Abstract
Ten members of the mezzettiaside family of natural products were synthesized and evaluated for anticancer and antibacterial activity. Complete anticancer (H460) and antibacterial (B. subtilis) activities for the ten natural products and four new analogues were found. Comparison to the cleistrioside and cleistetroside classes of natural products were made.
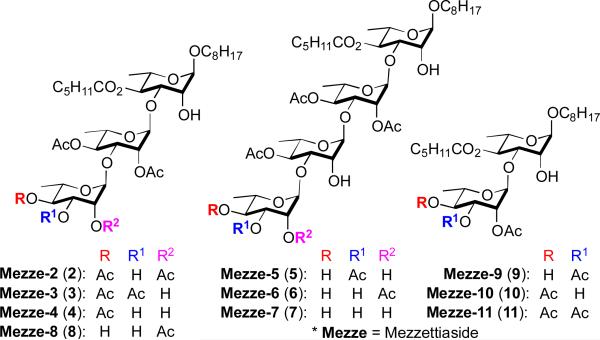
The mezzettiasides are a family of natural products discovered in 1986 and 1989,1 as part of the search for the active component associated with plant extracts from traditional folk medicines (Figure 1). The mezzettiasides 2-11 were isolated from the stem bark of Mezzettia leptopoda, which have been long used in the folk medicinal tradition of the Malaysian island of Borneo.2 The mezzettiasides are made up of a unique class of partially acetylated oligosaccharides. The carbohydrate core of these octyl di-, tri- and tetrasaccharide natural products consists of anα-linked 1,3-oligorhamnose motif. Because of limited supplies, not all the mezzettiasides were tested for biological activity, however, the ones that showed interesting anticancer activity against a panel of three human cancer cell lines are listed in Table 1.2
Figure 1.
The targeted mezzettiasides
Table 1.
Anticancer cytotoxicity activity (μM) for the mezzettiasides.a
| Cell lineb | M-2 | M-3 | M-4 | M-8 | M-9 | M-10 | M-11 |
|---|---|---|---|---|---|---|---|
| Lul | 10 | 14 | 25 | 25 | 9 | >20 | 9 |
| Co12 | 5 | 6 | 8 | 10 | >20 | >20 | 14 |
| KB | 7 | 17 | 19 | >20 | 19 | >20 | 20 |
Results are expressed as ED50 values (μM).
Lu1 = human lung cancer; Co12 = human colon cancer; KB human oral epidermoid carcinoma. M = Mezze
Previously, we have found success with the de novo synthesis3 and medicinal chemistry study of a related class of carbohydrate based natural products, the cleistriosides and cleistetrosides,4 using a Pd-catalyzed glycosylation.5 These studies demonstrated that both the cleistriosides and cleistetrosides possessed both antibacterial and anticancer activity across a range of cell lines (NCI panel of 60 cell lines).6 While a specific pharmacophore could not be identified, the activity was found to vary upon the length of the carbohydrate chain, as well as the degree and location of acylation. Thus the cleistetrosides were routinely more active than the cleistriosides in anticancer and antibacterial assays. Access to the mezzettiaside family of natural products allows us to expand the structure activity relationship (SAR) studies, as no biological data were reported for the tetrassacharide mezzettiasides 5-7. In addition, we wanted to explore analogues of the simplest disaccharide mezzettiasides 9-11, as these offered the greatest opportunity for structural modification. A review of the reported activities2 showed a great dependence on the degree of acylation (cf. M-10 to M-11, Table 1), so we decided to make C-3 ester analogues of mezzettiaside 10.
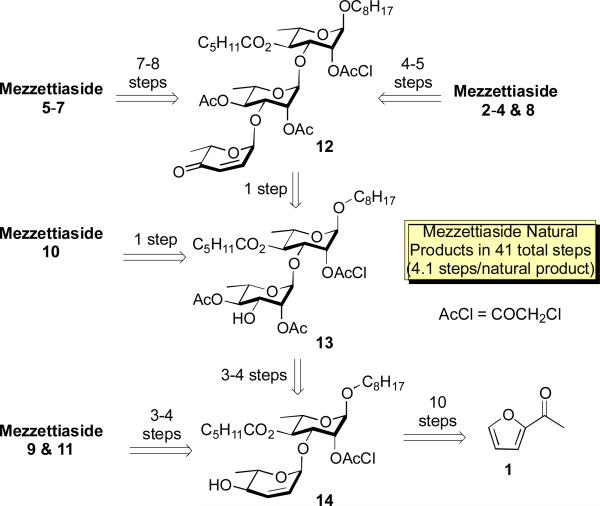
Recently, we reported a divergent de novo asymmetric synthesis of the entire family of the mezzettiaside natural products (Scheme 1).7 The overall efficiency of this approach is seen in that it provides access to all ten natural products in 41 total steps (on avg. 4.1 steps/natural product). This approach supplied both enough material for these biological studies as well as confirmed their absolute and relative stereochemistry. Retrosynthetically, both tetrasaccharide mezzettiaside (5-7) and trisaccharide (2-4 & 8) were obtained from a common intermediate 12, which in turn can be obtained from precursor 13. Disaccharide mezzettiaside (9-11) can be obtained from intermediate 13 or 14, which in turn can be prepared from acetyl furan 1,5,8,9 via an enantioselective Achmatowicz approach.10,11 In addition, disaccharide 13 was used as a starting point to synthesize analogues 15-18 (vide infra). Herein, we are enclosing the SAR studies of all ten natural products as well as four synthetic disaccharide analogues (15-18).
