Abstract
The heart adapts to exercise stimuli in a sex-dimorphic manner when mice are fed the traditional soy-based chow. Females undergo more voluntary exercise (4 wk) than males and exhibit more cardiac hypertrophy per kilometer run (18, 32). We have found that diet plays a critical role in cage wheel exercise and cardiac adaptation to the exercise stimulus in this sex dimorphism. Specifically, feeding male mice a casein-based, soy-free diet increases daily running distance over soy-fed counterparts to equal that of females. Moreover, casein-fed males have a greater capacity to increase their cardiac mass in response to exercise compared with soy-fed males. To further explore the biochemical mechanisms for these differences, we performed a candidate-based RT-PCR screen on genes previously implicated in diet- or exercise-based cardiac hypertrophy. Of the genes screened, many exhibit significant exercise, diet, or sex effects but only transforming growth factor-β1 shows a significant three-way interaction with no genes showing a two-way interaction. Finally, we show that the expression and activity of adenosine monophosphate-activated kinase-α2 and acetyl-CoA carboxylase is dependent on exercise, diet, and sex.
Keywords: cardiac hypertrophy, sex and exercise, diet, soy
the impact of physiological, pathological, and environmental factors on the ability of the heart to adapt has been of broad interest to many laboratories (7, 11, 28). We have also examined how these modifiers interact to ultimately dictate the course of cardiac remodeling (17, 18, 38). For example, in studying the interaction of biologic sex and exercise on cardiac adaptation, we found that female mice exposed to a voluntary cage wheel run more and exhibit approximately threefold greater percent increase in heart mass than their male counterparts when normalized to distance run (18). Furthermore, in studying the interaction of exercise and cardiac disease [hypertrophic cardiomyopathy (HCM)], we showed that not only is voluntary cage wheel exercise not harmful in the setting of heart disease but it is able to prevent and even reverse established HCM disease phenotypes (19).
Utilizing exercise to study the interaction of sex and disease and the molecular and cellular mechanisms mediating cardiac adaptation to exercise may lead to the identification of novel regulatory pathways and provide potential therapeutic targets for cardiac disease. This becomes especially significant considering that standard treatment strategies for cardiac rehabilitation employ regular exercise, particularly aerobic exercise, and that exercise reduces cardiovascular morbidity and mortality in these subjects (12, 34).
Still, utilizing cage wheel running to study the physiological impact of exercise on cardiac adaptation is not entirely straightforward. The last 15 years of research utilizing cage wheel running clearly indicates that wheel running is a complex behavior and is the net result of food availability, motivation and reward systems, and daily activity patterns (30). Interestingly, feral animals in nature display similar wheel-running habits as in laboratory animals (27). Consequently, when characterizing the response to cage wheel exercise, whether in a diseased or normal heart, the underlying factors driving cage wheel activity must be considered.
What becomes evident is that more integrative studies examining cardiac adaptation to physiological, pathological, and environmental stimuli, such as diet, are needed. Interestingly, it has been recognized that there are different dietary requirements for males and females undergoing physical activity (40, 41, 43). Furthermore, the relationship between dietary intake and cardiovascular disease has traditionally focused on the consumption of fat and its impact on blood triglycerides and cholesterol (14, 15, 39). However, such an approach is far too simplistic in that it de-emphasizes the ability of dietary nutrients, and the interaction of these nutrients, to impact cardiac adaptation on a global scale. Demonstrating the potential impact of diet on the HCM phenotype, we demonstrated that HCM male mice when switched from a soy-based diet to a calorically similar casein-based diet no longer show a poorer cardiac phenotype compared with females (38, 42).
Taken together, these data indicate that it becomes critical to understand how genomic (sex) and nongenomic (diet, exercise) modifiers interact to impact physiological (cage wheel running) cardiac adaptation. Yet, there are few data on how these differences impact cardiac adaptation. Moreover, the molecular mechanisms underlying these sex-dimorphic dietary impacts and, more importantly, sex-dependent cardiac adaptation remain obscure. Although our group has examined selective targets that putatively regulate cardiac adaptation in response to these stimuli, there are many potential candidates that may mediate such a complex phenotype. For example, histone deacytelases (HDACs) have been extensively studied in pathological cardiac hypertrophy, but there have been limited studies investigating the role of HDACs in exercise-induced cardiac adaptation (8, 24) and none in response to cage wheel exercise.
In all of our previous studies utilizing cage wheel exercise as a stimulus for cardiac adaptation, mice were eating the traditional soy-based chow. Considering the impact that a dietary switch had on HCM disease progression, we wished to build on our previous work examining cardiac adaptation and test the hypothesis that the sex-dimorphic impact of cage wheel running on cardiac adaptation is lost when male and female wild-type (WT) mice eating a soy-based are switched to casein-based (soy-free) chow. We chose cage wheel activity and cardiac adaptation as the primary endpoints for these studies. However, we also measured transcript and protein expression and activity of candidate targets that may contribute to sex- and diet-dependent differences.
MATERIALS AND METHODS
Voluntary Cage-Wheel Exercise
Voluntary running was performed by inbred male and female C57Bl/6J, age 12 wk at the start of cage wheel exposure. Individual animals were housed in a cage (47 × 26 × 14.5 cm) containing a free wheel for 21 days. The exercise wheels used have been previously described (1, 18). Briefly, this system consists of an 11.5-cm-diameter wheel with a 5.0-cm-wide running surface (model 6208; Petsmart, Phoenix, AZ) equipped with a digital magnetic counter (model BC 600; Sigma Sport, Olney, IL) that is activated by wheel rotation. For a given litter, mice were randomly assigned to a particular diet and to the sedentary or exercised group. All animals were given water and rodent chow ad libitum. Daily exercise values for time and distance run were recorded for each exercised animal throughout the duration of the exercise period. At the end of the specific exercise period, exercised and sedentary control animals mice were euthanized by cervical dislocation under inhaled anesthesia. Body mass was weighed and hearts were rapidly excised and washed with a modified ice-cold PBS solution (in mmol/l): 136.9 NaCl, 3.35 KCl, 12 NaH2PO4, and 1.84 KH2PO4 (pH 7.4). Left ventricles were dissected from whole hearts and frozen in isopentane cooled in liquid nitrogen. All experiments were performed using protocols that adhered to guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Arizona and the University of Colorado, and to 2003 National Institutes of Health Guidelines for Care and Use of Laboratory Animals.
Diets
Mice were fed ad libitum either a soy-based (Harlan Teklad 8640) or a casein-based (Research Diets D10001) rodent chow (38). All diets contain approximately equivalent macronutrient content. Briefly, the soy-based chow contained the following (in gram%/kcal%): crude protein (22/29), carbohydrate (N/A, 54), and fat (5.5/17). The casein-based chow contained the following (in gram%/kcal%): protein (20/21), carbohydrate (66, 68), and fat (5/11). For a detailed list of nutrient content please refer to http://www.harlan.com/ (8640) or http://www.researchdiets.com/(D10001).
