Abstract
Female sex is a risk factor for pulmonary arterial hypertension (PAH), yet females have better survival than males. We sought to determine if sex was associated with baseline haemodynamics in subjects with PAH, and whether age modified these relationships.
We conducted a pooled analysis from 11 randomised trials submitted to the US Food and Drug Administration. The study sample included 1211 subjects with idiopathic PAH, 25% of whom were males, and 489 subjects with connective tissue disease-associated PAH, 13% of whom were males.
After multivariable adjustment, right atrial pressure was 1.36 mmHg higher (95% CI 0.44–2.27, p=0.004), cardiac index was −0.14 L·min−1·m−2 lower (95% CI −0.23–0.04, p=0.01) and pulmonary vascular resistance was 1.23 Wood units higher (95% CI 0.18–2.27, p=0.02) in males compared with females. Younger males had 5.43 mmHg (95% CI 2.20–8.66, p=0.001) higher mean pulmonary arterial pressures than younger females, but these relationships were attenuated after age 45 years. In the subgroup of connective tissue disease-associated PAH, males may have had higher right atrial pressure.
These findings implicate age as a modifier and provide further evidence of sexual dimorphism in PAH.
Introduction
Female sex is the best established risk factor for pulmonary arterial hypertension (PAH) [1, 2]. Despite having greater disease burden, it appears that females have better right ventricular (RV) function and increased survival compared with males with PAH [3–6]. Haemodynamics, particularly indices of RV response to increased afterload, such as right atrial pressure (RAP) and cardiac output (CO), are well described predictors of outcome in PAH, but whether haemodynamic measures vary by sex, possibly contributing to sex-related differences in outcome, has not been clearly established [7–10].
Numerous basic science and clinical observations have implicated oestrogen in PAH disease pathogenesis [11–15]. Yet, oestrogen therapy has been shown to rescue both pulmonary vasculopathy and RV function in animal models of pulmonary hypertension [16, 17]. This paradoxical effect of oestrogen is, as yet, unexplained. Why females with PAH appear to fare better than males with the disease (and the role that other sex hormones may play in this process) is also poorly understood.
Recent observational data indicate that age may be an important modifier of disease risk and outcomes in pulmonary vascular disease [7, 18]. In systemic cardiovascular disease, male and female risk profiles converge following the menopausal transition, and much debate has surrounded hormone therapy and the impact of sex hormones on vascular homeostasis as individuals age [19–21]. We have shown that sex hormones are associated with RV size and function in health, and that females with PAH have greater responses to endothelin receptor antagonists than males with PAH, but the association of sex with haemodynamic burden in well-phenotyped patients is unknown [22, 23].
We sought to determine whether sexual dimorphism in PAH could in part be explained by differences in baseline haemodynamics, and whether age modified this relationship. We hypothesised that younger males would have higher mean RAP, mean pulmonary arterial pressures (mPAP) and pulmonary vascular resistance (PVR), and lower CO at baseline compared with young females with PAH, and that these differences would attenuate with age.
Methods
Study population
De-identified individual patient data from 12 placebo-controlled randomised trials of targeted PAH therapies was acquired from the US Food and Drug Administration (FDA) (Silver Springs, MD, USA). The trials, Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter Efficacy Studies (ARIES)-1 and −2 [24]; Bosentan-351 [25]); Bosentan: Randomized Trial of Endothelin receptor Antagonist Therapy (BREATHE)-1 [26]; Aerosolized Iloprost Randomized (AIR) [27]; AIR II [28]; Sildenafil Use in Pulmonary Hypertension (SUPER) [29]; Pulmonary Arterial Hypertension and Response to Tadalafil trial (PHIRST) [30]; Sitaxsentan To Relieve Impaired Exercise (STRIDE)-1 [31]; STRIDE-2 [32] and STRIDE-4 [33]; and the phase III subcutaneous treprostinil trial [34] compared active therapies (ambrisentan, bosentan, iloprost, sildenafil, tadalafil, sitaxsentan and subcutaneous treprostinil, respectively) with placebo. All the trials were found to have similar inclusion criteria and data collection processes [24–33]. We included subjects with a diagnosis of idiopathic or connective tissue disease (CTD)-associated PAH and excluded subjects with missing baseline data and those from the PHIRST trial, since some subjects in this trial had been treated with background bosentan therapy [30]. As idiopathic and CTD-associated PAH patients are known to have different haemodynamic profiles, we chose to analyse these two groups separately [35].
