Abstract
Stem-cell-mediated bone repair has been used in clinical trials for the regeneration of large craniomaxillofacial defects, to slow the process of bone degeneration in patients with osteonecrosis of the femoral head and for prophylactic treatment of distal tibial fractures. Successful regenerative outcomes in these investigations have provided a solid foundation for wider use of stromal cells in skeletal repair therapy. However, employing stromal cells to facilitate or enhance bone repair is far from being adopted into clinical practice. Scientific, technical, practical and regulatory obstacles prevent the widespread therapeutic use of stromal cells. Ironically, one of the major challenges lies in the limited understanding of the mechanisms via which transplanted cells mediate regeneration. Animal models have been used to provide insight, but these models largely fail to reproduce the nuances of human diseases and bone defects. Consequently, the development of targeted approaches to optimize cell-mediated outcomes is difficult. In this Review, we highlight the successes and challenges reported in several clinical trials that involved the use of bone-marrow-derived mesenchymal or adipose-tissue-derived stromal cells. We identify several obstacles blocking the mainstream use of stromal cells to enhance skeletal repair and highlight technological innovations or areas in which novel techniques might be particularly fruitful in continuing to advance the field of skeletal regenerative medicine.
Introduction
Bone has an innate propensity to regenerate following traumatic injury. Upon fracture, resident stromal, stem and progenitor cells work in tandem with pro-inflammatory and anti-inflammatory macrophages1,2 and circulating blood cells3 to orchestrate a complex signalling cascade that leads to scarless healing.4 In spite of this tremendous capability, a number of clinical indications remain that require therapeutic intervention to facilitate bone repair and regeneration. Autologous bone grafting, in which bone from another part of the body is transplanted to the defect site, remains the gold standard; however, this approach is associated with numerous drawbacks, including donor-site morbidity, the availability of limited grafting material and compromised bone quality in patients with osteoporosis.5 Bone-tissue engineering (BTE) has been developed as a potential alternative to overcome the critical shortcomings associated with autografts and allografts. In general, BTE involves the use of various combinations of cells, growth factors and/or cytokines, and bioactive carriers (scaffolds and/or hydrogels). Even though it has been ~30 years since the first efforts in this area,6 few BTE techniques have translated into clinical practice and none of them has become the standard of care in regenerative medicine.
This Review focuses specifically on the successes and challenges of using stromal or stem cells in the clinical translation of BTE techniques. Some controversy remains over the specification of adipose-tissue-derived and bone-marrow-derived progenitors as stem cells. Although the authors consider that each of the two descriptions has merits, these cells will be referred to in the remainder of this Review as stromal cells. Currently, the role of transplanted stromal cells in mediating regeneration remains poorly understood, particularly in the clinical trials that have been conducted. The original premise of many early in vitro and preclinical studies was that transplanted cells would undergo differentiation and morphogenesis to form the regenerated tissue; however, this paradigm has been challenged by experimental findings documenting that very few regenerative cells actually survive following transplantation.7 In spite of the clear benefits associated with cell delivery, the poor mechanistic understanding of stem-cell-mediated regeneration is an obstacle to optimizing regenerative approaches. Animal models have the potential to provide some insight; however, many of the available models do not effectively recapitulate the clinical situation, which is either due to the size of the defects or the timing of cell delivery relative to when the defect was created. In addition to the lack of mechanistic insight, logistical, regulatory and technical challenges continue to limit the clinical application of stromal and stem cells for skeletal regeneration. In this Review, we briefly discuss the history of stromal cells, their use in clinical trials, the challenges facing their widespread implementation and current approaches to bone regeneration that are based on stromal and stem cells. This Review also highlights novel technologies and future studies that are needed to establish stromal-cell-mediated and stem-cell-mediated BTE as a standard component of clinical care.
Stromal cells
Historical and developmental relationships
Pioneering reports in the 1960s by Alexander Friedenstein and colleagues at the University of Moscow laid the foundations for the modern era of multipotent-stromal-cell and mesenchymal-stem-cell (MSC) research.8–10 Friedenstein’s team was the first to demonstrate that bone marrow contains fibroblast-like stromal cells, termed mechanocytes, which are capable of osteogenic differentiation and are necessary for the creation of the haematopoietic microenvironment or niche.8 Additionally, the researchers demonstrated that similar cell populations are present in the thymus, liver and other organs. These findings were advanced in the 1980s by the demonstration that primary cultures of bone-marrow-derived stromal cells are adipogenic and chondrogenic.11,12 During the same period, techniques for the long-term culture of primary bone marrow cells were developed.13,14 Adherent stromal-cell populations, which are required to support proliferation and differentiation of haematopoietic progenitors and stem cells, were also shown to be capable of mediating adipogenesis in the presence of glucocorticoid-containing horse serum.15 In pursuit of these findings, haematologists across the globe developed clonal cell lines from adherent stromal cells in long-term cultures, which were isolated from bone marrow, spleen, liver and other tissues capable of supporting B-cell lymphopoiesis.16–18 When co-cultured in the presence of stromal cell clones, haematopoietic stem cells and progenitor cells (which routinely died in culture) were able to proliferate and differentiate as a result of the release of as yet unidentified growth factors. In many cases, stromal-cell clones went on to be used as critical reagents for the isolation and characterization of haematopoietic cytokines, such as IL-7 and IL-11.19–21
The stromal-cell field was advanced in the early 1990s by the adoption of the term ‘mesenchymal stem cells’ to classify the adherent bone marrow cells that are characterized by their ability to differentiate along the adipocyte, chondrocyte, osteoblast, skeletal myocyte and tenocyte pathways.22,23 Several research groups were among the first to generate monoclonal antibodies against the human bone-marrow-derived mesenchymal-stromal and stem-cell (MSC) surface antigens STRO-1, CD73 (which targets the Src homology [SH] 3 and SH4 domains) and CD105 (which targets the SH2 domain).24,25 Some researchers have argued that MSCs do not meet the scientific standards required to define them as stem cells, as no reports have documented their ability to be serially passaged through multiple recipients whilst retaining tissue-generating functionality. As a compromise, in 2006, the International Society for Cellular Therapy issued a consensus statement defining MSCs as ‘multipotent stromal cells’ on the basis of the following criteria: firstly, capability of plastic adherence and self-renewal in culture; secondly, staining positive for CD73, CD90 and CD105, and staining negative for CD11B or CD14, CD19 or CD79α, CD34, CD45 and HLA-DR; and thirdly, differentiating along the adipocyte, chondrocyte and osteoblast pathways in vitro.26 At present, these criteria have been used to define MSCs in the majority of the published literature; however, questions have been raised as to whether or not this in vitro evidence is sufficient for the characterization of MSCs as stem cells. The growing number of MSC filings at the FDA have employed an ever-widening array of surface antigenic biomarkers.27 The inconsistency of these characterizations has been complicated by the fact that an increasing percentage of MSC products are derived from tissues other than bone.27 The FDA consortium has suggested that additional bioactivity assays, which involve proteomic analysis of membrane proteins, and adipocyte differentiation be used to identify and define MSCs that are isolated from these various tissues.27–31 Some researchers have long advocated that the in vivo criteria should constitute the ‘gold standard’ for MSC definition.32 These researchers and others have documented the robust ability of bone marrow MSCs to form mineralized bone that contains a haematopoietic marrow after insertion on a hydroxyapatite or related scaffold and implantion subcutaneously in rats.32 Although the pharmaceutical and biotechnology industries continue to pursue in vitro surrogate assays, the in vivo assay of bone differentiation remains the definitive standard for many academic laboratories.33