Real-Time PCR
Total RNA was isolated from the left ventricles of WT and exercised mouse hearts using the mirVana miRNA isolation kit (Ambion) according to the manufacturer's protocol. Total cDNA was generated with NCode RNA First-Strand cDNA Synthesis Kit (Invitrogen). Maxima SYBR Green qPCR Master Mix (fermentas) was used for real-time PCR reaction. 18S ribosome RNA was used as internal control of mRNA for real time PCR. A full list of probe sets and gene and protein definitions are included in Table 1.
Table 1.
PCR primer list
| GOI | Forward | Reverse |
|---|---|---|
| Prkaa2 | GAGGCGGCCGAACATG | GCACGTAGTGTCCGATCTTCAC |
| Nppa | TGGGCTCCTTCTCCATCACC | GCCAAAAGGCCAGGAAGAGG |
| Myhc6 | CGCATCAAGGAGCTCACC | CCTGCAGCCGCATTAAGT |
| Cpt1b | GCCCATGTGCTCCTACCA | CTCTGAGAGGTGCTGTAGCAAG |
| Il6 | GCTACCAAACTGGATATAATCAGGA | CCAGGTAGCTATGGTACTCCAGAA |
| Mmp9 | CGACATAGACGGCATCCAG | CTGTCGGCTGTGGTTCAGT |
| Ppara | CACGCATGTGAAGGCTGTAA | CAGCTCCGATCACACTTGTC |
| Ppard | GGGCTGAGTGTCCATTGAAG | TCACTCTCTTGCCAAAGATCC |
| Sirt1 | TCGTGGAGAGACATTTTTAATCAGG | GCTTCATGATGGCAAGTGG |
| Tgfb1 | TGGAGCAACATGTGGAACTC | CAGCAGCCGGTTACCAAG |
| Tgfb2 | AGGAGGTTTATAAAATCGACATGC | TAGAAAGTGGGCGGGATG |
| Tgfb3 | CCCTGGACACCAATTACTGC | TCAATATAAAGGGGGCGTACA |
| Tnfa | TCTTCTCATTCCTGCTTGT | GGTCTGGGCCATAGAACTGA |
| 18S | GCAATTATTCCCCATGAACG | GGGACTTAATCAACGCAAGC |
PCR primer sets used for amplification of mRNA transcripts. GOI, gene of interest. Gene name, protein full name (protein abbreviation): Prkaa2, adenosine monophosphate-activated kinase α2 (AMPKα2); Nppa, atrial natiuretic peptide (ANP); Myh6, alpha myosin heavy chain (αMyHC); Cpt1b, carnitine palmitoyltransferase 1B (CPT1B); Il6, interleukin 6 (IL-6); Mmp9, matrix metallopeptidase 9 (MMP9); Ppara, peroxisome proliferator-activated receptor alpha (PPARα); Ppard, peroxisome proliferator-activated receptor delta (PPARδ); Sirt1, Sirtuin 1 (SIRT1); Tgfb1, transforming growth factor, beta 1 (TGF-β1); Tgfb2, Transforming growth factor, beta 2 (TGF-β2); Tgfb3, transforming growth factor, beta 2 (TGF-β3); Tnfa, Tumor necrosis factor-alpha (TNF-α).
Western Blot Analysis and Gel Electrophoresis
Preparation of heart samples for SDS-PAGE and subsequent Western immunoblotting for detection of proteins and particular sites of phosphorylation on each protein began by homogenization of left ventricles (minus the atria) in a protein extraction buffer (in mmol/l): 137 NaCl, 20 Tris(hydroxymethyl)-aminomethane, 10% vol/vol glycerol, and 1% vol/vol NP-40 (pH 7.4) (36). The homogenized tissue was then centrifuged at 12,000–14,000 g (Beckman J2-HS centrifuge) for 10 min at 4°C. The supernatant was removed and protein concentration was determined using the Bradford method. SDS-PAGE was performed on the heart extracts followed by the transfer to a membrane support [polyvinylidene difluoride (PVDF)]. The membranes were probed according to specifications and all antibodies [AMPKα, phosphorylated (p)-AMPKα, acetyl-CoA carboxylase (ACC), and pACC] were obtained commercially from Cell Signaling Technology (Beverly, MA) and used at 1/1,000 dilution except p-AMPK, which was used at 1/500 dilution. All immunoblot analysis was performed from the semiquantitation of individual blots (LabImage 1D software) and was not compared across blots according to accepted guidelines. Protein blots were normalized to total protein determined as detailed above and Ponceau S staining of PVDF membranes. Phosphorylated proteins were normalized to each respective unphosphorylated, total protein. All immunoblot images of a given target were cropped from the same blot to conserve figure space and redundancy.
HDAC Activity Assays
HDAC activity assays were performed as previously described (22). Class 1, IIa, and IIb HDAC substrates for the assay consist of lysine with three modifications: acetylation of the ε-amine, a protecting group on the α-amine, and conjugation of the carboxyl by the fluorophore 7-amino-4-methylcoumarin (AMC). HDAC activity from cell or tissue extracts removes the ε-acetyl group. Following incubation of the samples with substrate, trypsin is added, which is active only against deacetylated substrate. Trypsin releases AMC, resulting in increased fluorescence emission at 460 nm. Briefly, tissue extracts were prepared in PBS (pH 7.4) containing 0.5% Triton X-100, 300 mM NaCl, and protease/phosphatase inhibitor cocktail (Thermo Fisher) using a Bullet Blender homogenizer (Next Advance) followed by determination of protein concentration using a BCA Protein Assay Kit (Thermo Fisher). Tissue extracts were diluted into PBS buffer in 100-μl total volumes in 96-well plate (60 μg ventricular protein/well). AMC fluorescence was measured using a BioTek Synergy 2 plate reader, with excitation and emission filters of 360 and 460 nm, respectively (each with a bandwidth of 40 nm), along with a 400-nm dichroic top mirror. Background signals from buffer blanks were subtracted, and data were normalized as needed using appropriate controls. GraphPad Prism software was used to generate graphs and analyze data. ANOVA with Bonferroni's post test (P < 0.05) was used to determine statistical differences between groups.
Data and Statistical Analysis
Results are presented as means ± SE. The percent change in cardiac mass with exercise was determined by comparing the mass of each exercised animal to the mean cardiac mass of the sedentary group. The difference in cardiac mass was then expressed as a percent change from sedentary animals for each respective animal. Similarly, cardiac mass normalized to cage wheel activity was determined by subtracting the cardiac mass of each exercised animal from the mean cardiac mass of the sedentary group; this value was then normalized to the cage wheel activity of each corresponding animal. The differences between male and female groups were analyzed with a two-way ANOVA followed by a Tukey's honestly significant difference post hoc test; P <0.05 was considered as significant.
For analysis of RT-PCR, mixed effects ANOVA was used to test the differences among mean level of diet, exercise, and sex. Mixed effects models were used to account for the replicate runs for each mouse. First, a three-way interaction was tested, sex by diet by exercise. Secondly, two-way interactions were tested for any probes without a statistically significant three-way interaction, these included sex by diet, sex by exercise, and exercise by diet. Finally, differences in main effects were tested for all probes that did not demonstrate statistically significant differences in either a three-way or two-way interaction.