Variables
The dependent variables were baseline haemodynamic values from the trials as reported to the FDA. Individuals with missing RAP, mPAP, pulmonary capillary wedge pressure or CO were excluded from analyses. The independent variable was sex. Covariates (and effect modifiers) included age, race, height, weight and study.
Statistical analysis
Continuous variables were expressed as mean±SD. Categorical variables were expressed as percentages. Independent sample t-tests were used to compare continuous variables and Chi-squared was used to compare categorical variables in males and females. Multivariable linear regression was used to assess the relationship between sex and baseline haemodynamics. Results were expressed as adjusted effect estimates and least-square means. We assessed for a sex by age interaction, and stratified results by age group when the interaction was significant (p<0.05). Age groups (<45, 45–54, 55–64 and ≥65 years) were chosen a priori to correlate with typical changes in the hormonal milieu with age (e.g. menopause) [36, 37]. Main results were adjusted for age (if no sex by age interaction), race/ethnicity, height, weight and study. Models for cardiac index and pulmonary vascular resistance index (PVRI) were adjusted for age (if no sex by age interaction), race/ethnicity and study. Statistical significance was defined as p<0.05. Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
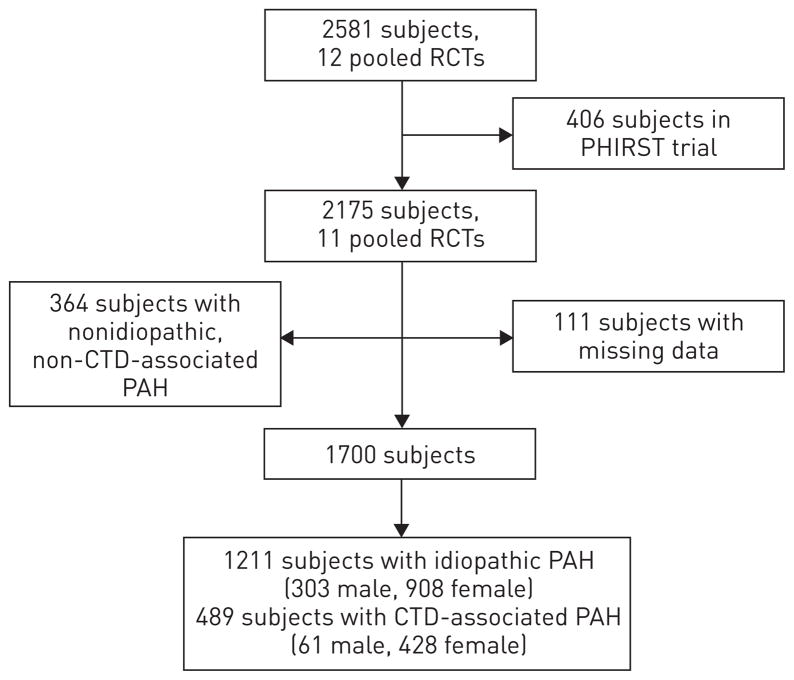
A total of 2581 subjects were enrolled in 12 randomised controlled trials, which were submitted for FDA approval (fig. 1). Of these, we excluded 406 (16%) from the PHIRST trial, 364 (17%) with nonidiopathic, non-CTD-associated PAH and 111 (5%) with missing data. The final study sample consisted of 1211 subjects with idiopathic PAH, 303 (25%) of whom were males, and 489 subjects with CTD-associated PAH, 61 (13%) of whom were males.
FIGURE 1.
Study sample. RCT: randomised controlled trial; PHIRST: Pulmonary Arterial Hypertension and Response to Tadalafil; CTD: connective tissue disease; PAH: pulmonary arterial hypertension.
