Abstract
The receptor tyrosine kinase human epidermal growth factor receptor-2 (HER2) is known to regulate pulmonary epithelial barrier function; however, the mechanisms behind this effect remain unidentified. We hypothesized that HER2 signaling alters the epithelial barrier through an interaction with the adherens junction (AJ) protein β-catenin, leading to dissolution of the AJ. In quiescent pulmonary epithelial cells, HER2 and β-catenin colocalized along the lateral intercellular junction. HER2 activation by the ligand neuregulin-1 was associated with tyrosine phosphorylation of β-catenin, dissociation of β-catenin from E-cadherin, and decreased E-cadherin-mediated cell adhesion. All effects were blocked with the HER2 inhibitor lapatinib. β-Catenin knockdown using shRNA significantly attenuated neuregulin-1-induced decreases in pulmonary epithelial resistance in vitro. Our data indicate that HER2 interacts with β-catenin, leading to dissolution of the AJ, decreased cell-cell adhesion, and disruption of the pulmonary epithelial barrier.
Keywords: human epidermal growth factor receptor-2, neuregulin-1, receptor tyrosine kinase, β-catenin, epithelial cell, permeability, adherens junction, cell adhesion
the pulmonary epithelium serves as the interface for the lung with the outside world and is responsible for numerous critical functions central to lung function. In the airways and alveoli, epithelial cells serve as a physical barrier against toxins and microorganisms and regulate solute and fluid flux. Numerous lung diseases are marked by injury to the pulmonary epithelium, leading to disruption of the epithelial barrier, and a rapid and efficient restoration of the epithelial barrier is essential to repair of the damaged lung (10, 46). However, understanding of the mechanisms that regulate cell-cell adhesion and epithelial barrier function in the lung is incomplete.
The epithelial cell barrier consists of epithelial cells joined by a belt-like apical junctional complex (AJC). The AJC is responsible for lateral adhesion between cells and acts as an obstacle to macromolecule diffusion through its major domains, tight junctions (TJ) and adherens junctions (AJ) (15, 29). The TJ acts as a gate, regulating solute flux, and a fence, preventing diffusion of proteins and lipids between the outer leaflet of the apical and basolateral plasma membrane domains. The AJ is essential for cell-cell adhesion. The AJ protein β-catenin connects the intracellular actin cytoskeleton to the transmembrane protein epithelial (E)-cadherin. E-cadherin, in turn, bridges extracellularly in trans with an E-cadherin molecule on an adjacent epithelial cell to establish cell-cell adhesion (22, 49). Thus β-catenin is a critical participant in maintaining cell-cell adhesion and has been linked to pulmonary epithelial barrier function (51).
Injury to the pulmonary epithelium by infection, inflammation, physical trauma (mechanical ventilation), inhaled particles (cigarette smoke), and allergic reactions (asthma) disrupts the AJ and epithelial barrier function. The net result is loss of cell-cell adhesion, increases in ion, molecule, and water permeability, epithelial cell apoptosis, necrosis, and sloughing followed by either regeneration of the epithelium or replacement with fibrous hyaline membranes. β-Catenin is central to the injury-repair process in the pulmonary epithelium, through both its structural capacity in regulating cell-cell adhesion and its signaling function, where it translocates to the nucleus and binds to the T cell factor (TCF) and lymphoid-enhancing factor 1 transcription factors to trigger gene transcription.
The tyrosine kinase receptor human epidermal growth factor receptor-2 (HER2) is expressed on the basolateral surface of airway and alveolar epithelial cells (44). A member of the epidermal growth factor receptor (EGFR or HER1) family of receptor tyrosine kinases (RTKs), HER2 is activated when the ligand neuregulin-1 (NRG-1) binds the receptor HER3, inducing HER2/3 heterodimerization, and activates the HER2 kinase domain, leading to HER2 autophosphorylation and initiation of subsequent HER2-dependent downstream signaling cascades (12). HER2 signaling has been demonstrated to participate in numerous cellular processes, including lung growth and development, cell proliferation, and migration (5, 33).
We have previously demonstrated that HER2 regulates pulmonary epithelial paracellular permeability in the context of inflammatory lung injury (11, 13, 14, 30). Although the mechanisms of HER2-mediated loss of epithelial barrier function remain elusive, existing evidence supports the notion that HER2 could signal through β-catenin to influence cell-cell adhesion; both HER2 and β-catenin localize to the basolateral membrane of cells, and β-catenin signaling is influenced by tyrosine phosphorylation, suggesting a possible interaction between these two signaling molecules.
We hypothesized that HER2 interacts with the AJ protein β-catenin in human pulmonary epithelial cells and that HER2 activation leads to AJ disruption, altered cell-cell adhesion, and pulmonary epithelial barrier function. We demonstrate that HER2 is physically associated with β-catenin at the AJC and that HER2 activation leads to β-catenin phosphorylation, β-catenin dissociation from E-cadherin, decreased E-cadherin-mediated cell-cell adhesion, and decreased transepithelial resistance (TER). In contrast, the ligand TGF-α induced HER2 and β-catenin phosphorylation, but the TGF-α effect on β-catenin was independent on HER2, indicating that the NRG-2 pathway is distinct from other signaling pathways activated by HER2.
MATERIALS AND METHODS
Reagents.
Antibodies targeting HER2 (C-18), phospho-HER2 (Tyr-1248), and GAPDH (6C5) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies directed against phospho-β-catenin (Tyr-654) (ab24925) and p-84 (ab487) were from Abcam (Cambridge, MA); β-catenin (no. 9562), E-cadherin (no. 3195), and Na,K-ATPase (no. 3010) were from Cell Signaling (Danvers, MA). The affinity-purified Alexa Fluor 488 donkey anti-rabbit (A21206) and Alexa Fluor 555 donkey anti-mouse (A31570) IgG were from Molecular Probes (Eugene, OR). Recombinant human NRG-1 and IL-1β were from R&D Systems (Minneapolis, MN) and Biosource (Camarillo, CA), respectively. Lapatinib ditosylate (GW-572016) was from Biovision (Milpitas, CA). Calcein-acetoxymethyl (AM) ester was from Life Technologies (Carlsbad, CA).
Cell culture and treatments.
