Abstract
Chlorine is a toxic gas used in a variety of industrial processes and is considered a chemical threat agent. High-level chlorine exposure causes acute lung injury, but the long-term effects of acute chlorine exposure are unclear. Here we characterized chronic pulmonary changes following acute chlorine exposure in mice. A/J mice were exposed to 240 parts per million-hour chlorine or sham-exposed to air. Chlorine inhalation caused sloughing of bronchial epithelium 1 day after chlorine exposure, which was repaired with restoration of a pseudostratified epithelium by day 7. The repaired epithelium contained an abnormal distribution of epithelial cells containing clusters of club or ciliated cells rather than the uniformly interspersed pattern of these cells in unexposed mice. Although the damaged epithelium in A/J mice was repaired rapidly, and minimal airway fibrosis was observed, chlorine-exposed mice developed pneumonitis characterized by infiltration of alveoli with neutrophils and prominent, large, foamy macrophages. Levels of CXCL1/KC, CXCL5/LPS-induced CXC chemokine, granulocyte colony-stimulating factor, and VEGF in bronchoalveolar (BAL) fluid from chlorine-exposed mice showed steadily increasing trends over time. BAL protein levels were increased on day 4 and remained elevated out to day 28. The number of bacteria cultured from lungs of chlorine-exposed mice 4 wk after exposure was not increased compared with sham-exposed mice, indicating that the observed pneumonitis was not driven by bacterial infection of the lung. The results indicate that acute chlorine exposure may cause chronic abnormalities in the lungs despite rapid repair of injured epithelium.
Keywords: pneumonitis, lung repair, foamy macrophage, cytokines
chlorine is a widely used industrial compound employed in water purification and bleaching operations and in the production of plastics and a variety of other chemicals. Chlorine is highly toxic when inhaled, producing irritation and injury to the respiratory tract. Human exposure leading to lung injury can occur through accidental release in industrial and household settings (48). Multiple train derailments leading to chlorine release and human casualties from chlorine inhalation have occurred (20, 46, 47). In addition, chlorine has been used as a war gas and is still considered a chemical threat agent, as large numbers of casualties could result if large amounts of the gas were released in an urban area (15).
Inhalation of chlorine produces an oxidative damage to the lung and at high enough levels results in acute lung injury characterized by the death of epithelial cells lining the respiratory tract, epithelial/endothelial barrier disruption, pulmonary edema, hypoxemia, and pneumonitis (48). Because of its highly reactive nature, chlorine preferentially damages the airways and typically produces alveolar injury only at higher doses. Chlorine inhalation can also produce delayed or chronic effects on the lung. In humans, many exposed individuals make a full recovery following chlorine-induced acute lung injury, whereas others develop chronic respiratory symptoms, dyspnea, and impaired pulmonary function (11, 24, 28, 37, 43). Development of this constellation of pulmonary abnormalities following an acute exposure to a high dose of a respiratory irritant has been termed reactive airways dysfunction syndrome or acute irritant-induced asthma (5, 28). The underlying processes by which chronic disease develops following recovery from exposure to chlorine or other irritant gases are largely unknown although some studies have provided evidence for long-lasting structural changes to the airways (3, 11, 43).
Studies in animal models have provided information on the repair of the respiratory tract following chlorine injury (10, 29–31, 45, 49). Repair of the pseudostratified airway epithelium is carried out by basal epithelial cells (29, 31, 49). Inadequate repair or chronic inflammation after chlorine exposure is associated with the development of airway fibrosis (29, 31, 49). We previously described differences in the efficiency of epithelial repair and susceptibility to chlorine-induced airway fibrosis in inbred mouse strains (29). After chlorine exposure, FVB/NJ mice showed inefficient repair of the pseudostratified epithelium of the larger airways, and these mice developed pronounced fibrosis of the distal trachea, mainstem bronchi, and lobar bronchi. In contrast, A/J mice had rapid epithelial repair and did not develop significant fibrosis. A/J mice had abundant basal cells in larger airways that mediated epithelial repair, whereas these cells were scarce in FVB/NJ mice at sites where fibrosis developed. In FVB/NJ mice, fibrosis developed rapidly and was progressive, which precluded study of long-term recovery in these animals. In the present study, recovery of the lungs after chlorine exposure in A/J mice was examined for up to 8 wk. The results show that, although epithelial injury was repaired quickly, multiple pulmonary abnormalities, including abnormal distribution of airway epithelial cells, pneumonitis, increased cytokine production, and plasma protein leakage, persisted for at least 8 wk after chlorine exposure.
MATERIALS AND METHODS
Mice and chlorine exposure.
All experiments involving animals were approved by the University of Louisville Institutional Animal Care and Use Committee and were performed in accordance with the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals (19). Mice were housed under specific pathogen-free barrier conditions. A/J, Confetti [Gt(ROSA)26Sortm1(CAG−Brainbow2.1)Cle/J; stock no. 013731] and cre transgenic [Gt(ROSA)26Sortm1(cre/ERT2)Tyj/J; stock no. 008463] mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Male A/J mice 8 wk of age were housed for 1–2 wk and were then randomly assigned to chlorine-exposed, sham-exposed, or unexposed groups. Chlorine exposure was performed as described in our previous publications (17, 18, 44). Mice were exposed to a target dose of 240 parts per million (ppm)-h chlorine in air (240 ppm for 1 h) followed by a 10-min period of airflow to purge the chamber before opening. The mean deviation between the target dose and the actual values was 2.7%. Sham exposure was performed using the same procedure except that the mice were exposed to air instead of chlorine.
Lung histology.
Mouse lung tissues were collected at days 7, 14, 28, and 56 after chlorine exposure and fixed by inflation with 10% neutral-buffered formalin at a pressure of 25 cm H2O. During embedding, the left lung and the inferior lobe of the right lung were oriented to produce cross sections of the main lobar bronchi, and the superior and middle lobes of the right lung were sectioned longitudinally. Sections were cut at a thickness of 5 μm and stained with hematoxylin and eosin.
Immunofluorescent staining.