With the ten mezzettiasides in hand, we measured their activity as anticancer and antibacterial agents. As the mezzettiasides were previously found2 to have the broadest range of activity against the lung cancer cell line (Lu1, Table 1), we chose to use the NCI H460 non-small cell lung cancer cell line for our evaluation. For the cleistetrosides/cleistriosides, we found the greatest antibacterial activity was seen with the Gram-(+) Bacillus subtilis (JH642) strain, so we chose to use that strain for our MIC screening of antibacterial activity.
Initial biological screening of these natural products began with the evaluation of the three disaccharide mezzettiasides 9-11 (Table 2). Only mezzettiaside-9 showed any appreciable anti-B. subtilis activity (16 μM). When we compared the anticancer activity of the disaccharides in H460 with that reported in Lu1, we saw a significant decrease in activity for mezzettiaside-9. Mezzettiaside-10 remained inactive and only a small decrease in activity was observed for mezzettiaside-11. Comparing the activities of the disaccharide mezzettiasides with the more complex cleistrioside and cleistetrosides finds comparable antibacterial activity for mezzettiaside-9 and anticancer activity for mezzettiaside-11.
Table 2.
Anticancer and bacterial activity (μM) for the mezzettiaside disaccharides.
MIC (μM) method was performed by the broth dilution method described by the National Committee for Clinical Laboratory Standard Methods (M7-A6, 2003).
The IC50 (|xM)value was measured by a 48 h treatment in an MTT assay, all values are an average of at least three independent experiments.
We next looked at the anticancer and antibacterial activity for the four trisaccharide mezzettiasides 2-4 and 8 (Table 3). Comparable anticancer activity was found for H460 as that reported2 in Lu1 cell line. In general, the trisaccharide mezzettiasides had improved anticancer and antibacterial activity over the disaccharides 9-11, with a broad range of anti-B. subtilis activity (8 to >128 μM) and good anticancer activity (9 to 24 μM). Of all the trisaccharides, mezzettiaside-4 showed the best activity in both assays.
Table 3.
Anticancer and bacterial activity (μM) for the mezzettiaside trisaccharides.
| R | R1 | R2 | MICa | IC50b | |
|---|---|---|---|---|---|
| B. subtilis | H460 | ||||
| Mezze-2 | Ac | H | Ac | >128 | 17 |
| Mezze-3 | Ac | Ac | H | >128 | 10 |
| Mezze-4 | Ac | H | H | 8 | 9 |
| Mezze-8 | H | H | Ac | 16 | 24 |
All values of MIC and IC50 (μM) are an average of at least three independent experiments
All values of MIC and IC50 (μM) are an average of at least three independent experiments.
We then looked at the three tetrasaccharide mezzettiasides 5-7 (Table 4). In contrast to the cleistrioside/cleistetrosides, where potency improved with increased chain length, we found a reduction in both anticancer and antibacterial activity when moving from the trisaccharide to the tetrasaccharide mezzettiasides. Thus, only mezzettiaside-7 had appreciable activity in both assays and mezzettiaside-5/mezzettiaside-6 significantly lost activity in one of the two assays.
Table 4.
Anticancer and bacterial activity (μM) for the mezzettiaside tetrasaccharides.
All values of MIC and IC50 (μM) are an average of at least three independent experiments.
All values of MIC and IC50 (μM) are an average of at least three independent experiments.
At the outset of these SAR-studies, we were concerned about the stability of the acetate groups towards either ester hydrolysis or migration in these assays. The stability of these acetate groups can be inferred from an analysis of the biological data. For example, if the C-2 acetate (R2) in mezzettiaside-6 were to remove (6 to 7) or to migrate to the C-3 position (6 to 5), it would have significantly improved anticancer activity (Table 4). This effect can similarly be seen in mezzettiaside-2 in the anti-B. subtilis assay (i.e., 2 to 4 leads to a significant improvement in activity).
