Abstract
Inner foveal thinning and intracellular alpha-synuclein were demonstrated in the retina in Parkinson disease. While pathognomonic alpha-synuclein is associated with embryonic dopaminergic (DA) neurons, postmortem studies in the nervous system and retina show prominent effect also in non-DA neurons. We evaluated foveal capillaries and foveal thickness in 23 Parkinson disease subjects and 13 healthy controls using retinal fluorescein angiography and optical coherence tomography. The size of the foveal avascular zone inversely correlates with foveal thinning. Foveal thinning highly correlates with motor impairment and also disease duration. Quantifying capillary and neuronal remodeling could serve as biological markers.
Introduction
Parkinson disease (PD) pathology is associated with misfolded protein alpha-synuclein (mAS) which causes toxicity in dopaminergic (DA) neurons of embryonic cell cultures.1 Postmortem studies in PD have demonstrated mAS aggregation in non-DA neurons in several locations including gut, vagus nerve, olfactory bulb, and the retina.2–4 In the retina, mAS is located in DA amacrine cells of the inner-plexiform layer and in non-DA ganglion cells.3 Here, we show an association of retinal thinning with capillary reorganization in PD.
Inner retinal capillaries are spread across the retina, in the nerve fiber layer, in the ganglion cell layer, and the inner nuclear layer. The centralmost fovea is free of capillaries, called the foveal avascular zone (FAZ). Foveal thinning in PD takes place in an annular zone around the FAZ.5,6 In this study, we quantify the centripetal expansion of the Perifoveolar capillary network and quantify neuronal remodeling with a measure of inner parafoveal to inner foveal thickness ratio (IPT/IFT). We further report that motor impairment correlates with this measure. Based on these results, we propose that capillary remodeling is a contributor to the determinants of pathology in PD.
Subjects and Methods
Study population
Twenty three PD subjects (46 eyes) and thirteen healthy controls (HC) (24 eyes) matched for age, gender, and ethnicity were prospectively enrolled in the study. The study protocol was approved by SUNY Downstate Medical Center Institutional Review Board and written informed consent was obtained from each subject.
All subjects underwent standard neurological examination. PD diagnosis was based on the UK brain bank criteria. Mean PD duration was 5.6 ± 2.5 years (range 1–10 years). Mean Hoehn and Yahr and UPDRS motor scores were 2.5 ± 0.5 and 30.9 ± 6.7, respectively. Out of 23 PD subjects, five had motor fluctuations. The UPDRS was conducted within 1–2 h after the last dose of medication for all subjects, that is presumably in “on” state. Exclusionary criteria were young-onset (<40 years old) PD, no response to levodopa or dopamine agonists, Mini-Mental State Exam score below 28, and diabetes mellitus. Comprehensive ophthalmological examination excluded subjects with subnormal visual acuity (<20/25), ocular hypertension, optic neuropathy, glaucoma, uveitis, pathologic myopia (>6 diopters), macular drusen or atrophy, and any form of retinal degeneration.
Retinal fluorescein angiography
Retinal fluorescein angiography was performed with 3 mL of 25% dye after pupil dilation. Consecutive digital fundus images were acquired by the Heidelberg Spectralis instrument in high-resolution mode. Highest quality images were chosen by a retina specialist (E. S.) for analysis. At the same visit, EMM5 macular scans were obtained using spectral-domain optical coherence tomography (OCT) (RTVue).
Quantifying of retinal thickness
Average IFT and IPT were obtained from OCT EMM5 scans (Fig.1A). A new derived parameter, “IPT/IFT ratio,” was obtained to normalize thickness data. This ratio quantifies foveal pit depth. A ratio of near 1 demonstrates decreased depth of the foveal pit.
Figure 1.
