic organelles; thus staining with the dye has been used to track and measure formation of acidic vacuoles such as endosomes, trans-Golgi vesicles, and lysosomes. Consistent with our electron microscopy data, LysoTracker Red staining confirmed that very low Baf-A1 concentration had no effect on the number of lysosomes. Similarly, using a lysosome-selective, pH-sensitive dye, we observed that low concentrations of Baf-A1 were without effect on vacuolar pH. In contrast, with higher concentrations of Baf-A1, the number of lysosomes as well as vacuolar pH was significantly reduced. Here we also demonstrate that Baf-A1 used at concentrations up to 1,000 times less than previously reported, and insufficient to fully inhibit V-ATPases, is sufficient to markedly limit influenza A replication in human lung epithelial host cells in vitro. This effect appears to occur without impact on lysosomal pH and is without cytotoxic effect. This suggests a novel antiviral mechanism for low-dose Baf-A1 and suggests this plecomacrolide antibiotic may offer future therapeutic potential in vivo.
Attachment to the host cell is the first crucial step in establishing a successful virus infection. Here we show that Baf-A1 treatment (at low and high concentrations) has no impact on IAV attachment to human lung epithelial cells. Although we show that both low and high concentrations of Baf-A1 disrupt nuclear import of IAV and attenuated virus replication and release, the mechanism of antiviral activity of a very low concentration of Baf-A1 at 0.1 nM did not involve pruning of acid lysosomes; indeed, this lower dose even appeared to limit infection-induced autophagosome formation. Conversely, markedly higher concentrations of Baf-A1 markedly attenuated lysosome number and autophagy flux, likely owing to inhibition of V-ATPase (4, 40, 58, 60), an effect that was concomitant with blocking IAV nuclear import and replication.
Reduced virus-induced LC3-II accumulation by low concentration of Baf-A1 could suggest an important role in lysosome-associated regulation of autophagy, a pathway that has been shown to be involved in IAV replication (61). Little is known about the molecular target(s) that low-dose Baf-A1 might affect. It is likely that low concentration of Baf-A1 may influence upstream factors that regulate autophagy such as mammalian TOR (mTOR) or hinder the Beclin 1/Bcl-2 interaction (42, 48). Additional research is warranted to further understand how low concentration of Baf-A1 limits autophagosome formation. In cerebellar granule neurons, Shacka and colleagues (47) showed that low concentration of Baf-A1 (≤1 nM) exhibits an inhibitory effect on chloroquine-induced caspase-3 activation and cell death in the absence of any effect on vacuolar acidification or autophagy. Similarly, this group has described a cytoprotective effect of ≤1 nM Baf-A1 in human neuroblastoma cells and Caenorhabditis elegans neuron cells treated with chloroquine, hydroxychloroquine amodiaquine, or staurosporine (43). Although this cytoprotective effect has been suggested to likely involve maintenance of the autophagy-lysosome pathway independent of V-ATPase inhibition (43), the exact mechanism of action remains unknown. Furthermore, similar to Pivtoraiko and colleagues' work (43), but in contrast to Shacka et al.'s study (47), our data suggest that, although there was no effect of 0.1 nM Baf-A1 on lysosome number in A549 cells, the autophagy pathway was affected by low and high concentrations of Baf-A1. The reported differences may be due to different cell types used in these studies.
The antiviral activity of Baf-A1 makes it a potentially suitable drug candidate for the treatment of viral infection. However, the ionophoric properties of ≥100 nM Baf-A1 cause mitochondrial damage and apoptosis, precluding the in vivo pharmacological use of this macrolide (17, 51). Consistent with studies in a number of different cell types (4, 39, 56), we found that 10 and 100 nM Baf-A1 treatment reduced A549 cell viability and induced apoptotic cell death. This effect appears to be linked to Baf-A1-mediated inhibition of the acidification of intracellular compartments that are required for vital cellular processes, such as membrane turnover, autophagy, and endocytotic uptake of nutrients, and severely limits the therapeutic use of Baf-A1 at a relatively high concentration (3, 18, 35). In a study conducted by Muller and colleagues (35), the investigators also found that low concentration of Baf-A1 (below 2 nM) could successfully inhibit viral replication. They also reported that three daily intraperitoneal injections of 350 ng/kg Baf-A1 in mice caused spleen and liver damage without impact on IAV infection. The effects of Baf-A1 at this dose could be attributed to the route of Baf-A1 administration and to processes of detoxification by the liver and kidney. Conceivably, other routes of drug administration such as nasal or inhalation would be more efficient to target the lung epithelium. Furthermore, it is not evident whether 350 ng/kg injection of Baf-A1 in mice is sufficient to inhibit V-ATPase activity. Other groups have used much higher concentrations of Baf-A1 to reach its inhibitory effect on V-ATPase. For instance, in a study conducted by Belibi and colleagues (2), mice were injected with 2 mg/kg Baf-A1 for 4 days, and in another study (41) 1 mg/kg of Baf-A1 was injected for 4 wk to reach inhibitory effects.
