Abstract
Cellular therapy via direct intratracheal delivery has gained interest as a novel therapeutic strategy for treating various pulmonary diseases including cystic fibrosis lung disease. However, concerns such as insufficient cell engraftment in lungs and lack of large animal model data remain to be resolved. This study aimed to establish a simple method for evaluating cell retention in lungs and to develop reproducible approaches for efficient cell delivery into mouse and pig lungs. Human lung epithelial cells including normal human bronchial/tracheal epithelial (NHBE) cells and human lung epithelial cell line A549 were infected with pSicoR-green fluorescent protein (GFP) lentivirus. GFP-labeled NHBE cells were delivered via a modified intratracheal cell instillation method into the lungs of C57BL/6J mice. Two days following cell delivery, GFP ELISA-based assay revealed a substantial cell-retention efficiency (10.48 ± 2.86%, n = 7) in mouse lungs preinjured with 2% polidocanol. When GFP-labeled A549 cells were transplanted into Yorkshire pig lungs with a tracheal intubation fiberscope, a robust initial cell attachment (22.32% efficiency) was observed at 24 h. In addition, a lentiviral vector was developed to induce the overexpression and apical localization of cystic fibrosis transmembrane conductance regulator (CFTR)-GFP fusion proteins in NHBE cells as a means of ex vivo CFTR gene transfer in nonprogenitor (relatively differentiated) lung epithelial cells. These results have demonstrated the convenience and efficiency of direct delivery of exogenous epithelial cells to lungs in mouse and pig models and provided important background for future preclinical evaluation of intratracheal cell transplantation to treat lung diseases.
Keywords: intratracheal delivery, cell transplantation, lung diseases, engraftment, lung epithelial cells
lung disease is one of the leading causes for mortality in the United States and worldwide (22, 57). For severe pulmonary diseases, lung transplantation could be the only therapeutic option, and tissue-engineering approaches have been developed to generate functional lungs that one day can be used in the clinic (41, 42). For other lung diseases, cellular therapies in which diseased lung epithelia could be replaced by normal exogenous cells through direct delivery into the airways might offer an alternative solution with less mortality and lower cost than whole lung transplantation (18, 55).
Because the lung contains multiple cell lineages and lung diseases are diverse, different types of cells, including bone marrow-derived stem cells, embryonic stem cells, fetal lung cells, alveolar epithelial type II cells, fibroblasts, and others, have been delivered into the lungs in animal models (2, 9, 10, 28, 31, 32, 44, 49, 59). Although these studies have shown cell engraftment in lungs after different periods of time, only a few of them have quantified the efficiency of cell retention, which will likely be essential for effective lung repair (36). In addition, most of these studies have used mouse or rat models to determine the potential of cellular therapies for treating lung diseases. Rodent models have been valuable to understand the mechanisms of diseases and are indispensable for studying new therapeutic approaches, but porcine models have been considered more appropriate to preclinically evaluate novel treatments for lung diseases such as cystic fibrosis (CF) (15, 46).
Many of the cell-based therapies that used stem cells to reconstitute lung epithelia have encountered various difficulties associated with low cell retention and, notably, inefficient differentiation of stem cells to lung epithelial cells in vivo (26, 30, 40). With the recent development in differentiating embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) to pulmonary epithelial cells or lung epithelial precursors in vitro (19–21, 33, 60), it seems that a new potential source of cells for treating lung diseases has emerged. For example, human alveolar epithelial type II cells have been derived from ESCs and have shown some treatment efficacy for lung fibrosis in mouse models (54). CF lung disease, which is caused by mutations in the CF transmembrane conductance regulator (CFTR) (14, 23) but has yet to see clinical success using gene-therapy approaches (1, 40), might also be treated with airway epithelial cells derived from patient-specific iPSCs (37, 52, 58) after ex vivo CFTR gene correction (38).
Previous studies have shown that transient lung injury can improve the pulmonary retention of exogenous cells, including ESCs (31), bone marrow-derived stem cells (10, 44), and alveolar epithelial type II cells (54). Among the different reagents used for inducing lung injury in animal models, polidocanol (PDOC) has been shown to temporarily remove the surface airway epithelial cells in mice and improve engraftment of stem cells or viral vectors (31, 34). In PDOC-treated lungs, the proliferation of normally quiescent epithelial cells was activated (5), probably in response to injury-induced cytokines (3). Therefore, PDOC might help to stimulate the retention (and proliferation) of airway epithelial cells for better outcome of the cell-based therapy.
The goal for this study was to establish a method to conveniently quantify cell retention following cell transplantation and to develop an approach to efficiently deliver nonprogenitor lung epithelial cells into mouse and pig lungs. These studies provide useful tools and fundamental background for future preclinical evaluation of intratracheal (instillational) cell transplantation to treat lung diseases.
MATERIALS AND METHODS
All animal procedures were approved by the Yale University Institutional Animal Care and Use Committee. All animal care complied with the Guide for the Care and Use of Laboratory Animals.
Cell culture.
Normal human bronchial/tracheal epithelial (NHBE) cells (Lonza, Walkersville, MD) were grown in bronchial epithelial cell growth medium (BEGM, Lonza) at 37°C, 5% CO2 and infected with pSicoR-green fluorescent protein (GFP) lentivirus (47) overnight at 37°C. Virus-containing medium was then removed, and cells were further incubated at 37°C for 3 days. In some studies, the human lung epithelial cell line A549 (ATCC) was grown in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C, 5% CO2 and infected with pSicoR-GFP lentivirus similarly to NHBE cells. Cells were examined for GFP under a fluorescence microscope or by flow cytometry.
Intratracheal administration of cells in mice.