Dependence on tissue of origin
Multiple independent studies have isolated cells with MSC-like characteristics from amniotic, placental and umbilical-cord tissues, as well as adult adipose, dental, dermal and skeletal muscle tissues.34–43 Tissue-derived MSCs have been isolated and/or identified, in part, on the basis of their adherence properties to tissue culture surfaces and by flow cytometric sorting on the basis of the surface antigens they express.24,43–45 Perivascular cells isolated from multiple tissues on the basis of their expression of CD146, chondroitin sulphate proteoglycan 4 (commonly known as NG2) and platelet-derived growth factor receptor β display clonal multipotency and express mesenchymal markers, which suggests that stromal cells and pericytes are functionally equivalent.46 A limited number of studies have directly compared stromal cells that were isolated from distinct tissues. Although failing to demonstrate substantial differences in immunophenotype or morphology, an initial comparison of cells that were isolated from adipose tissue, bone marrow and umbilical cord found a greater frequency of colony-forming units in adipose-tissue-derived cells than in bone-marrow-derived and umbilical-cord-derived cells and greater proliferative and inferior adipogenic capacities in umbilical-cord-derived cells than in adipose-tissue-derived and bone-marrow-derived cells.42 A later analysis determined that although adipose-tissue-derived stromal cells (ASCs) shared multiple features in common with their bone-marrow counterparts, ASCs display subtle differences in their immunophenotypic profile.47 These differences have been encapsulated in a joint International Society for Cellular Therapy and International Federation for Adipose Therapeutics and Science consensus statement.48 ASCs have demonstrated osteogenic potential in vitro and in vivo both in preclinical models and in clinical trials.34,49–55 In vitro studies have demonstrated that the extent of ASC osteogenesis can be enhanced by manipulating the concentrations of ascorbate and dexamethasone in cell-culture media.56 Nevertheless, some studies have highlighted concerns that the osteogenic capacity of ASCs is significantly lower than that of MSCs isolated from bone.57,58 In spite of this concern, the ease of accessibility and relative abundance of ASCs in comparison to that of MSCs confer practical advantages and have contributed to the continued interest in the clinical use of these cells in bone regeneration.52,54,59 Whether or not the tissue of origin regulates the epigenetic memory of MSCs and their subsequent differentiation potential remains to be determined.60
Skeletal-muscle-derived stem cells (MDSCs) are distinct from the resident satellite cells, which are stimulated upon muscle damage to repair the tissue. MDSCs, isolated from muscle tissues on the basis of expression of CD34 and apoptosis regulator Bcl-2, demonstrate robust osteogenic capacity and thus have potential for use in skeletal repair.61 Similarly to MSCs and ASCs, the MDSC population exhibits multipotency, can regenerate various tissues in vivo and can secrete a number of trophic factors that are capable of stimulating endogenous repair.62,63 However, as a therapy for bone defects, MDSCs lag behind ASCs and MSCs and they are not currently being investigated in clinical trials.
Skeletal regeneration
The field of skeletal regeneration continues to face multiple challenges that would potentially benefit from approaches that involve cell-based therapeutics. Of these challenges, the most common is acute trauma, which accounts for the majority of orthopaedic surgical procedures in the USA and internationally. The body responds to trauma by initiating a cascade of inflammatory and regenerative events. Sequentially, these actions include local and systemic release of proinflammatory cytokines, homing of immune cells to the site of injury, soft-tissue inflammation and oedema, mobilization of osteogenic progenitor cells, local release of bone morphogenetic proteins, callus formation, bone remodelling and eventual bone replacement. A rationale exists for introducing exogenous MSCs during one or more of these events. Immediately following the acute injury (hours to days), MSCs can dampen or modulate local and systemic inflammatory responses by producing immunosuppressive factors, such as transforming growth factor β, prostaglandin E2 and indoleamine 2,3-dioxygenase 1 (commonly known as IDO). Additionally, the release of stromal cell-derived factor 1 (also known as SDF-1) and other cytokines from MSCs can alter the types of immune cells that are recruited to the site of injury. During intermediate periods (from days to weeks) following the injury, MSCs can contribute to the repair process by differentiating into chondrocytes and osteoblasts, thereby augmenting the recruitment of local endogenous osteoprogenitor cells. Although whether exogenous MSCs have a substantial benefit when introduced late (from weeks to months) following acute trauma remains to be determined, at least one instance in which late delivery of MSCs can be beneficial is delayed union or non-union of bone.
Non-union can occur in up to 15% of cases of complex trauma as a result of mechanical factors, as seen in comminuted fractures with multiple bone fragments; infection, as seen with bacterial contamination of the injury site or a patient’s underlying viral diseases (for example, hepatitis and HIV); smoking and other tobacco-related or drug-related toxins; and endocrine disorders, such as type 2 diabetes mellitus, obesity, osteopenia and osteoporosis. Finally, exogenous MSCs have the potential to benefit treatment of bone-related tumours, such as Ewing sarcoma, osteosarcoma and metastatic bone disease. The introduction of a healthy exogenous MSC population might enable bone metabolism to recover more rapidly following chemotherapy, radiation and/or surgical ablation of the tumour by improving the local microenvironment. Additionally, MSCs that are genetically modified to deliver specific proteins, radioisotopes or microRNAs can be used as antitumour vectors owing to their ability to home to sites of active primary or meta-static cancers.64–71 Nevertheless, as MSCs can promote the proliferation of breast, prostate and other tumours in vitro and in vivo,72,73 preclinical safety studies need to be performed before using native or genetically modified MSCs in the context of bone tumours. The safety concerns regarding the use of ASCs or MSCs, which include tumour formation in bone regeneration applications, are significantly lower than those regarding the use of pluripotent stem cells (embryonic stem cells or induced pluripotent stem cells). To date, MSCs and ASCs have been used in a small number of studies for bone regeneration in humans (Table 1). In the majority of studies, autologous cells (with or without prior expansion in vitro) were either directly injected into the defect site or injected with the aid of biomaterial carriers. Owing to the immunoprivileged characteristics of ASCs and MSCs, a number of clinical trials are also currently being performed with genetic cells.
Table 1.
Clinical trials in which stromal cells were used for skeletal regeneration
| Indication | Cell source | Cell processing and delivery | Clinical trial |
|---|---|---|---|
| MSC | |||
| Non-union of bone | Autologous | Direct injection | NCT00512434,134 NCT01206179,135 NCT01429012,136 NCT01788059137 |
| Implantation with carrier | NCT00250302,138 NCT01435434,139 NCT02177565,140 NCT01626625,141 NCT01842477,142 NCT01725698,143 NCT01958502144 | ||
| ONFH | Autologous | Direct injection | NCT02065167,145 NCT01700920,146 NCT01544712147 |
| Implantation with carrier | NCT01605383148 | ||
| Other (spine fusion, osteoarthritis) | Autologous | Direct injection | NCT01210950149 |
| Implantation with carrier | NCT01552707,150 NCT01389661151 | ||
| Allogeneic | Direct injection | NCT01603836,152 NCT02172885,153 NCT00186914154 | |
| Implantation with carrier | NCT00001391,155 NCT01207193,156 NCT00221130157 | ||
| ASC | |||
| Non-union of bone | Autologous | Implantation with carrier | NCT01532076158 |
| Allogeneic | Direct injection | NCT02140528159 | |
| ONFH | Autologous | Direct injection | NCT01643655160 |
| Other (spine fusion, osteoarthritis) | Autologous | Direct injection | NCT01501461,161 NCT01739504,162 NCT01585857,163 NCT01885819,164 NCT02241408,165 NCT01885832,166 NCT02142842,167 NCT01947348168 |
| Implantation with carrier | NCT01633892,169 NCT01645722,170 NCT01218945171 | ||
Abbreviations: ASC, adipose-tissue-derived stromal cell; MSC, bone-marrow-derived stromal cell; ONFH, osteonecrosis of the femoral head.