In an attempt to reduce its cytotoxic effects, others have examined the cellular effects of short-term (1 h) pretreatment with high-dose Baf-A1 (≥100 nM) (34, 58). It has been suggested that, at high concentrations and short exposure times, Baf-A1 can reduce acid endosome and lysosome numbers, cause fusion block, inhibit autophagy (25), and lead to the inhibition of IAV replication (40). We opted for a noncytotoxic Baf-A1 concentration and an extended pretreatment (24 h), and this strategy succeeded in significantly reducing IAV replication. Although the complete molecular mechanism underlying the antiviral properties of low-dose Baf-A1 is not fully evident from our work, our data do indicate that this is unlikely to involve the ability of Baf-A1 to inhibit vacuolar acidification. Detailed investigation of this mechanism is currently underway by our group to understand how low-dose Baf-A1 has antiviral properties.
In conclusion, overall, our results reveal that low-concentration Baf-A1 is an effective inhibitor of IAV replication, without impacting host cell viability. Furthermore, these results suggest a unique mode of action of low concentration Baf-A1 that does not compromise host cell viability and thus has potential for in vivo therapeutic applications.
GRANTS
This work was supported by grant MOP-77759 from the Canadian Institute of Health Research (CIHR) awarded to A. J. Halayko, and CIHR grants ROP-104906 and MOP-106713 to K. M. Coombs, and by grants from the Manitoba Institute of Child Health (MICH) and Canada Foundation for Innovation. S. Ghavami is supported by a Parker B. Francis Fellowship in Pulmonary Research. T. Klonisch acknowledges support by the Natural Sciences and Engineering Research Council of Canada (NSERC). B. Yeganeh is supported by a MHRC/MICH postdoctoral fellowship. A. J. Halayko holds a Canada Research Chair in Airway Cell and Molecular Biology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.Y., S.G., K.M.C., and A.J.H. conception and design of research; B.Y., S.G., A.L.K., T.H.M., and G.L.S. performed experiments; B.Y., S.G., T.K., K.M.C., and A.J.H. analyzed data; B.Y., S.G., T.K., K.M.C., and A.J.H. interpreted results of experiments; B.Y. and S.G. prepared figures; B.Y. and S.G. drafted manuscript; S.G., T.K., K.M.C., and A.J.H. edited and revised manuscript; S.G., T.K., K.M.C., and A.J.H. approved final version of manuscript.
REFERENCES
- 1.Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine—Marshfield, Wisconsin, 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 57: 393–398, 2008. [PubMed] [Google Scholar]
- 2.Belibi F, Zafar I, Ravichandran K, Segvic AB, Jani A, Ljubanovic DG, Edelstein CL. Hypoxia-inducible factor-1α (HIF-1α) and autophagy in polycystic kidney disease (PKD). Am J Physiol Renal Physiol 300: F1235–F1243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA 85: 7972–7976, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25: 1025–1040, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown EG. Increased virulence of a mouse-adapted variant of influenza A/FM/1/47 virus is controlled by mutations in genome segments 4, 5, 7, and 8. J Virol 64: 4523–4533, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bui M, Whittaker G, Helenius A. Effect of M1 protein and low pH on nuclear transport of influenza virus ribonucleoproteins. J Virol 70: 8391–8401, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinni SR, Shisheva A. Arrest of endosome acidification by bafilomycin A1 mimics insulin action on GLUT4 translocation in 3T3–L1 adipocytes. Biochem J 339: 599–606, 1999. [PMC free article] [PubMed] [Google Scholar]