NuLi-1 cells (ATCC, Manassas, VA), a nontransformed, human airway epithelial cell line (50), normal human bronchial epithelial (NHBE) cells, and primary human type II (ATII) and type I-like (ATI) alveolar epithelial cells (45) were maintained as previously described (13). A549 and NCI-H292 cells derived from a human lung adenocarcinoma were obtained from ATCC.
Western blotting.
Western blotting was performed as previously described (11, 30). Protein lysates for membrane and nuclear fractions were prepared using FractionPrep Cell Fractionation Kit per the manufacturer's instructions (Biovision).
Fluorescence and confocal microscopy.
Cells were transfected with a HER2 yellow fluorescent protein, cultured overnight in reduced serum condition (DMEM/F12 with 1% FBS), exposed to NRG-1 (20 mM) or vehicle with or without Lapatinib (2 μM, 30-min pretreatment), fixed in ice-cold methanol, washed with PBS, and blocked with 1% BSA prepared in PBS. Cells were then stained overnight with β-catenin primary antibody. The following day, cells were washed and incubated with Alexa Fluor 555-tagged secondary antibody. Immunofluorescence images were obtained using a LSM 700 confocal microscope.
Adherence assay.
Pulmonary epithelial cell adherence to recombinant E-cadherin Fc chimera-coated plates was measured as described previously (48). Briefly, the recombinant E-cadherin Fc chimera (R&D Systems) was incubated overnight in 96-well plates at 4°C. Calcein-AM-labeled cells were then added to each well. After 45 min to allow adherence, cells were treated with or without Lapatinib (2 μM, 30-min pretreatment), and then exposed to NRG-1 (20 nM); fluorescence was read at an excitation wavelength of 480 nm and an emission wavelength of 530 nm.
Electrical resistance measurement.
Electrical resistance was determined using an ohmmeter (World Precision Instruments, Sarasota, FL) as previously described (13, 44).
Generation of HER2, EGFR, and β-catenin-null cell line.
NuLi-1 cells were infected with a control, nontargeting (NT), or specific HER2, EGFR, or β-catenin-targeting short hairpin RNA (shRNA) in lentivirus (2 × 106 plaque-forming units) containing a puromycin resistance gene obtained from Functional Genomics Core, University of Colorado, Boulder, CO and selected with puromycin.
Statistical analysis.
Values are expressed as means ± SE. Groups were compared using ANOVA followed by Bonferroni's posttest. P < 0.05 was considered significant.
RESULTS
HER2 and β-catenin are physically associated in pulmonary epithelia.
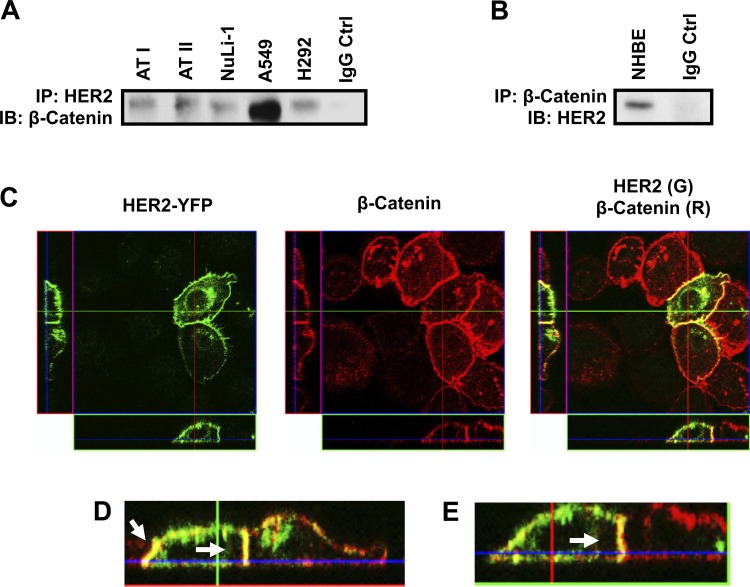
As they are both localized to the basolateral membrane in polarized epithelial cells, we sought to determine whether a physical association existed between HER2 and β-catenin. Immunoprecipitation of HER2 from primary NHBE as well as ATI and ATII epithelial cells followed by β-catenin immunoblotting revealed a HER2-β-catenin association under basal conditions (Fig. 1, A and B). This association was also observed in NuLi-1 cells, an immortalized, nontransformed airway epithelial cell line, as well as the lung adenocarcinoma epithelial cell lines A549 and H292, indicating that the HER2-β-catenin association is consistent across different types of pulmonary epithelial cells. Immunofluorescence imaging of NuLi-1 cells transfected with a HER2 yellow fluorescent protein plasmid and stained for β-catenin confirmed colocalization of HER2 and β-catenin along the lateral aspect of the cell-cell junction (Fig. 1, C–E) as well as some costaining at the cell surface.
Fig. 1.
Human epidermal growth factor receptor-2 (HER2) is associated with β-catenin in unstimulated pulmonary epithelial cells. Primary human alveolar type I and II (ATI and II), NuLi-1, A549, and H292 (A) and normal human bronchial epithelial (NHBE) cells (B) were grown at an air-fluid interface (ALI) and lysed, and HER2 was immunoprecipitated (IP) followed by Western immunoblotting (IB) performed for β-catenin. IgG served as a negative control. C: NuLi-1 cells were transfected with a HER2 yellow fluorescent protein (YFP) construct, and HER2-β-catenin was assessed by confocal microscopy for HER2 (green) and β-catenin (red). D and E: enlarged images of merged HER2-β-catenin staining (E, yellow). Arrows identify merged HER2-β-catenin staining along the basolateral membrane. n ≥ 3.
HER2 activation leads to β-catenin phosphorylation.