To observe the repair of bronchial epithelium after chlorine exposure, immunofluorescent staining for antigens specific for airway epithelial cell types was performed. Scgb1a1 was used as a marker for club (Clara) cells, acetylated tubulin (AcTub) was used as a marker for ciliated cells, and keratin 5 (K5) was used as a marker for basal cells. Staining was performed as described previously (29) using the following primary antibodies: goat antibody to Scgb1a1 (1:1,000; kind gift of Dr. Gurmukh Singh, VA Medical Center, Pittsburgh, PA); mouse antibody to AcTub (1:20,000; cat. no. T7451; Sigma-Aldrich, St. Louis, MO); and rabbit antibody to K5 (1:1,000; cat. no. PRB-160P; Covance, Princeton, NJ). Secondary antibodies labeled with Alexa Fluor dyes were purchased from Invitrogen Life Technologies (Grand Island, NY) and used at a dilution of 1:500. Slides were mounted with Prolong Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole dihydrochloride (Invitrogen Life Technologies). Labeled sections were viewed by epifluorescence or by laser-scanning confocal microscopy.
Clonal analysis of repaired airway epithelium was performed by breeding Confetti mice containing cre-activatable fluorescent protein genes with transgenic mice expressing cre from a ubiquitous promoter. Confetti/cre mice were treated with tamoxifen (5 mg/mouse ip, every 3–4 days for a total of 5 injections), exposed to chlorine, and analyzed 7 days after chlorine exposure. Lungs were inflated with 1 ml of PBS containing 1% paraformaldehyde and 1% low-melting-point agarose. After 5 min at room temperature, the lungs were excised and immersed in PBS containing 1% paraformaldehyde at 4°C. After 4 h, the solution was changed to PBS containing 30% sucrose, and the tissues were placed at 4°C overnight. The lungs were frozen in optimal cutting temperature compound and stored at −80°C until 10-μm frozen sections were cut. Sections were immunostained for Scgb1a1 and AcTub as described above and then imaged for the immunolabeled proteins and activated fluorescent proteins. Analysis was restricted to activation of the tdimer2(12) red fluorescent protein, as this reporter provided the most robust signal. The green, yellow, and blue fluorescent proteins faded rapidly, so these signals were removed by photobleaching before imaging immunostained Scgb1a1 labeled with Alexa Fluor 350 or AcTub labeled with Alexa Fluor 488. The cre induction procedure labeled only a small fraction (<0.5%) of lung epithelial cells with tdimer2(12), so clusters of adjacent labeled cells could be assumed to be clonally derived.
Morphometric analysis.
The expression of Scgb1a1 and AcTub in the epithelium of lobar bronchi and the volume fraction of lung parenchyma with chronic inflammation in the A/J mice were quantified by morphometric analysis. Images containing the entire circumference of cross sections of lobar bronchi from the left lung were digitally captured and analyzed using Image J (http://imagej.nih.gov/ij/) to determine the total area of Scgb1a1 or AcTub immunostaining normalized to the perimeter of the airway lumen. The volume fraction of lung parenchyma involved with chronic inflammation was measured by overlaying a grid of points over images captured from sections stained with hematoxylin and eosin, counting points over inflamed areas of lung parenchyma, and dividing by the total number of points over lung parenchyma. The parenchyma was defined as lung tissue (including air spaces) not containing airways or blood vessels.
BAL and BAL fluid analysis.
Bronchoalveolar lavage (BAL) was performed on days 4, 7, 14, and 28 after chlorine exposure as described previously (44). The protein concentration in BAL fluid was measured by Bradford method using Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). The levels of cytokines/chemokines in BAL fluid were measured using MILLIPLEX MAP Mouse Cytokine/Chemokine kit (Millipore, St. Charles, MO) by which 32 mouse cytokines and chemokines can be quantified simultaneously based on the Luminex xMAP technology. Cells in BAL fluid were counted using a hemacytometer, and cell differential was assessed following staining of cytospin slides with Diff-Quik (Dade Behring, Newark, DE). Macrophages with enlarged and engorged cytoplasm and with a foamy and vacuolated appearance were counted as foamy macrophages.
Bacterial culture and species identification.
Mouse lung tissue was collected at day 28 after chlorine exposure, and the whole lung was homogenized for bacterial culture using aseptic technique. Lungs were homogenized in 1 ml sterile PBS, and the homogenate was sieved using tissue filters. Aliquots (25 μl) of the homogenates were plated on Luria-Bertani (LB) agar, Brucella agar, brain-heart infusion agar, heart infusion agar, tryptic soy agar supplemented with 5% defibrinated sheep blood, or blood agar no. 2 plus 5% defibrinated sheep blood. LB and Brucella plates were grown for 48 h at 37°C at ambient CO2, whereas the other plates were grown at 37°C with 5% CO2. To recover any intracellular microbes, aliquots of the homogenates were brought to a concentration of 1% Triton X-100 before plating separate cultures. Following enumeration of colonies with different morphologies, chromosomal DNA was prepared for amplification of 16S ribosomal DNA. Sequencing of amplified DNA was used to identify bacterial species. Data are presented for culture on LB medium in air without Triton X-100, but similar results were obtained for cultures under the other conditions.
Data analysis.
Data from bacterial culture experiments were analyzed by Kruskal-Wallis test. Other data were analyzed using ANOVA with Bonferroni correction for multiple comparisons (GraphPad Prism; GraphPad Software, La Jolla, CA). If necessary, transformation of data was used to achieve normally distributed data before ANOVA analysis. The criterion for statistical significance was set at P < 0.05.
RESULTS
Repair of injured airway epithelium in A/J mice by basal cells.