- 8.Chu VC, Whittaker GR. Influenza virus entry and infection require host cell N-linked glycoprotein. Proc Natl Acad Sci USA 101: 18153–18158, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries E, Tscherne DM, Wienholts MJ, Cobos-Jimenez V, Scholte F, Garcia-Sastre A, Rottier PJ, de Haan CA. Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog 7: e1001329, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreux M, Gastaminza P, Wieland SF, Chisari FV. The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci USA 106: 14046–14051, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J Exp Biol 200: 1–8, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem 281: 36303–36316, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Gagliardi S, Gatti PA, Belfiore P, Zocchetti A, Clarke GD, Farina C. Synthesis and structure-activity relationships of bafilomycin A1 derivatives as inhibitors of vacuolar H+-ATPase. J Med Chem 41: 1883–1893, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Ghavami S, Yeganeh B, Stelmack GL, Kashani HH, Sharma P, Cunnington R, Rattan S, Bathe K, Klonisch T, Dixon IM, Freed DH, Halayko AJ. Apoptosis, autophagy and ER stress in mevalonate cascade inhibition-induced cell death of human atrial fibroblasts. Cell Death Dis 3: e330, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glogowska A, Pyka J, Kehlen A, Los M, Perumal P, Weber E, Cheng SY, Hoang-Vu C, Klonisch T. The cytoplasmic domain of proEGF negatively regulates motility and elastinolytic activity in thyroid carcinoma cells. Neoplasia 10: 1120–1130, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Polo RA, Boya P, Pauleau AL, Jalil A, Larochette N, Souquere S, Eskelinen EL, Pierron G, Saftig P, Kroemer G. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci 118: 3091–3102, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hall TJ. Cytotoxicity of vacuolar H+-ATPase inhibitors to UMR-106 rat osteoblasts: an effect on iron uptake into cells? Cell Biol Int 18: 189–193, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Hanada H, Moriyama Y, Maeda M, Futai M. Kinetic studies of chromaffin granule H+-ATPase and effects of bafilomycin A1. Biochem Biophys Res Commun 170: 873–878, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Hill AW, Guralnick RP, Wilson MJ, Habib F, Janies D. Evolution of drug resistance in multiple distinct lineages of H5N1 avian influenza. Infect Genet Evol 9: 169–178, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Hurt AC, Ernest J, Deng YM, Iannello P, Besselaar TG, Birch C, Buchy P, Chittaganpitch M, Chiu SC, Dwyer D, Guigon A, Harrower B, Kei IP, Kok T, Lin C, McPhie K, Mohd A, Olveda R, Panayotou T, Rawlinson W, Scott L, Smith D, D'Souza H, Komadina N, Shaw R, Kelso A, Barr IG. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antiviral Res 83: 90–93, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J 26: 313–324, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson LA. Using surveillance to evaluate influenza vaccine effectiveness. J Infect Dis 199: 155–158, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 337: 199–204, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, Ahn HJ, Ait-Mohamed O, Ait-Si-Ali S, Akematsu T, Akira S, Al-Younes HM, Al-Zeer MA, Albert ML, Albin RL, Alegre-Abarrategui J, Aleo MF, Alirezaei M, Almasan A, Almonte-Becerril M, Amano A, Amaravadi R, Amarnath S, Amer AO, Andrieu-Abadie N, Anantharam V, Ann DK, Anoopkumar-Dukie S, Aoki H, Apostolova N, Arancia G, Aris JP, Asanuma K, Asare NY, Ashida H, Askanas V, Askew DS, Auberger P, Baba M, Backues SK, Baehrecke EH, Bahr BA, Bai XY, Bailly Y, Baiocchi R, Baldini G, Balduini W, Ballabio A, Bamber BA, Bampton ET, Banhegyi G, Bartholomew CR, Bassham DC, Bast RC Jr, Batoko H, Bay BH, Beau I, Bechet DM, Begley TJ, Behl C, Behrends C, Bekri S, Bellaire B, Bendall LJ, Benetti L, Berliocchi L, Bernardi H, Bernassola F, Besteiro S, Bhatia-Kissova I, Bi X, Biard-Piechaczyk M, Blum JS, Boise LH, Bonaldo P, Boone DL, Bornhauser BC, Bortoluci KR, Bossis I, Bost F, Bourquin JP, Boya P, Boyer-Guittaut M, Bozhkov PV, Brady NR, Brancolini C, Brech A, Brenman JE, Brennand A, Bresnick EH, Brest P, Bridges D, Bristol ML, Brookes PS, Brown EJ, Brumell JH, Brunetti-Pierri N, Brunk UT, Bulman DE, Bultman SJ, Bultynck G, Burbulla LF, Bursch W, Butchar