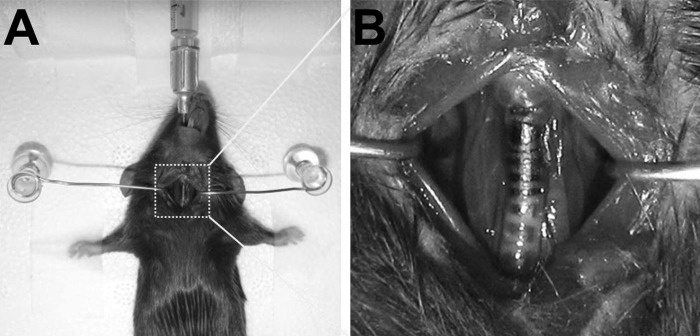
C57BL/6J mice (female, 10–12 wk; Charles River Laboratories, Wilmington, MA) were used. Mice were anesthetized with an intraperitoneal ketamine/xylazine injection and placed supine. A small incision was made on the neck area to visualize trachea. Mice were then intubated with a sterile blunt-end catheter (Covidien, Mansfield, MA) (Fig. 1). NHBE cells (0.5 × 106) infected with pSicoR-GFP lentivirus were resuspended in 50 μl BEGM and injected directly into the lungs. An aliquot of GFP-labeled NHBE cells was saved for later analysis. For the lung preinjury experiments, 20 μl of 2% PDOC (Sigma-Aldrich, St. Louis, MO) in PBS was intratracheally injected into the lungs 24 h before cell delivery. At the indicated time, mice were euthanized with an intraperitoneal lethal injection of pentobarbital sodium, and their lungs were harvested. The left lungs and trachea were fixed with formalin, and the right lungs were flash frozen.
Fig. 1.
Illustration of intratracheal delivery of cells to mouse lungs. A: mouse with an intubation needle positioned in the trachea. A small incision was made in the neck for tracheal visualization. B: the tip of the intubation needle is painted dark for illustration purpose only.
Transplantation of cells into pig lungs.
Yorkshire pigs (male, 25–30 kg; Earle Parsons & Sons, Hadley, MA) were anesthetized with an intraperitoneal telazol/ketamine/xylazine injection. A tracheal intubation fiberscope (Olympus) was used to ensure transplantation of cells into the right lower lung lobe of each animal. A549 cells (100 × 106) infected with pSicoR-GFP lentivirus were resuspended in 10 ml DMEM/10% FBS and injected directly into the pig lung through the fiberscope. An aliquot of GFP-labeled A549 cells was saved for later analysis. Twenty-four hours following cell transplantation, pigs were euthanized with an intraperitoneal lethal injection of pentobarbital sodium, and the lungs were harvested. The right lower lobe of the lungs was dissected from the whole lung, cut into pieces of ∼1 cm2, and formalin-fixed or flash frozen for later analysis.
ELISA for GFP.
pSicoR-GFP lentivirus-infected NHBE cells or A549 cells saved at the time of cell transplantation were lysed using Triton X-100 lysis buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS) containing freshly added proteinase inhibitors (Roche Diagnostics, Mansfield, Germany). Proteins were extracted, and the amount of GFP in the cell lysate was determined using a GFP ELISA kit (Cell Biolaboratories, San Diego, CA) according to the manufacturer's instructions. The average GFP amount per cell was obtaining by dividing total GFP amount by cell numbers counted directly from culture flasks. To determine whether ELISA quantification of GFP can be used to estimate the number of cells when engrafted in lungs, lung tissues that were harvested from normal C57BL/6J mice were spiked with pSicoR-GFP lentivirus-infected NHBE cells at twofold serial dilutions and lysed, and the amount of GFP was determined by ELISA.
Determining cell-retention efficiency in lungs.
Frozen lung tissues (i.e., right lungs from mice and lung pieces from pigs) were homogenized and lysed, and proteins were extracted. The amount of GFP in lung lysate was determined by ELISA, and the number of cells was calculated by dividing GFP amount with the average GFP amount per cell as follows: number of cells = total GFP amount/average GFP amount per cell. Each sample was analyzed in duplicate. To calculate the total number of cells retained in both mouse lungs for a single animal, the number of engrafted cells in the right lung was divided by 0.6, a ratio of the volume of whole lung to the volume right lung (35), assuming fairly similar cell distribution in both lungs using the intratracheal route (12), as follows: number of cells in whole mouse lung = number of engrafted cells in right lung/0.6. The total number of cells engrafted in the whole lung lobe of pigs was approximated by dividing the number of cells in each lung piece by a ratio of weight of individual lung piece to total weight of lung lobe, as follows: number of cells in whole pig lung lobe = number of engrafted cells in lung piece/(weight of lung piece/total weight of lung lobe). The cell retention efficiency was determined by dividing the number of cells engrafted in vivo by the number of cells delivered into the lungs, as follows: cell retention efficiency = (number of engrafted cells/number of delivered cells) × 100%.
Histological analysis and immunofluorescence staining.
Formalin-fixed lungs were paraffin embedded, cut into 5-μm tissue sections, and stained with hematoxylin and eosin (H&E). To differentiate from the background autofluorescence of lungs, lung sections were incubated with rabbit polyclonal antibody to GFP (1:250; Abcam, Cambridge, MA) followed by Alexa Fluor 555-conjugated goat-anti-rabbit IgG (1:500; Invitrogen, Carlsbad, CA) to visualize GFP-positive cells. Slides were counterstained with DAPI and mounted. Images were obtained using a Zeiss Axiovert 200M microscope equipped with Hamamatsu 1394 ORCA-ERA with software Volocity (PerkinElmer, Waltham, MA).
Construction of CFTR-GFP lentivector.
