Abstract
Asthma is an inflammatory disease of the lung characterized by airways hyper-responsiveness (AHR), inflammation, and mucus hyperproduction. Current mainstream therapies include bronchodilators that relieve bronchoconstriction and inhaled glucocorticoids to reduce inflammation. The small molecule hormone and neurotransmitter serotonin has long been known to be involved in inflammatory processes; however, its precise role in asthma is unknown. We have previously established that activation of serotonin 5-hydroxytryptamine (5-HT)2A receptors has potent anti-inflammatory activity in primary cultures of vascular tissues and in the whole animal in vasculature and gut tissues. The 5-HT2A receptor agonist, (R)-2,5-dimethoxy-4-iodoamphetamine [(R)-DOI] is especially potent. In this work, we have examined the effect of (R)-DOI in an established mouse model of allergic asthma. In the ovalbumin mouse model of allergic inflammation, we demonstrate that inhalation of (R)-DOI prevents the development of many key features of allergic asthma, including AHR, mucus hyperproduction, airways inflammation, and pulmonary eosinophil recruitment. Our results highlight a likely role of the 5-HT2 receptors in allergic airways disease and suggest that 5-HT2 receptor agonists may represent an effective and novel small molecule-based therapy for asthma.
Keywords: serotonin, inflammation, 5-HT2 receptor, 5-HT2A receptor, asthma, DOI
serotonin [5-hydroxytryptophan (5-HT)] is a ubiquitous, small hormone molecule, present in nearly all eukaryotes, that mediates a wide spectrum of physiological processes. In mammals, it exerts its action through 14 different receptor subtypes that comprise seven distinct families (5-HT1–7) (34). All but one family, the ligand-gated 5-HT3 receptor ion channel, are G-protein-coupled receptors (34). The 5-HT2A receptor is known primarily for its role in mediating complex cognitive behaviors within the central nervous system and for mediating physiological processes, such as vasoconstriction, in the periphery (32, 34). Interestingly, the 5-HT2A receptor is the primary target of classic hallucinogenic drugs, such as lysergic acid diethylamide, which produces intoxicating effects. Although 5-HT2A receptor mRNA is expressed at higher levels in immune-related tissues, such as spleen, thymus, and peripheral-circulating lymphocytes, compared with other serotonin receptor subtypes (i.e., 5-HT1A, 5-HT1D, 5-HT2C, 5-HT4, 5-HT5A, and 5-HT5B) (42), its precise role in inflammatory processes is not well defined. With regard to the potential role of serotonin in asthma, 5-HT2A receptors are functionally expressed in activated CD4+ T cells, alveolar macrophages, eosinophils, and lung epithelial and smooth muscle cells (8, 20, 21, 23, 30). In fact, migration of eosinophils in allergic asthma has been shown recently to be dependent on 5-HT2A receptor activation (21), and 5-HT2 receptors have been implicated in platelet function relevant to allergic asthma (13).
We reported recently that 5-HT2A receptor agonists potently inhibit inflammation in vitro (53). The anti-inflammatory effects of one particular 5-HT2A receptor agonist, (R)-2,5-dimethoxy-4-iodoamphetamine [(R)-DOI], is extremely potent, with an EC50 of ∼15 pM. Through activation of the 5-HT2A receptor, (R)-DOI blocks the expression and activation of proinflammatory markers, including expression of chemokines [e.g., monocyte chemotactic protein-1 (MCP-1)], cellular adhesion molecules (ICAM1 and VCAM1), cytokines (e.g., IL-6), nitric oxide synthase, and activation/nuclear translocation of NF-κB, in a variety of cell types, including primary aortic smooth muscle cells (53). We have translated these in vitro findings to a whole animal mouse model of inflammation by demonstrating that (R)-DOI, also through 5-HT2A receptor activation, has potent anti-inflammatory effects when administered before systemically administered TNF-α. These effects are most pronounced in the vasculature and the gut, where preadministration of (R)-DOI blocks TNF-α-induced increases in the proinflammatory gene and protein expression, including circulating IL-6 (33).
In an effort to extend our findings to the potential use of (R)-DOI as a therapeutic in inflammatory airways disease, herein, we examine the ability of (R)-DOI to block the key features of allergic asthma in the well-established mouse model of ovalbumin (OVA)-induced allergic asthma. In this model, mice are sensitized and challenged with inhaled chicken OVA peptide to induce a phenotype resembling human asthma, including airways hyper-responsiveness (AHR) in response to methacholine (MeCh), mucus hyperproduction, and pulmonary inflammation characterized by eosinophilia (5). We show here that inhaled (R)-DOI blocks AHR, recruitment of eosinophils to the lung, mucus hyperproduction, and inflammatory airway remodeling. We speculate that 5-HT2 receptor agonism may represent a novel therapeutic strategy for asthma.
MATERIALS AND METHODS
Drugs and reagents.
(R)-DOI was generously provided by Dr. David E. Nichols (Purdue University, West Lafayette, IN) and was dissolved in sterile physiological saline before use. OVA and MeCh were purchased from Sigma-Aldrich (St. Louis, MO).
Animals.
For the inhalation/asthma experiments, specific pathogen-free, wild-type BALB/c mice were purchased from Harlan Laboratories (Indianapolis, IN). Mice were maintained in the animal care facility at the Louisiana State University Health Sciences Center (New Orleans, LA) in ventilated cages, housed in a pathogen-free animal facility with free access to food and water. Animal protocols were prepared in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Louisiana State University Health Sciences Center.
Induction of allergic inflammatory airways disease, i.e., the OVA mouse model of asthma.
Mice (male; 6–8 wk old) were sensitized and challenged with chicken OVA Grade V (Sigma-Aldrich), as described previously (4). Briefly, mice were sensitized by an intraperitoneal injection (100 μl) of 20 μg OVA, emulsified in 2 ml Imject Alum [Al(OH)3/Mg(OH)2; Pierce, Rockford, IL] on days 0 and 14. Mice were subsequently challenged with an OVA aerosol, generated using an ultrasonic nebulizer (Pari Proneb nebulizer, Midlothian, WA) with a 1% (wt/vol) OVA solution in saline for 20 min on days 24–26. Thirty minutes before each OVA challenge, each mouse was treated with one of two different concentrations of (R)-DOI (nose-only inhalation of 0.01 or 1.0 mg/kg) or vehicle control using an ultrasonic nebulizer (Aerogen, Galway, Ireland).