JP, Buzgariu W, Bydlowski SP, Cadwell K, Cahova M, Cai D, Cai J, Cai Q, Calabretta B, Calvo-Garrido J, Camougrand N, Campanella M, Campos-Salinas J, Candi E, Cao L, Caplan AB, Carding SR, Cardoso SM, Carew JS, Carlin CR, Carmignac V, Carneiro LA, Carra S, Caruso RA, Casari G, Casas C, Castino R, Cebollero E, Cecconi F, Celli J, Chaachouay H, Chae HJ, Chai CY, Chan DC, Chan EY, Chang RC, Che CM, Chen CC, Chen GC, Chen GQ, Chen M, Chen Q, Chen SS, Chen W, Chen X, Chen YG, Chen Y, Chen YJ, Chen Z, Cheng A, Cheng CH, Cheng Y, Cheong H, Cheong JH, Cherry S, Chess-Williams R, Cheung ZH, Chevet E, Chiang HL, Chiarelli R, Chiba T, Chin LS, Chiou SH, Chisari FV, Cho CH, Cho DH, Choi AM, Choi D, Choi KS, Choi ME, Chouaib S, Choubey D, Choubey V, Chu CT, Chuang TH, Chueh SH, Chun T, Chwae YJ, Chye ML, Ciarcia R, Ciriolo MR, Clague MJ, Clark RS, Clarke PG, Clarke R, Codogno P, Coller HA, Colombo MI, Comincini S, Condello M, Condorelli F, Cookson MR, Coombs GH, Coppens I, Corbalan R, Cossart P, Costelli P, Costes S, Coto-Montes A, Couve E, Coxon FP, Cregg JM, Crespo JL, Cronje MJ, Cuervo AM, Cullen JJ, Czaja MJ, D'Amelio M, Darfeuille-Michaud A, Davids LM, Davies FE, De Felici M, de Groot JF, de Haan CA, De Martino L, De Milito A, De Tata V, Debnath J, Degterev A, Dehay B, Delbridge LM, Demarchi F, Deng YZ, Dengjel J, Dent P, Denton D, Deretic V, Desai SD, Devenish RJ, Di Gioacchino M, Di Paolo G, Di Pietro C, Diaz-Araya G, Diaz-Laviada I, Diaz-Meco MT, Diaz-Nido J, Dikic I, Dinesh-Kumar SP, Ding WX, Distelhorst CW, Diwan A, Djavaheri-Mergny M, Dokudovskaya S, Dong Z, Dorsey FC, Dosenko V, Dowling JJ, Doxsey S, Dreux M, Drew ME, Duan Q, Duchosal MA, Duff K, Dugail I, Durbeej M, Duszenko M, Edelstein CL, Edinger AL, Egea G, Eichinger L, Eissa NT, Ekmekcioglu S, El-Deiry WS, Elazar Z, Elgendy M, Ellerby LM, Eng KE, Engelbrecht AM, Engelender S, Erenpreisa J, Escalante R, Esclatine A, Eskelinen EL, Espert L, Espina V, Fan H, Fan J, Fan QW, Fan Z, Fang S, Fang Y, Fanto M, Fanzani A, Farkas T, Farre JC, Faure M, Fechheimer M, Feng CG, Feng J, Feng Q, Feng Y, Fesus L, Feuer R, Figueiredo-Pereira ME, Fimia GM, Fingar DC, Finkbeiner S, Finkel T, Finley KD, Fiorito F, Fisher EA, Fisher PB, Flajolet M, Florez-McClure ML, Florio S, Fon EA, Fornai F, Fortunato F, Fotedar R, Fowler DH, Fox HS, Franco R, Frankel LB, Fransen M, Fuentes JM, Fueyo J, Fujii J, Fujisaki K, Fujita E, Fukuda M, Furukawa RH, Gaestel M, Gailly P, Gajewska M, Galliot B, Galy V, Ganesh S, Ganetzky B, Ganley IG, Gao FB, Gao GF, Gao J, Garcia L, Garcia-Manero G, Garcia-Marcos M, Garmyn M, Gartel AL, Gatti E, Gautel M, Gawriluk TR, Gegg ME, Geng J, Germain M, Gestwicki JE, Gewirtz DA, Ghavami S, Ghosh P, Giammarioli AM, Giatromanolaki AN, Gibson SB, Gilkerson RW, Ginger ML, Ginsberg HN, Golab J, Goligorsky MS, Golstein P, Gomez-Manzano C, Goncu E, Gongora C, Gonzalez CD, Gonzalez R, Gonzalez-Estevez C, Gonzalez-Polo RA, Gonzalez-Rey E, Gorbunov NV, Gorski S, Goruppi S, Gottlieb RA, Gozuacik D, Granato GE, Grant GD, Green KN, Gregorc A, Gros F, Grose C, Grunt TW, Gual P, Guan JL, Guan KL, Guichard SM, Gukovskaya AS, Gukovsky I, Gunst J, Gustafsson AB, Halayko AJ, Hale AN, Halonen SK, Hamasaki M, Han F, Han T, Hancock MK, Hansen M, Harada H, Harada M, Hardt SE, Harper JW, Harris AL, Harris J, Harris SD, Hashimoto M, Haspel JA, Hayashi S, Hazelhurst LA, He C, He YW, Hebert MJ, Heidenreich KA, Helfrich MH, Helgason GV, Henske EP, Herman B, Herman PK, Hetz C, Hilfiker S, Hill JA, Hocking LJ, Hofman P, Hofmann TG, Hohfeld J, Holyoake TL, Hong MH, Hood DA, Hotamisligil GS, Houwerzijl EJ, Hoyer-Hansen M, Hu B, Hu CA, Hu HM, Hua Y, Huang C, Huang J, Huang S, Huang WP, Huber TB, Huh WK, Hung TH, Hupp TR, Hur GM, Hurley JB, Hussain SN, Hussey PJ, Hwang JJ, Hwang S, Ichihara A, Ilkhanizadeh S, Inoki K, Into T, Iovane V, Iovanna JL, Ip NY, Isaka Y, Ishida H, Isidoro C, Isobe K, Iwasaki A, Izquierdo M, Izumi Y, Jaakkola PM, Jaattela M, Jackson GR, Jackson WT, Janji B, Jendrach