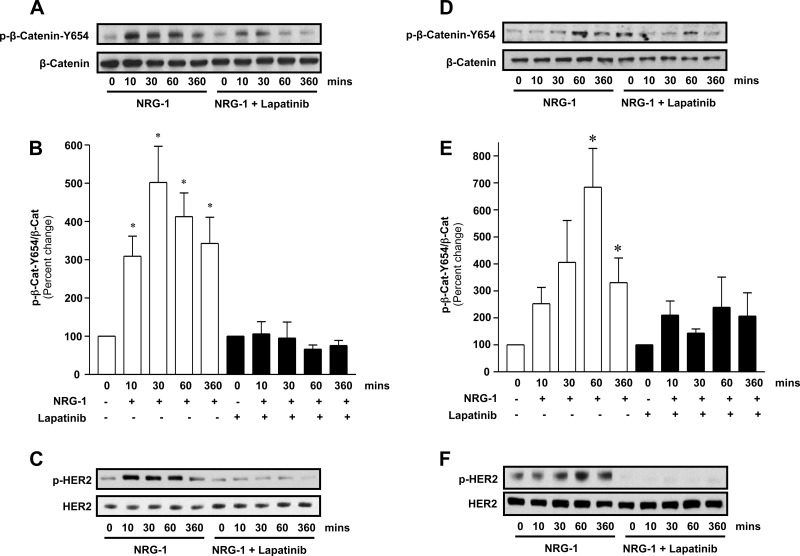
The physical association between the tyrosine kinase HER2 and β-catenin suggests a possible functional interaction. Tyrosine phosphorylation of β-catenin regulates function and integrity of the AJ (27, 28). As HER2 is a tyrosine kinase, we next determined whether HER2 activation led to tyrosine phosphorylation of β-catenin. In silico analysis of β-catenin using Phospho.ELM predicted that β-catenin tyrosine (Y)-654 is readily phosphorylated, and this site has been linked to cell-cell adhesion (34). Therefore, we sought to determine whether NRG-1-HER2 signaling led to Y-654 phosphorylation. As can be seen in Fig. 2, A and B, in NuLi-1 cells grown to confluence at air-liquid interface (ALI), NRG-1 led to a fivefold increase in Y-654 β-catenin phosphorylation (P < 0.05, NRG-1 vs. control 30 min), which was blocked by lapatinib, a small-molecule inhibitor that blocks the ATP binding site of the HER2 kinase (Fig. 2C), confirming a requirement for HER2 activation in β-catenin phosphorylation by NRG-1. This signaling was confirmed in primary NHBE cells grown to confluence at ALI. Similar to NuLi-1 cells, NRG-1 increased β-catenin Y-654 phosphorylation in NHBE cells almost sevenfold at 60 min (P < 0.01), which was blocked by lapatinib (Fig. 2, D–F). Although there were differences in the kinetics of β-catenin phosphorylation between NuLi-1 and NHBE cells, tyrosine phosphorylation peaked between 10 and 60 min and persisted for up to 6 h in both cell types.
Fig. 2.
HER2 activation results in Y-654 phosphorylation of β-catenin. A: Western blot for p-Y-654 β-catenin and total β-catenin from confluent NuLi-1 cells grown at ALI stimulated with neuregulin-1 (NRG-1) (20 nM) with and without lapatinib. B: densitometry of p-Y-654 β-catenin/total β-catenin relative to corresponding control in NuLi-1 cells. C: Western blot for p-HER2 and total HER2 from confluent NuLi-1 cells grown at ALI stimulated with NRG-1 (20 nM) with and without lapatinib. D: Western blot for p-Y-654 β-catenin and total β-catenin from confluent NHBE cells grown at ALI stimulated with NRG-1 (20 nM) with and without lapatinib. E: densitometry of p-Y-654 β-catenin/total β-catenin relative to corresponding control in NHBE cells. F: Western blot for p-HER2 and total HER2 from confluent NHBE cells grown at ALI stimulated with NRG-1 (20 nM) with and without lapatinib. Data are presented as means ± SE. *P < 0.05, experiments, n ≥ 3.
β-catenin-E-cadherin disassembly following HER2 activation.
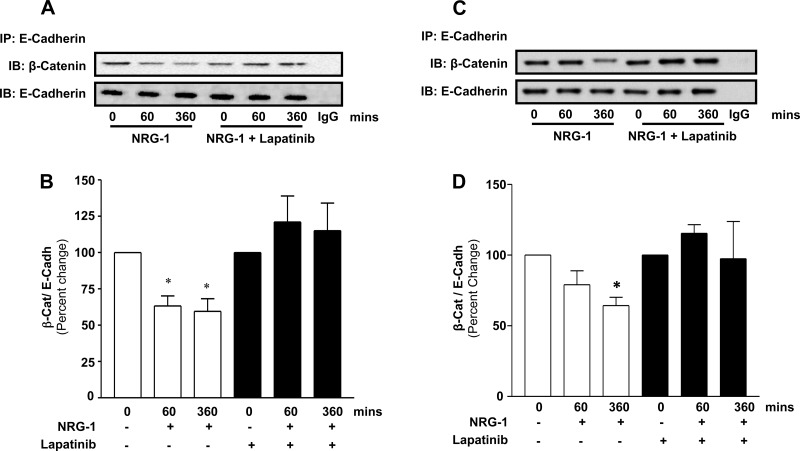
Phosphorylation of β-catenin at Y-654 has been reported to disrupt its association with E-cadherin and has been linked to disassembly of the AJ (34). We therefore next assessed the β-catenin-E-cadherin association following NRG-1-induced HER2 activation in NuLi-1 and NHBE cells. As expected, coimmunoprecipitation studies confirmed that E-cadherin and β-catenin were physically associated in NuLi-1 (Fig. 3A, time 0) and NHBE (Fig. 3C, time 0) cells at baseline. However, following HER2 activation with NRG-1, there was a significant decrease in E-cadherin-β-catenin association. Kinetic studies in NuLi-1 cells revealed that NRG-1 resulted in an early decrease in β-catenin-E-cadherin association evident at 10 min and 30 min that became significant at 60 min (35% decrease, P < 0.05) and 360 min (33% decrease, P < 0.05). Importantly, NRG-1-mediated dissociation was completely blocked with lapatinib. These findings were confirmed in NHBE cells exposed to NRG-1 with and without lapatinib. NRG-1 resulted in a HER2-dependent 36% decrease in β-catenin-E-cadherin association at 360 min in NHBE (P < 0.05).
Fig. 3.
HER2 activation is associated with β-catenin-E-cadherin dissociation. A: NuLi-1 cells grown at ALI were stimulated with NRG-1 (20 nM) with and without lapatinib, and E-cadherin immunoprecipitation followed by β-catenin and E-cadherin Western blotting was performed. B: densitometry of E-cadherin/β-catenin association by coimmunoprecipitation in NuLi-1 cells treated with and without lapatinib relative to corresponding control. C: NHBE cells grown at ALI were treated with NRG-1 and lapatinib followed by immunoprecipitation for E-cadherin and Western blotting for E-cadherin and β-catenin. D: densitometry of E-cadherin/β-catenin association by coimmunoprecipitation in NHBE cells treated with and without lapatinib relative to corresponding control. Data are presented as means ± SE. *P < 0.05, n ≥ 3.