Characteristics of the study sample with idiopathic PAH are shown in table 1. Males were older, taller and heavier, and had greater 6-min walking distance (6MWD) than females. In our study sample, more males than females were from AIR and ARIES-2 (12.2% of the males versus 6.3% of the females and 12.2% of the males versus 8.5% of the females, respectively), whereas more females than males were from ARIES-1 (10.5% versus 5%, respectively). Comparing unadjusted haemodynamic values, males had higher CO and lower PVR than females; however, females had higher cardiac index (p=0.01). There was no difference in PVRI between males and females.
TABLE 1.
Baseline characteristics of the study sample with idiopathic pulmonary arterial hypertension, by sex
| Variable | Males | Females | p-value |
|---|---|---|---|
| Subjects | 303 | 908 | |
| Age years | 50.5±15.2 | 46.6±14.5 | <0.001 |
| Race | 0.07 | ||
| Caucasian | 265 (87.5) | 726 (80.0) | |
| African-American | 9 (3.0) | 41 (4.5) | |
| Asian | 4 (1.3) | 19 (2.1) | |
| Hispanic | 24 (7.9) | 114 (12.6) | |
| Other | 1 (0.3) | 8 (0.9) | |
| Height cm | 173.9±8.3 | 160.7±7.2 | <0.001 |
| Weight kg | 81.6±17.0 | 72.1±17.8 | <0.001 |
| BMI kg·m−2 | 26.9±4.7 | 27.9±6.6 | 0.18 |
| Warfarin use | 167 (66.0) | 496 (61.5) | 0.19 |
| Study | 0.002 | ||
| AIR | 37 (12.2) | 57 (6.3) | |
| AIR-II | 9 (3.0) | 22 (2.4) | |
| ARIES-1 | 15 (5.0) | 95 (10.5) | |
| ARIES-2 | 37 (12.2) | 77 (8.5) | |
| Bosentan-351 | 4 (1.3) | 22 (2.4) | |
| BREATHE-1 | 31 (10.2) | 107 (11.8) | |
| STRIDE-1 | 25 (8.3) | 68 (7.5) | |
| STRIDE-2 | 37 (12.2) | 91 (10.0) | |
| STRIDE-4 | 11 (3.6) | 52 (5.7) | |
| SUPER | 41 (13.5) | 118 (13.0) | |
| Treprostinil | 56 (18.5) | 199 (21.9) | |
| NYHA functional class | 0.20 | ||
| I | 11 (3.6) | 26 (2.9) | |
| II | 110 (36.4) | 372 (41.2) | |
| III | 160 (53.0) | 464 (51.4) | |
| IV | 21 (7.0) | 40 (4.4) | |
| Baseline 6MWD m | 362.6±91.4 | 344.5±83.2 | 0.001 |
| Baseline haemodynamics | |||
| Right atrial pressure mmHg | 9.6±5.8 | 9.1±5.6 | 0.12 |
| Mean pulmonary artery pressure mmHg | 54.4±15.5 | 55.2±14.5 | 0.15 |
| Cardiac output L·min−1 | 4.3±1.3 | 4.1±1.4 | 0.02 |
| Cardiac index L·min−1·m−2 | 2.2±0.6 | 2.3±0.8 | 0.01 |
| Pulmonary capillary wedge pressure mmHg | 9.3±3.6 | 9.1±3.5 | 0.41 |
| Pulmonary vascular resistance Wood units | 12.0±6.2 | 13.1±6.9 | 0.004 |
| Pulmonary vascular resistance index Wood units·m−2 | 23.2±11.4 | 22.9±11.6 | 0.90 |
Data are shown as n, mean±SD or n (%), unless otherwise stated. BMI: body mass index; AIR: Aerosolized Iloprost Randomized; ARIES: Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter Efficacy Studies; BREATHE: Bosentan: Randomized Trial of Endothelin receptor Antagonist Therapy; STRIDE: Sitaxsentan To Relieve Impaired Exercise; SUPER: Sildenafil Use in Pulmonary Hypertension; NYHA: New York Heart Association; 6MWD: 6-min walking distance.