RESULTS
Cage Wheel Running
We previously demonstrated that 4-mo-old female C57Bl/6J mice exhibited enhanced wheel-running performance over their male counterparts as measured by both distance and duration when eating the standard, soy-based rodent chow (18). In the current study, we found a significant interaction between sex and diet in distance run. In addition, we found similar sex-specific running characteristics; female (C57Bl/6J; 12 wk at the start of cage wheel activity) mice ran significantly further than male mice as indicated in Fig. 1A, which shows that female mice ran an average of 6.5 ± 1.3 km while male mice ran 3.9 ± 0.2 km during each 24-h period, again when fed a soy-based diet. To complete these distances, females spent 5.0 ± 0.8 h on the wheel, which was greater than the 3.5 ± 0.2 h spent on the wheels by the males during each 24-h period, although this did not reach significance (Fig. 1B). Following a similar trend, wheel-running speeds were higher but not significant in female mice (1.24 ± 0.09 km/h) compared with male mice (1.17 ± 0.05 km/h; data not shown) when averaged over the 3-wk time period (Fig. 2, A and B).
Fig. 1.
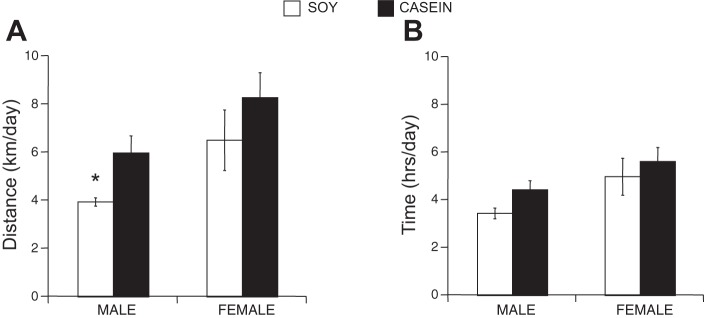
Voluntary cage wheel exercise in male and female mice eating a soy- or casein-based diet. A: average running distance (km/day) for every 24 h over the 21-day study period. B: average running time (h/day) for every 24 h over the 21-day study period. ANOVA interaction of sex × diet: P < 0.05, *P < 0.05, from males eating the casein-based diet and from females eating the soy-based diet.
Fig. 2.
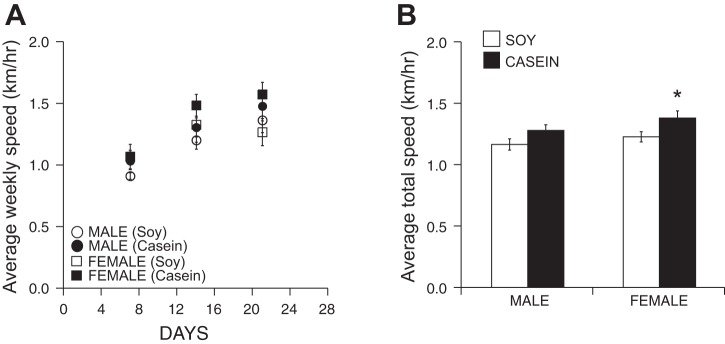
Voluntary cage running speed in male and female mice eating a soy- or casein-based diet. A: average weekly running speed (km/h) for every 24-h period over the 21-day study period. B: total average running speed (km/h) for every 24 h over the 21-day study period. ANOVA main effect of diet: P < 0.01, *P < 0.05, females eating the soy-based diet.
Considering our previous finding that diet plays a pivotal role in the progression of cardiac disease in a sex-specific manner (38), we tested the hypothesis that diet similarly modifies cage wheel exercise. Interestingly, male mice fed a casein-based chow elevated their running distance to 6.0 ± 0.7 km per 24-h period, a significant increase compared with their soy-fed counterparts. Moreover, this dietary change eliminated the sex difference (Fig. 1A) such that this increase in running distance by males was not different from females eating a casein-based diet (8.3 ± 1.0 km). Females eating the casein-based diet also showed a slight but insignificant increase over running distances of females eating the soy-based diet. Although there was a main effect of diet on wheel running speed, there were no differences in time (Fig. 1B) or speed (Fig. 2, A and B) on the wheel between male mice eating the casein-based and soy-based chow. Wheel running speed in female mice, however, significantly higher in those eating the casein-based diet.
Cardiac Mass/Adaptation
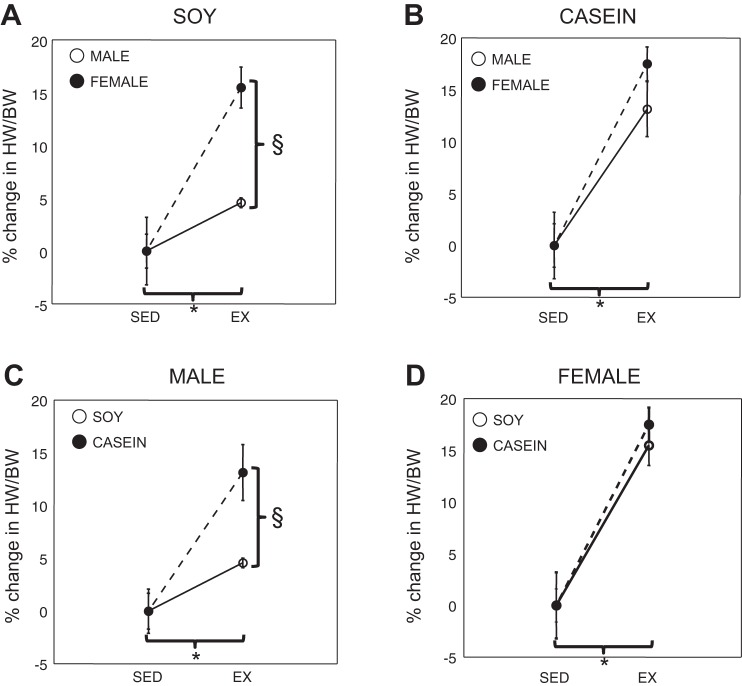
We and others have previously shown that hearts of mice increase in mass following exposure to a cage wheel and that the extent of the increase depends on the sex of the animal (1, 18–20, 23, 33). The results of these investigations are summarized in Table 2. Male and female mice demonstrated a sex-dimorphic increase in cardiac mass following cage wheel exposure when normalized to body weight (HW/BW in mg/g) and when eating the soy-based chow, similar to that previously reported (18). Accordingly, also as reported previously (1), when examined as a proportional increase in normalized cardiac mass (Fig. 3A), the percent increase in heart size was significantly greater in female mice (15.5 ± 1.9%) compared with their male counterparts (4.6 ± 0.5%).
Table 2.