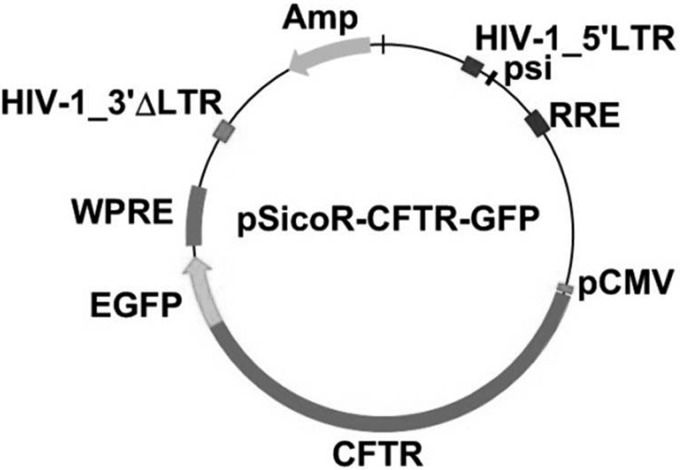
The lentiviral vector, designated pSicoR-CFTR-GFP, was constructed by inserting human CFTR full-length cDNA into the pSicoR-GFP vector (gift from Dr. Jiangbing Zhou, Yale University). Briefly, the CFTR cDNA was amplified from construct pBQ4.7V (gift from Drs. Wanda O'Neal and Scott Randell, University of North Carolina) by PCR using two cloning site-tagged primers CFTR forward (5′-gcattcgctattcagctagcaaccatgcagaggtcgcctctgg) and CFTR reverse (5′-tactgtcgttcgaagtaccggtgaaagccttgtatcttgcacctctt). The CFTR fragment was cloned into the NheI and AgeI sites of pSicoR-GFP to generate pSicoR-CFTR-GFP (Fig. 2). The COOH terminus of CFTR was fused to GFP in pSicoR-CFTR-GFP. The inserted CFTR cDNA was fully sequenced and verified. Sequencing was performed by the W. M. Keck Biotechnology Resource Laboratory at Yale University.
Fig. 2.
Schematic structure of pSicoR-cystic fibrosis transmembrane conductance regulator (CFTR)-green fluorescent protein (GFP) vector. Enhanced GFP (EGFP) gene was directly conjugated to the COOH terminus of CFTR in the vector.
Confocal microscopy.
NHBE cells were infected with pSicoR-CFTR-GFP lentivirus overnight at 37°C and cultured for 3 days. Cells were then fixed with 4% paraformaldehyde and incubated with anti-CFTR antibody (1:500, Ab570, courtesy of Dr. John Riodon, University of North Carolina) followed by Alexa Fluor 555-conjugated goat anti-mouse IgG (1:500, Invitrogen). Cells were examined by confocal microscopy (Leica TCS SP5; Leica Microsystem, Buffalo Grove, IL). Imaging analysis was performed using LAS AF (Leica).
Statistics.
Data are expressed as means ± SE of at least three samples. Statistical significance was determined by unpaired t-test. Difference (P value) <0.05 was considered significant.
RESULTS
Infecting cells with pSicoR-GFP lentivirus and quantifying cells by ELISA.
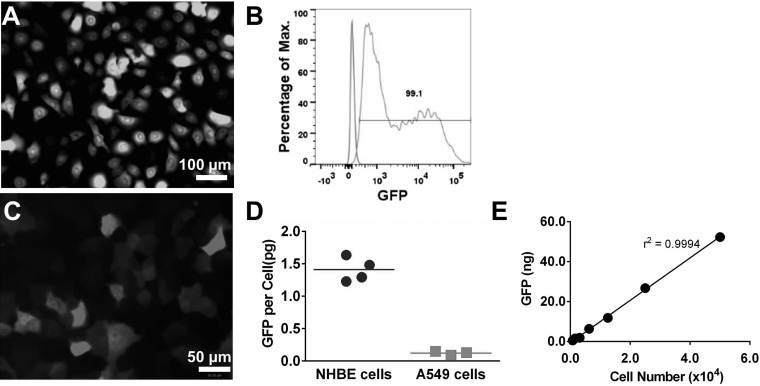
NHBE cells were infected with pSicoR-GFP lentivirus overnight. Three days after the infection, almost all cells expressed GFP, as examined by a fluorescence microscope and quantified by flow cytometry (Fig. 3, A and B). Similarly, the majority of A549 cells expressed GFP following the infection with pSicoR-GFP lentivirus (Fig. 3C) although the GFP intensity was much lower compared with that in NHBE cells. In agreement with the fluorescence images, the average GFP protein content per cell as determined by ELISA was 1.41 ± 0.09 pg for NHBE cells and 0.12 ± 0.02 pg for A549 cells following pSicoR-GFP lentivirus-mediated infection (Fig. 3D).
Fig. 3.
Labeling cells with GFP. A: normal human bronchial/tracheal epithelial (NHBE) cells were infected with pSicoR-GFP lentivirus overnight and examined after 3 days. B: flow cytometry analysis of NHBE cells infected by pSicoR-GFP lentivirus shows 99.1% GFP-positive cells. C: A549 cells were infected with pSicoR-GFP lentivirus overnight and examined after 3 days. D: average GFP mass per cell, 3 days after infection with pSicoR-GFP from at least 3 experiments. E: linear correlation between the amount of GFP and the number of GFP-labeled NHBE cells spiked into mouse lung tissue from 1 representative experiment.