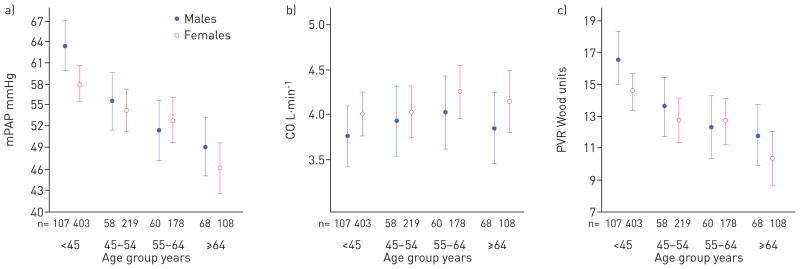
Adjusted associations between sex and baseline haemodynamics for those with idiopathic PAH are shown in table 2. Adjustment for height and weight in the multivariate models made it unnecessary to index the parameters to body surface area, as the former also accounts for differences in body size. After adjustment for age, race, height, weight and study, males had 1.36 mmHg higher RAP (95% CI 0.44–2.27 mmHg, p=0.004) compared with females. There was a significant interaction between sex and age for mPAP (p<0.05). Young males (age <45 years) had higher mPAP compared with young females (5.43 mmHg difference, 95% CI 2.20–8.66 mmHg, p=0.001) after adjustment for race, height, weight and study. However, at ages >45 years there were no significant differences in mPAP between males and females. There were no significant interactions between sex and age for the remainder of the haemodynamic measures (all p-values for interaction were >0.05). Males had higher PVR (1.23 Wood units, 95% CI 0.18–2.27 Wood units, p=0.02) on average compared with females. Males also tended to have lower CO than females (with adjustment for body size), but this association did not reach statistical significance (−0.21 L·min−1 difference, 95% CI −0.43–0.01; p=0.06). The results for cardiac index and PVRI without adjustment for height and weight were similar. Males had lower cardiac index compared with females, with a mean difference of −0.14 L·min−1·m−2 (95% CI −0.23–0.04 L·min−1·m−2; p=0.01). There was a sex by age interaction for PVRI (p<0.05), such that young males (age <45 years) had higher PVRI compared with young females (3.16 Wood units·m2 difference, 95% CI 0.77–5.55 Wood units·m2; p=0.01) without associations in the older ages. Figure 2 depicts estimated baseline mPAP, CO and PVR by multivariable linear regression, by sex and age group (age <45, 45–54, 55–64 and ≥65 years). In both males and females with idiopathic PAH, haemodynamic burden, particularly mPAP and PVR, tended to decrease with age.
TABLE 2.
Associations between sex and baseline haemodynamics in subjects with idiopathic pulmonary arterial hypertension, stratified by age group for mean pulmonary artery pressure
| Variable | Difference between males and females | 95% CI | p-value |
|---|---|---|---|
| Right atrial pressure mmHg | 1.36 | 0.44–2.27 | 0.004 |
| Mean pulmonary arterial pressure mmHg | |||
| Age <45 years | 5.43 | 2.20–8.66 | 0.001 |
| Age 45–54 years | 1.37 | −2.64–5.37 | 0.50 |
| Age 55–64 years | −1.45 | −5.70–2.81 | 0.50 |
| Age ≥65 years | 3.06 | −1.31–7.43 | 0.17 |
| Cardiac output L·min−1 | −0.21 | −0.43–0.01 | 0.06 |
| Pulmonary capillary wedge pressure mmHg | 0.19 | −0.38–0.77 | 0.51 |
| Pulmonary vascular resistance Wood units | 1.23 | 0.18–2.27 | 0.02 |
Adjusted for age (if no sex–age interaction), race, height, weight and study.
FIGURE 2.
Least-square means of baseline haemodynamics a) mean pulmonary artery pressure (mPAP), b) cardiac output (CO) and c) pulmonary vascular resistance (PVR) by sex and age with adjustment for race, height, weight and study in subjects with idiopathic pulmonary arterial hypertension. Markers show the point estimates and whiskers the 95% confidence interval.