Measurement of airway inflammation, pulmonary mechanics, and BALF cellularity.
Pulmonary function testing, bronchoalveolar lavage (BAL), and tissue harvests were performed on day 28 (when mice were 10–12 wk of age). For the forced oscillation method, pulmonary resistance was measured as described previously (11). In brief, anesthetized animals were mechanically ventilated with a tidal volume of 10 ml/kg and a frequency of 2.5 Hz using a computer-controlled piston ventilator (flexiVent; SCIREQ, Montreal, Canada). Bronchial tone was determined in response to increasing concentrations of the aerosolized bronchoconstrictor MeCh (at 0, 6.25, 12.5, 25, and 50 mg/ml in isotonic saline). The single compartment model was used to calculate airway resistance values, and peak values obtained after each MeCh challenge were plotted (17). On protocol day 28, BAL fluid (BALF) was harvested after pulmonary-function testing and analyzed for cellularity, as described previously (3). Differential cell counts were performed by two blinded observers using standard morphological criteria to classify individual leukocyte populations. All mice from each group were used for these analyses, and >200 cells were counted per animal. For the whole-body method, AHR to MeCh (0, 3.125, 6.25, 12.5, 25, 50, and 100 mg/ml in isotonic saline) was measured using whole-body plethysmography (Buxco Electronics, Troy, NY, and EMKA Technologies, Falls Church, VA) and performed as described previously (51). Mice were exposed to aerosolized MeCh for 1 min at each dose, and peak enhanced pause (PenH) response was recorded for 3 min. The maximum PenH was averaged for each dose, and data were plotted as percent change from vehicle controls.
Lung histopathology.
Lungs were isolated and prepared as described previously (52). Sections (4 μm) were cut from paraffin-embedded lungs and stained with periodic acid-Schiff staining to visualize mucus and imaged as described previously (52). Adjacent sections were stained with hematoxylin and eosin to visualize airway morphology and cellular inflammation.
Measurement of total protein in BALF.
Total protein was measured from BALF, isolated on day 28, using the Thermo Scientific Pierce bicinchoninic acid (BCA) protein assay kit (#23228), following the manufacturer's directions.
Measurement of total IgE and OVA-specific IgE.
Whole blood was taken via cardiac puncture by a 23-gauge needle on protocol day 28. Whole blood was placed into plasma separator tubes coated in lithium heparin (Becton Dickinson, Franklin Lakes, NJ). Plasma was isolated from whole blood following the manufacturer's protocols. Total mouse IgE in the isolated plasma was determined using the ELISA MAX Deluxe kit (Cat. No. 432404) and OVA-specific IgE was determined using LEGEND MAX Mouse OVA Specific IgE ELISA Kit (Cat. No. 439807), purchased from BioLegend (San Diego, CA).
Cytokine and chemokine analysis by qRT-PCR.
Lungs were harvested 48 h after the final OVA exposure, and expression levels of cytokines were determined using reverse transcription and quantitative real-time PCR (qRT-PCR). For all lung tissues, RNA was extracted with TRIzol reagent, purchased from Life Technologies (Carlsbad, CA), following the manufacturer's instructions. RNA was processed into first-strand cDNA using the ImProm-II cDNA synthesis kit (Promega, Madison, WI), following the manufacturer's instructions. The input cDNA for each reaction was 500 ng total RNA. Cytokine and chemokine mRNA expression, examined by probe-based qRT-PCR, included the following: Il-4, Il-5, Il-6, Il-10, Il-13, Tnfα, Mcp-1, and granulocyte macrophage colony-stimulating factor (Gm-csf). Primers were designed to be compatible with the Universal ProbeLibrary system using the Universal ProbeLibrary Assay Design Center (Roche Diagnostics, Indianapolis, IN) and synthesized by Integrated DNA Technologies (Coralville, IA). Primer sequences used in this study are as listed: Il-4 forward 5′-catcggcattttgaacgag-3′ and reverse 5′-cgagctcactctctgtggtg-3′; Il-5 forward 5′-acattgaccgccaaaaagag-3′ and reverse 5′-caccatggagcagctcag-3′; Il-6 forward 5′-tctaattcatatcttcaaccaagagg-3′ and reverse 5′-tggtccttagccactccttc-3′; Il-10 forward 5′-cagagccacatgctcctaga-3′ and reverse 5′-tgtccagctggtcctttgtt-3′; Il-13 forward 5′-cctctgacccttaaggagcttat-3′ and reverse 5′-cgttgcacaggggagtct-3′; Tnfα forward 5′-tcttctcattcctgcttgtgg-3′ and reverse 5′-ggtctgggccatagaactga-3′; Mcp-1 forward 5′-tcactgaagccagctctctct-3′ and reverse 5′-gatcatcttgctggtgaatgagt-3′; Gm-csf forward 5′-gcatgtagaggccatcaaaga-3′ and reverse 5′-cgggtctgcacacatgtta-3′. Probes used were from the Universal ProbeLibrary (Roche Diagnostics, Indianapolis, IN) and are listed with the following universal probe numbers: U2, U97, U78, U41, U17, U49, U22, and U79 for Il-4, Il-5, Il-6, Il-10, Il-13, Tnfα, Mcp-1, and Gm-csf, respectively. Quantification of gene expression (see Fig. 6) was performed on a Roche LightCycler 480 Instrument II LC (Roche Diagnostics). Gene-expression levels were calculated using the comparative threshold cycle method and normalized to internal Gapdh expression, as determined using the Mouse GAPD Gene Assay (Cat. no. 05046211001; Roche Diagnostics) in multiplex format.