Clinical trials
Craniofacial bone regeneration
Ectopic vascularized bone formation
The treatment of large craniofacial defects presents unique challenges owing to the complex 3D geometry of bone. The current gold standard for the treatment of large bone defects is autologous bone transplantation, which can be vascularized or nonvascularized. In nonvascularized bone, the lack of vasculature can lead to graft resorption with resultant loss of the geometric structure of bone. To transplant vascularized, autologous bone, the surgeon has to painstakingly dissect out suitable portions of the patient’s own iliac crest, fibula or ribs, shape them into an approximate anatomical shape and then use microsurgical techniques to restore the blood supply. This immediate supply of oxygen and nutrients is critical for graft survival and long-term integration. Consequently, one clinical approach to utilize BTE grafts incorporates an in vivo cultivation period. The grafts are implanted into large, highly vascularized muscle tissues (for example, latissimus dorsi74 or rectus abdominis75) for several months to facilitate vascular ingrowth and the development of a vascular paedicle suitable for microsurgical anastomosis. To achieve this vascular growth, a preshaped titanium mesh is used to enclose mineralized matrix (for example, autograft cancellous bone chips or xenograft bone blocks), osteoinductive recombinant human (rh) growth factors (such as rhBMP-2 or rhBMP-7) and cells (Figure 1). Both bone-marrow aspirates for mandibular reconstruction74 and ASCs expanded using good manufacturing practice (GMP) standards to treat maxillary defects75 have been successfully used to regenerate craniofacial bone with this methodology. The resulting bone had sufficient structural integrity to support dental implants 4 months following surgical reconstruction of the defect with the BTE graft.75
Figure 1.
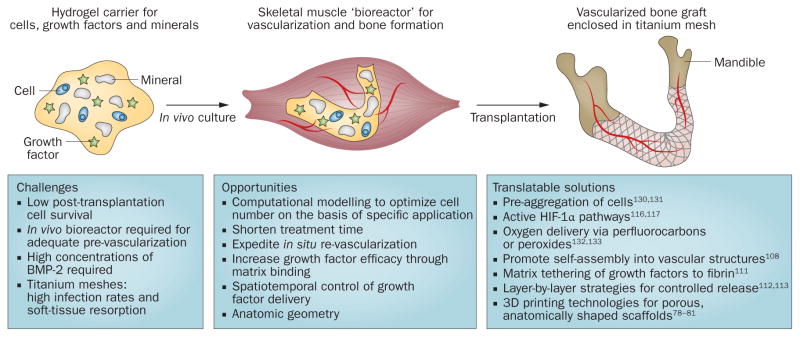
Generalized clinical approach for stem-cell-based regeneration of large craniofacial bone defects. Adipose-tissue-derived stromal and/or stem cells are mixed with growth factors (such as BMP-2) and combined with mineral blocks in a preshaped titanium mesh, cultured in vivo and transplanted to repair the bone defect (the mandible is shown as an example). Abbreviations: BMP-2, bone morphogenetic protein 2; HIF-1α, hypoxia-inducible factor 1α.
In situ bone formation
In spite of the considerable successes of the two-step process described above, ectopic bone formation requires additional surgeries, which increases the risk of comorbidities and involves a substantial investment in time to facilitate new mineral deposition in the graft. Therefore, several groups have taken a single-step approach to form new, structurally sound bone matrix in orthotopic sites. Treatment of a 10 cm anterior mandibular defect (left after tumour excision) using additive manufacturing recreated the exact geometry of a patient’s mandible from radiographic images.76 A titanium mesh was then prefabricated with the customized patient geometry and filled with β-tricalcium phosphate (β-TCP) granules that were soaked in rhBMP-2 for 48 h before implantation of ASC cultures (expanded under GMP conditions). By 10 months post-surgery, sufficient new bone had developed to support dental implants. A similar in situ approach was used to treat calvarial defects in a child with multiple fractures.77 The surgical team applied milled bone from the iliac crest, ASCs isolated from fat tissue harvested from the gluteal region and autologous fibrin glue, which was used to hold the cells and milled bone grafts in place. The patient displayed bone regeneration within 3 months of surgery. Although the patient numbers were limited and the studies were not randomized with double-blinded controls, these case reports indicate that MSCs can be used successfully to repair defects in nonweight-bearing craniofacial bones.76,77 In both cases, a titanium mesh was used to provide the regenerated bone with the appropriate anatomical geometry, whereas the granules, cancellous bone chips or bone blocks were osteoconductive and osteoinductive. Advanced biomaterial scaffolds capable of integrating these structural elements and biological signals can also be used to guide new bone growth (discussed elsewhere78–81).
Distal tibial non-union
In addition to the treatment of craniofacial defects, which requires highly invasive procedures, the clinical relevance of stromal cells in minimally invasive procedures has also been assessed. Specifically, non-unions and delayed fracture healing, in which a deficiency of fracture repair exists, are ideal situations in which to harness the regenerative potential of stromal and/or stem cells. In a randomized controlled clinical study, which included 24 patients who were considered to be at low risk of non-unions of the tibia, the prophylactic effects of MSCs in expediting fracture healing were assessed.82 Autologous MSCs (~108) isolated from the iliac crest and peripheral blood were injected into the fracture site together with platelet-rich plasma (containing 1.1 × 109 platelets) and allograft demineralized bone matrix. This treatment resulted in a significant reduction in the time to union, from 3.0 months to 1.5 months in the intervention group of patients who received the biological composite compared with the control group of patients who did not receive this treatment. Additionally, subcutaneous grafting of a portion of the injectable composites into immunodeficient mice resulted in bone formation; however, as the authors were unable to assess the origin of the newly formed bone in vivo, the precise role of MSCs in promoting the improved response remains unclear.