HER2 decreases epithelial cell binding to E-cadherin.
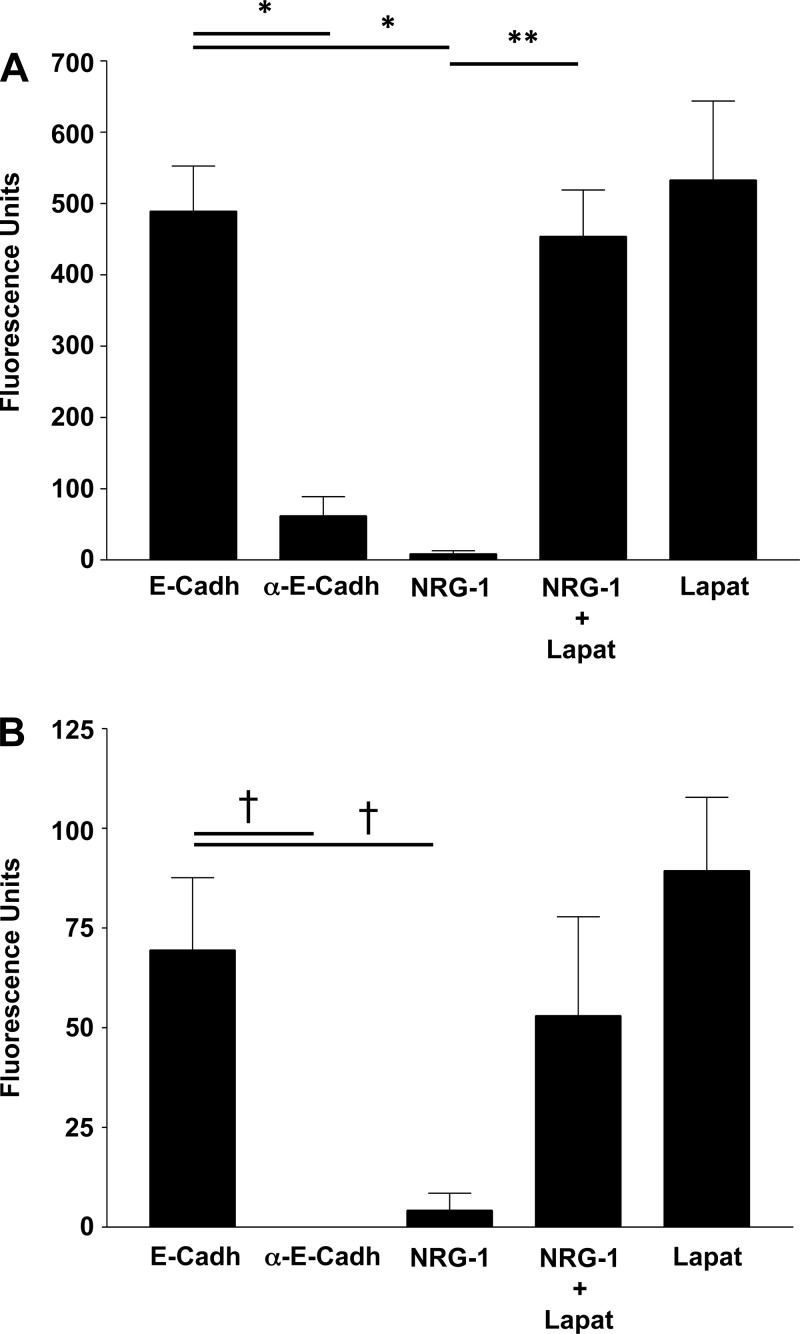
Epithelial cell-cell adhesion is maintained partially through homotypic interactions between extracellular E-cadherin domains of neighboring cells. β-Catenin regulates this connection by anchoring the intracellular domain of E-cadherin to the actin cytoskeleton. HER2-mediated tyrosine phosphorylation of β-catenin with dissociation of the E-cadherin-β-catenin complex suggests that HER2 activation would decrease epithelial cell interaction with a neighboring epithelial cell E-cadherin protein. To assess this, we directly measured E-cadherin-dependent cell adhesion using an E-cadherin-binding assay. NHBE or NuLi-1 cells were loaded with calcein-AM and seeded onto E-cadherin-coated plates. Treatment with an E-cadherin antibody, a positive control to confirm that binding was through E-cadherin and not through a nonspecific interaction with the plate, decreased epithelial cell binding (Fig. 4A). NRG-1 exposure significantly decreased E-cadherin-mediated cell adhesion at 2 h (P < 0.0001). In contrast, lapatinib prevented NRG-1-induced decreases in E-cadherin-mediated cell adhesion. Similarly, in NHBE, NRG-1 induced a 94% decrease in HER2-dependent E-cadherin-mediated adhesion (P < 0.004, Fig. 4B), confirming the importance of NRG-1-HER2 signaling in regulating AJ-mediated cell adhesion.
Fig. 4.
HER2 activation results in decreased E-cadherin-mediated pulmonary epithelial cell adherence. A: adherence of calcein-acetoxymethyl (AM)-loaded NuLi-1 cells seeded onto rh-E-cadherin-Fc chimera protein-bound plates. NuLi-1 cells were loaded with calcein and seeded onto plates coated with an rh-E-cadherin Fc. Cells were treated with NRG-1 with and without lapatinib and lapatinib alone. Use of an E-cadherin-blocking antibody served as a control to demonstrate binding of cells to the plate through E-cadherin. B: adherence of calcein-AM-loaded NHBE cells in the same fashion as NuLi-1 cells. Data are presented as means ± SE. *P < 0.0001, **P < 0.001, †P < 0.005, n > 3.
NRG-1-HER2-mediated increased TER is β-catenin dependent.