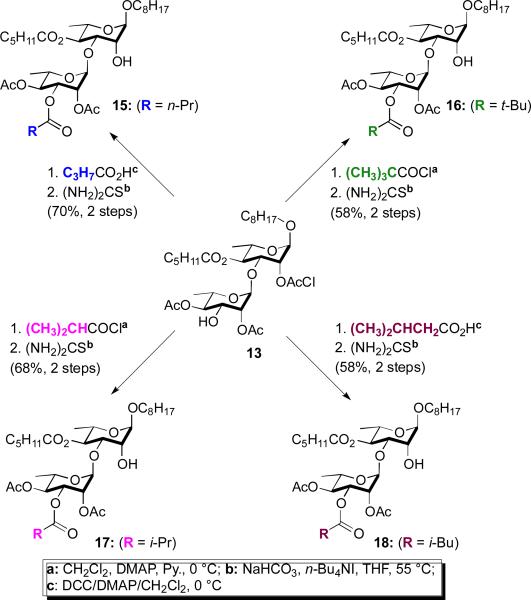
In all three types of mezzettiasides (di-, tri- and tetrasaccharides) a strong dependency upon the degree and location of acylation on activity can be seen. To further explore this effect, we decided to prepare analogues of the disaccharide mezzettiasides (Scheme 2), as these were the most abundant. Our analogue design focused on the dramatic change in activity upon substituting mezzetttiaside-10 with an acetate at the C-3 position. When this substitution is an acetylation (10 to 11), there is a significant increase in anticancer activity (>500 to 15 μM). Similar improvements in activity can be seen upon glycosylation with a mono- (10 to 2-4 or 8) or disaccharide (10 to 5-7). Thus, we decided to explore the substitution of the C-3 position of mezzettiaside-10 with larger acetyl groups (e.g., n-butyrate 15, pivalate 16, i-butyrate 17, and isovalerate 18).
The synthetic route for the four unnatural disaccharide analogues (15-18), began with disaccharide 13, the key pivot point in our divergent synthesis of the mezzettiasides. As outlined in Scheme 2, disaccharide 13 could be converted into the desired four analogues (15-18) in only two steps (acylation and deprotection).12
Analogues 15-18 were tested in both the H460 and B. subtilis assays and the results are outlined in Table 5. As with the acetate substitution of 10 to 11, the acylation of 10 to 15-18 showed consistent improvement in anticancer activity and similarly no effect on anti-B. subtilis activity. The smaller the ester group the greater the improvement in activity, as mezzettiaside-11 showed the best anticancer activity and analogue 15 with the unbranched n-Pr side chain had the second best activity (32 μM). For comparison, when the substituent is the significantly larger acylated sugar, as in trisaccharides mezzettiaside 2-4 and 8, improved anticancer was seen. In addition, both 4 and 8 had also improved antibacterial activity (Table 3).
Table 5.
Anticancer and bacterial activity (μM) for the mezzettiaside R1 ester disaccharide analogues.
| R | R1 | MICa | IC50b | |
|---|---|---|---|---|
| B. subtilis | H460 | |||
| Mezze-10 | Ac | H | >128 | >500 |
| Mezze-11 | Ac | Ac | >128 | 15 |
| 15 | Ac | n-PrCO | >128 | 32 |
| 16 | Ac | t-BuCO | >128 | 141 |
| 17 | Ac | i-PrCO | >128 | 47 |
| 18 | Ac | i-BuCO | >128 | 79 |
All values of MIC and IC50 (μM) are an average of at least three independent experiments.
All values of MIC and IC50 (μM) are an average of at least three independent experiments.
The five mezzettiasides (4, 6-9) with best anti-B. subtilis (JH642) activity were further screened for activity against a broader range of both Gram-(-) and Gram-(+) organisms. We chose a modified E. coli strains (Δimp), and two S. aureus strains (HG003 and MW2), as these are the most common gram-(−) and gram-(+) pathogens.13,14 In addition, we screened E. faecium and E. faecalis as they are the most common Enterococcus species.15 Consistent with our findings for cleistriosides and cleistetrosides, we found all the mezzettiasides were inactive against the E. coli16 and the two S. aureus strains. Only the most active mezzettiaside, mezzettiaside-4, showed admirable activity against E. faecium and E. faecalis (16 μM) (Table 6).
Table 6 Antibacterial activity (μM) for the mezzettiasides 4 & 6-9.