Inhalation of a dose of 240 ppm-h chlorine in mice produces a severe injury to larger airways and the sloughing of tracheal and bronchial epithelium. This results in the loss of virtually all club and ciliated cells, which is evident 1 day after chlorine exposure. We showed previously that A/J mice exhibited rapid repair of tracheal and bronchial epithelium, which appeared to be carried out by surviving airway epithelial basal cells with restoration of a pseudostratified epithelium by 7 days after exposure (29). Here we provide additional evidence for the growth and differentiation of basal cells during repair of airway epithelium after chlorine injury by examining transitional stages of differentiation on days 5 and 6 after chlorine exposure. Basal cells in uninjured lungs are small, pyramidal-shaped cells that reside close to the basement membrane and are not exposed to the luminal epithelial surface. During repair after chlorine injury, cells expressing the basal cell marker K5 initially spread and form squamous cells covering the basement membrane. Subsequently, strong K5 staining is observed in cuboidal proliferating cells with both basal and luminal locations by day 4 after chlorine exposure (29). Dual immunofluorescent staining for K5 in conjunction with Scgb1a1 or AcTub was used to probe for the presence of transitional cells expressing both basal and club or ciliated cell markers (Fig. 1). In unexposed mice, Scgb1a1 and AcTub staining was observed in cells having luminal locations, with K5 staining present in underlying basal cells (Fig. 1, A and B). Day 5 after chlorine exposure was the first time at which cells staining for Scgb1a1 or AcTub could be observed in regenerating epithelium, and these were all in luminal locations expected for club and ciliated cells (Fig. 1, C and 1D). At this stage, K5 staining was observed in cells with both basal and luminal locations; some luminal K5-stained cells appeared to have faint Scgb1a1 or AcTub staining. At day 6 after chlorine exposure, Scgb1a1 staining was stronger and was present in cells with the typical shape and location of club cells (Fig. 1E). Some luminal cells with typical club cell morphology clearly showed colocalization of K5 and Scgb1a1, suggestive of a transitional stage of differentiation between basal progenitor and club cells. Luminal cells that appeared to be stained with both K5 and AcTub were also observed at this time (Fig. 1F). At day 7 after chlorine exposure, injured airways were typically repaired to a pseudostratified epithelium with abundant club and ciliated cells (29), but some areas with lagging repair, as evidenced by residual luminal K5 staining, were observed (Fig. 1, G and H). In these areas, luminal K5-stained cells with apparent colocalization with Scgb1a1 or AcTub were still observed. These observations provide additional evidence supporting basal cells as progenitor cells that carry out epithelial repair after chlorine injury.
Fig. 1.
Immunofluorescent staining for epithelial markers showing transitional stages during repair of chlorine injury. Lung sections from chlorine-exposed and unexposed A/J mice were stained for the basal cell marker keratin 5 (K5) in conjunction with the club cell marker Scgb1a1 (A, C, E, or G) or the ciliated cell marker acetylated tubulin (AcTub) (B, D, F, and H). Staining was performed in sections from unexposed mice (A and B) or from mice 5 days (C and D), 6 days (E and F), or 7 days (G and H) after exposure. Images were captured by confocal microscopy. Arrows indicate cells with both K5 and either Scgb1a1 or AcTub staining in the same cell. Arrowheads indicate examples of lagging repair at day 7, where K5 staining is still observed in luminal locations. E shows example of strong K5 staining that occurs in some areas during the reparative stage after chlorine exposure (29). Scale bar in H represents 20 μm for all panels.
Airway epithelial structure after extended recovery.
Airway epithelial structure was examined after longer periods of recovery to determine whether there were any long-term abnormalities that persisted. Epithelium of lobar bronchi from unexposed mice showed a normal pseudostratified epithelium (Fig. 2, A and F). At day 7 following chlorine exposure, the injured epithelium was repaired, but areas with alternating thick and thin epithelial zones could be observed (Fig. 2B). These gradually resolved, and, by 56 days after exposure, the airway epithelium appeared normal by hematoxylin and eosin staining (Fig. 2, C–E). To garner a more detailed assessment of epithelial structure during this time, immunostaining for Scgb1a1 and AcTub was performed. Simultaneous labeling for both antigens revealed that, in the epithelium from unexposed mice, club and ciliated cells were uniformly interspersed (Fig. 3, A and G). In contrast, many areas of the epithelium 7 days after exposure contained clusters of cells containing only ciliated (Fig. 3B) or club (Fig. 3C) cells. The clustered distribution of club and ciliated cells was more prominent at day 7 after chlorine exposure, with a trend toward reversion to a normal distribution over time (Fig. 3, D–F). However, even at day 56 after exposure, some abnormal clustering of cells was still observed (Fig. 3F). Morphometric analysis was used to assess the total amount of Scgb1a1 and AcTub staining (Fig. 4). At day 7 after exposure, no difference in the total amount of Scgb1a1 or AcTub staining was observed. This indicated that the altered grouping of cells did not affect the overall amount of staining; hence areas with more ciliated cells appeared to be counterbalanced by other areas with more club cells. This analysis showed a trend of increased Scgb1a1 and AcTub staining from day 7 to day 28, but, by day 56 after exposure, these were returning toward normal values.
Fig. 2.
Time course of histology in lobar bronchi of chlorine-exposed mice. A/J mice were exposed to chlorine, and histological analysis was performed on lungs collected 7 days (B), 14 days (C), 28 days (D), and 56 days (E) after exposure. To control for possible age effects, lungs from unexposed mice the same ages as day 7 chlorine-exposed (A) and day 56 chlorine-exposed (F) were analyzed. Lung sections were stained with hematoxylin and eosin. As early as 7 days after chlorine exposure, the injured epithelium was repaired, and the airway was lined with a pseudostratified epithelium. Scale bar in F represents 40 μm for all panels.
Fig. 3.
Dual immunofluorescence staining for Scgb1a1 and AcTub in lobar bronchi after chlorine exposure. A/J mice were exposed to chlorine, and dual immunofluorescent staining was performed on sections from lungs collected from mice 7 days (B and C), 14 days (D), 28 days (E), and 56 days (F) days after exposure. Lungs from unexposed mice the same ages as day 7 chlorine-exposed mice (A) and day 56 chlorine-exposed mice (G) were analyzed as controls. Arrows in B show areas with clustered ciliated cells, and arrowheads in C show areas with clustered club cells. Repaired airways continued to display an abnormal distribution of club and ciliated cells up to 56 days after chlorine exposure. Scale bar in G represents 40 μm for all panels.