We examined the smaller group of patients with CTD-associated PAH. Baseline characteristics for 61 males and 428 females are presented in online supplementary table S1. The CTD-associated PAH group was similar to the cohort with idiopathic PAH, except that those with CTD used less warfarin and tended to have lower mPAP and PVR, as has been previously described [38]. While limited by smaller sample size, male sex seemed to be associated with higher RAP in CTD-associated PAH (1.60 mmHg difference, 95% CI −0.04–3.25; p=0.06). There were no significant associations between sex and the remainder of the haemodynamic indices (online supplementary table E2). There were no obvious trends in baseline haemodynamic values across age, although the small number of patients (particularly males) in each age group limited the precision of these estimates (online supplementary fig. E1).
Discussion
We have shown that males have higher RAP and PVR compared with females with idiopathic PAH and may have lower CO and cardiac index after adjustment for covariates. Age appears to modify the relationship between sex and mPAP and PVRI. Younger males with PAH had higher mPAP and PVRI than females, but these differences were attenuated at age ≥45 years. Both males and females with idiopathic PAH tended to have lower mPAP and PVR in older age as compared with younger age. In the smaller cohort of patients with CTD-associated PAH, males appeared to have higher RAP than females. Taken together, these observations suggest that males with PAH enrolled in clinical trials have worse haemodynamics than females in these trials and that age may modify sex differences in pulmonary vascular disease. While the effect sizes are modest, the differences in baseline haemodynamic profiles between the sexes (higher RAP, mPAP, PVR/PVRI and lower CO/cardiac index in males as compared with females) may translate to significant differences in the risk of death [4–6]. For example, the differences in RAP and CO between males and females found in this study could be associated with 5–8% increases in mortality risk [4].
A recent registry of PAH patients also showed that males had higher RAP and mPAP at diagnosis, and that males had worse survival, especially in older age [5, 7]. Male sex has also been associated with mortality in European registries [4, 6]. It is biologically plausible that sex and sex hormones influence not only the development of pulmonary vascular disease, but also disease severity (i.e. haemodynamic impairment) and outcomes [15]. In fact, in animal models of pulmonary hypertension, females have less vascular remodelling and haemodynamic compromise than males and aged females develop less severe pulmonary vascular changes than male and younger animals, respectively [39–41]. As in our study, greater haemodynamic burden has been observed in younger patients (age <50 years) as compared with older patients in the UK and Ireland, although similar haemodynamics were noted in males and females in this cohort [42].
We considered age as a continuous measure (unless there was significant effect modification) in multivariable modelling, which is a different methodological approach than that used in prior studies. In the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL), Benza et al. [5] identified age >60 years in males as a significant predictor which was then validated as part of a risk stratification equation. Based on this work, Shapiro et al. [7] stratified the REVEAL cohort by age at enrolment (≤60 years versus >60 years), and then confirmed poorer 2-year survival among older males in the registry and worse haemodynamics among males in general. In that study, survival curves started to diverge at age 50 years, while we demonstrated an attenuation of sex differences in mPAP after age 45 years and less haemodynamic burden in aged males and females overall. Of course, it is difficult to compare our results to those from REVEAL and other registries, given the inherent differences between the populations sampled (i.e. registry participants with group 1 PAH from various aetiologies versus idiopathic patients from pooled clinical trials).
While there has been much focus on the role of oestrogen in explaining sex differences in PAH, androgens (namely, testosterone) may also be important. Higher levels of testosterone may be more detrimental to males compared with females, and testosterone is associated with maladaptive RV hypertrophy and fibrosis in murines and greater RV mass and volumes in an epidemiological cohort [22, 39, 40, 43, 44]. While androgen replacement in testosterone-deficient males has been studied in heart failure, a recently terminated trial of testosterone administration in aged males demonstrated an increased risk of cardiovascular events, highlighting the uncertainties that remain [45–47]. Therefore, sex differences in haemodynamics in pulmonary vascular disease may not be explained by oestrogen signalling alone.