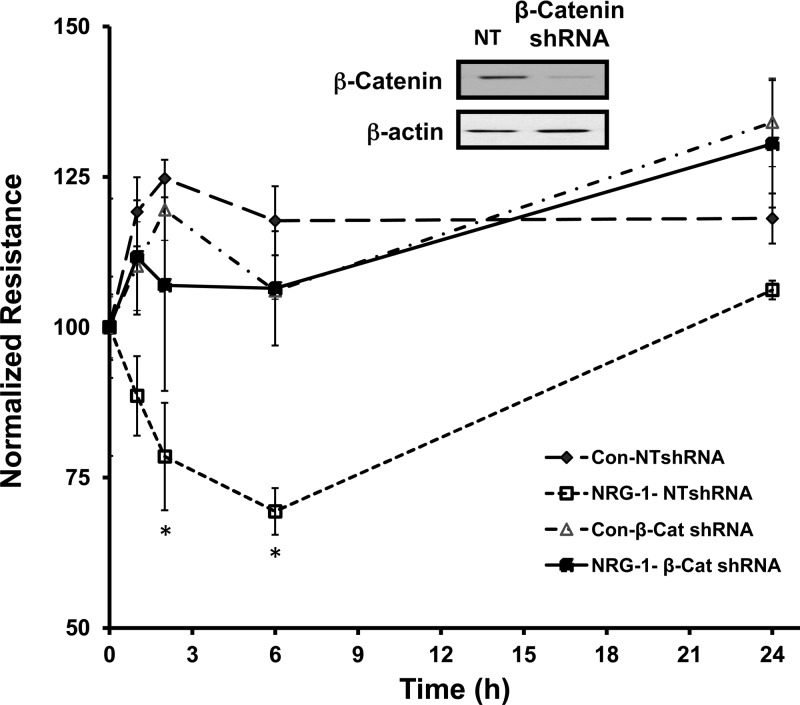
We have previously demonstrated that NRG-1 leads to HER2-dependent decreases in pulmonary epithelial resistance and increased large-molecule permeability (13). However, the mechanisms behind this are unknown. To assess whether the effect of HER2 on pulmonary barrier function might involve signaling through β-catenin, we generated a β-catenin-null NuLi-1 cell line using a β-catenin shRNA, which was compared with a cell line infected with a control, NT shRNA. Effective β-catenin knockdown was confirmed by Western blotting (Fig. 5, inset). β-Catenin-null and NT cell lines were then grown to confluence at ALI. Importantly, both β-catenin- and HER2-null cells achieved a baseline resistance of 1,000 Ohms. β-Catenin knockdown did not alter baseline resistance of the monolayer, a finding consistent with other reports (51). NRG-1 exposure induced a significant drop in TER in NT NuLi-1 cells with a 48% decrease in resistance compared with controls at 6 h (P < 0.05). However, in β-catenin-null cells, NRG-1 did not significantly alter TER at any time point (P = 0.18). These findings confirm that NRG-1-HER2-mediated changes in resistance are β-catenin dependent.
Fig. 5.
HER2-mediated changes in epithelial resistance require β-catenin. β-Catenin Western blotting in NuLi-1 cells transfected with a β-catenin shRNA or a nontargeting (NT) shRNA. Normalized resistance in NuLi-1 cells transfected with a β-catenin shRNA and a NT shRNA were grown to confluence at ALI and exposed to NRG-1. Data are presented as means ± SE. *P < 0.05, n ≥ 3.
HER2-dependent signaling is ligand specific.
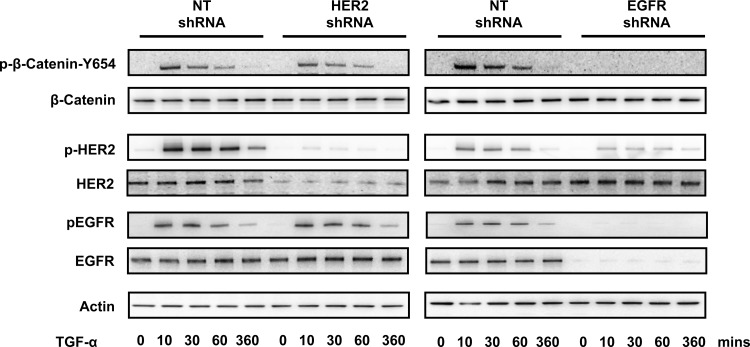
Previous reports have identified a physical association between the HER2/EGFR heterodimer and β-catenin phosphorylation after treatment with the EGFR ligand TGF-α in certain cancer cell lines (38). However, whether this occurs in nontransformed epithelial cells and whether TGF-α-mediated β-catenin phosphorylation requires HER2 have been unstudied (23, 31, 37, 38). To assess this, NuLi-1 cells transfected with a HER2- or EGFR-specific shRNA were exposed to TGF-α, and β-catenin Y-654 phosphorylation was measured by Western blot. TGF-α induced strong β-catenin, HER2, and EGFR phosphorylation in cells transfected with a NT shRNA (Fig. 6). In EGFR-depleted cells, there was a dramatic decrease in both HER2 and β-catenin phosphorylation. In contrast, TGF-α-induced β-catenin phosphorylation was maintained in HER2 knockdown cells, demonstrating that TGF-α signaling to β-catenin is through EGFR and independent of HER2. These findings indicate that, whereas NRG-1 and TGF-α can both activate HER2 and induce β-catenin phosphorylation, these ligands signal through distinct pathways, depending on the dimerization partner of HER2, namely HER3 vs. EGFR.
Fig. 6.
HER2-dependent signaling is ligand specific. NuLi-1 cells transfected with NT HER2 or EGFR shRNA were exposed to TGF (20 ng/ml), and Western blotting was performed for p-Y-654 β-catenin, total β-catenin, p-HER2, total HER2, p-EGFR, total EGFR, and actin. Representative blots are shown. n ≥ 3.
DISCUSSION
In this study, we identify mechanisms by which the RTK HER2 regulates cell-cell adhesion and pulmonary epithelial barrier function. We determined that HER2 is physically associated with the AJ protein β-catenin and that HER2 activation leads to β-catenin phosphorylation and disruption of β-catenin-E-cadherin-mediated cell-cell adhesion. We also confirmed that the effect of HER2 on pulmonary epithelial resistance is β-catenin dependent. Finally, we demonstrated that the interaction of HER2 with β-catenin is ligand specific with NRG-1 inducing HER2/3 dimerization and HER2-dependent phosphorylation of β-catenin, whereas TGF-α induces HER2/EGFR dimers and HER2-independent phosphorylation of β-catenin. The association of HER2 with β-catenin deepens our understanding of how these two molecules signal and has implications for numerous processes beyond cell adhesion, including cell migration, proliferation, and epithelial-to-mesenchymal transition, all of which are relevant to a range of pulmonary diseases, including asthma, the acute respiratory distress syndrome (ARDS), pulmonary fibrosis, and lung cancer.