Fig. 4.
Analysis of Scgb1a1 and AcTub staining. The area of Scgb1a1 (A) and AcTub (B) staining in images of the bronchial epithelium from the left lung and the inferior lobe of the right lung was measured and was normalized to airway lumen perimeter. Values are means ± SE for n = 8–13 mice per group; aP < 0.001 vs. unexposed (unexp).
One mechanism that could produce the observed cell clustering would be if contiguous groups of club and ciliated cells were clonally derived from surviving basal cells whose progeny became committed to the same cell type. This mechanism would predict that clonally derived cell groups similar in the size to the contiguous groups of cells would be observed and that these would contain only club or only ciliated cells. To investigate this, we performed an analysis of clonally derived cells using Confetti mice (41). These mice contain fluorescent protein transgenes whose expression can be stochastically activated via tamoxifen-inducible cre production to perform clonal analysis. Confetti/cre mice were treated with tamoxifen and exposed to chlorine, and then lungs were collected for analysis 7 days after exposure; unexposed Confetti/cre mice were treated in parallel (Fig. 5). The airway epithelium of unexposed Confetti/cre mice treated with tamoxifen contained single cells that were fluorescently labeled, indicative of the low turnover and proliferation rate in uninjured lung epithelium (Fig. 5A). In the airway epithelium of chlorine-exposed mice, clusters of labeled cells were commonly seen, reflecting clonal expansion of surviving basal cells during epithelial repair (Fig. 5, B and C). These clusters were small, usually consisting of section profiles containing 2–8 cells, and no clones as large as the typical contiguous groups of club or ciliated cells seen following epithelial repair were observed. Simultaneous immunostaining for Scgb1a1 and AcTub revealed the presence of clones containing club cells without ciliated cells (7% of clones), ciliated cells without club cells (29%), or mixed clones containing both cell types (63%). The results indicate that the contiguous clusters of club and ciliated cells observed after repair of chlorine-induced epithelial injury are not clonally derived.
Fig. 5.
Clonal analysis in repairing epithelium after chlorine injury. Confetti/cre mice were treated with tamoxifen to activate stochastic expression of fluorescent proteins and then were exposed to chlorine. Unexposed Confetti/cre mice that received the same tamoxifen treatment were used as controls. 7 days after exposure, frozen lung sections were prepared and immunostained for Scgb1a1 and AcTub. Sections were imaged for red fluorescent protein (RFP) to visualize clusters of clonally derived cells. A: 1 RFP-positive ciliated cell in a section from an unexposed mouse. B: RFP-labeled clonal cluster of club cells in a lobar bronchus from a mouse 7 days after chlorine exposure. C: RFP-labeled clonal cluster containing both ciliated and club cells in a lobar bronchus from a mouse 7 days after chlorine exposure. Arrows indicate locations of RFP-labeled cell clusters. Dashed lines are drawn along the basement membrane so the location of the epithelial cell layer can be visualized. Scale bar in C represents 20 μm for all panels.
Chronic inflammation following recovery from airway injury.
Although a pseudostratified bronchial epithelium was reestablished by 7 days after chlorine exposure, chronic inflammation developed with focal infiltration of macrophages and polymorphonuclear leukocytes (PMNs) into alveolar spaces and peribronchial or peribronchiolar areas (Fig. 6). Infiltrating macrophages were enlarged with a vacuolated cytoplasm and appeared similar to what have been termed foamy macrophages (25). Some areas of pneumonitis contained primarily or exclusively macrophages (Fig. 6, C and D), whereas others showed a mixed infiltrate with PMNs (Fig. 6, E and F). In some mice, granulomatous lesions (Fig. 6G) and bronchiolization of alveoli (Fig. 6H) were also observed. Pneumonitis was observed in most animals examined at 7, 14, 28, and 56 days after exposure, but this was not observed in unexposed or sham-exposed mice. There appeared to be a tendency for increased involvement of multiple lobes with time after exposure. Morphometric analysis of inflammation in the right lower lobe, which was the lobe most affected, showed increasing involvement through 28 days but no further increase at 56 days (Fig. 7).
Fig. 6.
Chronic pneumonitis in chlorine-exposed mice. Lung tissue sections from A/J mice were stained with hematoxylin and eosin. A: lung tissue from an unexposed mouse. B: lung tissue from a sham-exposed mouse 28 days after exposure. C and D: lung tissue from a chlorine-exposed mouse 56 days after exposure, showing abundant enlarged and foamy macrophages (arrows) in the alveolar spaces. E and F: lung tissue from chlorine-exposed mice 14 days (E) and 56 days (F) after exposure, showing numerous macrophages (arrows) and polymorphonuclear leukocyte infiltration (arrowheads) in peribronchial and alveolar areas. G: lung tissue from a chlorine-exposed mouse 56 days after exposure showing a granulomatous lesion. H: lung tissue from a chlorine-exposed mouse 28 days after exposure, showing thickened alveolar septa and bronchiolization of the alveoli (arrow). Bar in A represents 40 μm for all left panels; bar in B represents 40 μm for all right panels.
Fig. 7.
Extent of pneumonitis in chlorine-exposed mice. A/J mice were exposed to chlorine, and lung tissue was collected 7, 14, 28, and 56 days after exposure for histological examination. Both sham-exposed and unexposed mice were used as controls. The volume fraction of inflamed lung parenchyma in the right lower lobe was measured at different times after exposure. Values are means ± SE for n = 5–13 mice per group; aP < 0.05 vs. sham.
Lavage fluid parameters.