Body and cardiac mass
| Body Mass, g | Cardiac Mass, mg | HW/BW, mg/g | |
|---|---|---|---|
| Soy | |||
| Sedentary | |||
| Males (n = 8) | 29.5 ± 0.6 | 146.9 ± 5.1 | 4.89 ± 0.08 |
| Females (n = 8) | 23.2 ± 0.4 | 115.1 ± 2.2 | 4.96 ± 0.08 |
| Exercised | |||
| Males (n = 5) | 27.4 ± 0.8 | 139.8 ± 4.0 | 5.11 ± 0.02† |
| Females (n = 5) | 21.2 ± 0.7 | 121.1 ± 4.7 | 5.72 ± 0.10† |
| Casein | |||
| Sedentary | |||
| Males (n = 9) | 27.4 ± 0.9 | 142.3 ± 3.8 | 4.91 ± 0.10 |
| Females (n = 14) | 22.6 ± 0.8 | 116.8 ± 2.9* | 5.23 ± 0.17 |
| Exercised | |||
| Males (n = 8) | 26.4 ± 1.2 | 155.1 ± 6.1 | 5.90 ± 0.15† |
| Females (n = 10) | 21.9 ± 0.6 | 134.6 ± 3.8*† | 6.15 ± 0.07† |
Values are means ± SE. Summary of morphometric data from sedentary and exercised C57Bl/6J male and female mice eating a soy- or casein-based chow as indicated. Heart rate/body weight (HW/BW) was determined by dividing cardiac mass (in mg) by body weight (in g).
P < 0.05, from values obtained for males; †P < 0.05, from values obtained in respective sedentary counterparts.
Fig. 3.
Cardiac adaptation in sedentary and exercised male and female mice eating a soy- or casein-based diet. The impact of cardiac adaption is displayed to show the impact of sex (A and B) or diet (C and D). A: cardiac adaptation expressed as percent change from respective mean sedentary normalized heart weight comparing males and females eating a soy-based diet. B: cardiac adaptation comparing males and females eating a casein-based diet. C: cardiac adaptation expressed as percent change from respective mean sedentary normalized heart weight comparing males eating a soy- or casein-based diet. D: cardiac adaptation comparing females eating a soy- or casein-based diet. *P < 0.05, from values obtained sedentary animals. §P < 0.05.
Because diet dramatically impacted running performance in males, we hypothesized that diet would differentially affect cardiac adaptation (size) and that this adaptation would be modified by sex. Male mice eating the casein-based diet demonstrated an increase in cardiac mass (13.2 ± 2.7%) that was similar to that in female mice eating either the casein-based (17.5 ± 1.6%; Fig. 3B) or soy-based diet. Moreover, this percent increase was greater than male mice eating the soy-based diet (Fig. 3C). Diet did not affect cardiac adaptation in response to exercise in female mice (Fig. 3d).
We previously reported in soy-fed mice that when the increase in normalized cardiac mass was adjusted for cage wheel activity, C57Bl/6J female mice showed a significantly greater increase in heart mass for kilometers of distance run or hour spent on the wheel compared with males (18). Similar results were found in this study. When the gain in cardiac mass was normalized to cage wheel activity in mice eating a soy-based diet, female mice demonstrated significantly greater hypertrophy when normalized to distance run (2.7 ± 0.5 vs. 1.2 ± 0.1 HW·BW−1·km−1 in a 24-h period; Fig. 4) compared with males, respectively. We also found a significant interaction between sex and diet in this parameter.
Fig. 4.
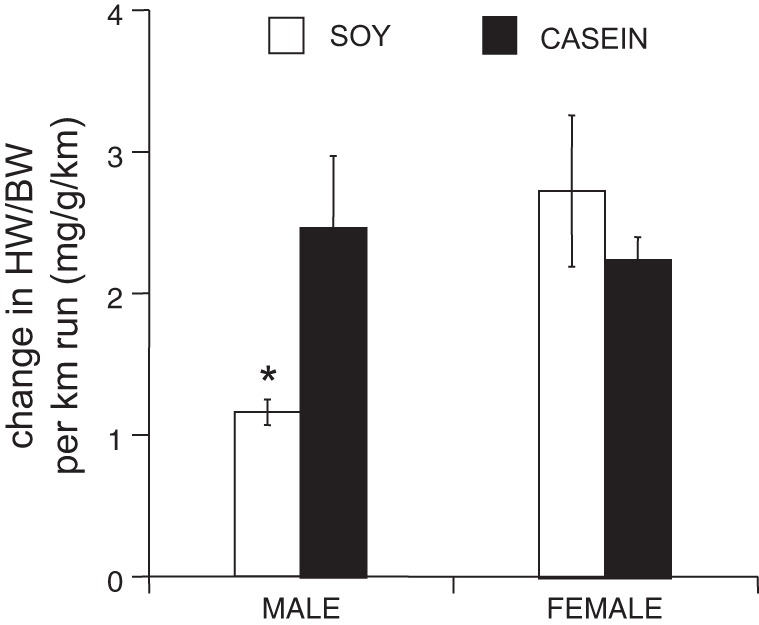
Cardiac adaptation normalized to activity. Normalized heart weight (HW/BW) divided by mean distance (HW/distance) run in a given 24-h period. ANOVA interaction of sex × diet: P < 0.05, *P < 0.05, from males eating the casein-based diet and from females eating the soy-based diet.
Feeding male mice a casein-based diet increased this ratio (2.5 ± 0.5 mg HW·BW−1·km−1 in a 24-h period; Fig. 4) such that they were no longer different from females eating the soy- or casein-based diet (2.3 ± 0.2 mg HW·BW−1·km−1 in a 24-h period). As expected, there were no differences between female runners eating the soy-based or casein-based diets.
mRNA analysis.
We recently reported that diet differentially impacts the genetic landscape of the heart in the context of sex and disease (25). Consequently, we hypothesized that a differential genetic expression pattern may underlie the ability of the heart to respond to a physiological hypertrophic stimulus such as exercise. Therefore, we explored the hypothesis that diet will similarly impact exercise-induced cardiac gene expression in a sex-specific manner. As an initial examination of this hypothesis, we selected 11 candidates including hypertrophic, inflammatory, and metabolic genes and performed quantitative RT-PCR analysis on the mRNA. There was a significant three-way interaction among diet, sex, and exercise for transforming growth factor-β1 (TGF-β1). Interestingly, we detected no significant two-way interactions for any probes. Therefore, we evaluated differences in main effects for the remaining probe sets. From this analysis we revealed significant differences between nonrunners and runners for the following genes: MyHCα, TNF-α, ANF, CPT1, MMP, PPARα, PPAR-β, SIRT1, and TGF-β3. A sex dimorphism was found for all probes except IL-6. Finally, a diet effect was seen for MyHCα, TNF-α, adenosine monophosphate-activated kinase (AMPKα2), and SIRT1. Table 3 summarizes the directional change of each gene demonstrating significant differences in main effects.
Table 3.