To determine whether the number of cells engrafting into lungs can be estimated based on GFP quantification, various numbers of pSicoR-GFP lentivirus-infected NHBE cells were spiked into mouse lung tissue, and the GFP amount was determined. There was a linear correlation between the quantity of GFP and the number of cells that were spiked into mouse lung tissue (Fig. 3E). The slope was 1.06, indicating an average 1.06 pg GFP/cell in this particular experiment, and thus the number of cells was calculated by dividing total GFP quantity (in picograms) by a factor of 1.06. GFP has been routinely used as a reporter of gene expression; however, here we show that its quantity can be used to quantify cells in tissues.
Cell transplantation into mouse lungs.
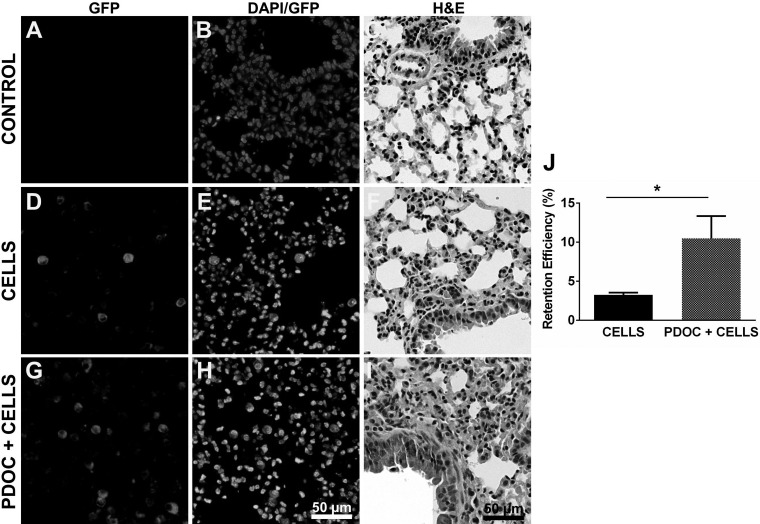
To reliably deliver cells into mouse lungs, we developed a modified intratracheal instillation method (Fig. 1) and directly injected 0.5 × 106 GFP-labeled NHBE cells in a 50-μl volume into the mouse lungs. Two days after cell transplantation, GFP+ cells were distributed in various regions of the lungs as shown by immunofluorescence staining (Fig. 5, D and E). There was no nonspecific staining in the control lungs (Figs. 5A and 6A). Using the ELISA quantification method described above, the average cell-retention efficiency (i.e., the fraction of cells delivered that were counted in the lungs) was estimated to be 3.26 ± 0.28% (n = 6) at 48 h after instillation (Fig. 5J), with little variation between different experiments. These findings indicate that nonprogenitor human airway epithelial cells can engraft in mouse lungs for at least 2 days. Moreover, our intratracheal instillation approach can generate relatively reproducible results for repeated studies in mice.
Fig. 5.
Cell retention in mouse lungs. 0.5 × 106 GFP-labeled NHBE cells were delivered to mouse lungs, and the retention of delivered cells was determined after 2 days. A–C: lung sections from control mice with no cell delivery. D–F: lung sections from animals with cell delivery only. G–I: lung sections from animals received cell delivery 24 h following pretreatment with 2% PDOC. Lung sections were immunostained with rabbit polyclonal antibody to GFP followed by Alexa Fluor 555-conjugated IgG (A, D, and G) and counterstained with DAPI (B, E, and H) or stained for H&E (C, F, and I). J: cell-retention efficiency was determined by GFP ELISA. CELLS, cell delivery only; PDOC+CELLS, cell delivery following lung pretreatment with PDOC. *P < 0.05.
Fig. 6.
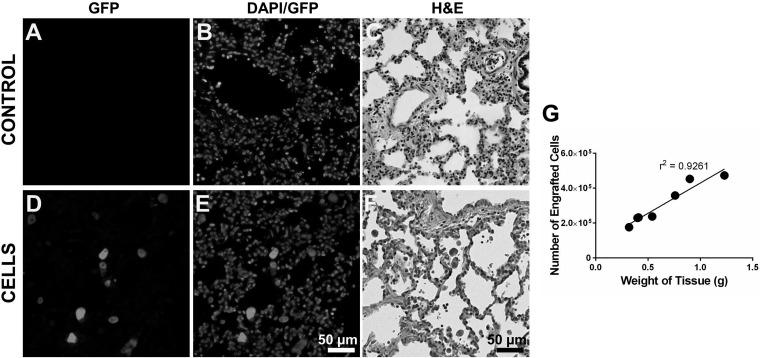
Retention of cells in pig lungs. 100 × 106 GFP-labeled A549 cells were delivered to 1 lobe of the pig lung, and the cell retention was determined after 24 h. A–C: lung sections from control pigs. D–F: lung sections from animals received cell delivery. Lung sections were immunostained with rabbit polyclonal antibody to GFP followed by Alexa Fluor 555-conjugated IgG (A and D) and counterstained with DAPI (B and E) or stained for H&E (C and F). G: number of engrafted cells in random sections of the pig lung of various weights.