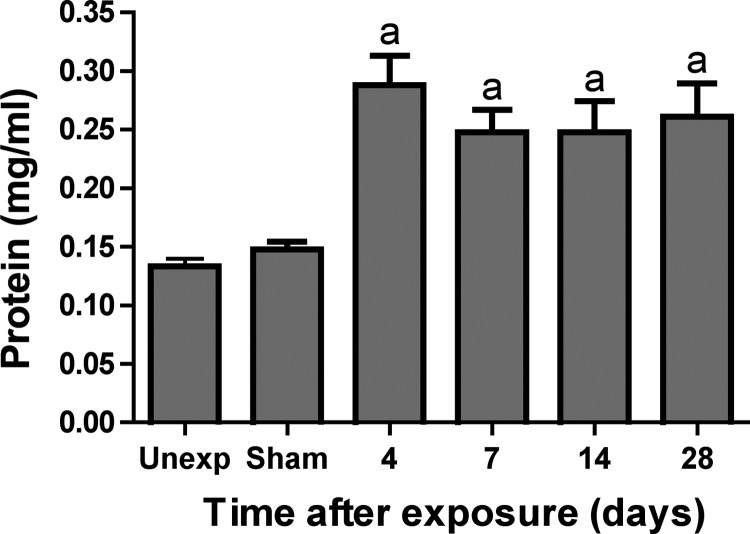
BAL was performed in mice at different times after chlorine exposure to measure inflammatory cells and soluble mediators of inflammation. The total number of macrophages did not differ between chlorine-exposed and unexposed mice 14 or 28 days after exposure. Foamy macrophages were only observed in significant numbers in chlorine-exposed groups and were present in similar numbers at both time points (Fig. 8A). We showed previously that the number of BAL neutrophils in chlorine-exposed A/J mice peaked at day 4 after exposure and began to decline by day 7 although they remained significantly higher than in unexposed mice (29). BAL neutrophils continued to be elevated in chlorine-exposed mice 14 and 28 days after exposure (Fig. 8B), which was consistent with the presence of PMNs observed histologically at these times. BAL protein content, indicative of injury and loss of epithelial-endothelial barrier function, was increased at day 4 and remained elevated at substantially similar levels through day 28 (Fig. 9). This result suggests the existence of a sustained chlorine-induced injury that is not repaired over the course of 28 days following exposure. A panel of 32 cytokines was measured in BAL fluid recovered from chlorine-exposed mice 4, 7, 14, and 28 days after exposure. Most of the analytes [eotaxin, granulocyte-macrophage colony-stimulating factor, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IP-10, leukemia inhibitory factor, monocyte chemoattractant protein-1, macrophage colony-stimulating factor, monokine induced by IFN, macrophage inflammatory protein (MIP)-1α, MIP-1β, MIP-2, RANTES, and TNF-α] did not change significantly at these times following chlorine exposure (not shown). Levels of CXCL1/KC, CXCL5/LPS-induced CXC chemokine, granulocyte colony-stimulating factor (G-CSF), and VEGF all steadily increased over time to 28 days in concert with a sustained pattern of inflammation and injury after chlorine exposure (Fig. 10).
Fig. 8.
Bronchoalveolar lavage (BAL) macrophages (A) and neutrophils (B) in chlorine-exposed mice. A/J mice were exposed to chlorine, and cells were counted in BAL fluid collected from mice 14 and 28 days after exposure. Values are means ± SE for n = 5–7 mice per group; aP < 0.01 vs. sham.
Fig. 9.
BAL protein in chlorine-exposed mice. A/J mice were exposed to chlorine, and total protein was measured in BAL fluid collected from mice 4, 7, 14, and 28 days after exposure. Values are means ± SE for n = 5–12 mice per group; aP < 0.01 vs. sham.
Fig. 10.
BAL cytokines in chlorine-exposed mice. A/J mice were exposed to chlorine, and 32 cytokines were measured in BAL fluid collected from mice 4, 7, 14, and 28 days after exposure. Results are shown for the 4 cytokines that were significantly increased after chlorine exposure. Values are means ± SE for n = 5–12 mice per group; aP < 0.01 vs. sham. LIX, LPS-induced CXC chemokine; G-CSF, granulocyte colony-stimulating factor.
Bacterial burden in the lungs of chlorine-exposed mice.
The high dose of chlorine used in this model produces substantial injury to airway epithelium and temporarily disrupts the barrier that normally protects the respiratory tract. Thus we considered it possible that colonization of the lung by opportunistic microorganisms might produce the sustained inflammation that was observed. To address this possibility, lungs were removed from chlorine-exposed mice and homogenized using aseptic technique, and the homogenates were cultured to detect the presence of bacteria in the lungs. Four distinct bacterial colony morphologies were observed, and these were identified as Staphylococcus xylosus, Lactobacillus johnsonii, Klebsiella oxytoca, and Enterobacter sp. through sequencing of PCR-amplified 16S rDNA sequences. No significant differences were observed in overall or species-specific bacterial numbers between chlorine-exposed and sham-exposed mice, indicating that the chronic inflammation observed following chlorine exposure was not caused by increased bacterial colonization of the lungs (Fig. 11).
Fig. 11.
Bacteria cultured from chlorine-exposed mice. Lungs were collected from chlorine- or sham-exposed mice 28 days after exposure and homogenized. The number of bacterial colonies of different types that could be cultured from the homogenates is shown. Ir, iridescent (Klebsiella oxytoca); sm, small (Staphylococcus xylosus); pin, pinpoint (Lactobacillus johnsonii); tran, translucent (Enterobacter sp.). Horizontal lines indicate median values; error bars indicate interquartile ranges. No significant differences between chlorine- and sham-exposed groups were detected.
DISCUSSION
Chlorine is considered a chemical threat agent that could be intentionally released to produce acute lung injury. Chlorine is also a widely used industrial chemical, and accidental releases of chlorine have resulted in human exposures and lung injury. Many victims of chlorine inhalation, even those requiring hospitalization for lung injury, recover normal lung function (20). However, other individuals exposed to a single, high dose of chlorine appear to develop chronic respiratory symptoms and functional impairment known as acute irritant-induced asthma (11, 27, 28, 43). Long-term pulmonary function abnormalities that have been observed following chlorine exposure include decreased FEV1 (1, 11, 27), increased airway resistance (22), airway hyperreactivity (3, 11, 27, 28, 43), and decreased residual volumes (37). In addition to producing chronic effects on pulmonary function, chlorine exposure may also cause long-lasting inflammation (11, 24, 38) and structural changes in the lungs, including epithelial desquamation and airway fibrosis (3, 11, 24, 38). The long-term consequences of chlorine exposure are likely governed by multiple factors, such as severity and duration of exposure, genetic susceptibility, preexisting pulmonary disease, and exposures to other pulmonary toxins (e.g., cigarette smoking). We have identified inbred mouse strains in which the ability to repair chlorine-induced airway injury and the susceptibility to airway fibrosis appear to be under genetic control (29). The present study shows that A/J mice, which after chlorine exposure show a rapid and widespread restoration of a pseudostratified epithelium and do not develop airway fibrosis, nevertheless exhibit pulmonary abnormalities that are not resolved 8 wk after exposure. Investigation of these processes may shed light on potential mechanisms by which lung disease in patients with irritant-induced asthma is initiated and maintained.