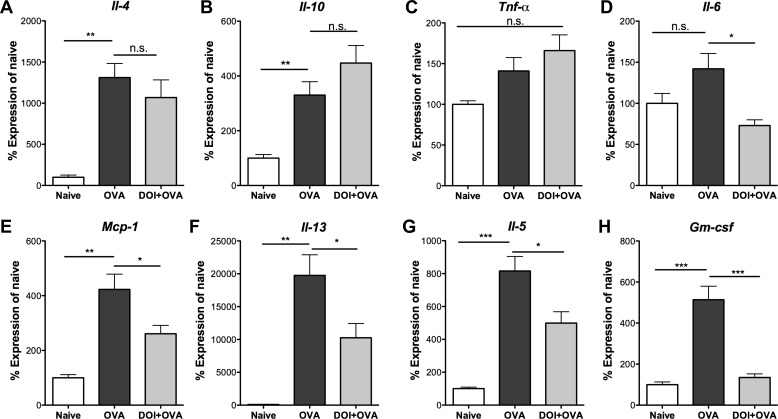
Fig. 6.
Inhaled (R)-DOI (1.0 mg/kg) inhibits proinflammatory gene expression in the whole lung. Quantitative RT-PCR measurement of mRNA expression levels of several inflammatory markers is shown. OVA produces a significant increase in the mRNA levels of Il-4 (A), Il-10 (B), monocyte chemotactic protein-1 (Mcp-1; E), Il-13 (F), Il-5 (G), and granulocyte macrophage colony-stimulating factor (Gm-csf; H) compared with naïve. No significant effect of OVA was observed on Tnfα (C) or Il-6 (D) expression. (R)-DOI produces significant inhibition of the OVA-induced increases in the mRNA expression of Mcp-1, Il-13, Il-5, and Gm-csf. Although Il-6 levels were not statistically different between naïve and OVA groups, (R)-DOI elicited a significant decrease in Il-6 expression levels when administered before OVA exposure compared with OVA alone. ***P < 0.0001, **P < 0.01, and *P < 0.05; n.s. = no significance; n = 4 animals for the Naïve group, and n = 10 animals for the OVA and DOI + OVA treatment groups; error bars represent ± SE; ANOVA with Tukey post hoc test.
Statistics.
All statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA). See figure legends for specific data.
RESULTS
Pulmonary administration of (R)-DOI is effective in preventing AHR in a mouse model of allergic asthma.
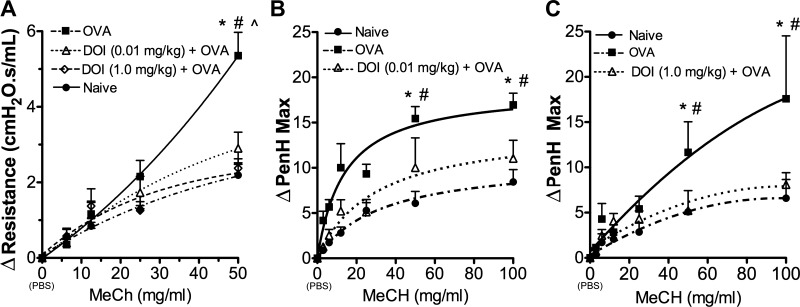
Following OVA sensitization and challenge, we measured airways resistance by two different methods in separate groups of mice. For the first method, we used the forced oscillation technique and in the second method, whole-body plethysmography in awake, freely moving mice. As expected, mice receiving only OVA develop significant AHR in both methods (Fig. 1). Mice pretreated with inhaled (R)-DOI at 0.01 (Fig. 1, A and B) or 1.0 mg/kg (Fig. 1, A and C), before OVA challenge, display airways responsiveness not significantly different from naïve, as measured by either method.
Fig. 1.
(R)-2,5-Dimethoxy-4-iodoamphetamine [(R)-DOI] prevents the development of airways hyperresponsiveness (AHR). A: in forced oscillation-resistance measurements (flexiVent; SCIREQ), naïve mice and those treated nose only with 0.01 and 1.0 mg/kg (R)-DOI during the sensitization process exhibited significantly different resistances from the ovalbumin (OVA)-only-treated group at 50 mg/ml methacholine (MeCh) and were not significantly different from naïve, saline-treated mice [resistance = average of the fractional difference (Δ) of the value measured vs. the individual baseline values]. B and C: results from whole-body plethysmography experiments in awake, freely moving mice are consistent with the forced oscillation results: pretreatment with (B) 0.01 mg/kg (R)-DOI nose only and (C) 1.0 mg/kg (R)-DOI nose only significantly reduced the development of airways resistance. In all figure panels, DOI represents (R)-DOI at the indicated dose. *P < 0.05 OVA vs. Naïve, #P < 0.05 OVA vs. (R)-DOI (1.0 mg/kg) + OVA, and ∧P < 0.05 OVA vs. (R)-DOI (0.01 mg/kg) + OVA; n = 5–9 animals/treatment group; error bars represent ± SE; 2-way ANOVA with Bonferroni post hoc test. Peak enhanced pause maximum (PenH Max) values represent baseline-normalized values.
(R)-DOI prevents pulmonary inflammation and mucus hyperproduction.
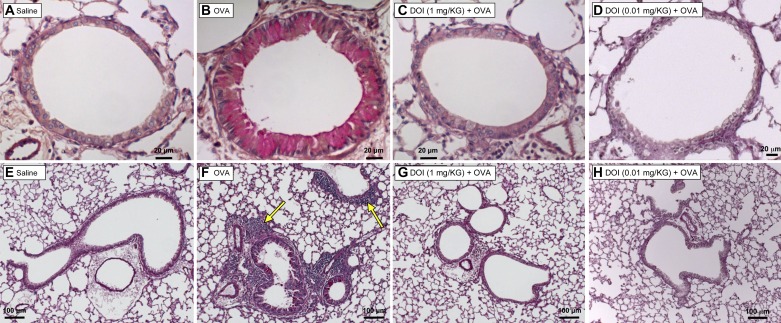
Histopathological analysis of lung sections from the different treatment groups demonstrated that as expected, OVA mice develop significant pulmonary inflammation and mucus. Animals treated with (R)-DOI (1.0 mg/kg) before OVA exposure exhibit very little peribronchial inflammation or mucus. Mice treated with two orders of magnitude less (R)-DOI (0.01 mg/kg) demonstrate significantly reduced inflammation and mucus production compared with the OVA-only-exposed lungs (Figs. 2 and 3).