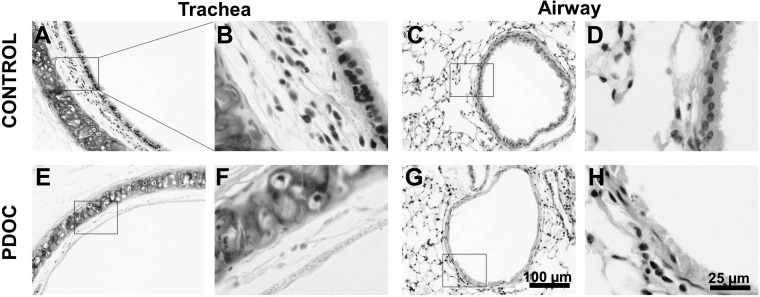
It has been reported that transient lung injury enhances cell retention in lungs (10, 31, 44, 54). We thus explored the effect of lung preinjury with PDOC on cell retention in mice. Twenty-four hours following intratracheal administration of 2% PDOC into mouse lungs, the removal of surface epithelial cells in both the trachea and the airways was observed (Fig. 4, E–H), similar to what has been previously reported (5, 31). GFP-labeled NHBE cells were then intratracheally delivered into mouse lungs. After 48 h, GFP+ cells were found in various sections of the lung tissues (Fig. 5, C and F), and a significant increase in cell-retention efficiency (10.48 ± 2.86%, n = 7) was achieved in the preinjured lungs (PDOC+ CELLS) compared with the nonpretreated lungs (CELLS, Fig. 5J). These findings indicate the augmenting effect of 2% PDOC on airway epithelial cell retention and are in good agreement with other previous studies (31).
Fig. 4.
Partial depletion of epithelium in mouse lungs by polidocanol (PDOC). A–D: hematoxylin and eosin (H&E) images of trachea (A and B) and airway (C and D) from animals without treatment (control). E–H: H&E images of trachea (E and F) and airway (G and H) from animals treated with 20 μl of 2% PDOC for 24 h.
Cell engraftment in pig lungs.
We next determined whether nonprogenitor lung epithelial cells can also engraft in porcine lungs through instillational transplantation. For this proof-of-concept study, A549 cells that can be easily obtained in large numbers were used. With the use of a tracheal intubation fiberscope, 100 × 106 GFP-labeled A549 cells were delivered into the lower right lobe of the pig lung in a 10-ml volume. Twenty-four hours after cell administration, the lung lobe was harvested and analyzed. Extensive distribution of GFP+ cells was found in various locations of the lung as determined by immunofluorescence staining for GFP (Fig. 6). A linear correlation between the number of cells engrafted (determined using the GFP ELISA quantification method) and the weight of lung tissue was observed (Fig. 6G), indicating nearly uniformed distribution of the delivered cells. There was an average 22.32% retention efficiency at 24 h following instillational cell delivery into the pig lungs.
Lentiviral vector-induced overexpression of CFTR-GFP.
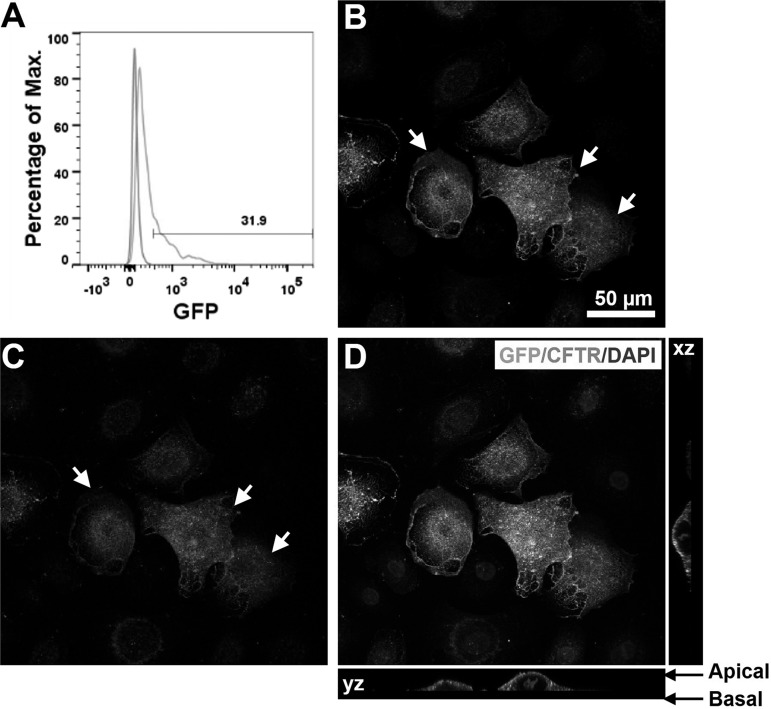
Persistent expression and correct apical localization of CFTR channels are required to restore normal function in differentiated lung epithelial cells from patients with CF (45, 62). We thus constructed a lentiviral vector, designated pSicoR-CFTR-GFP, in which the COOH terminus of CFTR was fused to GFP (Fig. 2). To determine whether pSicoR-CFTR-GFP lentivirus can be used to ex vivo correct the CFTR defect in lung epithelial cells, as a proof-of-concept study, we infected NHBE cells with the lentivirus overnight. Three days after the infection, at least 30% of the cells were found to express GFP (Fig. 7A). The relatively lower infection efficiency of pSicoR-CFTR-GFP lentivirus compared with pSicoR-GFP lentivirus is mostly due to the large size of the CFTR-GFP gene (5.2 kb) (29) and may be improved by increasing viral titration as described (13). In addition, sorting for GFP+ cells could potentially allow for production of a pure population of cells expressing CFTR-GFP. To determine the cellular localization of CFTR-GFP fusion proteins, pSicoR-CFTR-GFP lentivirus-infected NHBE cells were then stained using an antibody for CFTR (27) and examined using confocal microscopy. Stronger immunostaining for CFTR was observed in cells that also expressed enhanced GFP signals, indicating overexpression of CFTR-GFP proteins (Fig. 7, B–E). Furthermore, CFTR-GFP fusion proteins were dominantly localized at the apical surface of the cells (Fig. 7E), indicating that COOH-terminal tagging with GFP did not affect the correct trafficking of CFTR in NHBE cells.
Fig. 7.