Main effects of mRNA transcripts
| GOI | Exercise | Sex | Diet | Direction |
|---|---|---|---|---|
| Prkaa2 | n.s. | <0.0001 | <0.05 | ↓male, ↓soy |
| Nppa | <0.01 | <0.0001 | n.s. | ↓runner, ↓male |
| Myh6 | <0.05. | <0.0001 | <0.05 | ↓sed, ↓male, ↓soy |
| Cpt1b | <0.05 | <0.0001 | n.s | ↓runner, ↓male |
| Il6 | n.s. | n.s. | n.s. | |
| Mmp9 | <0.0001 | <0.0001 | n.s. | ↓runner, ↓male |
| Ppara | <0.01 | <0.0001 | n.s. | ↓runner, ↓male |
| Ppard | <0.05 | <0.0001 | n.s. | ↓runner, ↓male |
| Sirt1 | <0.05 | <0.0001 | <0.01 | ↓runner, ↓male, ↓soy |
| Tgfb1 | Three-way interaction | |||
| Tgfb2 | n.s. | <0.0001 | n.s. | ↓male |
| Tgfb3 | <0.01 | <0.0001 | n.s. | ↓runner, ↓male |
| Tnfa | <0.05. | <0.0001 | <0.05 | ↓sed, ↓male, ↓soy |
Summary of mRNA transcript analysis of the RT-PCR data from sedentary and exercised C57Bl/6J male and female mice eating a soy- or casein-based chow. Directional change and group is indicated. Tgfb1 (TGF-β1) demonstrated a three-way interaction. Abbreviations are listed in Table 1.
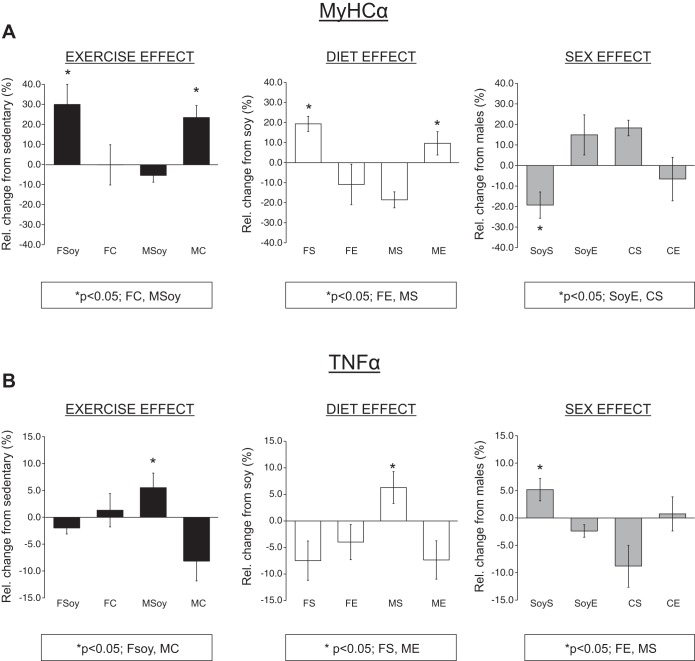
Two of these genes (MyHCα and TNF-α) were significantly impacted by diet, exercise, and sex. To identify more specifically the relative impact of each of these factors, MyHCα and TNF-α expression for each experimental group was represented as a relative (percent) change and compared as an effect of either diet, exercise or sex. When looking at the impact of exercise, there was an ∼35% increase in MyHCα following exercise in female mice on the soy-based diet whereas no change was observed following exercise in casein-fed females or soy-fed males (Fig. 5A, left). This exercise effect in females on the soy-based diet was significant compared with females fed the casein-based diet and males fed the soy-based diet. Casein-fed males demonstrated an ∼30% increase in MyHCα expression following exercise, similar to soy-fed females (Fig. 5A). This increase was, again, similar to soy-fed females, significantly greater than females fed the casein-based diet and males fed the soy-based diet.
Fig. 5.
The impact of exercise, diet, or sex on mRNA transcripts MyHCα and TNF-α. A: bar graph representation of MyHCα transcript expression as impacted by exercise, diet, or sex. B: bar graph representation of TNF-α transcript expression as impacted by exercise, diet, or sex. Experimental groups as follows: FSoy, female soy; FC, female casein; MSoy, male soy; MC, male casein; FS, female sedentary; FE, female exercise; MS, male sedentary; ME, male exercise; SoyS, soy sedentary; SoyE, soy exercise; CS, casein sedentary; CE, casein exercise.
Looking at the impact of diet on the relative expression of MyHCα, Fig. 5A, middle, shows that MyHCα expression was elevated in casein-fed compared with soy-fed females in the sedentary group only. This diet effect in the female sedentary group was significantly greater compared with the female exercise or male sedentary group. In addition, the diet effect was greater in the male exercise group compared with both female exercise or male sedentary groups. Comparing MyHCα expression based on sex, sedentary soy-fed females showed a decrease in expression compared with soy-fed exercised and casein-fed sedentary females (Fig. 5A, right).
Interestingly, cage wheel exercise elevated TNF-α, an inflammatory cytokine in soy-fed males only, over that of the reduced levels in soy-fed females and casein-fed males. Also, sedentary male mice fed the casein-based diet demonstrated increased TNF-α expression compared with sedentary females and exercised males where casein feeding reduced TNF-α expression. Finally, TNF-α expression was higher in sedentary females fed a soy-based diet compared with sedentary females on the casein-based diet (Fig. 5B).
HDAC catalytic activity.
Although the role of HDACs has been extensively studied in pathological cardiac hypertrophy, there have been limited studies investigating the role of HDACs in exercise-induced cardiac adaptation (8, 24) and none in response to cage wheel exercise. Accordingly, to address the possible involvement of specific HDAC classes in exercise-induced cardiac remodeling, we performed an enzymatic HDAC activity assay that quantifies class I, IIa, or IIb HDAC catalytic activity in cardiac extracts from each of the experimental groups (22). Although some trends emerged within each experimental group, no significant differences in class I, IIa, or IIb HDAC activities were detected (data summarized in Fig. 6 and Table 4).
Fig. 6.
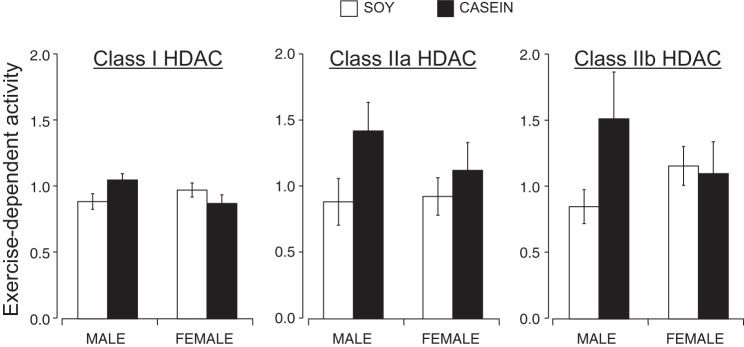
Quantification of class I, IIa, and IIb histone deacetylase (HDAC) catalytic activity in cardiac tissue homogenates from sedentary and exercised male and female mice eating a soy- or casein-based diet. Cardiac left ventricular extracts from each experimental group was incubated with different HDAC substrates. Each bar graph represents exercise-dependent HDAC activity such relative to sedentary control counterparts. Left: class I-selective substrate and corresponding HDAC activity data. Middle: class IIa substrate and corresponding HDAC activity data. Right: Class IIb substrate and corresponding activity data.