Osteonecrosis of the femoral head
Another potential minimally invasive application of stromal cells is the treatment of osteonecrosis of the femoral head (ONFH).83–86 Nontraumatic ONFH is a debilitating skeletal disorder that can lead to collapse of the femoral head and the need for total hip replacement. In 2004, a double-blind nonrandomized study was conducted to assess the effect of delivering autologous bone marrow mononuclear cells (following core decompression of the lesion) to patients with stage I or stage II ONFH.84 Cells were injected directly into the defect site via a trephine without any scaffold or hydrogel to enhance retention at the site. Within 3 months, a statistically significant reduction in the lesion-volume:femoral-head-volume ratio was observed in the cell-grafting group compared with the untreated control group. Further decreases in this volume ratio occurred by 24 months, which indicated the possibility of a slight progression in healing over time. At 60 months of follow-up, the number of patients who progressed to fracture in the cell-grafting group was markedly decreased compared with those in the control group;83 however, the use of core decompression might have introduced additional trauma to the region. A less-invasive approach, in which iliac-crest-derived bone marrow mononuclear cells (BMMCs) are delivered through the medial circumflex femoral artery (using fluoroscopy to locate the injection site) was subsequently developed.85 The 62 patients in this study were all treated with autologous BMMCs, which enabled the safety, but not the efficacy, of the treatment to be assessed. One limiting factor in these approaches is the low concentration of MSCs in bone-marrow aspirates from the iliac crest. This limitation was overcome and the safety and efficacy of the approach was demonstrated in a randomized trial of 100 patients by use of culture-expanded autologous BMMCs that were isolated from the subtrochanteric region (aspirated through the decompression tunnel) together with iliac-crest-derived BMMCs.86
Limitations, challenges and opportunities
Animal models
The available clinical data strongly support an enhanced regenerative effect of stromal cell delivery to the site of bone defects. However, advancing these approaches in order to bring them into the realm of standard clinical care requires insight into the underlying mechanisms of cell-mediated effects. Animal models provide the best proxy to investigate cellular mechanisms. Small rodent models are, traditionally, the first choice to assess in vivo responses. Cranial defects in mice have been used to demonstrate bone regeneration following the delivery of ASCs.87–89 By use of fluorescent in situ hybridization staining of female (donor) chromosomes in male recipient mice,89 up to 99% of new bone was seen to be formed by the transplanted cells. An important consideration, however, is that the mouse calvarium is <500 μm thick and, therefore, oxygen gradients throughout the graft that might lead to the generation of hypoxic regions in the core are not a complication. Consequently, cell survival in this model is not heavily dependent on revascularization, which does not mimic the reality of the clinical situation. The model of femoral defects in rats shows greater reliance on sufficient revascularization to facilitate bone healing than that in mice.90 Using this model, transplanted cells were confirmed to enhance regeneration even though they are not incorporated into the new bone.7 Such reports provide indirect evidence that the primary mechanism through which transplanted stem cells mediate tissue repair might involve the secretion of paracrine factors that stimulate the recruitment and activation of endogenous stem cells.91,92
The critical dependence on trophic factors to facilitate regeneration rather than to direct differentiation, tissue morphogenesis and integration of transplanted cells is further supported by studies in which blocking VEGF signalling impaired stem-cell-mediated bone repair.93–95 Hypoxia, acting through the hypoxia-inducible factor 1 (HIF-1) transcription factor, reduced the expression of vascular endothelial growth factor receptor 1 (VEGFR-1; commonly known as FLT-1) on bone marrow MSCs.94 Similarly, in vitro studies using murine muscle-derived stem cells showed that overexpression of soluble FLT-1, a VEGF antagonist, promoted chondrogenesis in pellet cultures.95 Overexpression of soluble FLT-1 improved articular cartilage repair in vivo, whereas overexpression of VEGF-A165 (the main biological isoform of VEGF-A) led to arthritic changes in the joint, which were consistent with hypertrophic cartilage formation.95 Furthermore, gain-of-function and loss-of-function expression studies in the mouse embryo have shown that VEGF, released by the limb bud mesenchyme, is required for the development of the skeletal vasculature.93
Long-bone defects in larger animal models such as dogs and sheep might be better approximations of the clinical scenario than similar defects in mouse. Treatment of 3 cm full-thickness, segmental defects in 6–7-year-old sheep by use of polycaprolactone–tricalcium phosphate (PCL–TCP) scaffolds with nonautologous, culture-expanded MSCs or rhBMP-7 has been reported.96 In this study, rhBMP-7 elicited the greatest new bone formation, whereas sheep that received MSCs showed similar healing to the group that received PCL-TCP only. Large animal models might also be used to assess bone healing in skeletally immature animals. For example, a porcine model was used to evaluate the effect of BMP-7 delivery on bone healing in an immature pig.97 The porcine model has also been used to assess the effect of direct or indirect ASC delivery to noncritical-sized bone defects in the mandible.98 None of these models precisely mimics the challenges involved in regeneration of large craniofacial defects, non-unions or ONFH in humans. At this time, no single species has been identified as the ‘gold standard’ preclinical animal model for human skeletal regeneration. Consequently, future studies to investigate the mechanisms of stromal-cell-mediated bone repair will continue to rely on a variety of small and large animal models.
Cell survival and vascular integration
Widespread adoption of BTE grafts as the clinical standard of care for the treatment of massive bone defects requires the development of simple and effective techniques to rapidly vascularize grafts and enhance the survival of transplanted stromal cells. To address this need, multiple groups have investigated methods of prevascularization to harness the proangiogenic potential of endothelial cells, which are co-cultured with pro- osteogenic stromal cells. Endothelial cells have the ability to self-assemble into primitive capillary-like networks in response to proangiogenic growth factors in permissive hydrogel environments. Ideally, these nascent networks can anastomose with blood vessels, which infiltrate the defect site from its periphery. Once perfused, the resulting blood flow stimulates maturation and subsequent pruning of the vasculature. In purely vascular tissue-engineering systems, these nascent networks have been shown to remain viable for up to 1 year after intervention.99 Several groups have employed this technique using endothelial cells and either MSCs or ASCs to engineer vascularized bone grafts in preclinical models.100–104 The main limitation of this technique is the extensive in vitro manipulation and precultivation required to facilitate blood vessel development and mineralization. Angiogenesis and osteogenesis are tightly coupled processes during bone development and healing. However, the development of vascularized osteogenic grafts has resulted in the requirement for separate cultivation conditions for cells, followed by a reintegration phase. The extensive manipulation and use of multiple culture conditions and growth factors might continue to substantially limit the clinical utility of this approach. Cell pre-aggregation methods, which enhance cell survival following transplantation105–107 and harness the potential of stromal cells to self-assemble into complex tissues,4,108 might facilitate translation of this approach into clinical practice. These techniques could be combined with the development of biomaterials capable of time-released, serial delivery of angiogenic factors followed by delivery of osteogenic factors; biomaterials with these attributes have already been described.109–113
Rapid vascularization is essential for graft viability. In the absence of an immediate vascular supply to BTE grafts in the defect site (as was the case in the clinical trials conducted to date), transplanted cells are immediately exposed to severe hypoxia and ischaemia. To minimize the negative effect of hypoxia, several groups have investigated the potential of localized oxygen delivery using perfluorocarbons or peroxide-based scaffolds. Perfluorocarbons are capable of delivering oxygen for 2–3 h before becoming depleted.114 Revascularization occurs over a period of 1–2 weeks in clinically sized grafts and bone formation takes months. In spite of this obvious mismatch, delivery of oxygen for just a few hours after transplantation resulted in statistically significantly enhanced bone formation within 6 weeks in a murine model.115 In contrast to perfluorocarbons, oxygen-generating peroxide scaffolds are able to continuously supply oxygen for weeks to months. The main drawback of peroxides is that they produce reactive oxygen species (ROS), which are detrimental to cell viability. Thus, methods to improve biomaterial design for localized oxygen delivery remain a promising opportunity for future research to enhance the outcomes of cell-based therapies.
An alternative approach for delivering oxygen along with the scaffold is to prime cells to be resistant to low-oxygen conditions. MSCs and ASCs are known to be highly resilient in hypoxic conditions and, under these conditions, they upregulate expression of the HIF-1α pathway. The transcription factor HIF-1α controls both the production of angiogenic growth factors and cytokines and the ability of vascular cells to respond to these proteins. Additionally, HIF-1α activates the expression of multiple proangiogenic cytokines, including SDF-1, placenta growth factor and angiopoietins,116,117 which are required for physiological vascularization and which cannot be effectively stimulated by VEGF signalling through VEGFR-2 alone. HIF-1α modulates metabolic responses to hypoxia in order to maintain homeostatic levels of cellular energy, pH and the redox state. These responses include cellular adaptations regulated by downstream HIF-1α signalling that limit the production of ROS during periods of hypoxia; upregulation of levels of glucose transporters and glycolytic enzymes; and modified protein expression that facilitates pH homeostasis. Interestingly, the expression of HIF-1α in osteoblasts has been identified as a mechanism for coupling angiogenesis and osteogenesis in native bone.118–120 By culturing cells in low-oxygen conditions or chemically inducing upregulation of levels of HIF-1α before transplantation, cell survival and homing of the cells following transplantation (mediated by upregulation of levels of CXCR4) can be augmented, which thereby enhances the regenerative properties of the cells, as has been shown in cardiovascular applications.121,122
Cell dosing and optimal concentrations
Cell numbers for transplantation are often determined empirically through intuition on the basis of prior experience or from in vitro data. In clinical applications for which the size and geometry of the graft needs to be customized, it is impossible to ascertain optimal cell numbers a priori without knowledge of the mechanisms of cell-mediated bone regeneration. However, mechanistic data derived from preclinical models can be used to develop computational approaches that model bone repair as a function of initial bone condition.123 Computational models can be used predictively to rigorously define the number of cells and mode of delivery required for any specific application.