Expression and localization of CFTR-GFP in NHBE cells. A: flow cytometry analysis of NHBE cells infected by pSicoR-CFTR-GFP lentivirus. B–E: confocal images of NHBE cells infected with pSicoR-CFTR-GFP lentivirus. B: GFP fluorescence. C: CFTR immunofluorescence. CFTR was detected with mouse monoclonal anti-CFTR followed by Alexa Fluor 555-conjugated IgG (red). D and E: overlay of GFP fluorescence, CFTR immunofluorescence, and DAPI staining. Arrows, overexpression of CFTR-GFP.
DISCUSSION
In this study, we showed the feasibility of labeling human lung epithelial cells with GFP and the convenience of using a GFP ELISA-based assay for evaluating cell retention in lungs. We developed a repeatable, instillational cell-delivery approach for mice and pigs and achieved robust initial cell engraftment in mouse and porcine lungs based on immunofluorescence staining and ELISA quantification. We also constructed a lentiviral vector for CFTR to induce the overexpression of CFTR-GFP proteins at the apical surface of human airway epithelial cells for future ex vivo gene therapy of cells with CFTR mutations.
Lentiviral-based vectors can transfect nondividing cells and integrate into the cell genome (39), making them attractive vectors to target airway epithelial cells for persistent gene expression (39). Here we showed efficient infection of NHBE cells and A549 cells with pSicoR-GFP lentivirus to induce the expression of GFP. GFP labeling, not only allowed us to directly detect and sort cells using fluorescence, but also provided a simple cell quantification method based on ELISA. Because of the linear correlation between GFP quantity and cell number, retention of exogenous GFP-labeled cells in lung tissues can be easily quantified, assuming that the average GFP per cell after engraftment in lung remained the same as before delivery. Although the lacZ reporter gene is also commonly used to label cells, unlike with GFP labeling, lacZ-labeled cells cannot be directly detected using fluorescence-activated cell sorting. In addition, the presence of endogenous β-galactosidase activity in lung tissue might cause inaccurate quantification of lacZ-expressed exogenous cells (56). On the other hand, GFP labeling for ELISA-based cell quantification did not require the donor-recipient sex mismatch as needed for PCR-based quantification used by others (10, 49). Although only NHBE cells and A549 cells have been tested in this study, and it is also possible that GFP signal from some nonviable cells (51) has been included for the estimation of cell retention, our results undoubtedly indicate that lentivirus-mediated GFP labeling is a simple and reliable method to allow the detection and quantification of exogenous cells in lungs.
The most direct route to deliver therapeutic reagents (such as cells and viruses) into the lungs is through the trachea (9, 25). The two intratracheal methods commonly used in rodents include tracheotomy and intubation (48). Although the intubation method has been used by many groups, it requires special equipment or techniques (4, 11). Here, we introduced a modified intratracheal delivery approach that combined the advantages of these aforementioned methods and showed robust cell engraftment in mouse lungs 2 days after the delivery of NHBE cells. There was little variation in cell retention efficiency between different animals and different experiments, suggesting the reproducibility of this technique for delivering cells into mouse lungs. In addition, we have achieved over 10% cell-retention efficiency in PDOC-preinjured mouse lungs using this approach. The removal of surface lung epithelial cells by PDOC might have allowed for more initial attachment of the delivered cells as shown by others (31). Although PDOC-induced injury has been shown to cause inflammation in lungs and increase the proliferation of lung epithelial cells in vivo (5), we observed little proliferation of GFP+ cells in PDOC-treated lungs (data not shown), suggesting that the threefold increase in cell retention might be mainly attributable to enhanced initial adhesion. Hence, our results suggest the feasibility of efficiently transplanting nonprogenitor (relatively differentiated) lung epithelial cells into the lungs of mice.
In our proof-of-concept studies, we delivered GFP-labeled human airway epithelial A549 cells into one of the lung lobes in a pig using an intubation fiberscope and observed extensive cell retention after 24 h with an average retention efficiency of over 20%. These data suggest that airway epithelial cells can be efficiently delivered into larger animal models, beyond rodent models, and tracheal intubation fiberscope provides a simple delivery approach that may be easily translated into clinical applications. It is worth noting, however, that A549 cells are relatively more resilient than NHBE cells; thus some nonadherent cells in the pig lungs might have also been counted toward the retention efficiency. We will apply whole lung lavage to remove nonadherent cells before harvest in future studies. Although delivery of viruses to pig airways has been reported by several groups (6, 50), this is the first time that human lung epithelial cells were delivered and retained in pig lungs. As in mouse models, pretreatment of pig lungs with PDOC might further improve cell attachment. Whereas efficient initial engraftment of delivered cells in lungs is critical, sustained retention and function of these cells is of clinically importance for treating lung diseases. Future studies therefore will be performed to determine the long-term outcome of cells in porcine lungs.
For cell-based therapy, the ideal cells would be of autologous origin to avoid immune rejection from cell transplantation and should be easily obtained and expanded in vitro to provide large quantities for sufficient cell replacement and possible therapeutic effects. Current development in generating clinical-grade human iPSCs (17, 61) and differentiating (patient-specific) iPSCs to lung epithelial cells (19–21, 33, 60) has suggested a new cell source for potentially treating lung diseases with cell transplantation. For CF-iPSC-derived lung epithelial cells, correction of the CFTR mutation is required before cell transplantation into lungs. We therefore constructed a lentivector pSicoR-CFTR-GFP and showed its efficacy in inducing the correct localization of the CFTR-GFP fusion protein at the apical surface of NHBE cells. In our preliminary studies, we found that bronchial epithelial cells from patients with CF (CFBE cells, Courtesy of Dr. M. Egan, Yale University) can be similarly infected with pSicoR-CFTR-GFP lentivector although the infection efficiency was lower for CFBE cells than for NHBE cells (data not shown). Future studies will need to focus on improving the infection efficiency of pSicoR-CFTR-GFP lentivirus and determining whether this lentivector can similarly infect CF-iPSCs-derived lung epithelial cells to ex vivo restore the CFTR defect. Alternatively, lung epithelial cells derived from CF iPSCs can be corrected with small molecules (43, 58) and labeled with GFP with lentivirus before delivery into lungs.