The associations seen here pertain not only to disease burden in the pulmonary vascular bed (i.e. mPAP and PVR/PVRI), but to RV function specifically (i.e. RAP and CO/cardiac index). Haemodynamic and morphological parameters of RV function are important predictors of survival in PAH, and females tend to have more favourable RV indices than males [3, 8–10]. In health, males have accelerated cardiomyocyte loss with ageing, lower RV ejection fraction and larger age-related decrements in RV mass as compared with females, although age-related changes in RV function have not been studied in a large-scale manner in PAH [22, 48–50].
Age and the menopausal transition have long been implicated as potential risk modifiers in systemic cardiovascular disease [19, 51, 52]. Oestrogen has variable effects on the vasculature dependent on age, which may explain why the risk/benefit ratio of hormone therapy in post-menopausal females varies depending upon the age of the population studied (the so-called “timing hypothesis”) [20, 53]. Differences in tissue-specific oestrogen receptor expression, oestrogen-mediated genetic modifications and oestrogen metabolism have all been proposed as possible mechanisms to explain the time-dependent vascular effects of oestrogen [17, 54–58]. While we do not have specific data on menopausal status of the study subjects, our results suggest that young females tend to have higher mPAP and PVR as compared with older females but less haemodynamic impairment as compared with young males. Yuan et al. [17] have recently demonstrated an endogenous oestrogen deficiency following monocrotaline injury, leading to alterations in oestrogen receptor signalling and metabolism and a pro-proliferative, anti-apoptotic state that is rescued by exogenous oestradiol. These findings have interesting implications when considered in the context of female menopause and waning oestrogen levels, and given that our previous work demonstrated that hormone therapy use is associated with improved RV ejection fraction in healthy post-menopausal females [22]. In PAH, it is unknown whether the hormonal milieu before mid-life increases risk of disease, or perhaps favourably impacts RV adaptation and/or response to PAH therapy. Prior work using a subset of this same data source showed that females were more responsive to endothelin receptor antagonists than were males, although age did not modify the strength of this relationship [23].
This study has limitations. First, the pooled data analysed here are subject to limitations of the clinical trials. Measurement error in haemodynamics (if nondifferential with respect to sex and age) would bias toward the null, so that the true associations of sex and age with haemodynamics may be stronger than we have shown. There were some differences in the inclusion criteria (i.e. upper- and lower- limits for 6MWD) and PAH severity (e.g. functional class) across trials [24–34]. However, neither distance walked nor functional class would impact the exposure variable (sex) and so are unlikely to confound the exposure–outcome (sex–haemodynamic) relationship in the pooled analysis. It is possible that sex differences and the haemodynamic trends across age groups observed were subject to survivor or lead time bias, i.e. that young patients with the greatest haemodynamic impairment succumb early and are therefore not recruited into clinical trials, leaving only the older patients with less haemodynamic derangement. This could be especially true for males, given that sex has been associated with a diagnostic delay in other chronic pulmonary diseases, although sex and timing of diagnosis do not appear to be related in PAH [59, 60]. Our results may be subject to selection bias introduced by the enrolment of certain subjects in clinical trials (i.e. younger patients) over others (i.e. older patients with advanced PAH), although there were reasonable numbers of patients in the ≥65 years age group (fig. 2 and online supplementary fig. E1). Registry data suggest that younger age is associated with a delay in PAH diagnosis, but how this may impact subjects enrolled into clinical trials is not known [60]. Finally, the relatively small numbers of CTD-associated patients, particularly males, limits our ability to draw firm conclusions about the relationships between sex, age and haemodynamics in CTD-associated PAH, and it is important to note that age has previously been shown to amplify the risk of PAH and to worsen disease severity in systemic sclerosis [61, 62].
In conclusion, we have shown that males with idiopathic PAH have higher RAP, mPAP and PVR compared with females, and lower CO/cardiac index. Future work exploring sexual dimorphism in PAH should consider the impact of age on the sex hormone–pulmonary vascular relationship, and define the impact of androgens in males with PAH.
Supplementary Material
Acknowledgments
Support statement: Funding was provided by the American Heart Association (grant number 11FTF7400032), ASPIRE Pulmonary Vascular Disease Young Investigator Research Award (grant number WS1952812) and National Institutes of Health (grant number K24 HL103844).