Fig. 2.
OVA-induced lung inflammation and mucus hyperproduction are inhibited by nose-only (R)-DOI. Representative sections of airways (4 μm) stained with the periodic acid-Schiff (PAS) technique are shown in this figure to highlight mucus (bright pink color). Saline-treated animals have normal airway morphology and no mucus or inflammation (A and E). OVA-alone-treated animals have thickened airways with a significant amount of mucus present (B), as well as peribronchial inflammation (F; arrows indicate inflammatory cells). Animals pretreated with (R)-DOI (1.0 mg/kg and 0.01 mg/kg nose only) demonstrate normal airway morphology, with little to no detectable mucus or inflammation (C and G and D and H, respectively). A–D, 40× objective; E–H, 10× objective.
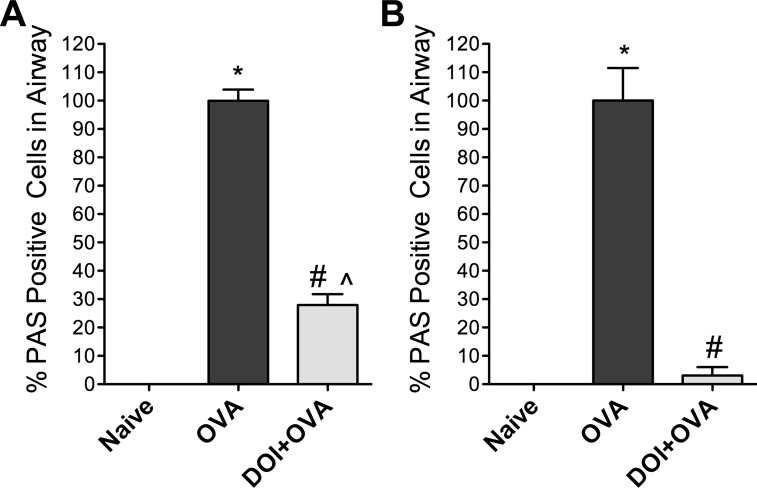
Fig. 3.
Inhaled (R)-DOI reduces mucus cell hyperplasia and mucus production in the airway. The fraction of airway cells containing mucus, as determined by PAS staining (see Fig. 2), was determined for 2 doses of (R)-DOI in 2 separate experiments. Results are presented as normalized to OVA = 100%. Naïve airways did not contain PAS-positive cells, and OVA sensitization dramatically increases mucus production (OVA). A: the number of airway cells containing mucus is reduced significantly by administration of aerosolized (R)-DOI before OVA challenge (0.01 mg/kg DOI + OVA). B: the number of airway cells containing mucus is nearly abolished by inhaled (R)-DOI treatment at 1.0 mg/kg (DOI + OVA). *P < 0.0001 vs. Naïve, #P < 0.0001 vs. OVA, and P̂ < 0.001 vs. Naïve error bars represent ± SE; ANOVA with Tukey post hoc test. All airways/section, which included both lungs, were scored by an unbiased observer for each of 3 animals/treatment (n = 3).
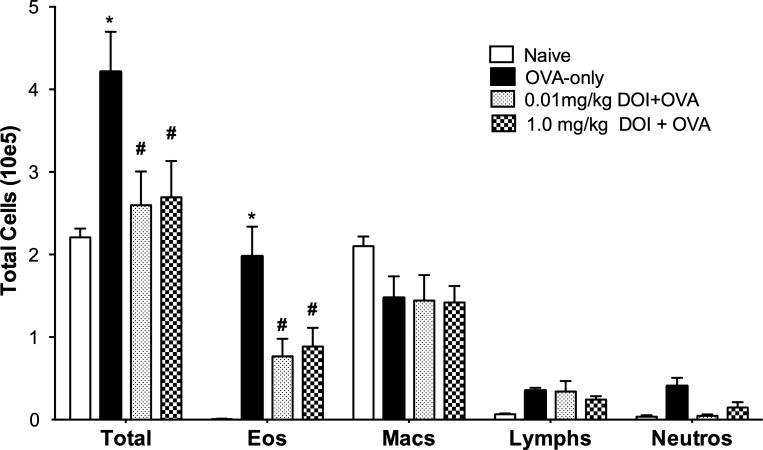
(R)-DOI reduces pulmonary inflammation and BALF eosinophilia.
Pulmonary inflammation is a common feature of asthma and is partly responsible for increased AHR (15). To associate (R)-DOI treatment and decreased AHR, as well as normal-appearing histological results with lack of inflammation, we performed cell-differential counts on BALF cell populations for each mouse in each group. As expected, OVA induced a significant increase in the total number of cells recovered in the BALF compared with naïve and (R)-DOI-treated animals. A large fraction of the BALF cellularity was due to elevated numbers of eosinophils (Fig. 4). Total BALF cell numbers and eosinophil numbers for naïve, 0.01 mg/kg DOI + OVA, and 1.0 mg/kg DOI + OVA were significantly lower than the OVA-only mice (Fig. 4). Although the eosinophil numbers for the (R)-DOI-treated mice were greater than those of naïve mice, they were not significantly different. There is a trend for a decrease in the neutrophil numbers in (R)-DOI-treated mice compared with OVA-treated mice; however, the difference was not significant.
Fig. 4.
Bronchoalveolar lavage fluid (BALF) cellularity is altered in mice exposed to OVA but not in mice exposed to OVA and treated with (R)-DOI. BAL cellularities and differentials are expressed as the product of the total number of cells recovered and the percentages of each cell type derived from differentials. The total number of cells is nearly double in the OVA-alone-treated mice compared with naïve (Total). (R)-DOI treatment, before OVA exposure, reduced the total cellularity back to naïve levels. This difference was primarily due to a significant reduction in eosinophils (Eos) in the mice treated with (R)-DOI. No significant differences in the numbers of macrophages (Macs), lymphocytes (Lymphs), or neutrophils (Neutros) were observed between treatment groups. *OVA-only vs. Naïve (P < 0.001) and #OVA-only vs. (R)-DOI + OVA (P < 0.01); n = 5–6 animals/treatment group; error bars represent ± SE; 2-way ANOVA with Bonferroni post hoc test. Total cells (10e5) = y-axis value multiplied by 1 × 105.