In vitro expansion
In cell culture, the term hypoxia is used to describe oxygen levels lower than the 20% found in the atmosphere in which cells are cultured ex vivo. However, average oxygen levels within tissues in the body can be as low as 5%. Consequently, culturing cells ex vivo exposes them to hyperoxic conditions, which might lead to elevated levels of intracellular ROS production. Another drawback of extended ex vivo culture is the potential for development of genetic and epigenetic mutations as a consequence of rapid cell division. Thus, methods that employ culture-expanded allogenic or autologous cell sources require robust assays to monitor and validate biological changes that might negatively influence the safety and subsequent efficacy of cell-based therapies.
Genetic modification of stromal and stem cells
Stromal and stem cells can be transduced to overexpress growth factors (in particular, BMPs124) and transcription factors (such as sonic hedgehog125) in order to enhance the efficacy of promoting new bone formation. This approach has been shown to be efficacious in small-animal models and its potential has been reviewed elsewhere.126,127 However, genetically modified stromal cells and stem cells remain a highly experimental model, which has proven difficult to advance through the regulatory process as a result of substantial safety concerns.
Approved cell-based products
The most established commercial cell product available for orthopaedic disabilities is Carticel® (Genzyme, USA), which uses culture-expanded, autologous chondrocytes for the treatment of degenerated articular cartilage. The use of MSCs or ASCs rather than chondrocytes can be advantageous owing to the potential to obtain larger quantities of cells and because it is associated with less donor-site morbidity. Although studies assessing the role of cell adhesion in improving chondrogenic outcomes with MSCs have been reported,128 no comparable commercially available cell products for bone regeneration exist. Osteocel® (NuVasive, USA), which retains viable MSCs within bone allografts, is now available for spine fusion applications. Trinity® Evolution™ (Orthofix, Netherlands Antilles) is a similar product for spine and other orthopaedic conditions. Lately, culture-expanded allograft MSCs have been used in a clinical trial to treat meniscectomies.129 Such clinical successes might herald the routine use of allograft cell-based products for bone regeneration.
To achieve widespread usage, cell-based products must be readily available at all levels of the healthcare system. This advance will require the development, validation and standardization of guidelines and protocols for the shipment and storage of cell therapeutics. Fortunately, the decades of experience obtained, and the advances made, by blood and transfusion centres serve as a foundation for the nascent MSC field. Nevertheless, multiple questions remain to be addressed. At present, no national or international infrastructure of facilities to manufacture and ship cells for skeletal regeneration to points of care exists. Few hospitals or universities are equipped with certified current GMP laboratories that are suitable for the isolation, expansion, characterization and processing of MSCs. Although private current GMP contract research organizations have emerged to fill this gap, their numbers and locations are limited. Furthermore, cells are being produced, stored and shipped under multiple environmental conditions, ranging from room temperature to −196° C (by use of liquid nitrogen); however, not all hospitals or outpatient surgical centres have the capability of storing cell products reliably at low temperatures. Owing to the fact that regulatory authorities and clinical research societies have not yet agreed on definitions for the quality assurance and control of MSC products, the distribution of skeletal regenerative cells across national borders is difficult. Such issues related to international standardization remain an obstacle to the continued growth of the cell-therapy field. Another difficulty relates to obtaining regulatory approval from the FDA for combination products, such as cells combined with growth factors and other bioactive biomaterials (for example, osteoinductive scaffolds). Without appropriate precedence, the safety standards of these approaches remain undefined, a challenge compounded when one considers the huge costs associated with animal testing.
Conclusions
Stem-cell-mediated bone regeneration provides a number of potential therapeutic advantages to the use of autograft tissues. Currently, there is a large amount of preclinical and clinical data that support the delivery of stromal and stem cells to defect sites to enhance bone repair and regeneration. The successful clinical applications of MSCs and ASCs firmly establish proof-of-concept of the clinical feasibility of complex, multistage surgical processes, as well as the indications requiring minimally invasive approaches. The number of ongoing clinical trials with both MSCs and ASCs (Table 1) bodes well for the future of cell-based therapies. Therefore, what are the obstacles limiting the more extensive use of stromal cells and stem cells in the clinic and what steps need to be taken in order to bring ASC-mediated and MSC-mediated bone repair up to the standard of care currently reserved for autografts? In the near term, more prevalent therapeutic use of stromal and stem cells for skeletal regeneration does not require any major changes in methodology. The techniques for cell delivery involving hydrogel encapsulation in combination with a mineralized component have been well established over the past 30 years. The critical restriction at this stage is the development of internationally recognized, standardized regulatory guidelines that define the minimum safety criteria and, consequently, robust methods for cell expansion, storage and shipping that minimize or eliminate potentially harmful changes in the cells’ genetic make-up whilst retaining their potency. The growing number of cell products on the market will facilitate movements in this direction.
However, in the longer term (that is, beyond the next 10 years), elevating stromal-cell-mediated and stem-cell-mediated bone regeneration to the standard of clinical care requires technological advances that maximize cell retention, viability, homing (for systemic delivery), vascular network formation, osteogenic differentiation capacity and tissue assembly properties. These advances require a fundamental understanding of the functioning of cells following transplantation. Put simply, are transplanted cells actively undergoing tissue morphogenesis and integration or are they merely mediators, emitting signals to recruit and stimulate their endogenous counterparts into action? The answer might be context-dependent and simple techniques, such as cell aggregation (reviewed elsewhere130,131) might profoundly influence the fate of cells after transplantation. Similarly, continued research into cell–biomaterial interactions (particularly in vivo interactions in immunocompetent animals) that spawn novel techniques for oxygen delivery132,133 or growth-factor tethering, retention and presentation111 might profoundly enhance regenerative outcomes. Most critically, systematic studies that deepen our level of understanding to an extent that we can model predicted regenerative outcomes on the basis of specific input parameters will ultimately facilitate the creation of customized therapies that are founded on rational design and usher in a new standard of care in the field of regenerative medicine.
Key points.
Stromal cells and/or stem cells can be isolated from different tissues on the basis of plastic adherence and surface-antigen profiles, thereby providing opportunities for bone regeneration
The regenerative potential of therapies that are based on adipose-tissue-derived and bone-marrow-derived mesenchymal stromal or stem cells is being tested clinically for the treatment of craniofacial bone defects, tibial non-unions and osteonecrosis of the femoral head
Although most approaches in this area use autologous cells, allogeneic sources that include commercially available allograft cell-based products are being investigated
Widespread use of cell-based products requires the development and standardization of guidelines and protocols for the shipment and storage of cell therapeutics
Despite strong clinical data, which indicates enhanced regenerative outcomes following stromal-cell or stem-cell transplantation, further insight is needed into the mechanisms of action of these strategies
Opportunities exist to develop technologies that improve cell survival, morphogenesis and functionality to advance cell therapy as standard care for the treatment of bone defects
Review criteria.