The authors would like to thank Norman Stockbridge at the US Food and Drug Administration (Silver Spring, MD, USA) for providing us with the data to make this study possible.
Footnotes
Conflict of interest: Disclosures can be found alongside the online version of this article at www.erj.ersjournals.com
This article has supplementary material available from www.erj.ersjournals.com
References
- 1.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 3.Kawut SM, Al-Naamani N, Agerstrand C, et al. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest. 2009;135:752–759. doi: 10.1378/chest.08-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 5.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry To Evaluate Early And Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 6.Escribano-Subias P, Blanco I, Lopez-Meseguer M, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J. 2012;40:596–603. doi: 10.1183/09031936.00101211. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro S, Traiger GL, Turner M, et al. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the Registry To Evaluate Early And Long-Term Pulmonary Arterial Hypertension Disease Management. Chest. 2012;141:363–373. doi: 10.1378/chest.10-3114. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 9.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 10.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 11.Austin ED, Cogan JD, West JD, et al. Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J. 2009;34:1093–1099. doi: 10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835–842. doi: 10.1164/rccm.200809-1472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahm T, Crisostomo PR, Markel TA, et al. Selective estrogen receptor-alpha and estrogen receptor-beta agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1486–R1493. doi: 10.1152/ajpregu.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White K, Johansen AK, Nilsen M, et al. Activity of the estrogen-metabolizing enzyme cytochrome p450 1b1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 15.Paulus JK, Roberts KE. Oestrogen and the sexual dimorphism of pulmonary arterial hypertension: a translational challenge. Eur Respir J. 2013;41:1014–1016. doi: 10.1183/09031936.00021713. [DOI] [PubMed] [Google Scholar]
- 16.Umar S, Iorga A, Matori H, et al. Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med. 2011;184:715–723. doi: 10.1164/rccm.201101-0078OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan P, Wu WH, Gao L, et al. Oestradiol ameliorates monocrotaline pulmonary hypertension via NO, prostacyclin and endothelin-1 pathways. Eur Respir J. 2013;41:1116–1125. doi: 10.1183/09031936.00044112. [DOI] [PubMed] [Google Scholar]
- 18.Hyduk A, Croft J, Ayala C, et al. Pulmonary hypertension surveillance – United States, 1980–2002. MMWR Surveill Summ. 2005;54:1–28. [PubMed] [Google Scholar]
- 19.Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–2641. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- 20.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 21.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 22.Ventetuolo CE, Ouyang P, Bluemke DA, et al. Sex hormones are associated with right ventricular structure and function: the MESA-Right Ventricle study. Am J Respir Crit Care Med. 2011;183:659–667. doi: 10.1164/rccm.201007-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabler NB, French B, Strom BL, et al. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest. 2012;141:20–26. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the Ambrisentan In Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy (ARIES) Study 1 And 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 25.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 26.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 27.Olschewski H, Simonneau G, Galié N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 28.Olschewski H, Hoeper MM, Behr J, et al. Long-term therapy with inhaled iloprost in patients with pulmonary hypertension. Respir Med. 2010;104:731–740. doi: 10.1016/j.rmed.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 30.Galié N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 31.Barst RJ, Langleben D, Frost A, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 32.Barst RJ, Langleben D, Badesch D, et al. Treatment of pulmonary arterial hypertension with the selective endothelin-a receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006;47:2049–2056. doi: 10.1016/j.jacc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 33.Sandoval J, Torbicki A, Souza R, et al. Safety and efficacy of sitaxsentan 50 and 100 mg in patients with pulmonary arterial hypertension. Pulm Pharmacol Ther. 2012;25:33–39. doi: 10.1016/j.pupt.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Simonneau G, Barst RJ, Galié N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 35.Fisher MR, Mathai SC, Champion HC, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 36.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103–115. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 37.Haring R, Hannemann A, John U, et al. Age-specific reference ranges for serum testosterone and androstenedione concentrations in women measured by liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2012;97:408–415. doi: 10.1210/jc.2011-2134. [DOI] [PubMed] [Google Scholar]