(R)-DOI does not alter lung leak or plasma IgE levels.
Increased protein content of the BALF is a hallmark of asthma and the OVA model (46). Analysis of BALF total protein by BCA assay from different treatment groups revealed a significant increase between naïve and OVA groups but showed no difference between mice treated with (R)-DOI + OVA and those animals that were treated with OVA only (Fig. 5). The OVA model characteristically produces increased serum levels of IgE and OVA-specific IgE (19, 28, 56); therefore, we tested the effects of (R)-DOI on total IgE and OVA-specific IgE. In both cases, we measured a significant increase between naïve and OVA-treated groups. (R)-DOI treatment, however, had no effect on either total IgE or OVA-specific IgE as induced by OVA (Fig. 5).
Fig. 5.
Total protein and IgE levels are not affected by (R)-DOI (1.0 mg/ml, nose only). A: the total of protein content in the BALF, as measured by bicinchoninic acid assay, is increased significantly in the OVA-only-treated lungs compared with naïve. (R)-DOI does not alter total BALF protein induced by OVA. B: total plasma IgE, as measured by ELISA, is increased significantly by OVA treatment. (R)-DOI, administered before OVA challenge, has no effect on total plasma IgE. C: OVA-specific plasma IgE, as measured by ELISA, is increased significantly by OVA treatment. (R)-DOI, administered before OVA challenge, has no effect on OVA-specific plasma IgE. ***P < 0.001 vs. OVA; n.s. = no significance vs. OVA; n = 7–17 animals/treatment group; error bars represent ± SE; ANOVA with Tukey post hoc test.
(R)-DOI suppresses expression of genes involved in the T cell and innate-immune cell response.
A panel of cytokines and chemokines typically involved in asthma and the OVA model (Il-4, Il-5, Il-6, Il-10, Il-13, Tnfα, Mcp-1, and Gm-csf) was examined in the lungs by qRT-PCR (6, 10, 17, 29, 36, 45, 49). There were, as anticipated, significant increases in mRNA for Il-4, Il-5, Il-10, Il-13, Mcp-1, and Gm-csf with OVA treatment compared with naïve mice. There was a trend that did not reach significance for Il-6 and Tnfα expression. (R)-DOI had no effect on the increased expression levels of OVA-induced Il-4 or Il-10. Interestingly, (R)-DOI treatment significantly repressed the OVA-induced increases in mRNA expression for Mcp-1, Il-13, and Il-5 and completely blocked the increase in Gm-csf (Fig. 5). Although Il-6 expression was not up-regulated significantly in the OVA group compared with vehicle control, (R)-DOI did significantly reduce Il-6 expression levels in OVA-treated mice, as expected from our previous studies in different inflammatory models (Fig. 6).
DISCUSSION
To determine if serotonin 5-HT2 receptor activation with (R)-DOI is an effective mechanism to treat a pathological inflammatory disease, we investigated the effects of the highly selective 5-HT2 receptor agonist (R)-DOI in a mouse model of allergic asthma. By building upon our earlier in vitro and in vivo studies, we demonstrate here that inhaled (R)-DOI has potent anti-inflammatory effects and blocks the development of allergic asthma in the OVA mouse model. Importantly, we have already established that the anti-inflammatory effects of (R)-DOI in vitro and in vivo are mediated through activation of the serotonin 5-HT2A receptor subtype (33, 53). Here, we tested two different doses of (R)-DOI. The 1.0-mg/kg dose is in the range of that used in typical behavioral experiments (41). The very low dose of 0.01 mg/kg was chosen to test the super potency of (R)-DOI, predicted by our previous cellular studies (53). Anti-inflammatory effects of this very low dose were also observed in our recent in vivo study examining the ability of (R)-DOI to block the effects of systemic administration of TNF-α (33). Because activation of the 5-HT2A receptor subtype and not the 5-HT2C receptor subtype was found to be necessary for the anti-inflammatory effects of (R)-DOI in our previous studies, we hypothesized that the effects of (R)-DOI against allergic asthma were also mediated through 5-HT2A receptor activation. Although we were not able to validate this here, we have confirmed the presence of 5-HT2A receptor mRNA on whole-lung tissue (33). Furthermore, the expression of 5-HT2A receptors has been reported in airway smooth muscle cells (2) and alveolar macrophages (30), and although naïve T cells do not express high levels of the 5-HT2A receptor, activated T cells do express high levels of 5-HT2A receptor mRNA (23). We suggest that the site of therapeutic action is directly on the pulmonary tissues, including resident-activated T cell populations and/or innate-immune cells.
The major components of allergic asthma in humans include AHR, pulmonary inflammation, and mucus hyperproduction (7). In addition, eosinophils, which release cytotoxic mediators and leukotrienes, are recruited in large numbers to the lungs of asthmatic individuals (37). Eosinophil production, chemotaxis, and survival are controlled by regulated on activation, normal T cell expressed and secreted (CC chemokine ligand 5), macrophage inflammatory protein 1α, eotaxins, IL-5, and GM-CSF (18, 25, 35, 43, 47). IL-5 and GM-CSF are derived from activated pulmonary epithelial cells, eosinophils themselves, and activated T-lymphocytes (1, 27, 50). IL-5 and GM-CSF are molecules important in the development of asthma and are increased in serum and BALF of asthmatics in the clinic (12, 44). Significantly, our data show that both genes are suppressed by administration of (R)-DOI in the OVA mouse model.
The role of eosinophils in asthma is both direct, causing bronchoconstriction and destruction to airways, and indirect by provoking degranulation of mast cells and basophils (7). We demonstrate here that (R)-DOI blocks recruitment of eosinophils to the lung, prevents mucus hyperproduction, blocks AHR, and represses T helper cell 2 (Th2) and innate-immune cell gene expression (e.g., Il-5 and Mcp-1). We delivered (R)-DOI directly to the lung using inhalation techniques in these experiments, and it remains to be determined whether systemically injected (R)-DOI has the same or similar effects on the development of asthma. Importantly, effective levels of (R)-DOI, administered by this route (inhalation), are orders of magnitude less than those necessary to produce either behavioral intoxication, as indicated by the classical head-twitch response (9), or airways constriction in mice (>10 mg/kg inhaled; data not shown).