We identify HER2 as a kinase that mediates critical tyrosine phosphorylation of β-catenin. Phosphorylation of β-catenin influences cell-cell adhesion and signaling in a site-specific manner. Serine/threonine phosphorylation of β-catenin leads to its degradation by the axin-adenomatous polyposis coli complex. In contrast, tyrosine phosphorylation leads to β-catenin dissociation from E-cadherin. Consistent with our findings, Y-654 phosphorylation has been associated with alteration of the COOH terminus of β-catenin, freeing it to bind the TCF transcription factor and increase gene transcription (9, 34). We identified that HER2 effects on barrier function require β-catenin (Fig. 5) and that HER2 alters β-catenin-E-cadherin interaction and E-cadherin-E-cadherin binding (Figs. 3–4). It is reasonable to suppose that these facts are related, but we do not definitely test this. Although it is very possible that these two events are independent of each other and that HER2 impacts cell adhesion through a process independent of E-cadherin, a role for β-catenin in regulating E-cadherin cell-cell adhesion is supported by existing literature. β-Catenin phosphorylation can be associated with a decrease in β-catenin-E-cadherin interaction and E-cadherin-mediated cell-cell adhesion (36, 47). Indeed Winter et al. (47) demonstrated that G protein receptors influence E-cadherin-mediated adhesion though a process dependent on β-catenin tyrosine phosphorylation. Although the exact mechanisms of how loss of β-catenin interaction with E-cadherin regulates its adhesive properties is unknown and not a focus of our study, it has been postulated that loss of catenin-cadherin binding leads to a conformational change in E-cadherin that alters the extracellular E-cadherin-E-cadherin bond (19). Alternatively, loss of β-catenin binding might allow for other intracellular proteins to bind E-cadherin and influence dimerization with E-cadherin on an adjacent cell. HER2 joins a relatively small list of kinases that phosphorylate β-catenin, including Src (36), BCR-Abl (8), Met (53), and TGF-β (24, 43). Although it is possible that the HER2-β-catenin association is part of a larger macromolecular complex or that an intermediary kinase, activated by HER2, is responsible for β-catenin phosphorylation after NRG-1 exposure or that HER2 activation results in the synthesis or secretion of Wnts, our findings that β-catenin and HER2 are physically associated suggest that HER2 interacts with β-catenin in a functional manner.
HER2 can enhance as well as attenuate pulmonary epithelial barrier function, and our findings linking HER2 and β-catenin provide new insights into the mechanisms behind HER2-mediated barrier regulation. The proinflammatory cytokine IL-1β induces NRG-1 shedding and HER2 activation in both airway and alveolar epithelia, and both NRG-1 and HER2 (but not EGFR) are required for IL-1β-mediated changes in epithelial barrier function (13). Similarly, NRG-1 activates HER2 in cooperation with HER3 and increases epithelial barrier disruption (13). In contrast, using a scratch model, Vermeer and colleagues (44) demonstrated that NRG-1-HER2 signaling potentiated wound closure in injured pulmonary epithelial cells. Whether HER2 is a barrier-disruptive factor vs. barrier-protective factor possibly depends on the state of the epithelium. Our data demonstrate that, in quiescent cells, HER2 activation leads to mobilization of the AJ and subsequent loss of cell-cell adhesion and barrier function. In injured cells, HER2 activation promotes cell migration and reforms epithelial contacts, yet whether this process involves β-catenin remains unknown. However, the role of β-catenin in reformation of a competent monolayer in pulmonary epithelia after neutrophil exposure (51) combined with our current findings suggest that β-catenin could be involved in the barrier-disruptive as well as barrier-restorative functions of HER2.
The classic paradigm of epithelial barrier function posits that the TJ is largely responsible for paracellular resistance (40) although this concept is evolving and AJ proteins are now recognized to influence both cell-cell adhesion and epithelial barrier function. E-cadherin-mediated cell-cell adhesion is required for establishment of a competent TJ (6, 16, 18). Loss of E-cadherin results in decreased zonnula occludens-1 expression and decreased epithelial resistance in pulmonary airway epithelial cells (20). In vivo, conditional depletion of epidermal E-cadherin resulted in aberrant localization of TJ proteins, decreased resistance, and increased permeability to water (42). Our data linking β-catenin to epithelial barrier function are consistent with other findings that identify β-catenin as a regulator of barrier function. Zemans et al. (51) have demonstrated that β-catenin is required to establish the epithelial barrier after neutrophil transmigration, attributable, at least in part, to β-catenin-dependent transcription of Cyr61 and Wnt-inducible signaling pathway protein 1 (52). Whether other mechanisms are involved in HER2 regulation of epithelial resistance, such as changes in calcium flux or alterations of TJ proteins, is unknown. However, our data indicate that HER2-mediated changes in barrier function require an interaction with β-catenin.
Alterations in cell-cell adhesion and epithelial barrier function have been linked to a profibrotic response (1, 2, 7, 39), and, perhaps not surprisingly, both HER2 and β-catenin signaling have been tied to models of pulmonary fibrosis. In a 21-day, murine bleomycin-induced pulmonary fibrosis model, we observed alveolar shedding of NRG-1 as well as persistent activation of pulmonary HER2 (30). Fibrosis was attenuated using a pharmacological HER2 inhibitor in wild-type mice (11) and in mice deficient in ATII HER2 signaling (30). Similarly, β-catenin signaling modulates the fibrotic response. TGF-β induces Y-654 phosphorylation of β-catenin, which then interacts with Smad2 to promote epithelial-to-mesenchymal transition in pulmonary epithelial cells (43). Several in vivo studies support β-catenin signaling, particularly phosphorylated Y-654 β-catenin and its role as a transcription factor, as profibrotic in the lung (3, 4, 21, 25, 26, 43). In contrast, Tanjore and colleagues (41) have recently demonstrated that ATII cell deletion of the β-catenin gene promotes fibrosis in a murine bleomycin model. Although the exact reasons for the opposing findings between these studies are unknown, one postulated mechanism is that a critical amount of β-catenin signaling is protective in an injured lung, whereas exaggerated β-catenin signaling exacerbates the fibrogenic response. Whether the profibrotic effect of HER2 activation involves β-catenin is currently unknown.