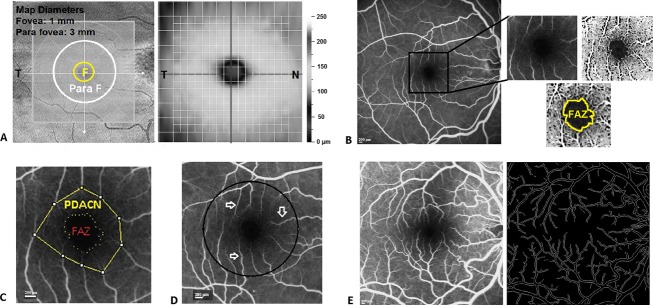
OCT and fluorescein angiography. (A) Retinal thickness measured by RTtvue includes foveal thickness, considered as the central area with 1 mm diameter, and parafoveal thickness, considered as the cocentered annular area with 3 mm diameter. The IPT/IFT ratio was calculated by dividing the average inner parafoveal thickness with the average inner foveal thickness. This normalized ratio quantifies foveal pit depth. (B) FAZ area measurements based on angiography images of the retina acquired by Heidelberg OCT-angiograph. The image contrast was adjusted by the image J software to enhance the FAZ. The border of FAZ was delineated and the area within the FAZ was calculated using the software (scale, 200 μm); (C) PDACN area was measured by delineating the area within the tip of radial capillaries and then subtracting the cocentered FAZ area. (D) Radial capillaries (arrows) were counted within the central foveal area with 3 mm diameter (area within black circle). (E) Macular fractal dimensions (FD) analysis using a peak phase angiographic image of the macula. The binary image of the skeletonized macular vasculature was produced using ImageJ software, and the Standard Box-Counting method (scale, 200 μm). F, fovea; S, superior; T, temporal; N, nasal; I, inferior; OCAT, optical coherence tomography; IFT, inner foveal thickness; IPT, Inner parafoveal thickness; FAZ, foveal avascular zone; PDACN, Perifoveolar diffuse annular capillary network.
Retinal capillary bed
Angiography images were semiautomatically analyzed by Image J NIH software ver.1.45 (National Institutes of Health, Bethesda, MD).
FAZ area
FAZ area was calculated by two blinded observers using the following steps (Fig.1B): adjusting image contrast to enhance the FAZ; encircling the FAZ borders and measuring the area within this border. The mean of reported measures was considered for each subject. FAZ radius was measured in each quadrant (superior, inferior, temporal, nasal) centered at the foveola.
Perifoveolar diffuse annular capillary network
Perifoveolar diffuse annular capillary network (PDACN) area was determined by subtracting the FAZ area from the cocentered area delineated by the tip of radial capillaries (Fig.1C).
Perifoveolar radial capillaries
Perifoveolar radial capillaries were counted manually within the central foveal area with 3 mm diameter (Fig.1D).
Macular fractal dimensions
Macular fractal dimensions (FD) were quantified in 25 PD and in 17 control eyes. The images were matched at peak angiography phase. ImageJ software was applied for tracing retinal capillaries in order to automatically calculate the FD using Standard Box-Counting method. Twenty five boxes were chosen for all images (Fig.1E). Larger FD values represent a more complex branching pattern.
Statistical analysis
Statistical Analysis was performed using IBM SPSS: Chicago, IL, United States statistical software version 21.0. P < 0.05 and correlation coefficients with 95% confidence intervals were considered statistically significant.
Results
Thinning of the inner fovea
The average IPT/IFT ratio is significantly smaller in PD subjects compared to controls (1.71 ± 0.30 vs. 1.97 ± 0.28; P = 0.002). The IPT/IFT ratio strongly correlates with UPDRS motor score (R = −0.564; P = 0.0001) and disease duration (R = −0.329, P = 0.029) (Fig.2A).
Figure 2.
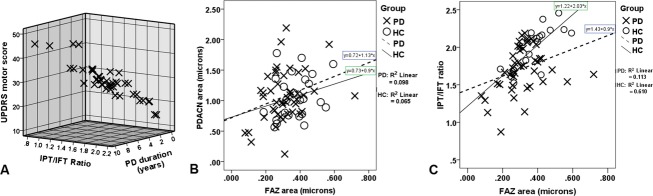
(A) The negative correlation of the IPT/IFT ratio with UPDRS motor score and disease duration. Notice that subjects with higher UPRDS motor score and longer disease duration have lower IPT/IFT ratio. (B) The correlation between the FAZ area and the PDACN area. A smaller FAZ area correlates with smaller PDACN area in PD. (C) The correlation between FAZ area and IPT/IFT ratio in PD and HC subjects. Notice the decreased IPT/IFT ratio in PD subjects with smaller FAZ area. Most HC have higher thickness ratio and larger FAZ. The regression line demonstrates the correlation between thickness ratio and FAZ area, separately in PD and controls. OCAT, optical coherence tomography; IFT, inner foveal thickness; IPT, Inner parafoveal thickness; FAZ, foveal avascular zone; PDACN, Perifoveolar diffuse annular capillary network.