M, Jeon JH, Jeung EB, Jiang H, Jiang JX, Jiang M, Jiang Q, Jiang X, Jimenez A, Jin M, Jin S, Joe CO, Johansen T, Johnson DE, Johnson GV, Jones NL, Joseph B, Joseph SK, Joubert AM, Juhasz G, Juillerat-Jeanneret L, Jung CH, Jung YK, Kaarniranta K, Kaasik A, Kabuta T, Kadowaki M, Kagedal K, Kamada Y, Kaminskyy VO, Kampinga HH, Kanamori H, Kang C, Kang KB, Kang KI, Kang R, Kang YA, Kanki T, Kanneganti TD, Kanno H, Kanthasamy AG, Kanthasamy A, Karantza V, Kaushal GP, Kaushik S, Kawazoe Y, Ke PY, Kehrl JH, Kelekar A, Kerkhoff C, Kessel DH, Khalil H, Kiel JA, Kiger AA, Kihara A, Kim DR, Kim DH, Kim EK, Kim HR, Kim JS, Kim JH, Kim JC, Kim JK, Kim PK, Kim SW, Kim YS, Kim Y, Kimchi A, Kimmelman AC, King JS, Kinsella TJ, Kirkin V, Kirshenbaum LA, Kitamoto K, Kitazato K, Klein L, Klimecki WT, Klucken J, Knecht E, Ko BC, Koch JC, Koga H, Koh JY, Koh YH, Koike M, Komatsu M, Kominami E, Kong HJ, Kong WJ, Korolchuk VI, Kotake Y, Koukourakis MI, Kouri Flores JB, Kovacs AL, Kraft C, Krainc D, Kramer H, Kretz-Remy C, Krichevsky AM, Kroemer G, Kruger R, Krut O, Ktistakis NT, Kuan CY, Kucharczyk R, Kumar A, Kumar R, Kumar S, Kundu M, Kung HJ, Kurz T, Kwon HJ, La Spada AR, Lafont F, Lamark T, Landry J, Lane JD, Lapaquette P, Laporte JF, Laszlo L, Lavandero S, Lavoie JN, Layfield R, Lazo PA, Le W, Le Cam L, Ledbetter DJ, Lee AJ, Lee BW, Lee GM, Lee J, Lee JH, Lee M, Lee MS, Lee SH, Leeuwenburgh C, Legembre P, Legouis R, Lehmann M, Lei HY, Lei QY, Leib DA, Leiro J, Lemasters JJ, Lemoine A, Lesniak MS, Lev D, Levenson VV, Levine B, Levy E, Li F, Li JL, Li L, Li S, Li W, Li XJ, Li YB, Li YP, Liang C, Liang Q, Liao YF, Liberski PP, Lieberman A, Lim HJ, Lim KL, Lim K, Lin CF, Lin FC, Lin J, Lin JD, Lin K, Lin WW, Lin WC, Lin YL, Linden R, Lingor P, Lippincott-Schwartz J, Lisanti MP, Liton PB, Liu B, Liu CF, Liu K, Liu L, Liu QA, Liu W, Liu YC, Liu Y, Lockshin RA, Lok CN, Lonial S, Loos B, Lopez-Berestein G, Lopez-Otin C, Lossi L, Lotze MT, Low P, Lu B, Lu Z, Luciano F, Lukacs NW, Lund AH, Lynch-Day MA, Ma Y, Macian F, MacKeigan JP, Macleod KF, Madeo F, Maiuri L, Maiuri MC, Malagoli D, Malicdan MC, Malorni W, Man N, Mandelkow EM, Manon S, Manov I, Mao K, Mao X, Mao Z, Marambaud P, Marazziti D, Marcel YL, Marchbank K, Marchetti P, Marciniak SJ, Marcondes M, Mardi M, Marfe G, Marino G, Markaki M, Marten MR, Martin SJ, Martinand-Mari C, Martinet W, Martinez-Vicente M, Masini M, Matarrese P, Matsuo S, Matteoni R, Mayer A, Mazure NM, McConkey DJ, McConnell MJ, McDermott C, McDonald C, McInerney GM, McKenna SL, McLaughlin B, McLean PJ, McMaster CR, McQuibban GA, Meijer AJ, Meisler MH, Melendez A, Melia TJ, Melino G, Mena MA, Menendez JA, Menna-Barreto RF, Menon MB, Menzies FM, Mercer CA, Merighi A, Merry DE, Meschini S, Meyer CG, Meyer TF, Miao CY, Miao JY, Michels PA, Michiels C, Mijaljica D, Milojkovic A, Minucci S, Miracco C, Miranti CK, Mitroulis I, Miyazawa K, Mizushima N, Mograbi B, Mohseni S, Molero X, Mollereau B, Mollinedo F, Momoi T, Monastyrska I, Monick MM, Monteiro MJ, Moore MN, Mora R, Moreau K, Moreira PI, Moriyasu Y, Moscat J, Mostowy S, Mottram JC, Motyl T, Moussa CE, Muller S, Munger K, Munz C, Murphy LO, Murphy ME, Musaro A, Mysorekar I, Nagata E, Nagata K, Nahimana A, Nair U, Nakagawa T, Nakahira K, Nakano H, Nakatogawa H, Nanjundan M, Naqvi NI, Narendra DP, Narita M, Navarro M, Nawrocki ST, Nazarko TY, Nemchenko A, Netea MG, Neufeld TP, Ney PA, Nezis IP, Nguyen HP, Nie D, Nishino I, Nislow C, Nixon RA, Noda T, Noegel AA, Nogalska A, Noguchi S, Notterpek L, Novak I, Nozaki T, Nukina N, Nurnberger T, Nyfeler B, Obara K, Oberley TD, Oddo S, Ogawa M, Ohashi T, Okamoto K, Oleinick NL, Oliver FJ, Olsen LJ, Olsson S, Opota O, Osborne TF, Ostrander GK, Otsu K, Ou JH, Ouimet M, Overholtzer M, Ozpolat B, Paganetti P, Pagnini U, Pallet N, Palmer GE, Palumbo C, Pan T, Panaretakis T, Pandey UB, Papackova Z, Papassideri I, Paris I, Park J, Park OK, Parys JB, Parzych KR, Patschan S, Patterson C, Pattingre S, Pawelek JM, Peng J, Perlmutter