Exposure to chemicals that produce cellular damage to the respiratory tract appears to trigger a common repair pathway to restore the pseudostratified airway epithelium. This process, which has been shown to occur following exposure to naphthalene (8, 16), sulfur dioxide (36), and chlorine (29–31), involves the spreading of surviving basal cells to cover denuded areas of the basement membrane, the proliferation of basal cells, and differentiation to produce club and ciliated cells. Following injury caused by naphthalene (8, 16) or sulfur dioxide (36), the newly repaired epithelium contains club and ciliated cells shown to be derived from basal cells by lineage-tracing techniques. Here we provide evidence for transitional cells that express both basal cell and club or ciliated cell markers during epithelial repair consequent to chlorine-induced airway injury. This occurred during a narrow temporal window 5–6 days after chlorine exposure. Although epithelial repair was efficient in the sense that a pseudostratified epithelium was restored by 7 days after exposure, the resulting epithelium was not normal in that it contained contiguous clusters of club and ciliated cells rather than the uniformly interspersed distribution observed in uninjured lungs. Any functional consequences of this abnormally repaired epithelium are unknown at this time. The airway epithelium not only provides a barrier function for the respiratory tract but also serves multiple other functions related to immune responses, microbial defense, and response to injuries; any of these potentially could be compromised by abnormal epithelial structure. Results from our lung culture experiments indicated that at least one function, viz. the ability to prevent bacterial colonization of the lung under laboratory housing conditions, was not impaired in A/J mice following chlorine exposure.
Abnormal clusters of club and ciliated cells were observed in repaired airway epithelium 7 days after chlorine exposure. The processes controlling the production of club and ciliated cells from basal progenitor cells during repair of epithelial injury are not well understood. Notch (35) and Wnt/β-catenin (4, 40) pathways have been implicated, but information about specific molecular mechanisms or other potential signaling pathways is lacking. One way that clusters of the same cell type may be generated is by clonal expansion of progenitors committed to that particular cell fate. Results of clonal analysis using Confetti mice were inconsistent with this possibility, as none of the observed clones were large enough to encompass the size of the clusters that were typically observed, and most labeled clones in repaired airway epithelium contained both club and ciliated cells. These observations are consistent with lineage-tracing studies showing that basal cells proliferate and generate club and ciliated cells after injury (8, 16, 36). Previous studies have also provided evidence that both club and ciliated cells can be produced directly from basal cells (13). Thus the clusters of club and ciliated cells noted in the current study could have been produced directly from basal cell progenitors under local influences that drive cell differentiation toward club or ciliated cells. In vitro studies suggest that this may be regulated by paracrine influences from neighboring cells (14, 40), but the identity of secreted factors involved in this process is not known at this time. We observed that, between 7 and 56 days after chlorine exposure, the abnormal epithelial cell distribution tended to become less pronounced, indicating a process toward normalization of the epithelial structure. This could potentially occur through continued differentiation of the repaired areas of the epithelium. Such a process was documented after naphthalene injury, in which the nature of lineage-tagged clones derived from keratin 14-expressing basal cells changed significantly between days 22 and 62 after injury (13).
Despite the restoration of a pseudostratified epithelium in larger airways after chlorine exposure, A/J mice developed a chronic state of injury and inflammation in the lung. This was characterized by neutrophils and large, foamy macrophages in the airspaces as well as increased levels of protein and cytokines in BAL fluid. Increased levels of cytokines involved in granulocyte mobilization and chemotaxis (CXCL1, CXCL5, and G-CSF) were consistent with the neutrophilic inflammation in the lungs of chlorine-exposed A/J mice. Neutrophilic inflammation is associated with microvascular leakage, which may contribute to the observed increases in BAL protein levels. The development of foamy macrophages appears to be a relatively nonspecific response of the lung to injury (7, 32, 34, 50), infection (23, 33, 39), the presence of foreign substances (25), or deficiency of host factors (21, 26, 51). Types of injuries that promote the development of foamy macrophages include phosgene (32), wood smoke (34), welding fumes (50), and ionizing radiation (7). “[L]arge mononuclear cells with watery, vacuolated protoplasm” reminiscent of foamy macrophages were also described in early studies of the chronic effects of chlorine exposure in dogs (49). The underlying cause of chronic inflammation in chlorine-exposed A/J mice was not apparent. Pneumonitis in lung parenchyma tended to be in areas near larger airways, which are injured by chlorine inhalation in this model. This observation suggests that injury from chlorine exposure may be propagated from the airways to the surrounding lung parenchyma, either by oxidizing species derived from chlorine (42) or by soluble factors such as inflammatory mediators or cytokines.
Culture experiments showed that the number of bacteria that could be isolated from the lungs of A/J mice was not affected by chlorine exposure. This finding ruled out increased colonization of the lung by opportunistic bacteria as a cause of the chronic pneumonitis that was observed. Previous work has shown that C57BL/6 mice exposed to chlorine were deficient in the ability to clear Aspergillus fumigatis that had been experimentally introduced into the lung (12). In chlorine-exposed mice, neutrophils migrated into the lung in response to Aspergillus challenge but exhibited impaired production of superoxide, which is necessary for effective fungal killing. Our results in A/J mice indicate either that neutrophil function is not impaired or that any impaired function is dispensable for controlling bacterial infection under normal laboratory conditions. Although the immune system is thought to maintain a sterile environment within the lung, we observed numerous bacteria in the lungs of sham-exposed mice. Bacteria are small enough that, when they are inhaled, a significant fraction may bypass the upper airways and reach the lung. Healthy host defense mechanisms will effect the rapid clearance of isolated bacteria before extensive proliferation and clinical infection result. Thus, at any given time, inhaled bacteria that have reached the lung but have yet to be cleared may be present, as we observed in both chlorine- and sham-exposed mice. These observations are consistent with recent studies in humans showing extensive diversity of bacterial species in the lungs of healthy individuals (2, 6).