Foveal capillary bed remodeling
FAZ area in PD subjects (0.308 ± 0.11 mm2) is significantly smaller than in HC (0.372 ± 0.10 mm2) (P = 0.02). The mean FAZ radius is also smaller in PD compared to controls (297 ± 57 vs. 327 ± 46 μm; P = 0.028), especially in the inferior quadrant (283 ± 72 vs. 322 ± 66 μm; P = 0.031), while the radius does not vary with quadrants in controls. Mean PDACN area is nearly the same in PD and controls (1.068 ± 0.41 vs. 1.065 ± 0.36 mm2; P = 0.98). FAZ area significantly correlates with PDACN area (R = 0.285, P = 0.017) (Fig.2B).
FAZ area correlates with the IPT/IFT ratio in both PD (R = 0.336, P = 0.026) and HC (R = 0.781, P = 0.0001) (Fig.2C). FAZ area has a significant negative correlation with both IFT and IPT in HC (R = −0.7, P = 0.0001). In PD, FAZ area negatively correlates only with IFT (R = −0.5, P = 0.0001), not with IPT (R = −0.180, P = 0.243). FAZ is not correlated with age (R = 0.075, P = 0.537), PD duration (R = 0.086, P = 0.571), disease stage (R = 0.109, P = 0.470), or UPDRS motor score (R = 0.138, P = 0.359). FAZ area was 0.288 ± 0.07 mm2 in 10 eyes with 1–3 years disease duration, 0.322 ± 0.06 mm2 in 16 eyes with 4–6 years of disease duration, and 0.308 ± 0.15 mm2 in 20 eyes with 7–10 years of disease duration.
Macular FD is the same in PD and controls (1.58 ± 0.03 vs. 1.58 ± 0.03, respectively; P = 0.91). The Perifoveolar radial capillary count does not statistically differ between PD and HC (14.1 ± 1.8 vs. 13.4 ± 1.9; P = 0.11).
Discussion
The inner retina in PD is thinned in an annular zone about 0.5–1.5 mm radius.5,6 The encroachment of the FAZ by Perifoveolar capillaries originates in this annular zone (Fig.3A).4 In our study, FAZ mean radius in controls was 0.32 ± 0.04 and 0.29 ± 0.05 mm in PD. Our data suggest that a smaller FAZ in PD is due neither to capillary branching, as there is no difference in the fractal dimension between PD and HC; nor to the proliferation of the number of radial capillaries.
Figure 3.
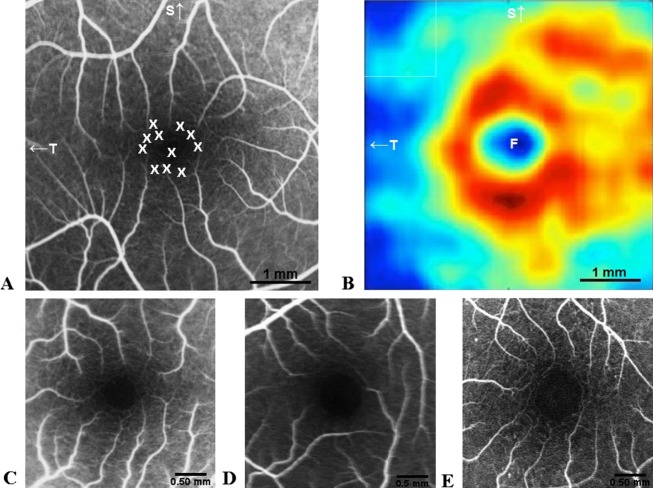
(A) Area of Perifoveolar thickness attenuation in PD patients (After Spund et al.5). (B) Fluorescein angiography image of the retina showing the distribution of dopaminergic neurons represented by X in the FAZ, according to topography of DA neurons in the FAZ, after Savy et al.7 (C) FAZ in PD; (D) Healthy control; and a (E) DM subject. Note the smaller FAZ surrounded by richer Perifoveolar annular capillary network in PD compared to HC and DM. F, FAZ; OD, optic disc; S, superior; T, Temporal; PD, Parkinson disease; FAZ, foveal avascular zone; DM, diabetes mellitus; DA, dopaminergic; HC, healthy controls.