The articles selected for this Review were identified by searching PubMed, ISI Web of Knowledge and ClinicalTrials.gov. Search terms used included, but were not limited to: “stromal cells”, “stem cells”, “MSCs”, “ASCs”, “muscle-derived stem cells”, “pericytes”, “bone tissue engineering”, “oxygen”, “hypoxia”, “VEGF”, “BMP”, “HIF”, “angiogenesis”, “osteogenesis”, “craniofacial bone”, “non-union”, “osteonecrosis”, “spine” and “gene delivery”. Often, the names of renowned researchers within the field were combined with key words in Boolean searches. Articles spanning the late 1960s to the mid 1990s were used to write the brief history of the field. Known clinical trial reports were researched and articles from those reference lists were used to find other clinical trial reports. Major advances that continue to affect current paradigms were cited to describe the development of the field over time without considerations of publication date. A major focus of the article concerns developing trends and novel technologies. Hence, many of the studies cited were published within the past 5 years. In citing other review articles on specific topics, the most recent reviews were used. All cited articles were published in the English language.
Acknowledgments
The authors acknowledge funding from the Maryland Stem Cell Research Fund (2014-MSCRFI-0699), NSF CAREER award (CBET 1350554), Johns Hopkins University Centre for Musculoskeletal Research, and the American Society for Bone and Mineral Research (2013CEA13). The authors also thank D. Hutton for assistance with the artwork.
Footnotes
Competing interests
J.M.G. is co-founder, co-owner and Chief Scientific Officer of LaCell, a biotechnology company focusing on the clinical translation of stromal-cell and stem-cell science. The other authors declare no competing interests.
Author contributions
W.L.G., B.P.H. and J.M.G. researched data for the article and wrote the article. W.L.G., B.A.B., E.M., T.F. and J.M.G. made substantial contributions to discussions of the content. All authors reviewed and/or edited the manuscript before submission.
References
- 1.Raggatt LJ, et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 2014;184:3192–3204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Das A, Segar CE, Hughley BB, Bowers DT, Botchwey EA. The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages. Biomaterials. 2013;34:9853–9862. doi: 10.1016/j.biomaterials.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuroda R, et al. Clinical impact of circulating CD34-positive cells on bone regeneration and healing. Tissue Eng Part B Rev. 2014;20:190–199. doi: 10.1089/ten.teb.2013.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutton DL, Grayson WL. Stem cell-based approaches to engineering vascularized bone. Curr Opin Chem Eng. 2014;3:75–82. [Google Scholar]
- 5.Neovius E, Engstrand T. Craniofacial reconstruction with bone and biomaterials: review over the last 11 years. J Plast Reconstr Aesthet Surg. 2010;63:1615–1623. doi: 10.1016/j.bjps.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Casserbette M, Murray AB, Closs EI, Erfle V, Schmidt J. Bone-formation by osteoblast-like cells in a 3-dimensional cell-culture. Calcif Tissue Int. 1990;46:46–56. doi: 10.1007/BF02555824. [DOI] [PubMed] [Google Scholar]
- 7.Dupont KM, et al. Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci USA. 2010;107:3305–3310. doi: 10.1073/pnas.0905444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 9.Friedenstein AJ, Lalykina KS. Thymus cells are inducible to osteogenesis. Eur J Immunol. 1972;2:602–603. doi: 10.1002/eji.1830020624. [DOI] [PubMed] [Google Scholar]
- 10.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 11.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 12.Ashton BA, et al. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980;151:294–307. [PubMed] [Google Scholar]
- 13.Allen TD, Dexter TM. Cellular interrelationships during in vitro granulopoiesis. Differentiation. 1976;6:191–194. doi: 10.1111/j.1432-0436.1976.tb01486.x. [DOI] [PubMed] [Google Scholar]
- 14.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 15.Lanotte M, Scott D, Dexter TM, Allen TD. Clonal preadipocyte cell lines with different phenotypes derived from murine marrow stroma: factors influencing growth and adipogenesis in vitro. J Cell Physiol. 1982;111:177–186. doi: 10.1002/jcp.1041110209. [DOI] [PubMed] [Google Scholar]
- 16.Hunt P, et al. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987;48:997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- 17.Pietrangeli CE, Hayashi S, Kincade PW. Stromal cell lines which support lymphocyte growth: characterization, sensitivity to radiation and responsiveness to growth factors. Eur J Immunol. 1988;18:863–872. doi: 10.1002/eji.1830180606. [DOI] [PubMed] [Google Scholar]
- 18.Whitlock CA, Tidmarsh GF, Muller-Sieburg C, Weissman IL. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987;48:1009–1021. doi: 10.1016/0092-8674(87)90709-4. [DOI] [PubMed] [Google Scholar]
- 19.Namen AE, et al. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 20.Lee G, Namen AE, Gillis S, Kincade PW. Recombinant interleukin-7 supports the growth of normal B lymphocyte precursors. Curr Top Microbiol Immunol. 1988;141:16–18. doi: 10.1007/978-3-642-74006-0_3. [DOI] [PubMed] [Google Scholar]
- 21.Paul SR, et al. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci USA. 1990;87:7512–7516. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 23.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 24.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 25.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 26.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 27.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14:141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Lo Surdo J, Bauer SR. Quantitative approaches to detect donor and passage differences in adipogenic potential and clonogenicity in human bone marrow-derived mesenchymal stem cells. Tissue Eng Part C Methods. 2012;18:877–889. doi: 10.1089/ten.tec.2011.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo Surdo JL, Millis BA, Bauer SR. Automated microscopy as a quantitative method to measure differences in adipogenic differentiation in preparations of human mesenchymal stromal cells. Cytotherapy. 2013;15:1527–1540. doi: 10.1016/j.jcyt.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mindaye ST, Ra M, Lo Surdo J, Bauer SR, Alterman MA. Improved proteomic profiling of the cell surface of culture-expanded human bone marrow multipotent stromal cells. J Proteomics. 2013;78:1–14. doi: 10.1016/j.jprot.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Mindaye ST, Ra M, Lo Surdo JL, Bauer SR, Alterman MA. Global proteomic signature of undifferentiated human bone marrow stromal cells: evidence for donor-to-donor proteome heterogeneity. Stem Cell Res. 2013;11:793–805. doi: 10.1016/j.scr.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Bianco P, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robey PG, Kuznetsov SA, Riminucci M, Bianco P. Bone marrow stromal cell assays: in vitro and in vivo. Methods Mol Biol. 2014;1130:279–293. doi: 10.1007/978-1-62703-989-5_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 35.De Coppi P, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell KE, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 37.Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCS) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Usas A, Huard J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials. 2007;28:5401–5406. doi: 10.1016/j.biomaterials.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feisst V, Brooks AE, Chen CJ, Dunbar PR. Characterization of mesenchymal progenitor cell populations directly derived from human dermis. Stem Cells Dev. 2014;23:631–642. doi: 10.1089/scd.2013.0207. [DOI] [PubMed] [Google Scholar]
- 41.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 42.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 43.Qu-Petersen Z, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gronthos S, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 46.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Pachon-Pena G, et al. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226:843–851. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bourin P, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halvorsen YD, et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 50.Hicok KC, et al. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371–380. doi: 10.1089/107632704323061735. [DOI] [PubMed] [Google Scholar]
- 51.Justesen J, Pedersen SB, Stenderup K, Kassem M. Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivo. Tissue Eng. 2004;10:381–391. doi: 10.1089/107632704323061744. [DOI] [PubMed] [Google Scholar]