Previous studies in animal models have revealed pulmonary pathology following recovery from chlorine-induced acute lung injury. Dogs that were exposed to chlorine and then allowed to recover for 15 to 193 days exhibited chronic inflammation and bronchiolitis obliterans (49). In these studies, it was suggested that this chronic lung disease resulted from bacterial infection of the injured lung. This contrasted with our results, which showed chronic inflammation in the absence of increased bacterial colonization of the lung. In studies using rats, some animals that recovered from chlorine exposure retained abnormal lung function, including increased resistance and airway hyperreactivity, for 1–3 mo (9). Chlorine exposure in A/J mice was previously shown to produce a host of acute effects, most of which normalized by 7 days after exposure (29, 45). Parameters that remained altered 7–10 days after exposure (the longest recovery times examined in these studies) were increased collagen and smooth muscle surrounding airways and increased BAL macrophages and neutrophils (29, 45). Rats examined 7 days after chlorine exposure had thickened airways, increased mucus production, and airway hyperreactivity, indicative of structural and functional abnormalities in the airways (10). We previously showed that FVB/NJ mice exhibited inefficient epithelial repair after chlorine injury and rapidly developed severe airway fibrosis. These mice appear to be at one extreme in terms of susceptibility to delayed effects of chlorine. A/J mice had better epithelial repair and developed minimal fibrosis yet still showed manifestations of chronic injury and inflammation. Human populations show great variability in long-term outcomes after chlorine-induced lung injury, ranging from complete recovery to persistent abnormalities in pulmonary function and airway structure. Further understanding of the spectrum of delayed lung disease in A/J and other strains of mice may provide insight for how chronic lung pathology is maintained after recovery from acute chlorine lung injury and how chronic lung disease resulting from chlorine exposure may be treated.
GRANTS
This work was supported by the National Institutes of Health CounterACT Program through the Office of the Director and the National Institute of Environmental Health Sciences (awards R21 ES020123 and U01 ES022564 to G. Hoyle). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.M., J.M.W., and G.W.H. conception and design of research; Y.M., J.C., D.M.H., R.A.F., J.M.W., and G.W.H. performed experiments; Y.M., J.C., D.M.H., R.A.F., and G.W.H. analyzed data; Y.M., R.A.F., J.M.W., and G.W.H. interpreted results of experiments; Y.M., J.C., D.M.H., R.A.F., and G.W.H. prepared figures; Y.M. and G.W.H. drafted manuscript; Y.M., J.C., D.M.H., R.A.F., J.M.W., and G.W.H. edited and revised manuscript; Y.M., J.C., D.M.H., R.A.F., J.M.W., and G.W.H. approved final version of manuscript.
REFERENCES
- 1.Bherer L, Cushman R, Courteau JP, Quevillon M, Cote G, Bourbeau J, L'Archeveque J, Cartier A, Malo JL. Survey of construction workers repeatedly exposed to chlorine over a three to six month period in a pulpmill: II. Follow up of affected workers by questionnaire, spirometry, and assessment of bronchial responsiveness 18 to 24 mo after exposure ended. Occup Environ Med 51: 225–228, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blainey PC, Milla CE, Cornfield DN, Quake SR. Quantitative analysis of the human airway microbial ecology reveals a pervasive signature for cystic fibrosis. Sci Transl Med 4: 153ra130, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulet LP, Laviolette M, Turcotte H, Cartier A, Dugas M, Malo JL, Boutet M. Bronchial subepithelial fibrosis correlates with airway responsiveness to methacholine. Chest 112: 45–52, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Brechbuhl HM, Ghosh M, Smith MK, Smith RW, Li B, Hicks DA, Cole BB, Reynolds PR, Reynolds SD. beta-Catenin dosage is a critical determinant of tracheal basal cell fate determination. Am J Pathol 179: 367–379, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest 88: 376–384, 1985. [DOI] [PubMed] [Google Scholar]
- 6.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 184: 957–963, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang CS, Liu WC, Jung SM, Chen FH, Wu CR, McBride WH, Lee CC, Hong JH. Compartmental responses after thoracic irradiation of mice: strain differences. Int J Radiat Oncol Biol Phys 62: 862–871, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal basal cells: A facultative progenitor cell pool. Am J Pathol 177: 362–376, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demnati R, Fraser R, Ghezzo H, Martin JG, Plaa G, Malo JL. Time-course of functional and pathological changes after a single high acute inhalation of chlorine in rats. Eur Respir J 11: 922–928, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, Matalon S. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol 46: 599–606, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautrin D, Boulet LP, Boutet M, Dugas M, Bherer L, L'Archeveque J, Laviolette M, Cote J, Malo JL. Is reactive airways dysfunction syndrome a variant of occupational asthma? J Allergy Clin Immunol 93: 12–22, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Gessner MA, Doran SF, Yu Z, Dunaway CW, Matalon S, Steele C. Chlorine gas exposure increases susceptibility to invasive lung fungal infection. Am J Physiol Lung Cell Mol Physiol 304: L765–L773, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, Runkle CM, Reynolds SD. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol 45: 403–410, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh M, Helm KM, Smith RW, Giordanengo MS, Li B, Shen H, Reynolds SD. A single cell functions as a tissue-specific stem cell and the in vitro niche-forming cell. Am J Respir Cell Mol Biol 45: 459–469, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homeland Security Council. The Homeland Security Council, Planning Scenarios: Executive Summaries (2004). http://www.globalsecurity.org/security/library/report/2004/hsc-planning-scenarios-jul04_08.htm [August 17, 2010].