Although the presence of 5-HT2A receptor mRNA has been demonstrated in pulmonary tissues by our lab and others, the role of this receptor in the lung has remained largely undefined. A few reports have suggested that the 5-HT2A receptor mediates AHR in allergic asthma (14, 40, 54). However, these studies used the antagonist ketanserin, which is nonselective in rodents for 5-HT2 receptors and also has high affinity for histamine H1 and α-adrenergic receptors, to block the effects of serotonin. This makes it difficult to interpret results using ketanserin. In any case, these reports indicated that serotonin activation of 5-HT2A receptors contributes to AHR rather than preventing it. Serotonin itself has been implicated in airways inflammation in allergic asthma by acting as a critical factor to recruit inflammatory cells and prime Th2 responses by activation of bone marrow-derived dendritic cells, although the receptor(s) mediating these effects remain unknown (13). Conversely, blockade of serotonin receptors with a nonselective antagonist for multiple subtypes has demonstrated antiasthma effects in the OVA model (24, 40). Why then, if serotonin appears to have a proinflammatory effect in the lung, does activation of 5-HT2 receptors with (R)-DOI have an anti-inflammatory effect? One possibility is that selective activation of 5-HT2 receptors with (R)-DOI avoids activation of other serotonin receptor types responsible for the inflammatory response. A more likely explanation is that (R)-DOI, which has a much higher affinity for the 5-HT2 receptors than serotonin, is acting as a functionally selective ligand and recruiting anti-inflammatory effector pathways that serotonin itself does not (26, 31). Significantly, (R)-DOI has already been shown to activate different signaling pathways than serotonin at the 5-HT2A receptor in vivo (38, 39).
It is unlikely that the therapeutic mechanistic site of action of (R)-DOI is on the B cell or the antigen-presenting cell, as (R)-DOI has no effect on OVA-induced Il-4 gene expression. Recent reports indicate that IgE-dependent mast cell activation is involved in the development of AHR (28). The fact that (R)-DOI has no measureable effect on humoral IgE production, yet prevents AHR, suggests (R)-DOI is acting on activated rather than naïve T cells to block AHR through nonmast cell-dependent mechanisms. Because (R)-DOI blocks Mcp-1 and Gm-csf mRNA production, the therapeutic target may also include innate immune cells. There is also the possibility that (R)-DOI may be acting on the naïve CD4+ population; however, naïve T cells do not express high levels of 5-HT2A receptor mRNA until activated. Our data demonstrate that (R)-DOI treatment significantly inhibits the OVA-induced expression of Th2-related genes that include Il-13, Il-5, and Gm-csf in the lung. Interestingly, vascular (or more likely, epithelial) permeability is not improved with (R)-DOI, as total protein in the BALF is not reduced compared with OVA alone.
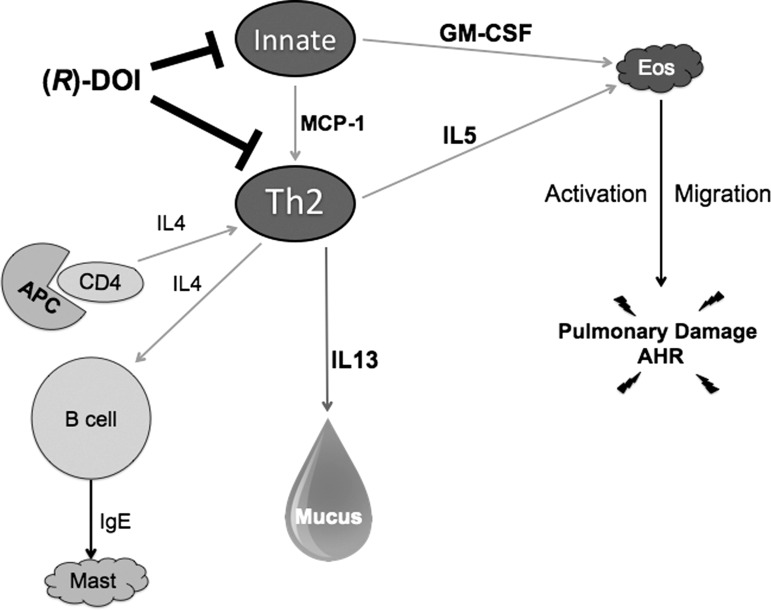
We propose a model, shown in Fig. 7, where the pool of 5-HT2A receptors activated by (R)-DOI that responds with anti-inflammatory properties could reside on activated Th2 cells and/or innate immune cells. In this proposed model, 5-HT2A receptor activation would lead to reduced IL-5, GM-CSF, and MCP-1 secretion, in turn, decreasing eosinophil recruitment, Th2 polarization, and IL-13 production (16, 22, 48, 55). Overall, these effects would combine to reduce inflammation and AHR. The precise cellular signaling pathways, however, remain to be elucidated.
Fig. 7.
A proposed therapeutic mechanism of (R)-DOI. The presented data show that (R)-DOI has no effect on Il-4 gene expression, as well as no effect on humoral IgE production. These data provide evidence that the therapeutic action of (R)-DOI is not on the B cell, the antigen-presenting cell (APC), and/or the naïve CD4+ population. Importantly, we show that (R)-DOI treatment significantly inhibits expression of T helper cell 2 (Th2)-related genes, including Mcp-1, Il-13, Il-5, and Gm-csf compared with asthmatic animals. Taken together, we suggest that (R)-DOI exerts its therapeutic action in the OVA asthma model by activating anti-inflammatory signaling pathways through the serotonin 5-hydroxytryptamine 2A receptors on T cells and/or innate immune cells, leading to a decrease in secretion of Il-13, resulting in a decrease in mucus production; a decrease in Il-5 and Gm-csf secretion, leading to a decrease in eosinophilia recruitment; and a decrease in Mcp-1 production, leading to a decrease in Th2 polarization. These changes contribute to a general decrease in both inflammation and AHR.