The cause for lengthening of foveal capillaries is not known. DA neurons are located in the FAZ in the human retina (Fig.3B).7 MPTP Methyl Phenyl Tetrahydro Pyridine, a compound for induction of a monkey model of PD, causes retinal capillary endothelial damage around DA neurons.8 It is conceivable that in PD a damage of foveal DA neurons,9 promotes vasculogenesis from the capillary surround.
This role of DA neurons in promoting longer capillaries rather than ganglion cells is supported by studies in glaucoma. In glaucoma, the primary damage is to ganglion cells and there are no changes in the FAZ.10 In PD, an altered angio-structure of the substantia nigra (SN) was first suggested by Finley.11 The FAZ is surrounded by microglial cells,12 and its etiological role in vascular remodeling may be similar in the retina and SN in PD. Faucheux et al.13 and Barcia et al.14 noted an increased number and density of blood vessels and VEGF Vascular Endothelial Growth Factor expressing neurons in the SN of postmortem PD and MPTP primate model. It was suggested that there is an increased transferring of toxic compounds or iron accumulation to DA neurons,13 with consequent loss in the SN.14 Microvascular changes in PD were also reported in cingulate cortex, putamen, and locus coeruleus,15–17 but not in other areas in which alpha-synuclein deposition has been noted including dorsal motor nucleus of the vagus, olfactory bulb, enteric nervous system.
The present study is the first to quantify capillary remodeling in the PD retina.18 The clinical specificity of our results in PD is noteworthy. Neither in HC,19 nor in PD did we find a correlation between the FAZ area and age. We did not find the any relationship between the FAZ size and the disease duration. The smaller FAZ in PD was observed even in patients with 1–3 years of disease duration. Our results in the retina are consistent with early microvascular changes reported in the SN in PD and incidental lewy body disease.16
There is variability in FAZ diameter in healthy retina.19 In the healthy human retina, a smaller FAZ is associated with thicker fovea and narrower pit.19,20 In contrast to HC, in PD a smaller FAZ correlates with thinner and flatter foveal pit. Both background and proliferative diabetic retinopathy are associated with larger FAZ area, presumably due to capillary occlusions (Fig.3C–E).21 Branching asymmetry and altered capillary pattern were suggested as retinal biomarker for Alzheimer's disease.22 Increased retinal microvascular FD is associated with stroke.23 Macular FD is unchanged in PD.
Whether DA cell loss leads to capillary changes or conversely capillary pathology affects DA neurons is unknown. In either case, our results suggest that in addition to intracellular oxidative stress,18 common to many neurodegenerative diseases, local capillary changes contribute to neuronal loss in PD.
In summary, from retinal thickness we derived a normalized parameter of foveal pit morphology, the IPT/IFT ratio. The ratio correlates with UPDRS motor score and duration of the disease. We quantified a smaller FAZ area in PD, as the capillary network invades from an annular zone surrounding the foveola. The fact that in PD non-dopaminergic neurons are also affected, may have a microvascular etiology. Specifying retinal microvascular changes opens a new approach to establish a biomarker for PD.
Acknowledgments
This work was supported indirectly by the Parkinson Study Group, the Michael J. Fox Foundation, and by the Research to Prevent Blindness. We thank Galina Glazman, Pawel Kuczaj, and Bella Berger for help in data collection; Bill Lytton, MD and Olie Westheimer, MA for editing the paper.
Author Contributions
The authors contributed equally to this work.
Conflict of Interest
None declared.