It is unclear exactly how many cells are required for lung engraftment to treat various diseases. Nevertheless, in vitro studies suggest that correction of only 6–10% of the CF-affected epithelial cells is sufficient to restore normal chloride channel function (24), suggesting that cell transplantation-based therapy could at least alleviate the symptoms of CF when efficient retention of cells with normal CFTR has been guaranteed. In addition, whether the airway epithelial cells should be fully differentiated before transplantation and the fate of the initially adhered exogenous cells in the lungs remain uncertain. Regardless of these concerns, our studies have shed light on the feasibility of delivering nonprogenitor relatively differentiated airway epithelial cells into the lungs for potential treatment of lung epithelial diseases.
GRANTS
This work is supported by National Institutes of Health U01 HL111016 and HL098220 (both to L. Niklason) and Connecticut Stem Cell Research 12-SCA-YALE-26 (to J. Zhou; Co-PI, L. Gui).
DISCLOSURES
L. Niklason has a financial interest in Humacyte, a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein. No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L. Gui and L.E.N. conception and design of research; L. Gui, H.Q., K.A.R., and L. Grecu performed experiments; L. Gui analyzed data; L. Gui, H.Q., K.A.R., and L. Grecu interpreted results of experiments; L. Gui prepared figures; L. Gui drafted manuscript; L. Gui and L.E.N. edited and revised manuscript; L. Gui, H.Q., K.A.R., L. Grecu, and L.E.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Drs. Wanda O'Neal and Scott Randell for the full-length human CFTR cDNA, Dr. Jiangbing Zhou for the pSicoR-GFP lentivector, Dr. Marie Egan for the human bronchial epithelial cells from patients with CF, and Dr. John Riodon for the CFTR antibody. We also thank Dr. Jiasheng Zhang, Mr. Liping Zhao, and Dr. Marlene Typaldos Sanchez for the excellent technical assistance in mouse surgery. We also thank Dr. Jing Zhou for support with confocal microscopy.
REFERENCES
- 1.Alton EW, Boyd AC, Cheng SH, Cunningham S, Davies JC, Gill DR, Griesenbach U, Higgins T, Hyde SC, Innes JA, Murray GD, Porteous DJ. A randomised, double-blind, placebo-controlled phase IIB clinical trial of repeated application of gene therapy in patients with cystic fibrosis. Thorax 68: 1075–1077, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Andrade CF, Wong AP, Waddell TK, Keshavjee S, Liu M. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol 292: L510–L518, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bals R, Hiemstra PS. Innate immunity in the lung: How epithelial cells fight against respiratory pathogens. Eur Respir J 23: 327–333, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bivas-Benita M, Zwier R, Junginger HE, Borchard G. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur J Pharm Biopharm 61: 214–218, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 24: 662–670, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Cao H, Machuca TN, Yeung JC, Wu J, Du K, Duan C, Hashimoto K, Linacre V, Coates AL, Leung K, Wang J, Yeger H, Cutz E, Liu M, Keshavjee S, Hu J. Efficient gene delivery to pig airway epithelia and submucosal glands using helper-dependent adenoviral vectors. Mol Ther Nucleic Acids 2: e127, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellani S, Conese M. Lentiviral vectors and cystic fibrosis gene therapy. Viruses 2: 395–412, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copreni E, Penzo M, Carrabino S, Conese M. Lentivirus-mediated gene transfer to the respiratory epithelium: a promising approach to gene therapy of cystic fibrosis. Gene Ther 11, Suppl 1: S67–S75, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Crisanti MC, Koutzaki SH, Mondrinos MJ, Lelkes PI, Finck CM. Novel methods for delivery of cell-based therapies. J Surg Res 146: 3–10, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchesneau P, Wong AP, Waddell TK. Optimization of targeted cell replacement therapy: A new approach for lung disease. Mol Ther 18: 1830–1836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc 4: 1064–1072, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger C, Cannet C, Gerard C, Jarman E, Jarai G, Feige A, Suply T, Micard A, Dunbar A, Tigani B, Beckmann N. Administration of bleomycin via the oropharyngeal aspiration route leads to sustained lung fibrosis in mice and rats as quantified by UTE-MRI and histology. PLoS One 8: e63432, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis BL, Potts PR, Porteus MH. Creating higher titer lentivirus with caffeine. Hum Gene Ther 22: 93–100, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelhardt JF, Zepeda M, Cohn JA, Yankaskas JR, Wilson JM. Expression of the cystic fibrosis gene in adult human lung. J Clin Invest 93: 737–749, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher JT, Zhang Y, Engelhardt JF. Comparative biology of cystic fibrosis animal models. Methods Mol Biol 742: 311–334, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flotte TR, Ng P, Dylla DE, McCray PB Jr, Wang G, Kolls JK, Hu J. Viral vector-mediated and cell-based therapies for treatment of cystic fibrosis. Mol Ther 15: 229–241, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci 85: 348–362, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia O, Carraro G, Navarro S, Bertoncello I, McQualter J, Driscoll B, Jesudason E, Warburton D. Cell-based therapies for lung disease. Br Med Bull 101: 147–161, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, Niklason LE. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest 123: 4950–4962, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaedi M, Mendez JJ, Bove PF, Sivarapatna A, Raredon MS, Niklason LE. Alveolar epithelial differentiation of human induced pluripotent stem cells in a rotating bioreactor. Biomaterials 35: 699–710, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green MD, Chen A, Nostro MC, d'Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol 29: 267–272, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyert DL, Xu J. Deaths: Preliminary data for 2011. Natl Vital Stat Rep 61: 1–52, 2012. [PubMed] [Google Scholar]