In conclusion, we have identified an important and new functional role of 5-HT2 receptors in the lung. (R)-DOI activation of serotonin 5-HT2 receptors potently prevents the development of a clinically relevant mouse model of allergic asthma at drug levels far below those necessary to invoke adverse cardiovascular or behavioral effects. Based on our previous in vitro and in vivo studies, we predict that it is the 5-HT2A receptor that is the therapeutic target of (R)-DOI in our model. Our results demonstrate that activation of 5-HT2 receptors differentially regulates Th2 signaling, innate cytokine responses, and other relevant inflammatory effector pathways and that selective activation with (R)-DOI, or perhaps other 5-HT2A agonists in its class, represents a novel, small molecule-based therapeutic strategy for the treatment of asthma.
GRANTS
Support for this work was provided by the National Heart, Lung, and Blood Institute Grant R21HL095961, the American Asthma Foundation, and The Heffter Research Institute (to C. D. Nichols); the National Institute of Allergy and Infectious Diseases (Grant R01AI090059) and the National Institute of Environmental Health Sciences (Grants R01ES015050 and P42ES013648; to S. A. Cormier); and the National Heart, Lung, and Blood Institute (Grant T35HL105350 to J. Miller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.N., J.M., T.A., K.I.H., S.A.C., and C.D.N. conception and design of research; F.N., J.M., J.S., T.A., B.Y., and C.D.N. performed experiments; F.N., J.M., T.A., B.Y., S.A.C., and C.D.N. analyzed data; F.N., B.Y., K.I.H., S.A.C., and C.D.N. interpreted results of experiments; F.N., T.A., and C.D.N. prepared figures; C.D.N. drafted manuscript; F.N., K.I.H., S.A.C., and C.D.N. edited and revised manuscript; F.N., S.A.C., and C.D.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Amy Weinburg, Vi Tran, and David Martin for technical assistance.
Present address of S. A. Cormier: Dept. of Pediatrics, University of Tennessee Health Sciences Center, 50 North Dunlap St., and Children's Foundation Research Institute, Le Bonheur Children's Hospital, Memphis, TN 38103.
REFERENCES
- 1.Arm JP, Lee TH. The pathobiology of bronchial asthma. Adv Immunol 51: 323–382, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Bai Y, Zhang M, Sanderson MJ. Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways. Am J Respir Cell Mol Biol 36: 122–130, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balakrishna S, Saravia J, Thevenot P, Ahlert T, Lominiki S, Dellinger B, Cormier SA. Environmentally persistent free radicals induce airway hyperresponsiveness in neonatal rat lungs. Part Fibre Toxicol 8: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becnel D, You D, Erskin J, Dimina DM, Cormier SA. A role for airway remodeling during respiratory syncytial virus infection. Respir Res 6: 122, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blyth D, Pedrick M, Savage T, Hessel E, Fattah D. Lung inflammation and epithelial changes in a murine model of atopic asthma. Am J Respir Cell Mol Biol 14: 425–438, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol 89: 958–967, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Busse WW, Lemanske RF. Asthma. N Engl J Med 344: 350–362, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Campos-Bedolla P, Vargas MH, Segura P, Carbajal V, Calixto E, Figueroa A, Flores-Soto E, Barajas-López C, Mendoza-Patiño N, Montaño LM. Airway smooth muscle relaxation induced by 5-HT(2A) receptors: role of Na(+)/K(+)-ATPase pump and Ca(2+)-activated K(+) channels. Life Sci 83: 438–446, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4: 556–576, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung F. Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-γ. Mediators Inflamm 10: 51–59, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cormier SA, Yuan S, Crosby JR, Protheroe CA, Dimina DM, Hines EM, Lee NA, Lee JJ. T(H)2-mediated pulmonary inflammation leads to the differential expression of ribonuclease genes by alveolar macrophages. Am J Respir Cell Mol Biol 27: 678–687, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Corrigan CJ, Haczku A, Gemou-Engesaeth V, Doi S, Kikuchi Y, Takatsu K, Durham SR, Kay AB. CD4 T-lymphocyte activation in asthma is accompanied by increased serum concentrations of interleukin-5. Effect of glucocorticoid therapy. Am Rev Respir Dis 147: 540–547, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Dürk T, Duerschmied D, Müller T, Grimm M, Reuter S, Vieira RP, Ayata K, Cicko S, Sorichter S, Walther DJ, Virchow JC, Taube C, Idzko M. Production of serotonin by tryptophan hydroxylase 1 and release via platelets contribute to allergic airway inflammation. Am J Respir Crit Care Med 187: 476–485, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Rodriguez S, Broadley KJ, Ford WR, Kidd EJ. Increased muscarinic receptor activity of airway smooth muscle isolated from a mouse model of allergic asthma. Pulm Pharmacol Ther 23: 300–307, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TNC, Proudfoot A, Martinez -AC, Dorf M, Bjerke T, Coyle AJ, Gutierrez-Ramos JC. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med 188: 157–167, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404: 407–411, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Hamelmann E, Takeda K, Schwarze J, Vella AT, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am J Respir Cell Mol Biol 21: 480–489, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Hamid QA, Minshall EM. Molecular pathology of allergic disease: I: lower airway disease. J Allergy Clin Immunol 105: 20–36, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Honda K, Marquillies P, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptor γ is expressed in airways and inhibits features of airway remodeling in a mouse asthma model. J Allergy Clin Immunol 113: 882–888, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Okazaki T, Kitazono T, Mizushima M, Omata M, Ozaki S. Regulation of antigen-specific CTL and Th1 cell activation through 5-Hydroxytryptamine 2A receptor. Int Immunopharmacol 11: 67–73, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Kang BN, Ha SG, Bahaie NS, Hosseinkhani MR, Ge XN, Blumenthal MN, Rao SP, Sriramarao P. Regulation of serotonin-induced trafficking and migration of eosinophils. PLoS One 8: e54840, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 8: 885–889, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Leon-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 109: 3139–3146, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima C, Souza VMO, Soares AL, Macedo MS, Tavares-de-Lima W, Vargaftig BB. Interference of methysergide, a specific 5-hydroxytryptamine receptor antagonist, with airway chronic allergic inflammation and remodelling in a murine model of asthma. Clin Exp Allergy 37: 723–734, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 338: 436–445, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28: 390–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makino S, Fukuda T. Eosinophils and allergy in asthma. Allergy Proc 16: 13–21, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Mayr SI, Zuberi RI, Zhang M, de Sousa-Hitzler J, Ngo K, Kuwabara Y, Yu L, Fung-Leung WP, Liu FT. IgE-dependent mast cell activation potentiates airway responses in murine asthma models. J Immunol 169: 2061–2068, 2002. [DOI] [PubMed] [Google Scholar]