DH, Perrotta I, Perry G, Pervaiz S, Peter M, Peters GJ, Petersen M, Petrovski G, Phang JM, Piacentini M, Pierre P, Pierrefite-Carle V, Pierron G, Pinkas-Kramarski R, Piras A, Piri N, Platanias LC, Poggeler S, Poirot M, Poletti A, Pous C, Pozuelo-Rubio M, Praetorius-Ibba M, Prasad A, Prescott M, Priault M, Produit-Zengaffinen N, Progulske-Fox A, Proikas-Cezanne T, Przedborski S, Przyklenk K, Puertollano R, Puyal J, Qian SB, Qin L, Qin ZH, Quaggin SE, Raben N, Rabinowich H, Rabkin SW, Rahman I, Rami A, Ramm G, Randall G, Randow F, Rao VA, Rathmell JC, Ravikumar B, Ray SK, Reed BH, Reed JC, Reggiori F, Regnier-Vigouroux A, Reichert AS, Reiners JJ Jr, Reiter RJ, Ren J, Revuelta JL, Rhodes CJ, Ritis K, Rizzo E, Robbins J, Roberge M, Roca H, Roccheri MC, Rocchi S, Rodemann HP, Rodriguez de Cordoba S, Rohrer B, Roninson IB, Rosen K, Rost-Roszkowska MM, Rouis M, Rouschop KM, Rovetta F, Rubin BP, Rubinsztein DC, Ruckdeschel K, Rucker EB 3rd, Rudich A, Rudolf E, Ruiz-Opazo N, Russo R, Rusten TE, Ryan KM, Ryter SW, Sabatini DM, Sadoshima J, Saha T, Saitoh T, Sakagami H, Sakai Y, Salekdeh GH, Salomoni P, Salvaterra PM, Salvesen G, Salvioli R, Sanchez AM, Sanchez-Alcazar JA, Sanchez-Prieto R, Sandri M, Sankar U, Sansanwal P, Santambrogio L, Saran S, Sarkar S, Sarwal M, Sasakawa C, Sasnauskiene A, Sass M, Sato K, Sato M, Schapira AH, Scharl M, Schatzl HM, Scheper W, Schiaffino S, Schneider C, Schneider ME, Schneider-Stock R, Schoenlein PV, Schorderet DF, Schuller C, Schwartz GK, Scorrano L, Sealy L, Seglen PO, Segura-Aguilar J, Seiliez I, Seleverstov O, Sell C, Seo JB, Separovic D, Setaluri V, Setoguchi T, Settembre C, Shacka JJ, Shanmugam M, Shapiro IM, Shaulian E, Shaw RJ, Shelhamer JH, Shen HM, Shen WC, Sheng ZH, Shi Y, Shibuya K, Shidoji Y, Shieh JJ, Shih CM, Shimada Y, Shimizu S, Shintani T, Shirihai OS, Shore GC, Sibirny AA, Sidhu SB, Sikorska B, Silva-Zacarin EC, Simmons A, Simon AK, Simon HU, Simone C, Simonsen A, Sinclair DA, Singh R, Sinha D, Sinicrope FA, Sirko A, Siu PM, Sivridis E, Skop V, Skulachev VP, Slack RS, Smaili SS, Smith DR, Soengas MS, Soldati T, Song X, Sood AK, Soong TW, Sotgia F, Spector SA, Spies CD, Springer W, Srinivasula SM, Stefanis L, Steffan JS, Stendel R, Stenmark H, Stephanou A, Stern ST, Sternberg C, Stork B, Stralfors P, Subauste CS, Sui X, Sulzer D, Sun J, Sun SY, Sun ZJ, Sung JJ, Suzuki K, Suzuki T, Swanson MS, Swanton C, Sweeney ST, Sy LK, Szabadkai G, Tabas I, Taegtmeyer H, Tafani M, Takacs-Vellai K, Takano Y, Takegawa K, Takemura G, Takeshita F, Talbot NJ, Tan KS, Tanaka K, Tang D, Tanida I, Tannous BA, Tavernarakis N, Taylor GS, Taylor GA, Taylor JP, Terada LS, Terman A, Tettamanti G, Thevissen K, Thompson CB, Thorburn A, Thumm M, Tian F, Tian Y, Tocchini-Valentini G, Tolkovsky AM, Tomino Y, Tonges L, Tooze SA, Tournier C, Tower J, Towns R, Trajkovic V, Travassos LH, Tsai TF, Tschan MP, Tsubata T, Tsung A, Turk B, Turner LS, Tyagi SC, Uchiyama Y, Ueno T, Umekawa M, Umemiya-Shirafuji R, Unni VK, Vaccaro MI, Valente EM, Van den Berghe G, van der Klei IJ, van Doorn W, van Dyk LF, van Egmond M, van Grunsven LA, Vandenabeele P, Vandenberghe WP, Vanhorebeek I, Vaquero EC, Velasco G, Vellai T, Vicencio JM, Vierstra RD, Vila M, Vindis C, Viola G, Viscomi MT, Voitsekhovskaja OV, von Haefen C, Votruba M, Wada K, Wade-Martins R, Walker CL, Walsh CM, Walter J, Wan XB, Wang A, Wang C, Wang D, Wang F, Wang G, Wang H, Wang HG, Wang HD, Wang J, Wang K, Wang M, Wang RC, Wang X, Wang YJ, Wang Y, Wang Z, Wang ZC, Wansink DG, Ward DM, Watada H, Waters SL, Webster P, Wei L, Weihl CC, Weiss WA, Welford SM, Wen LP, Whitehouse CA, Whitton JL, Whitworth AJ, Wileman T, Wiley JW, Wilkinson S, Willbold D, Williams RL, Williamson PR, Wouters BG, Wu C, Wu DC, Wu WK, Wyttenbach A, Xavier RJ, Xi Z, Xia P, Xiao G, Xie Z, Xu DZ, Xu J, Xu L, Xu X, Yamamoto A, Yamashina S, Yamashita M, Yan X, Yanagida M, Yang DS, Yang E, Yang JM, Yang SY, Yang W, Yang WY, Yang Z, Yao MC, Yao TP, Yeganeh B, Yen WL, Yin JJ, Yin XM, Yoo OJ, Yoon G, Yoon SY, Yorimitsu T, Yoshikawa Y, Yoshimori T, Yoshimoto K, You HJ, Youle RJ, Younes A, Yu L, Yu SW, Yu WH, Yuan ZM, Yue Z, Yun CH, Yuzaki M, Zabirnyk O, Silva-Zacarin E, Zacks D, Zacksenhaus E, Zaffaroni N, Zakeri Z, Zeh HJ 3rd, Zeitlin SO, Zhang H, Zhang HL, Zhang J, Zhang JP, Zhang L, Zhang MY, Zhang XD, Zhao M, Zhao YF, Zhao Y, Zhao ZJ, Zheng X, Zhivotovsky B, Zhong Q, Zhou CZ, Zhu C, Zhu WG, Zhu XF, Zhu X, Zhu Y, Zoladek T, Zong WX, Zorzano A, Zschocke J, and Zuckerbraun B. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8: 445–544, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy 4: 849–950, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Lakadamyali M, Rust MJ, Zhuang X. Endocytosis of influenza viruses. Microbes Infect 6: 929–936, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manabe T, Yoshimori T, Henomatsu N, Tashiro Y. Inhibitors of vacuolar-type H+-ATPase suppresses proliferation of cultured cells. J Cell Physiol 157: 445–452, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Marjuki H, Gornitzky A, Marathe BM, Ilyushina NA, Aldridge JR, Desai G, Webby RJ, Webster RG. Influenza A virus-induced early activation of ERK and PI3K mediates V-ATPase-dependent intracellular pH change required for fusion. Cell Microbiol 13: 587–601, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastrobattista E, Koning GA, van Bloois L, Filipe AC, Jiskoot W, Storm G. Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins. J Biol Chem 277: 27135–27143, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Mathapati BS, Mishra N, Rajukumar K, Nema RK, Behera SP, Dubey SC. Entry of bovine viral diarrhea virus into ovine cells occurs through clathrin-dependent endocytosis and low pH-dependent fusion. In Vitro Cell Dev Biol Anim 46: 403–407, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Matlin KS, Reggio H, Helenius A, Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol 91: 601–613, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meertens L, Bertaux C, Dragic T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J Virol 80: 11571–11578, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizushima N. Autophagy: process and function. Genes Dev 21: 2861–2873, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Mousavi SA, Kjeken R, Berg TO, Seglen PO, Berg T, Brech A. Effects of inhibitors of the vacuolar proton pump on hepatic heterophagy and autophagy. Biochim Biophys Acta 1510: 243–257, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Muller KH, Kainov DE, El Bakkouri K, Saelens X, De Brabander JK, Kittel C, Samm E, Muller CP. The proton translocation domain of cellular vacuolar ATPase provides a target for the treatment of influenza A virus infections. Br J Pharmacol 164: 344–357, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nawa M. Japanese encephalitis virus infection in Vero cells: the involvement of intracellular acidic vesicles in the early phase of viral infection was observed with the treatment of a specific vacuolar type H+-ATPase inhibitor, bafilomycin A1. Microbiol Immunol 41: 537–543, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139: 271–279, 1991. [DOI] [PubMed] [Google Scholar]
- 38.Niks M, Otto M. Towards an optimized MTT assay. J Immunol Methods 130: 149–151, 1990. [DOI] [PubMed] [Google Scholar]
- 39.Nishihara T, Akifusa S, Koseki T, Kato S, Muro M, Hanada N. Specific inhibitors of vacuolar type H+-ATPases induce apoptotic cell death. Biochem Biophys Res Commun 212: 255–262, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Ochiai H, Sakai S, Hirabayashi T, Shimizu Y, Terasawa K. Inhibitory effect of bafilomycin A1, a specific inhibitor of vacuolar-type proton pump, on the growth of influenza A and B viruses in MDCK cells. Antiviral Res 27: 425–430, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Ohta T, Arakawa H, Futagami F, Fushida S, Kitagawa H, Kayahara M, Nagakawa T, Miwa K, Kurashima K, Numata M, Kitamura Y, Terada