- 23.Jiang Q, Engelhardt JF. Cellular heterogeneity of CFTR expression and function in the lung: Implications for gene therapy of cystic fibrosis. Eur J Hum Genet 6: 12–31, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R, Boucher RC. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet 2: 21–25, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Kanaan SA, Kozower BD, Suda T, Daddi N, Tagawa T, Ritter JH, Mohanakumar T, Patterson GA. Intratracheal adenovirus-mediated gene transfer is optimal in experimental lung transplantation. J Thorac Cardiovasc Surg 124: 1130–1136, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 33: 328–334, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell 16: 2154–2167, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuang PP, Lucey E, Rishikof DC, Humphries DE, Bronsnick D, Goldstein RH. Engraftment of neonatal lung fibroblasts into the normal and elastase-injured lung. Am J Respir Cell Mol Biol 33: 371–377, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar M, Keller B, Makalou N, Sutton RE. Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther 12: 1893–1905, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Lau AN, Goodwin M, Kim CF, Weiss DJ. Stem cells and regenerative medicine in lung biology and diseases. Mol Ther 20: 1116–1130, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leblond AL, Naud P, Forest V, Gourden C, Sagan C, Romefort B, Mathieu E, Delorme B, Collin C, Pages JC, Sensebe L, Pitard B, Lemarchand P. Developing cell therapy techniques for respiratory disease: intratracheal delivery of genetically engineered stem cells in a murine model of airway injury. Hum Gene Ther 20: 1329–1343, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med 173: 171–179, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, Dowton AA, Serra M, Weiss DJ, Green MD, Snoeck HW, Ramirez MI, Kotton DN. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10: 398–411, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitomo K, Griesenbach U, Inoue M, Somerton L, Meng C, Akiba E, Tabata T, Ueda Y, Frankel GM, Farley R, Singh C, Chan M, Munkonge F, Brum A, Xenariou S, Escudero-Garcia S, Hasegawa M, Alton EW. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes. Mol Ther 18: 1173–1182, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitzner W, Brown R, Lee W. In vivo measurement of lung volumes in mice. Physiol Genomics 4: 215–221, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Moodley Y, Manuelpillai U, Weiss DJ. Cellular therapies for lung disease: A distant horizon. Respirology 16: 223–237, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, Rajagopal J. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 10: 385–397, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naldini L. Ex vivo gene transfer and correction for cell-based therapies. Nat Rev Genet 12: 301–315, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272: 263–267, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Oakland M, Sinn PL, McCray PB Jr. Advances in cell and gene-based therapies for cystic fibrosis lung disease. Mol Ther 20: 1108–1115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16: 927–933, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-engineered lungs for in vivo implantation. Science 329: 538–541, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pettit RS. Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment. Ann Pharmacother 46: 1065–1075, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Rejman J, Colombo C, Conese M. Engraftment of bone marrow-derived stem cells to the lung in a model of acute respiratory infection by Pseudomonas aeruginosa. Mol Ther 17: 1257–1265, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, McCann JD, Klinger KW, Smith AE, Welsh MJ. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 347: 358–363, 1990. [DOI] [PubMed] [Google Scholar]
- 46.Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB Jr, McLennan G, Meyerholz DK, Namati E, Ostedgaard LS, Prather RS, Sabater JR, Stoltz DA, Zabner J, Welsh MJ. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 295: L240–L263, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 33: 401–406, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev 58: 1030–1060, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 176: 1261–1268, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Sinn PL, Cooney AL, Oakland M, Dylla DE, Wallen TJ, Pezzulo AA, Chang EH, McCray PB Jr. Lentiviral vector gene transfer to porcine airways. Mol Ther Nucleic Acids 1: e56, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skillman LC, Sutherland IW, Jones MV, Goulsbra A. Green fluorescent protein as a novel species-specific marker in enteric dual-species biofilms. Microbiology 144: 2095–2101, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, Lafyatis R, Demierre MF, Weiss DJ, French DL, Gadue P, Murphy GJ, Mostoslavsky G, Kotton DN. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells 28: 1728–1740, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tate S, Elborn S. Progress towards gene therapy for cystic fibrosis. Expert Opin Drug Deliv 2: 269–280, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Wang D, Morales JE, Calame DG, Alcorn JL, Wetsel RA. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther 18: 625–634, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 8: 223–272, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss DJ, Liggitt D, Clark JG. In situ histochemical detection of beta-galactosidase activity in lung: assessment of X-Gal reagent in distinguishing lacZ gene expression and endogenous beta-galactosidase activity. Hum Gene Ther 8: 1545–1554, 1997. [DOI] [PubMed] [Google Scholar]
- 57.WHO. The Top 10 Causes Of Death (Online). http://www.who.int/mediacentre/factsheets/fs310/en/index.html [September 2, 2014]. [Google Scholar]
- 58.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30: 876–882, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong AP, Dutly AE, Sacher A, Lee H, Hwang DM, Liu M, Keshavjee S, Hu J, Waddell TK. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol 293: L740–L752, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Wong AP, Rossant J. Generation of lung epithelium from pluripotent stem cells. Curr Pathobiol Rep 1: 137–145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324: 797–801, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH, Dang YL, Vogel LN, McKay T, Mengos A, Boucher RC, Collins PL, Pickles RJ. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol 7: e1000155, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]