References
- Xu J, Kao SY, Lee FJ, et al. Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric α-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S. α-synuclein in the inner retina in parkinson disease. Ann Neurol. 2014;75:964–966. doi: 10.1002/ana.24182. [DOI] [PubMed] [Google Scholar]
- Beach TG, Carew J, Serrano G, et al. Phosphorylated α-synuclein-immunoreactive retinal neuronal elements in Parkinson's disease subjects. Neurosci Lett. 2014;571:34–38. doi: 10.1016/j.neulet.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spund B, Ding Y, Liu T, et al. Remodeling of the fovea in Parkinson disease. J Neural Transm. 2013;120:745–753. doi: 10.1007/s00702-012-0909-5. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Miri S, Glazman S. Venturing into the no-man's land of the retina in Parkinson disease. Mov Disord. 2014;29:15–22. doi: 10.1002/mds.25741. [DOI] [PubMed] [Google Scholar]
- Savy C, Simon A, Nguyen-Legros J. Spatial geometry of the dopamine innervation in the avascular area of the human fovea. Vis Neurosci. 1991;7:487–498. doi: 10.1017/s0952523800009779. [DOI] [PubMed] [Google Scholar]
- Adams JD, Pickford MS, Wong CG. The acute retinal histopathology of MPTP. Neurotoxicology. 1992;13:541–549. [PubMed] [Google Scholar]
- Djamgoz MBA, Hankins MW, Hirano J, Archer SN. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Res. 1997;37:3509–3529. doi: 10.1016/S0042-6989(97)00129-6. [DOI] [PubMed] [Google Scholar]
- Arend O, Remky A, Plange N, et al. Capillary density and retinal diameter measurements and their impact on altered retinal circulation in glaucoma: a digital fluorescein angiographic study. Br J Ophthalmol. 2002;86:429–433. doi: 10.1136/bjo.86.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley KH. Angio-architecture of the substantia nigra and its pathogenic significance. Arch Neurol Psych. 1936;36:118–127. [Google Scholar]
- Penfold PL, Provis JM. Antibodies to human leucocyte antigens indicate subpopulations of microglia in human retina. Vis Neurosci. 1991;7:383–388. doi: 10.1017/s0952523800004879. [DOI] [PubMed] [Google Scholar]
- Faucheux BA, Bonnet AM, Agid Y, Hirsch EC. Blood vessels change in the mesencephalon of patients with Parkinson's disease. Lancet. 1999;353:981–982. doi: 10.1016/S0140-6736(99)00641-8. [DOI] [PubMed] [Google Scholar]
- Barcia C, Bautista V, Sanchez-Bahillo A, et al. Changes in vascularization in substantia nigra pars compacta of monkeys rendered Parkinsonian. J Neural Transm. 2005;112:1237–1248. doi: 10.1007/s00702-004-0256-2. [DOI] [PubMed] [Google Scholar]
- Farkas E, De Jong GI, de Vos RA, et al. Pathological features of cerebral cortical capillaries are doubled in Alzheimer's disease and Parkinson's disease. Acta Neuropathol. 2000;100:395–402. doi: 10.1007/s004010000195. [DOI] [PubMed] [Google Scholar]
- Desai Bradaric B, Patel A, Schneider JA, et al. Evidence for angiogenesis in Parkinson's disease, incidental Lewy body disease, and progressive supranuclear palsy. J Neural Transm. 2012;119:59–71. doi: 10.1007/s00702-011-0684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Arai H, Takanashi M, et al. Expression levels of vascular endothelial growth factor and its receptors in Parkinson's disease. NeuroReport. 2006;17:705–709. doi: 10.1097/01.wnr.0000215769.71657.65. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Fernández-Sánchez L, Campello L, et al. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43C:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Dubis AM, Hansen BR, Cooper RF, et al. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012;53:1628–1636. doi: 10.1167/iovs.11-8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui TY, Zhong Z, Song H, Burns SA. Foveal avascular zone and its relationship to foveal pit shape. Optom Vis Sci. 2012;89:602. doi: 10.1097/OPX.0b013e3182504227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath J, Giorgi R, Raccah D, Ridings B. Foveal avascular zone in diabetic retinopathy: quantitative vs qualitative assessment. Eye. 2004;19:322–326. doi: 10.1038/sj.eye.6701456. [DOI] [PubMed] [Google Scholar]
- Frost S, Kanagasingam Y, Sohrabi H, et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer's disease. Transl Psychiatry. 2013;3:e233. doi: 10.1038/tp.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Liew G, Lindley RI, et al. Retinal fractals and acute lacunar stroke. Ann Neurol. 2010;68:107–111. doi: 10.1002/ana.22011. [DOI] [PubMed] [Google Scholar]