T, Ohkuma S. Bafilomycin A1 induces apoptosis in the human pancreatic cancer cell line Capan-1. J Pathol 185: 324–330, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–939, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Pivtoraiko VN, Harrington AJ, Mader BJ, Luker AM, Caldwell GA, Caldwell KA, Roth KA, Shacka JJ. Low-dose bafilomycin attenuates neuronal cell death associated with autophagy-lysosome pathway dysfunction. J Neurochem 114: 1193–1204, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahim MN, Selman M, Sauder PJ, Forbes NE, Stecho W, Xu W, Lebar M, Brown EG, Coombs KM. Generation and characterization of a new panel of broadly reactive anti-NS1 mAbs for detection of influenza A virus. J Gen Virol 94: 593–605, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1: 1458–1461, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Ritzwoller DP, Bridges CB, Shetterly S, Yamasaki K, Kolczak M, France EK. Effectiveness of the 2003–2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics 116: 153–159, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Shacka JJ, Klocke BJ, Shibata M, Uchiyama Y, Datta G, Schmidt RE, Roth KA. Bafilomycin A1 inhibits chloroquine-induced death of cerebellar granule neurons. Mol Pharmacol 69: 1125–1136, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Sinha S, Colbert CL, Becker N, Wei Y, Levine B. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy 4: 989–997, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun X, Whittaker GR. Role for influenza virus envelope cholesterol in virus entry and infection. J Virol 77: 12543–12551, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki T, Yamaya M, Sekizawa K, Hosoda M, Yamada N, Ishizuka S, Nakayama K, Yanai M, Numazaki Y, Sasaki H. Bafilomycin A1 inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1. Am J Physiol Lung Cell Mol Physiol 280: L1115–L1127, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Teplova VV, Tonshin AA, Grigoriev PA, Saris NE, Salkinoja-Salonen MS. Bafilomycin A1 is a potassium ionophore that impairs mitochondrial functions. J Bioenerg Biomembr 39: 321–329, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Tian Y, Sir D, Kuo CF, Ann DK, Ou JH. Autophagy required for hepatitis B virus replication in transgenic mice. J Virol 85: 13453–13456, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA 301: 1066–1069, 2009. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and Other Influenza Viruses. Herndon, VA: WHO Press, Stylus Publishing, 2010. [PubMed] [Google Scholar]
- 55.Wright P, Ng F, Kawaoka Y. Orthomyxoviruses. In: Fields Virology, edited by Knipe DM, Howley PM, Griffin DE. Philadelphia, PA: Wolters Kluwer, Lippincott Williams & Wilkins, 2007, p. 1691–1740. [Google Scholar]
- 56.Wu YC, Wu WK, Li Y, Yu L, Li ZJ, Wong CC, Li HT, Sung JJ, Cho CH. Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem Biophys Res Commun 382: 451–456, 2009. [DOI] [PubMed] [Google Scholar]
- 57.Wubbolts R, Fernandez-Borja M, Oomen L, Verwoerd D, Janssen H, Calafat J, Tulp A, Dusseljee S, Neefjes J. Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J Cell Biol 135: 611–622, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23: 33–42, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Yang M, Berhane Y, Salo T, Li M, Hole K, Clavijo A. Development and application of monoclonal antibodies against avian influenza virus nucleoprotein. J Virol Methods 147: 265–274, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 266: 17707–17712, 1991. [PubMed] [Google Scholar]
- 61.Zhou Z, Jiang X, Liu D, Fan Z, Hu X, Yan J, Wang M, Gao GF. Autophagy is involved in influenza A virus replication. Autophagy 5: 321–328, 2009. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Z, Xue Q, Wan Y, Yang Y, Wang J, Hung T. Lysosome-associated membrane glycoprotein 3 is involved in influenza A virus replication in human lung epithelial (A549) cells. Virol J 8: 384, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]