Table 4.
Effect of exercise on energetic/hypertrophic signaling pathways
| Male |
Females |
|||
|---|---|---|---|---|
| Soy | Casein | Soy | Casein | |
| AMPKα2 | n.s. | n.s. | ↓* | n.s. |
| p-AMPK172 | ↑* | ↑* | n.s. | n.s. |
| ACC | n.s. | n.s. | n.s. | n.s. |
| p-ACC | n.s. | n.s. | ↑* | n.s. |
| CLASS I HDAC | n.s. | n.s. | n.s. | n.s. |
| CLASS IIa HDAC | n.s. | n.s. | n.s. | n.s. |
| CLASS IIb HDAC | n.s. | n.s. | n.s. | n.s. |
Summary of hypertrophic signaling molecules. ACC, acetyl-CoA carboxylase; HDAC, histone deacytelase. Symbols represent changes from sedentary animals: n.s.: not statistically significant; ↑: increase; ↓: decrease.
Statistical significance at P < 0.05.
AMPK and ACC Expression and Activity
Endurance training enhances exercise performance, and we hypothesized that this may be partly mediated through activation of AMPK in skeletal muscle (29). Interestingly, a high soy-containing diet, similar to that used in this study, activates AMPK in several tissues (5). Moreover, metabolic alterations resulting from a dietary change depend on regulation by AMPK (3, 16). Therefore, we hypothesized that differential expression of AMPK would underlie the ability of these hearts to undergo physiological hypertrophy.
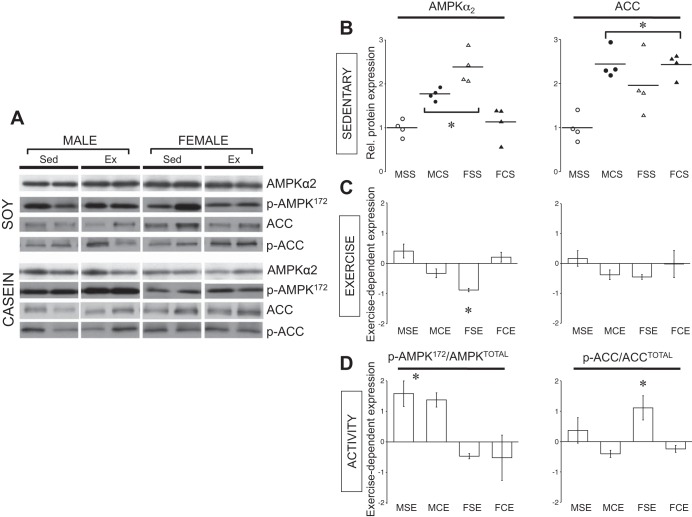
We performed Western blots from whole cardiac extracts of AMPKα2 (Fig. 7A), the predominant isoform of AMPK in the heart (37). As indicated in Fig. 7B, basal expression of AMPKα2 was significantly elevated in male sedentary mice eating a casein-based diet compared with sedentary males eating a soy-based diet. A sex dimorphism was found when sedentary males were compared with sedentary females both eating a soy-based diet; AMPKα2 expression levels were higher in females compared with males. Interestingly, AMPKα2 expression levels were similar between sedentary males eating a casein-based diet and sedentary females eating a soy-based diet. Sedentary females eating a casein-based diet demonstrated AMPKα2 expression levels that were significantly lower than casein-fed males and soy-fed females (both sedentary) but equal to soy-fed sedentary males.
Fig. 7.
AMPK and acetyl-CoA carboxylase (ACC) protein expression in sedentary and exercised male and female mice eating a soy- or casein-based diet. A: representative Western Blots of AMPKα, p-AMPK172, ACC, and p-ACC. B: sedentary: Western blots represented as dot blots of AMPKα (left) and ACC (right) in sedentary male (circles) and female (triangles) mice eating a soy (open symbols) or casein (closed symbols). Mean value represented by horizontal line. C: exercise: means ± SE fold change following the exercise protocol relative to each respective sedentary control. D: activity: means ± SE fold change following the exercise protocol relative to each respective sedentary control. *P < 0.05, from indicated groups. Experimental groups as follows: MSS, male soy sedentary; MCS, male casein sedentary; FSS, female soy sedentary; FCS, female casein sedentary; MSE, male soy exercise; MCE, male casein exercise; FSE, female soy exercise; FCE, female casein exercise.
We next wished to examine how AMPKα2 expression levels changed following 3 wk of cage wheel exercise under these different dietary conditions and if these changes were different between the sexes. To represent the directional impact of exercise in each sex under these different dietary conditions, the change in AMPKα2 expression relative to sedentary counterparts was determined. Figure 7C shows that 3 wk of cage wheel exposure did not change AMPKα2 expression except in females fed a soy-based diet, where exercise decreased AMPKα2 expression.
AMPK is regulated by direct phosphorylation at Thr-172 and/or inhibition of dephosphorylation (13) directly correlates with AMPK activity as measured previously by our group and others (6, 35). Therefore, to estimate the level of AMPK activity, we also performed Western blot analysis that detected AMPKα2 only when phosphorylated at Thr-172 (p-AMPK172). Figure 7D depicts the change in p-AMPK172 relative to the sedentary counterparts to better illustrate the exercise-dependent effect. Both male groups independent of diet experienced a significant relative increase in p-AMPK172 with exercise whereas both groups of females did not.
Activation of AMPK in the heart leads to direct phosphorylation of ACC (21). We therefore examined expression of total ACC and the phosphorylation of ACC. Sedentary males showed a diet effect of total ACC expression where males eating the casein-based diet had a 2.5-fold significant elevation of ACC relative to males eating the soy-based diet (Fig. 7B). Unlike males, the amount of total ACC measured in both sedentary female groups was not affected by diet. The level of total ACC expression in the females was significantly higher compared with sedentary males fed the soy-based diet but similar to males fed the casein-based diet (Fig. 7B). As illustrated in Fig. 7C, exercise did not impact the expression of ACC in any of the experimental groups. When examining the effect of exercise on p-ACC, only females eating a soy-based diet showed an increase relative to sedentary counterparts (Fig. 7D).
DISCUSSION
Running wheels have been used in a variety of contexts in rodent studies. Many of these studies are aimed at assessing energy balance, intake, and expenditure but are also used to determine brain reward stimuli, stress, and anxiety [see Novak et al. (30) for review]. Our studies utilizing cage wheel exercise are focused on addressing the adaptive and potentially protective mechanisms that underlie physiological cardiac hypertrophy in response to the exercise stimulus (1, 18, 19). The end goal of these studies is to harness this knowledge for the identification and development of novel treatment strategies against cardiovascular disease. What has emerged, however, is that cage wheel running is a complex behavior and that cardiac adaptation to wheel running is impacted by the factors that influence running behavior. Therefore, it becomes important to systematically address how environmental (diet) and genetic factors (sex) impact wheel running activity and cardiac adaptation as we have in this study.