- 52.Mesimaki K, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Levi B, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS ONE. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandor GK, et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl Med. 2014;3:530–540. doi: 10.5966/sctm.2013-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levi B, et al. Differences in osteogenic differentiation of adipose-derived stromal cells from murine, canine, and human sources in vitro and in vivo. Plast Reconstr Surg. 2011;128:373–386. doi: 10.1097/PRS.0b013e31821e6e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Girolamo L, Sartori MF, Albisetti W, Brini AT. Osteogenic differentiation of human adipose-derived stem cells: comparison of two different inductive media. J Tissue Eng Regen Med. 2007;1:154–157. doi: 10.1002/term.12. [DOI] [PubMed] [Google Scholar]
- 57.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi O, Katsube Y, Hirose M, Ohgushi H, Ito H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int. 2008;82:238–247. doi: 10.1007/s00223-008-9112-y. [DOI] [PubMed] [Google Scholar]
- 59.Wolff J, et al. GMP-level adipose stem cells combined with computer-aided manufacturing to reconstruct mandibular ameloblastoma resection defects: experience with three cases. Ann Maxillofac Surg. 2013;3:114–125. doi: 10.4103/2231-0746.119216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eilertsen KJ, Floyd Z, Gimble JM. The epigenetics of adult (somatic) stem cells. Crit Rev Eukaryot Gene Expr. 2008;18:189–206. doi: 10.1615/critreveukargeneexpr.v18.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, et al. Sustained release of bone morphogenetic protein 2 via coacervate improves the osteogenic potential of muscle-derived stem cells. Stem Cells Transl Med. 2013;2:667–677. doi: 10.5966/sctm.2013-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Usas A, et al. Skeletal muscle-derived stem cells: implications for cell-mediated therapies. Medicina. 2011;47:469–479. [PubMed] [Google Scholar]
- 63.Gao X, et al. Role of donor and host cells in muscle-derived stem cell-mediated bone repair: differentiation vs. paracrine effects. FASEB J. 2014;28:3792–3809. doi: 10.1096/fj.13-247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Studeny M, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 65.Katakowski M, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xin H, et al. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 67.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of trail can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belmar-Lopez C, et al. Tissue-derived mesenchymal stromal cells used as vehicles for anti-tumor therapy exert different in vivo effects on migration capacity and tumor growth. BMC Med. 2013;11:139. doi: 10.1186/1741-7015-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dwyer RM, et al. Mesenchymal stem cell-mediated delivery of the sodium iodide symporter supports radionuclide imaging and treatment of breast cancer. Stem Cells. 2011;29:1149–1157. doi: 10.1002/stem.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah K. Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev. 2012;64:739–748. doi: 10.1016/j.addr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao J, et al. Stem cell-mediated delivery of SPIO-loaded gold nanoparticles for the theranosis of liver injury and hepatocellular carcinoma. Nanotechnology. 2014;25:5101–5101. doi: 10.1088/0957-4484/25/40/405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang T, et al. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2014;4:70. doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo J, et al. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. 2013;33:2768–2778. doi: 10.1038/onc.2013.233. [DOI] [PubMed] [Google Scholar]
- 74.Warnke PH, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 75.Mesimaki K, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Sandor GK, et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J Oral Maxillofac Surg. 2013;71:938–950. doi: 10.1016/j.joms.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Lendeckel S, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–554. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shrivats AR, McDermott MC, Hollinger JO. Bone tissue engineering: state of the union. Drug Discov Today. 2014;19:781–786. doi: 10.1016/j.drudis.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 80.Bhumiratana S, Vunjak-Novakovic G. Personalized human bone grafts for reconstructing head and face. Stem Cells Transl Med. 2012;1:64–69. doi: 10.5966/sctm.2011-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tevlin R, et al. Biomaterials for craniofacial bone engineering. J Dent Res. 2014;93:1187–1195. doi: 10.1177/0022034514547271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liebergall M, et al. Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Mol Ther. 2013;21:1631–1638. doi: 10.1038/mt.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49:1005–1009. doi: 10.1016/j.bone.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 84.Gangji V, et al. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells—a pilot study. J Bone Joint Surg Am. 2004;86–A:1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Mao Q, et al. The efficacy of targeted intraarterial delivery of concentrated autologous bone marrow containing mononuclear cells in the treatment of osteonecrosis of the femoral head: a five year follow-up study. Bone. 2013;57:509–516. doi: 10.1016/j.bone.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao D, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–330. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Levi B, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS ONE. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levi B, et al. Dura mater stimulates human adipose-derived stromal cells to undergo bone formation in mouse calvarial defects. Stem Cells. 2011;29:1241–1255. doi: 10.1002/stem.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cowan CM, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 90.Boerckel JD, Uhrig BA, Willett NJ, Huebsch N, Guldberg RE. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc Natl Acad Sci USA. 2011;108:E674–E680. doi: 10.1073/pnas.1107019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 92.Dong F, Caplan AI. Cell transplantation as an initiator of endogenous stem cell-based tissue repair. Curr Opin Organ Transplant. 2012;17:670–674. doi: 10.1097/MOT.0b013e328359a617. [DOI] [PubMed] [Google Scholar]
- 93.Eshkar-Oren I, et al. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development. 2009;136:1263–1272. doi: 10.1242/dev.034199. [DOI] [PubMed] [Google Scholar]
- 94.Okuyama H, et al. Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem. 2006;281:15554–15563. doi: 10.1074/jbc.M602003200. [DOI] [PubMed] [Google Scholar]
- 95.Kubo S, et al. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum. 2009;60:155–165. doi: 10.1002/art.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reichert JC, et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci Transl Med. 2012;4:141ra93. doi: 10.1126/scitranslmed.3003720. [DOI] [PubMed] [Google Scholar]
- 97.Springer IN, et al. Bone graft versus BMP-7 in a critical size defect—cranioplasty in a growing infant model. Bone. 2005;37:563–569. doi: 10.1016/j.bone.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 98.Wilson SM, et al. Adipose-derived mesenchymal stem cells enhance healing of mandibular defects in the ramus of swine. J Oral Maxillofac Surg. 2012;70:E193–E203. doi: 10.1016/j.joms.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 99.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Correia C, et al. In vitro model of vascularized bone: synergizing vasculogenesis and osteogenesis. PLoS ONE. 2011;6:e28352. doi: 10.1371/journal.pone.0028352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Correia C, et al. Human adipose-derived cells can serve as a single-cell source for the in vitro cultivation of vascularized bone grafts. J Tissue Eng Regen Med. 2012;8:629–639. doi: 10.1002/term.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsigkou O, et al. Engineered vascularized bone grafts. Proc Natl Acad Sci USA. 2010;107:3311–3316. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rivron NC, et al. Sonic hedgehog-activated engineered blood vessels enhance bone tissue formation. Proc Natl Acad Sci USA. 2012;109:4413–4418. doi: 10.1073/pnas.1117627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fuchs S, Hofmann A, Kirkpatrick CJ. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007;13:2577–2588. doi: 10.1089/ten.2007.0022. [DOI] [PubMed] [Google Scholar]
- 105.Martineau L, Doillon CJ. Angiogenic response of endothelial cells seeded dispersed versus on beads in fibrin gels. Angiogenesis. 2007;10:269–277. doi: 10.1007/s10456-007-9079-8. [DOI] [PubMed] [Google Scholar]
- 106.Laib AM, et al. Spheroid-based human endothelial cell microvessel formation in vivo. Nat Protoc. 2009;4:1202–1215. doi: 10.1038/nprot.2009.96. [DOI] [PubMed] [Google Scholar]
- 107.Alajati A, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;5:439–445. doi: 10.1038/nmeth.1198. [DOI] [PubMed] [Google Scholar]