A HER2-β-catenin association has been identified during cancer pathogenesis, particularly in concert with EGFR heterodimerization or HER2 overexpression, leading to homodimerization. However, how this relates to NRG-1-HER2/3 signaling in nontransformed pulmonary epithelia, where EGFR is not activated (13), is not clear. HER family signaling is a multifaceted system governed by factors, such as dimerization partner, cell surface receptor density, cell type, and specific ligand. Although HER2 activation, β-catenin phosphorylation, and HER2-β-catenin association after administration of the EGFR ligand TGF-α have been observed in gastric cancer cells and breast cancer cell lines, whether β-catenin phosphorylation was HER2 dependent was not specifically investigated (23, 31, 37, 38). Although the HER2 blocker trastuzumab inhibited both HER2 activation and β-catenin phosphorylation in a breast cancer cell line (54), an effect on cell-cell adhesion was not investigated. Our data demonstrate that HER2 is not required for EGFR-mediated β-catenin phosphorylation in nontransformed pulmonary epithelial cells after TGF-α exposure. That TGF-α and NRG-1 can both result in HER2 phosphorylation with potentially different HER2-dependent effects on downstream signaling is notable but consistent with previous reports that different HER family heterodimer pairs (e.g., HER2/3 vs. HER2/EGFR) elicit different intracellular signaling and biological effects (35). For example, activated EGFR associates with and phosphorylates αCbl and Grb2 only when dimerized with HER2 but not when dimerized with other HER family receptors (17, 32). Additionally, although several ligands can bind and activate numerous HER receptors, each ligand elicits specific intracellular responses depending on cell type (e.g., transformed vs. nontransformed) and the availability of other HER receptors. In cells devoid of HER2, NRG-1 can induce EGFR/HER3 dimerization and activation. However, when HER2 is present, NRG-1 preferentially induces HER2/3 heterodimers to the exclusion of EGFR that results in activation of distinct downstream signaling events (13, 17). Additionally, the dimerization partner affects duration of receptor activation, further indicating that cell contexts, i.e., cell type, tissue, dimerization partner, and ligand, all influence the effect of HER family signaling. Our findings demonstrate that, unlike HER family ligands, NRG-1 influences β-catenin through HER2/3 and independent of EGFR in nontransformed pulmonary epithelial cells.
Although treatment strategies against HER2 are currently used in certain cancers, our data suggest that HER2 might serve as a therapeutic target in conditions marked by disruption of pulmonary epithelial cell-cell adhesion and increased epithelial permeability in nontransformed pulmonary epithelial cells. That NRG-1-HER2 signaling alters barrier disruption via β-catenin and that this can be blocked by the small-molecule inhibitor lapatinib might have translation potential. We have previously demonstrated that, in patients with ARDS, a disease marked by altered epithelial barrier function, the NRG-1-HER2/3 pathway is activated in the lung, suggesting that lapatinib might be beneficial in these patents, although further preclinical studies are needed.
GRANTS
This work was funded by NIH HL111674 (J. Finigan), Flight Attendants Medical Research Initiative (FAMRI) 113038 (J. Finigan), NIH P50 CA058187 (J. Finigan), and NIH HL106112.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.H.F. and J.A.K. conception and design of research; J.H.F., V.T.V., R.M., M.A.S., and R.J.M. analyzed data; J.H.F., V.T.V., J.V.T., R.J.M., and J.A.K. interpreted results of experiments; J.H.F., V.T.V., J.V.T., and R.M. prepared figures; J.H.F. drafted manuscript; J.H.F. and J.A.K. edited and revised manuscript; J.H.F., V.T.V., J.V.T., R.M., M.A.S., R.J.M., and J.A.K. approved final version of manuscript; V.T.V., J.V.T., R.M., and M.A.S. performed experiments.
ACKNOWLEDGMENTS
The authors acknowledge the Career Development Program of the University of Colorado Lung Cancer SPORE. The authors thank the National Jewish Primary Cell Core and Hong Wei Chu for expertise in primary airway epithelial cell isolation and culture to make these studies possible.
REFERENCES
- 1.Adamson IY, Hedgecock C, Bowden DH. Epithelial cell-fibroblast interactions in lung injury and repair. Am J Pathol 137: 385–392, 1990. [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson IY, Young L, Bowden DH. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol 130: 377–383, 1988. [PMC free article] [PubMed] [Google Scholar]
- 3.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun 3: 735, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aumiller V, Balsara N, Wilhelm J, Gunther A, Konigshoff M. WNT/beta-catenin signaling induces IL-1beta expression by alveolar epithelial cells in pulmonary fibrosis. Am J Respir Cell Mol Biol 49: 96–104, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Beckers J, Herrmann F, Rieger S, Drobyshev AL, Horsch M, Hrabé de Angelis M, Seliger B. Identification and validation of novel ERBB2 (HER2, NEU) targets including genes involved in angiogenesis. Int J Cancer 114: 590–597, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Behrens J, Birchmeier W, Goodman SL, Imhof BA. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol 101: 1307–1315, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitterman PB. Pathogenesis of fibrosis in acute lung injury. Am J Med 92: 39S–43S, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Coluccia AML, Vacca A, Dunach M, Mologni L, Redaelli S, Bustos VH, Benati D, Pinna LA, Gambacorti-Passerini C. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J 26: 1456–1466, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox RT, Pai LM, Kirkpatrick C, Stein J, Peifer M. Roles of the C terminus of armadillo in wingless signaling in drosophila. Genetics 153: 319–332, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298: L715–L731, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faress JA, Nethery DE, Kern EF, Eisenberg R, Jacono FJ, Allen CL, Kern JA. Bleomycin-induced pulmonary fibrosis is attenuated by a monoclonal antibody targeting HER2. J Appl Physiol 103: 2077–2083, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Finigan JH, Downey GP, Kern JA. HER receptor signaling in acute lung injury. Am J Respir Cell Mol Biol 47: 395–404, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finigan JH, Faress JA, Wilkinson E, Mishra RS, Nethery DE, Wyler D, Shatat M, Ware LB, Matthay MA, Mason R, Silver RF, Kern JA. Neuregulin-1-human epidermal receptor-2 signaling is a central regulator of pulmonary epithelial permeability and acute lung injury. J Biol Chem 286: 10660–10670, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finigan JH, Mishra R, Vasu VT, Silveira LJ, Nethery DE, Standiford TJ, Burnham EL, Moss M, Kern JA. BAL neuregulin-1 is elevated in acute lung injury and correlates with inflammation. Eur Respir J 41: 396–401, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 81: 1–44, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK). J Membr Biol 86: 113–125, 1985. [DOI] [PubMed] [Google Scholar]