We previously demonstrated how a dietary change dramatically impacts pathological cardiac adaptation and alters the course of cardiac disease progression (38). In that study, we reported that male mice with HCM fed the traditional soy-based diet deteriorate to severe, dilated cardiomyopathy (38, 42). However, simply changing the diet to a calorically similar casein-based diet prevents these phenotypes (38). Considering this relationship among diet, sex, and cardiac adaptation, we asked whether diet would have an impact on cardiac adaptation to an exercise stimulus. Our current observations demonstrate a clear sex-dependent response in cardiac adaptation to a dietary change and show that male mice eating a soy-based diet undergo physiological cardiac hypertrophy to a lesser extent than male mice eating a casein-based diet. This dietary change from soy to casein does not elicit such a response in females; the hearts of female mice experience hypertrophy in response to the exercise stimulus regardless of diet. Apart from the impact of this dietary change on cardiac hypertrophy, the extent of cardiac hypertrophy is also dependent on sex, similar to previous studies (18, 38).
This study was designed after an exercise protocol well established in our laboratory. Specifically, we gave male and female C57Bl/6 mice that were fed either a soy-based or a casein-based (soy-free) diet free access to a cage wheel (1). We first noted that feeding male mice a casein-based diet eliminated the soy-based differences between the sexes such that daily running distance matched that of female mice. Incidentally, the dietary switch in female mice had no effect on cage wheel running measured by time and distance on the cage wheel, and cage wheel running by each sex on the soy diet was similar to our previous observations (18).
Apart from the effect that diet has on cage wheel running, the adaptive response of cardiac and/or skeletal muscle to the exercise stimulus in the context of different diets or sex may underlie the observations of sex- and diet-dependent differences in exercise performance. In this study, we demonstrate that the response of the heart to the exercise stimulus depends on diet. Specifically, removal of soy, once again, eliminates any sex differences when measuring the increase in cardiac mass following exercise. Males eating the casein-based diet effectively doubled the proportional increase in normalized heart size compared with soy-fed counterparts. This increase was not different from casein-fed females who, on average, demonstrate a similar proportional increase in cardiac mass following exercise compared with soy-fed females. Again, there are no differences between cardiac adaptation in female mice on any of the diets.
It is likely that the soy diet directly alters muscle biochemistry and, potentially, exercise capacity. It is well documented that there are gender differences in muscle carbohydrate and lipid metabolism (40, 41). In another study, investigators demonstrated that skeletal muscle exhibits different amino acid uptake when subjects are fed either a soy or casein protein-based diet (26). However, the data were not distinguished by sex nor was there a difference in acute amino acid metabolism. We can predict that nutrient load from each diet, despite being calorically similar, is handled in a sex-specific manner. Moreover, by altering muscle biochemistry, the adaptive response of muscle, particularly cardiac muscle, to the exercise stimulus will be dependent on diet.
We initiated an initial examination of potential sex-, exercise-, and diet-dependent mediators in the hearts of both male and female mice from each exercise and diet group. Considering the complex interaction among diet, exercise, and sex, we wished to explore how specific mRNA transcripts were regulated. From the candidate screen, we show that regulation of TGF-β1 depends on the combinatorial interaction of these three factors. TGF-β1 is a multifunctional peptide that is gaining significance as a key player in cardiac disease progression particular during myocardial infarction and subsequent remodeling (2, 10). However, given the functional complexity of TGF signaling, a clear role during exercise-induced cardiac adaptation has not been investigated. Because examination of additional transcripts did not reveal any two-way interactions, we assessed these potential regulatory molecules for main effects of the three contributing factors. These data indicate primarily sex and exercise effects with little impact of diet. The suggestion from these data is that the degree to which sex and exercise impact gene regulation depends on the dietary context whereas diet as a central determinant of gene expression may be less predictive.
As mentioned above, carbohydrate and lipid metabolism has known sex differences, especially when it comes to exercise preparation and performance (4, 40, 43). Recently, it was discovered that a soy diet activates AMPK in white-adipose tissue, skeletal muscle, and liver (5). Therefore, we chose to examine AMPK expression in our control compared with our experimental groups. The most striking observation is the increased expression (and activity; see below) of AMPKα2, the predominant AMPK isoform in the heart (37), in sedentary male mice eating the casein diet compared with sedentary male mice eating the soy diet. This appears to be inconsistent with the aforementioned study showing that soy can increase AMPK activity (5). Furthermore, exercise did not impact AMPKα2 expression levels in male mice. Interestingly, AMPKα2 expression levels in soy-fed females are equally elevated but declined following 3–4 wk of cage wheel exposure similar to our recent publication (31). The suggestion is that AMPK expression in females may be uniquely sensitive to the soy diet compared with males. Moreover, how estrogen modifies this responsiveness may provide underlying mechanistic insight given the ability of estrogen to modify AMPK activity (9).
Because AMPK is a key regulator of ACC activity by direct phosphorylation (21), we examined total ACC and p-ACC and ACC protein expression in each experimental group. Total protein expression is lowest in males eating the soy-based diet compared with all other groups. Moreover, the expression levels did not significantly change with exercise. In addition, only females eating the soy-based diet showed a significant increase compared with sedentary controls in p-ACC following 3 wk of cage wheel exposure. The elevation of total ACC protein in casein-fed males and soy-fed females compared with soy-fed males follows the same pattern as AMPKα2 expression. On the other hand, casein-fed females show an increase ACC expression that is not paralleled by an increase in AMPKα2 expression. Furthermore, exercise resulted in increased p-AMPK172 in both male groups that did not result in a corresponding increase in p-ACC. Similarly, exercised, soy-fed females display an elevated p-ACC but not p-AMPK172. These data clearly demonstrate that the patterning of AMPK and ACC expression and activity can be uncoupled and may be differentially regulated under chronic exercise and dietary conditions.
The difficulty with the interpretation of this data set is underscored by the fact that the response of the heart to an exercise stimulus is impacted by multiple factors. While the biological mechanisms underlying these observations are certainly multifactorial, we cannot rule out the role of factors that impact cage wheel behavior such as motivation, stress, and reward systems. Previous work has demonstrated that cage wheel activity targets reward centers in the brain and interacts with other reinforcers (30). The implication is that the impact of sex and diet on cardiac adaptation in this study may be an indirect consequence of the factors that impact wheel-running behavior. From these data, it is apparent that the ability of the heart to respond to an exercise stimulus depends on both the diet and sex of the mouse. Table 4 summarizes the current data that were tested in this article. Our ability to translate these findings into putative therapeutic targets will be dependent on a clear elucidation of the molecular mechanisms responsible for the adaptive response. Moreover, a clear understanding of potential hormonal regulators, whether endocrine or autocrine, such as insulin, leptin, and adiponectin, may provide a more therapeutically viable approach. Perhaps more important is the ability to delineate treatment strategies based on sex. In previous studies, we have detailed models by which to investigate beneficial adaptive pathways. Here, we have extended this model to study the impact of diet as an environmental modifier of these pathways.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-50560 (to L. A. Leinwand) and HL-098256, National and Mentored Research Science Development Award K01-AR-052840, and National Heart, Lung, and Blood Institute Independent Scientist Award K02-HL-105799 (to J. P. Konhilas).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.P.K., E.D.L., and L.A.L. conception and design of research; J.P.K., H.C., E.D.L., L.A.M., J.R., T.A.M., and T.R.H. performed experiments; J.P.K., H.C., E.D.L., L.A.M., J.R., T.A.M., T.R.H., and B.L. analyzed data; J.P.K., H.C., P.A.W., B.L.S., Z.I.K., T.A.M., B.L., and L.A.L. interpreted results of experiments; J.P.K. and H.C. prepared figures; J.P.K. drafted manuscript; J.P.K., P.A.W., B.L.S., Z.I.K., and L.A.L. edited and revised manuscript; J.P.K. and L.A.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Destiny Lagrand and Kitsie Penick for assistance with manuscript preparation and submission.