- 38.Castelain V, Chemla D, Humbert M, et al. Pulmonary artery pressure–flow relations after prostacyclin in primary pulmonary hypertension. Am J Respir Crit Care Med. 2002;165:338–340. doi: 10.1164/ajrccm.165.3.2106033. [DOI] [PubMed] [Google Scholar]
- 39.McMurtry IF, Frith CH, Will DH. Cardiopulmonary responses of male and female swine to simulated high altitude. J Appl Physiol. 1973;35:459–462. doi: 10.1152/jappl.1973.35.4.459. [DOI] [PubMed] [Google Scholar]
- 40.Rabinovitch M, Gamble WJ, Miettinen OS, et al. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol Heart Circ Physiol. 1981;240:H62–H72. doi: 10.1152/ajpheart.1981.240.1.H62. [DOI] [PubMed] [Google Scholar]
- 41.Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol. 2001;280:L88–L97. doi: 10.1152/ajplung.2001.280.1.L88. [DOI] [PubMed] [Google Scholar]
- 42.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the Pulmonary Hypertension Registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 43.Hemnes AR, Maynard KB, Champion HC, et al. Testosterone negatively regulates right ventricular load stress responses in mice. Pulm Circ. 2012;2:352–358. doi: 10.4103/2045-8932.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Wu S, Wei H, et al. Effects of sex hormones and their balance on the proliferation of rat vascular endothelial cells. Horm Res Paediatr. 2002;58:16–20. doi: 10.1159/000063211. [DOI] [PubMed] [Google Scholar]
- 45.Khaw K, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 46.Toma M, McAlister FA, Coglianese EE, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012;5:315–321. doi: 10.1161/CIRCHEARTFAILURE.111.965632. [DOI] [PubMed] [Google Scholar]
- 47.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. New Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawut SM, Lima JAC, Barr RG, et al. Sex and race differences in right ventricular structure and function: the Multi-Ethnic Study Of Atherosclerosis-Right Ventricle Study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallat Z, Fornes P, Costagliola R, et al. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J Gerontol A Biol Sci Med Sci. 2001;56:M719–M723. doi: 10.1093/gerona/56.11.m719. [DOI] [PubMed] [Google Scholar]
- 50.Ventetuolo CE, Barr RG, Bluemke DA, et al. Selective serotonin reuptake inhibitor use is associated with right ventricular structure and function: the MESA-Right Ventricle Study. PLoS One. 2012;7:17. doi: 10.1371/journal.pone.0030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 52.Wellons M, Ouyang P, Schreiner PJ, et al. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 54.Wang JM, Hou X, Adeosun S, et al. A dominant negative ERβ splice variant determines the effectiveness of early or late estrogen therapy after ovariectomy in rats. PLoS One. 2012;7:13. doi: 10.1371/journal.pone.0033493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 56.Shearman AM, Cupples LA, Demissie S, et al. Association between estrogen receptor alpha gene variation and cardiovascular disease. JAMA. 2003;290:2263–2270. doi: 10.1001/jama.290.17.2263. [DOI] [PubMed] [Google Scholar]
- 57.Zhu Y, Bian Z, Lu P, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 58.Peter I, Kelley-Hedgepeth A, Huggins GS, et al. Association between arterial stiffness and variations in oestrogen-related genes. J Hum Hypertens. 2009;23:636–644. doi: 10.1038/jhh.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nick JA, Chacon CS, Brayshaw SJ, et al. Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2010;182:614–626. doi: 10.1164/rccm.201001-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown LM, Chen H, Halpern S, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL registry. Chest. 2011;140:19–26. doi: 10.1378/chest.10-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schachna L, Wigley FM, Chang B, et al. Age and risk of pulmonary arterial hypertension in scleroderma. Chest. 2003;124:2098–2104. doi: 10.1378/chest.124.6.2098. [DOI] [PubMed] [Google Scholar]
- 62.Hachulla E, Launay D, Mouthon L, et al. Is pulmonary arterial hypertension really a late complication of systemic sclerosis? Chest. 2009;136:1211–1219. doi: 10.1378/chest.08-3042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.