- 108.Hutton DL, Moore EM, Gimble J, Grayson WL. Platelet-derived growth factor and spatiotemporal cues induce development of vascularized bone tissue by adipose-derived stem cells. Tissue Eng Part A. 2013;19:2076–2086. doi: 10.1089/ten.tea.2012.0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev. 2012;64:1257–1276. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaigler D, Silva EA, Mooney DJ. Guided bone regeneration using injectable vascular endothelial growth factor delivery gel. J Periodontol. 2013;84:230–238. doi: 10.1902/jop.2012.110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martino MM, et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3:100ra89. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- 112.Shah NJ, et al. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials. 2011;32:6183–6193. doi: 10.1016/j.biomaterials.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shah NJ, et al. Surface-mediated bone tissue morphogenesis from tunable nanolayered implant coatings. Sci Transl Med. 2013;5:191ra183. doi: 10.1126/scitranslmed.3005576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spiess BD. Perfluorocarbon emulsions as a promising technology: a review of tissue and vascular gas dynamics. J Appl Physiol. 2009;106:1444–1452. doi: 10.1152/japplphysiol.90995.2008. [DOI] [PubMed] [Google Scholar]
- 115.Kimelman-Bleich N, et al. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials. 2009;30:4639–4648. doi: 10.1016/j.biomaterials.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 116.Kelly BD, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 117.Bosch-Marce M, et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y, et al. The hypoxia-inducible factor a pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y, Wan C, Gilbert SR, Clemens TL. Oxygen sensing and osteogenesis. Ann NY Acad Sci. 2007;1117:1–11. doi: 10.1196/annals.1402.049. [DOI] [PubMed] [Google Scholar]
- 120.Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu X, et al. Leptin signaling is required for augmented therapeutic properties of mesenchymal stem cells conferred by hypoxia preconditioning. Stem Cells. 2014;32:2702–2713. doi: 10.1002/stem.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang Q, et al. Remote ischemic postconditioning enhances cell retention in the myocardium after intravenous administration of bone marrow mesenchymal stromal cells. J Mol Cell Cardiol. 2013;56:1–7. doi: 10.1016/j.yjmcc.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 123.Carlier A, et al. Mosaic: a multiscale model of osteogenesis and sprouting angiogenesis with lateral inhibition of endothelial cells. PLoS Comput Biol. 2012;8:e10022724. doi: 10.1371/journal.pcbi.1002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie C, et al. Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering. Tissue Eng. 2007;13:435–445. doi: 10.1089/ten.2006.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang C, Tang M, Yehling E, Zhang X. Overexpressing sonic hedgehog peptide restores periosteal bone formation in a murine bone allograft transplantation model. Mol Ther. 2014;22:430–439. doi: 10.1038/mt.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosen V. Harnessing the parathyroid hormone, Wnt, and bone morphogenetic protein signaling cascades for successful bone tissue engineering. Tissue Eng Part B Rev. 2011;17:475–479. doi: 10.1089/ten.TEB.2011.0265. [DOI] [PubMed] [Google Scholar]
- 127.Bleich NK, et al. Gene therapy approaches to regenerating bone. Adv Drug Deliv Rev. 2012;64:1320–1330. doi: 10.1016/j.addr.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hung BP, Babalola OM, Bonassar LJ. Quantitative characterization of mesenchymal stem cell adhesion to the articular cartilage surface. J Biomed Mater Res A. 2013;101:3592–3598. doi: 10.1002/jbm.a.34647. [DOI] [PubMed] [Google Scholar]
- 129.Vangsness CT, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy. J Bone Joint Surg Am. 2014;96:90–98. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- 130.Sart S, Tsai AC, Li Y, Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B. 2014;20:365–380. doi: 10.1089/ten.teb.2013.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sart S, Ma T, Li Y. Preconditioning stem cells for in vivo delivery. Biores Open Access. 2014;3:137–149. doi: 10.1089/biores.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Oxygen producing biomaterials for tissue regeneration. Biomaterials. 2007;28:4628–4634. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 133.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30:757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 134.US National Library of Medicine. . ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT00512434?term=NCT00512434&rank=1.
- 135.US National Library of Medicine. . ClinicalTrials.gov. 2011 [online], http://clinicaltrials.gov/ct2/show/NCT01206179?term=NCT01206179&rank=1.
- 136.US National Library of Medicine. . ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT01429012?term=NCT01429012&rank=1.
- 137.US National Library of Medicine. . ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT01788059?term=NCT01788059&rank=1.
- 138.US National Library of Medicine. . ClinicalTrials.gov. 2011 [online], http://clinicaltrials.gov/ct2/show/NCT00250302?term=NCT00250302&rank=1.
- 139.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01435434?term=NCT01435434&rank=1.
- 140.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT02177565?term=NCT02177565&rank=1.
- 141.US National Library of Medicine. . ClinicalTrials.gov. 2012 [online], http://clinicaltrials.gov/ct2/show/NCT01626625?term=NCT01626625&rank=1.
- 142.US National Library of Medicine. . ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT01842477?term=NCT01842477&rank=1.
- 143.US National Library of Medicine. . ClinicalTrials.gov. 2012 [online], http://clinicaltrials.gov/ct2/show/NCT01725698?term=NCT01725698&rank=1.
- 144.US National Library of Medicine. . ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT01958502?term=NCT01958502&rank=1.
- 145.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT02065167?term=NCT02065167&rank=1.
- 146.US National Library of Medicine. . ClinicalTrials.gov. 2012 [online], http://clinicaltrials.gov/ct2/show/NCT01700920?term=NCT01700920&rank=1.
- 147.US National Library of Medicine. . ClinicalTrials.gov. 2012 [online], http://clinicaltrials.gov/ct2/show/NCT01544712?term=NCT01544712&rank=1.
- 148.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01605383?term=NCT01605383&rank=1.
- 149.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01210950?term=NCT01210950&rank=1.
- 150.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01552707?term=NCT01552707&rank=1.
- 151.US National Library of Medicine. . ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT01389661?term=NCT01389661&rank=1.
- 152.US National Library of Medicine. . ClinicalTrials.gov. 2012 [online], http://clinicaltrials.gov/ct2/show/NCT01603836?term=NCT01603836&rank=1.
- 153.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT02172885?term=NCT02172885&rank=1.
- 154.US National Library of Medicine. . ClinicalTrials.gov. 2008 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/ct2/show/NCT00186914?term=NCT00186914&rank=1. [DOI] [PubMed]
- 155.US National Library of Medicine. . ClinicalTrials.gov. 2010 [online], http://clinicaltrials.gov/ct2/show/NCT00001391?term=NCT00001391&rank=1.
- 156.US National Library of Medicine. . ClinicalTrials.gov. 2011 [online], http://clinicaltrials.gov/ct2/show/NCT01207193?term=NCT01207193&rank=1.
- 157.US National Library of Medicine. . ClinicalTrials.gov. 2009 [online], http://clinicaltrials.gov/ct2/show/NCT00221130?term=NCT00221130&rank=1.
- 158.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01532076?term=NCT01532076&rank=1.
- 159.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT02140528?term=NCT02140528&rank=1.
- 160.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01643655?term=NCT01643655&rank=1.
- 161.US National Library of Medicine. . ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT01501461?term=NCT01501461&rank=1.
- 162.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01739504?term=NCT01739504&rank=1.
- 163.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01585857?term=NCT01585857&rank=1.
- 164.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01885819?term=NCT01885819&rank=1.
- 165.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT02241408?term=NCT02241408&rank=1.
- 166.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01885832?term=NCT01885832&rank=1.
- 167.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT02142842?term=NCT02142842&rank=1.
- 168.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01947348?term=NCT01947348&rank=1.
- 169.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01633892?term=NCT01633892&rank=1.
- 170.US National Library of Medicine. . ClinicalTrials.gov. 2014 [online], http://clinicaltrials.gov/ct2/show/NCT01645722?term=NCT01645722&rank=1.
- 171.US National Library of Medicine. . ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT01218945?term=NCT01218945&rank=1.