- 16.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol 286: L643–L649, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Hoyle GW, Chang W, Chen J, Schlueter CF, Rando RJ. Deviations from Haber's law for multiple measures of acute lung injury in chlorine-exposed mice. Toxicol Sci 118: 696–703, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyle GW, Hoyle CI, Chen J, Chang W, Williams RW, Rando RJ. Identification of triptolide, a natural diterpenoid compound, as an inhibitor of lung inflammation. Am J Physiol Lung Cell Mol Physiol 298: L830–L836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute of Laboratory Animal Resources, Commission of Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press, 1996. [Google Scholar]
- 20.Jones RN, Hughes JM, Glindmeyer H, Weill H. Lung function after acute chlorine exposure. Am Rev Respir Dis 134: 1190–1195, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Korfhagen TR, Sheftelyevich V, Burhans MS, Bruno MD, Ross GF, Wert SE, Stahlman MT, Jobe AH, Ikegami M, Whitsett JA, Fisher JH. Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo. J Biol Chem 273: 28438–28443, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Kowitz TA, Reba RC, Parker RT, Spicer WS Jr. Effects of chlorine gas upon respiratory function. Arch Environ Health 14: 545–558, 1967. [DOI] [PubMed] [Google Scholar]
- 23.Le VL, Courtney CL, Steel J, Compans RW. Closely related influenza viruses induce contrasting respiratory tract immunopathology. PLoS One 8: e76708, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemiere C, Malo JL, Boutet M. Reactive airways dysfunction syndrome due to chlorine: sequential bronchial biopsies and functional assessment. Eur Respir J 10: 241–244, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Lewis DJ, Williams TC, Beck SL. Foamy macrophage responses in the rat lung following exposure to inhaled pharmaceuticals: a simple, pragmatic approach for inhaled drug development. J Appl Toxicol 34: 319–331, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Lyerla TA, Rusiniak ME, Borchers M, Jahreis G, Tan J, Ohtake P, Novak EK, Swank RT. Aberrant lung structure, composition, and function in a murine model of Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol 285: L643–L653, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Malo JL, Cartier A, Boulet LP, L'Archeveque J, Saint-Denis F, Bherer L, Courteau JP. Bronchial hyperresponsiveness can improve while spirometry plateaus two to three years after repeated exposure to chlorine causing respiratory symptoms. Am J Respir Crit Care Med 150: 1142–1145, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Malo JL, L'Archeveque J, Castellanos L, Lavoie K, Ghezzo H, Maghni K. Long-term outcomes of acute irritant-induced asthma. Am J Respir Crit Care Med 179: 923–928, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Mo Y, Chen J, Schlueter CF, Hoyle GW. Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L92–L102, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musah S, Chen J, Hoyle GW. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respir Res 13: 107, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Koren EG, Hogan BL, Gunn MD. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am J Respir Cell Mol Biol 49: 788–797, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauluhn J. Acute nose-only exposure of rats to phosgene. II. Concentration x time dependence of changes in bronchoalveolar lavage during a follow-up period of 3 months. Inhal Toxicol 18: 595–607, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, Daffe M, Emile JF, Marchou B, Cardona PJ, de Chastellier C, Altare F. Foamy macrophages from tuberculosis patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog 4: e1000204, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos C, Pedraza-Chaverri J, Becerril C, Cisneros J, Gonzalez-Avila G, Rivera-Rosales R, Sommer B, Medina-Campos ON, Montano M. Oxidative stress and lung injury induced by short-term exposure to wood smoke in guinea pigs. Toxicol Mech Methods 23: 711–722, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8: 639–648, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106: 12771–12775, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz DA, Smith DD, Lakshminarayan S. The pulmonary sequelae associated with accidental inhalation of chlorine gas. Chest 97: 820–825, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Shroff CP, Khade MV, Srinivasan M. Respiratory cytopathology in chlorine gas toxicity: a study in 28 subjects. Diagn Cytopathol 4: 28–32, 1988. [DOI] [PubMed] [Google Scholar]
- 39.Siracusa MC, Reece JJ, Urban JF Jr, Scott AL. Dynamics of lung macrophage activation in response to helminth infection. J Leukoc Biol 84: 1422–1433, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MK, Koch PJ, Reynolds SD. Direct and indirect roles for β-catenin in facultative basal progenitor cell differentiation. Am J Physiol Lung Cell Mol Physiol 302: L580–L594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: Reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol 299: L289–L300, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda N, Maghni K, Daigle S, L'Archeveque J, Castellanos L, Al-Ramli W, Malo JL, Hamid Q. Long-term pathologic consequences of acute irritant-induced asthma. J Allergy Clin Immunol 124: 975–981, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol 20: 783–793, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Tuck SA, Ramos-Barbon D, Campbell H, McGovern T, Karmouty-Quintana H, Martin JG. Time course of airway remodelling after an acute chlorine gas exposure in mice. Respir Res 9: 61, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med 27: 1–7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weill H, George R, Schwarz M, Ziskind M. Late evaluation of pulmonary function after acute exposure to chlorine gas. Am Rev Respir Dis 99: 374–379, 1969. [PubMed] [Google Scholar]
- 48.White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 7: 257–263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winternitz MC, Lambert RA, Jackson L, Smith GH. The pathology of chlorine poisoning. In: Collected Studies on the Pathology of War Gas Poisoning, edited by Winternitz MC. New Haven, CT: Yale University School of Medicine, 1920, p. 1–32. [Google Scholar]
- 50.Yang MJ, Yang YS, Sung JH, Kim JS, Cho KH, Lim CW, Chung YH, Kim HY, Yang JS, Yu IJ, Song CW. Recurrent exposure to welding fumes induces insufficient recovery from inflammation. Inhal Toxicol 21: 337–346, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Yatera K, Morimoto Y, Kim HN, Myojo T, Mukae H. Foam cell formation of alveolar macrophages in Clara cell ablated mice inhaling crystalline silica. Inhal Toxicol 23: 736–744, 2011. [DOI] [PubMed] [Google Scholar]