REFERENCES
- 1.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol 90, 1900–1908, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184–195, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11: 213–219, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab 280: E898–E907, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Cederroth CR, Vinciguerra M, Gjinovci A, Kuhne F, Klein M, Cederroth M, Caille D, Suter M, Neumann D, James RW, Doerge DR, Wallimann T, Meda P, Foti M, Rohner-Jeanrenaud F, Vassalli JD, Nef S. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 57: 1176–1185, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Untiveros GM, McKee LA, Perez J, Li J, Antin PB, Konhilas JP. Micro-RNA-195 and -451 regulate the LKB1/AMPK signaling axis by targeting MO25. PLoS One 7: e41574, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Feller GM, Barbato JC, Periyasamy S, Xie ZJ, Koch LG, Shapiro JI, Britton SL. Cardiac performance in inbred rat genetic models of low and high running capacity. J Physiol 535: 611–617, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox EJ, Marsh SA. Exercise and diabetes have opposite effects on the assembly and O-GlcNAc modification of the mSin3A/HDAC1/2 complex in the heart. Cardiovasc Diabetol 12: 101, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280: 35983–35991, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Doetschman T, Barnett JV, Runyan RB, Camenisch TD, Heimark RL, Granzier HL, Conway SJ, Azhar M. Transforming growth factor beta signaling in adult cardiovascular diseases and repair. Cell Tissue Res 347: 203–223, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubnov-Raz G, Pines A, Berry EM. Diet and lifestyle in managing postmenopausal obesity. Climacteric 10, Suppl 2: 38–41, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Devereaux PJ. A review of heart failure treatment. Mt Sinai J Med 71: 47–54, 2004. [PubMed] [Google Scholar]
- 13.Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J 338: 717–722, 1999. [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper L, Summerbell CD, Higgins JP, Thompson RL, Capps NE, Smith GD, Riemersma RA, Ebrahim S. Dietary fat intake and prevention of cardiovascular disease: systematic review. BMJ 322: 757–763, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper L, Summerbell CD, Higgins JP, Thompson RL, Clements G, Capps N, Davey S, Riemersma RA, Ebrahim S. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 3: CD002137, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Jeon BT, Lee DH, Kim KH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Ketogenic diet attenuates kainic acid-induced hippocampal cell death by decreasing AMPK/ACC pathway activity and HSP70. Neurosci Lett 453: 49–53, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Konhilas JP, Leinwand LA. The effects of biological sex and diet on the development of heart failure. Circulation 116: 2747–2759, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol 287: H2768–H2776, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, Luckey SW, Rosenberg P, Leinwand LA. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res 98: 540–548, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Konhilas JP, Widegren U, Allen DL, Paul AC, Cleary A, Leinwand LA. Loaded wheel running and muscle adaptation in the mouse. Am J Physiol Heart Circ Physiol 289: H455–H465, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta 1301: 67–75, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Lemon DD, Horn TR, Cavasin MA, Jeong MY, Haubold KW, Long CS, Irwin DC, McCune SA, Chung E, Leinwand LA, McKinsey TA. Cardiac HDAC6 catalytic activity is induced in response to chronic hypertension. J Mol Cell Cardiol 51: 41–50, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest 117: 3211–3223, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Muhlfeld C, Niemann B, Pan R, Li R, Hilfiker-Kleiner D, Chen Y, Rohrbach S. Mitochondrial biogenesis and PGC-1alpha deacetylation by chronic treadmill exercise: differential response in cardiac and skeletal muscle. Basic Res Cardiol 106: 1221–1234, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Luczak ED, Barthel KK, Stauffer BL, Konhilas JP, Cheung TH, Leinwand LA. Remodeling the cardiac transcriptional landscape with diet. Physiol Genomics 43: 772–780, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luiking YC, Engelen MP, Soeters PB, Boirie Y, Deutz NE. Differential metabolic effects of casein and soy protein meals on skeletal muscle in healthy volunteers. Clin Nutr 30: 65–72, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Meijer JH, Robbers Y. Wheel running in the wild. Proc Biol Sci 28: pii: 20140210, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell 134: 405–415, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev 36: 1001–1014, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez JN, Chen H, Regan JA, Emert A, Constantopoulos E, Lynn M, Konhilas JP. Effects of chemically induced ovarian failure on voluntary wheel-running exercise and cardiac adaptation in mice. Comp Med 63: 233–243, 2013. [PMC free article] [PubMed] [Google Scholar]
- 32.Regitz-Zagrosek V, Dworatzek E, Kintscher U, Dragun D. Sex and sex hormone-dependent cardiovascular stress responses. Hypertension 61: 270–277, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Ruppert C, Deiss K, Herrmann S, Vidal M, Oezkur M, Gorski A, Weidemann F, Lohse MJ, Lorenz K. Interference with ERK(Thr188) phosphorylation impairs pathological but not physiological cardiac hypertrophy. Proc Natl Acad Sci USA 110: 7440–7445, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S, Firoozi S, McKenna WJ. Value of exercise testing in assessing clinical state and prognosis in hypertrophic cardiomyopathy. Cardiol Rev 9: 70–76, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 101: 3329–3335, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J 19: 2537–2548, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271: 611–614, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Stauffer BL, Konhilas JP, Luczak ED, Leinwand LA. Soy diet worsens heart disease in mice. J Clin Invest 116: 209–216, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang JL, Armitage JM, Lancaster T, Silagy CA, Fowler GH, Neil HA. Systematic review of dietary intervention trials to lower blood total cholesterol in free-living subjects. BMJ 316: 1213–1220, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR. Gender differences in substrate for endurance exercise. J Appl Physiol 68: 302–308, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Tarnopolsky MA. Gender differences in substrate metabolism during endurance exercise. Can J Appl Physiol 25: 312–327, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Vikstrom KL, Factor SM, Leinwand LA. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Mol Med 2: 556–567, 1996. [PMC free article] [PubMed] [Google Scholar]
- 43.Wismann J, Willoughby D. Gender differences in carbohydrate metabolism and carbohydrate loading. J Int Soc Sports Nutr 3: 28–34, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]