- 29.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol 172: 2903–2908, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Mikulski Z, Zaslona Z, Cakarova L, Hartmann P, Wilhelm J, Tecott LH, Lohmeyer J, Kummer W. Serotonin activates murine alveolar macrophages through 5-HT2C receptors. Am J Physiol Lung Cell Mol Physiol 299: L272–L280, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther 321: 1054–1061, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Nagatomo T, Rashid M, Abul Muntasir H, Komiyama T. Functions of 5-HT2A receptor and its antagonists in the cardiovascular system. Pharmacol Ther 104: 59–81, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Nau F Jr, Yu B, Martin D, Nichols CD. Serotonin 5-HT2A receptor activation blocks TNF-α mediated inflammation in vivo. PLoS One 8: e75426, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev 108: 1614–1641, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Nickel R, Beck LA, Stellato C, Schleimer RP. Chemokines and allergic disease. J Allergy Clin Immunol 104: 723–742, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Ohkawara Y, Lei XF, Stämpfli MR, Marshall JS, Xing Z, Jordana M. Cytokine and eosinophil responses in the lung, peripheral blood, and bone marrow compartments in a murine model of allergen-induced airways inflammation. Am J Respir Cell Mol Biol 16: 510–520, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Rothenberg ME. Eosinophilia. N Engl J Med 338: 1592–1600, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. J Neurosci 30: 13513–13524, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA 105: 1079–1084, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segura P, Vargas MH, Córdoba-Rodríguez G, Chávez J, Arreola JL, Campos-Bedolla P, Ruiz V, García-Hernández LM, Méndez C, Montaño LM. Role of 5-HT2A, 5-HT4 and 5-HT7 receptors in the antigen-induced airway hyperresponsiveness in guinea-pigs. Clin Exp Allergy 40: 327–338, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Smith R, Barrett R, Sanders-Bush E. Discriminative stimulus properties of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(+/−)DOI] in C57BL/6J mice. Psychopharmacology (Berl) 166: 61–68, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Stefulj J, Jernej B, Cicin-Sain L, Rinner I, Schauenstein K. mRNA expression of serotonin receptors in cells of the immune tissues of the rat. Brain Behav Immun 14: 219–224, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Sulakvelidze I, Inman MD, Rerecich T, O'Byrne PM. Increases in airway eosinophils and interleukin-5 with minimal bronchoconstriction during repeated low-dose allergen challenge in atopic asthmatics. Eur Respir J 11: 821–827, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Till S, Li B, Durham S, Humbert M, Assoufi B, Huston D, Dickason R, Jeannin P, Kay AB, Corrigan C. Secretion of the eosinophil-active cytokines interleukin-5, granulocyte/macrophage colony-stimulating factor and interleukin-3 by bronchoalveolar lavage CD4+ and CD8+ T cell lines in atopic asthmatics, and atopic and non-atopic controls. Eur J Immunol 25: 2727–2731, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Tomkinson A, Duez C, Cieslewicz G, Pratt JC, Joetham A, Shanafelt MC, Gundel R, Gelfand EW. A murine IL-4 receptor antagonist that inhibits IL-4- and IL-13-induced responses prevents antigen-induced airway eosinophilia and airway hyperresponsiveness. J Immunol 166: 5792–5800, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Van Vyve T, Chanez P, Bernard A, Bousquet J, Godard P, Lauwerijs R, Sibille Y. Protein content in bronchoalveolar lavage fluid of patients with asthma and control subjects. J Allergy Clin Immunol 95: 60–68, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Warringa RA, Koenderman L, Kok PT, Kreukniet J, Bruijnzeel PL. Modulation and induction of eosinophil chemotaxis by granulocyte-macrophage colony-stimulating factor and interleukin-3. Blood 77: 2694–2700, 1991. [PubMed] [Google Scholar]
- 48.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev 202: 175–190, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, Lam CW. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol 125: 177–183, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xing Z, Braciak T, Ohkawara Y, Sallenave JM, Foley R, Sime PJ, Jordana M, Graham FL, Gauldie J. Gene transfer for cytokine functional studies in the lung: the multifunctional role of GM-CSF in pulmonary inflammation. J Leukoc Biol 59: 481–488, 1996. [DOI] [PubMed] [Google Scholar]
- 51.You D, Becnel D, Wang K, Ripple M, Daly M, Cormier SA. Exposure of neonates to respiratory syncytial virus is critical in determining subsequent airway response in adults. Respir Res 7: 107, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.You D, Ripple M, Balakrishna S, Troxclair D, Sandquist D, Ding L, Ahlert TA, Cormier SA. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J Immunol 181: 3486–3494, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu B, Becnel J, Zerfaoui M, Rohatgi R, Boulares AH, Nichols CD. Serotonin 5-hydroxytryptamine(2A) receptor activation suppresses tumor necrosis factor-alpha-induced inflammation with extraordinary potency. J Pharmacol Exp Ther 327: 316–323, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Cardell LO, Adner M. IL-1β induces murine airway 5-HT2A receptor hyperresponsiveness via a non-transcriptional MAPK-dependent mechanism. Respir Res 8: 29, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 103: 779–788, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuberi RI, Apgar JR, Chen SS, Liu FT. Role for IgE in airway secretions: IgE immune complexes are more potent inducers than antigen alone of airway inflammation in a murine model. J Immunol 164: 2667–2673, 2000. [DOI] [PubMed] [Google Scholar]