- 17.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J 16: 1647–1655, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: Identification of a uvomorulin-like polypeptide. J Cell Biol 102: 457–468, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6: 622–634, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Heijink IH, Brandenburg SM, Noordhoek JA, Postma DS, Slebos DJ, van Oosterhout AJM. Characterisation of cell adhesion in airway epithelial cell types using electric cell-substrate impedance sensing. Eur Respir J 35: 894–903, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Henderson WR Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA 107: 14309–14314, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 105: 391–402, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Kanai Y, Ochiai A, Shibata T, Oyama T, Ushijima S, Akimoto S, Hirohashi S. c-erbB-2 gene product directly associates with beta-catenin and plakoglobin. Biochem Biophys Res Commun 208: 1067–1072, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, Chapman HA. Integrin α3β1-dependent β-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol 184: 309–322, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Gunther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119: 772–787, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, Budinger GR, Feghali-Bostwick CA, Varga J, Gottardi CJ. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol 45: 915–922, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr Opin Cell Biol 17: 459–465, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Lilien J, Balsamo J, Arregui C, Xu G. Turn-off, drop-out: Functional state switching of cadherins. Dev Dyn 224: 18–29, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Nagafuchi A. Molecular architecture of adherens junctions. Curr Opin Cell Biol 13: 600–603, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Nethery DE, Moore BB, Minowada G, Carroll J, Faress JA, Kern JA. Expression of mutant human epidermal receptor 3 attenuates lung fibrosis and improves survival in mice. J Appl Physiol 99: 298–307, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Ochiai A, Akimoto S, Kanai Y, Shibata T, Oyama T, Hirohashi S. c-erbB-2 gene product associates with catenins in human cancer cells. Biochem Biophys Res Commun 205: 73–78, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Olayioye MA, Graus-Porta D, Beerli RR, Rohrer J, Gay B, Hynes NE. ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol Cell Biol 18: 5042–5051, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel NV, Acarregui MJ, Snyder JM, Klein JM, Sliwkowski MX, Kern JA. Neuregulin-1 and human epidermal growth factor receptors 2 and 3 play a role in human lung development in vitro. Am J Respir Cell Mol Biol 22: 432–440, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Piedra J, Martiýnez D, Castaño J, Miravet S, Duñach M, de Herreros AG. Regulation of β-catenin structure and activity by tyrosine phosphorylation. J Biol Chem 276: 20436–20443, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Riese DJ 2nd, van Raaij TM, Plowman GD, Andrews GC, Stern DF. The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol Cell Biol 15: 5770–5776, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem 274: 36734–36740, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Schroeder JA, Adriance MC, McConnell EJ, Thompson MC, Pockaj B, Gendler SJ. ErbB-beta-catenin complexes are associated with human infiltrating ductal breast and murine mammary tumor virus (MMTV)-Wnt-1 and MMTV-c-Neu transgenic carcinomas. J Biol Chem 277: 22692–22698, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Shomori K, Ochiai A, Akimoto S, Ino Y, Shudo K, Ito H, Hirohashi S. Tyrosine-phosphorylation of the 12th armadillo-repeat of beta-catenin is associated with cadherin dysfunction in human cancer. Int J Oncol 35: 517–524, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ, Simon RH. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med 181: 254–263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 20: 142–149, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Tanjore H, Degryse AL, Crossno PF, Xu XC, McConaha ME, Jones BR, Polosukhin VV, Bryant AJ, Cheng DS, Newcomb DC, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. beta-Catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am J Respir Crit Care Med 187: 630–639, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J 24: 1146–1156, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulsamer A, Wei Y, Kim KK, Tan K, Wheeler S, Xi Y, Thies RS, Chapman HA. Axin pathway activity regulates in vivo pY654-beta-catenin accumulation and pulmonary fibrosis. J Biol Chem 287: 5164–5172, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422: 322–326, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol 36: 661–668, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Winter MC, Shasby S, Shasby DM. Compromised E-cadherin adhesion and epithelial barrier function with activation of G protein-coupled receptors is rescued by Y-to-F mutations in β-catenin. Am J Physiol Lung Cell Mol Physiol 294: L442–L448, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Winter MC, Shasby SS, Ries DR, Shasby DM. PAR2 activation interrupts E-cadherin adhesion and compromises the airway epithelial barrier: Protective effect of β-agonists. Am J Physiol Lung Cell Mol Physiol 291: L628–L635, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Xu W, Kimelman D. Mechanistic insights from structural studies of beta-catenin and its binding partners. J Cell Sci 120: 3337–3344, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, Welsh M, Klingelhutz AJ. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am J Physiol Lung Cell Mol Physiol 284: L844–L854, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, Yang IV, De Langhe S, Reynolds SD, Mason RJ, Kahn M, Henson PM, Colgan SP, Downey GP. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci USA 108: 15990–15995, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zemans RL, McClendon J, Aschner Y, Briones N, Young SK, Lau LF, Kahn M, Downey GP. Role of β-catenin-regulated CCN matricellular proteins in epithelial repair after inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 304: L415–L427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng G, Apte U, Micsenyi A, Bell A, Monga SPS. Tyrosine residues 654 and 670 in β-catenin are crucial in regulation of Met-β-catenin interactions. Exp Cell Res 312: 3620–3630, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu H, Zhang G, Wang Y, Xu N, He S, Zhang W, Chen M, Liu M, Quan L, Bai J, Xu N. Inhibition of ErbB2 by Herceptin reduces survivin expression via the ErbB2-β-catenin/T cell factor (TCF)4-survivin pathway in ErbB2-overexpressed breast cancer cells. Cancer Sci 101: 1156–1162, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]