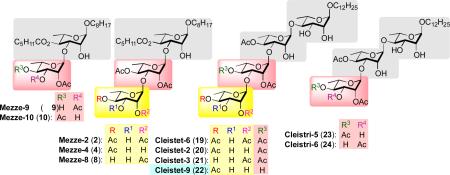
In an effort to gain insight into the SAR of these three structurally related families of natural products, we overlaid the structures to look for sites of similarity. This led us to discount the first sugar of the cleistriosides and cleistetrosides and overlap the second sugar with the first sugar of the mezzettiasides (Table 7).
Table 7.
Antibacterial and anticancer SAR-comparison of Mezzettiasides to the Cleistetrosides and Cleistriosides (μM).
| |||
|---|---|---|---|
| Mezze | MICa | IC50b | Cleistet or Cleistri |
| B. subtilis | H460 | ||
| Mezze-2 | >128 | 17 | |
| 4 | 8 | Cleistet-6 | |
| Mezze-4 | 8 | 9 | |
| 4 | 9 | Cleistet-2 | |
| 8 | 17 | Cleistet-9 | |
| Mezze-8 | 16 | 24 | |
| 8 | 13 | Cleistet-3 | |
| Mezze-10 | >128 | >500 | |
| 32 | 91 | Cleistri-5 | |
| Mezze-9 | 16 | 151 | |
| 8 | 13 | Cleistri-6 | |
All values of MIC and IC50 (μM) are an average of at least three independent experiments.
All values of MIC and IC50 (μM) are an average of at least three independent experiments.
This solely structural analysis identifies the trisaccharide mezzettiasides (2,4 and 8) to be most similar to the cleistetrosides (19, 20, 21 and 22). In general, these two groups are the most active members of their respective family of natural products. Focusing the comparison to the terminal sugar motifs (yellow and pink) of these two natural product families, points to the comparison of mezzettiaside-4 with cleistetroside-2, as they have identical acylation patterns and quite similar activities against B. subtilis and cancer cell lines. Just focusing on the terminal sugars (yellow) expands this pattern to cleistetroside-9, which shows similar activities. Applying this analysis to other acylation patterns leads to a comparison of mezzettiaside-8 with cleistetroside-3, which also possess similar activities. In contrast, the comparison of mezzettiaside-2 with cleistetroside-6 identifies pairs with similar anticancer activity but quite different antibacterial activity.
Finally, this analysis similarly leads to the comparisons of the disaccharide mezzettiasides (9 and 10) with the cleistriosides (23 and 24). Clearly, this comparison does not work as well with the disaccharide mezzettiasides. This breakdown in the comparison could be due to the closer proximity of the terminal sugar to the C-4 hexanoate, which is the most significant structural difference between these two classes of natural products.
In conclusion, we have demonstrated the utility of a highly divergent de novo asymmetric synthesis of the mezzettiaside family of natural products for SAR-type studies. Sufficient quantities of the ten natural products as well as four analogues were prepared and evaluated for both anticancer and antibacterial activities. It is worth noting that these efforts discovered previously unreported antibacterial activities for the mezzettiaside class of acylated oligo-rhamnoside natural products. Finally an initial SAR-analysis identifies structural similarities between the trisaccharide mezzettiasides and the cleistetrosides, likewise for the disaccharide mezzettiasides and the cleistriosides. Further SAR studies along these lines are ongoing and will be reported in due course.
Supplementary Material
Scheme 1.
Retrosynthetic analysis
Scheme 2.
Synthesis of disaccharide analogues 15-18
Acknowledgments
We are grateful to NIH (GM090259), NSF (CHE-1213596) and the American Cancer Society (RSG-12-161-01-DMC) for their financial support of this research.
References
- 1.a Etse JT, Gray AI, Lavaud C, Massiot G, Nuzillard JM, Waterman PG. J. Chem. Soc., Perkin Trans. 1991;1:861. [Google Scholar]; b Powell DA, York WS, Halbeek HV, Etse JT, Gray AI, Waterman PG. Can. J. Chem. 1990;68:1044. [Google Scholar]
- 2.Cui B, Chai H, Santisuk T, Reutrakul V, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. J. Nat. Prod. 1998;61:1535. doi: 10.1021/np980270j. [DOI] [PubMed] [Google Scholar]
- 3.a Ko SY, Lee AWM, Masamune S, Reed LA, Sharpless KB. Science. 1983;220:949. doi: 10.1126/science.220.4600.949. [DOI] [PubMed] [Google Scholar]; b Northrup AB, MacMillan DWC. Science. 2004;305:1752. doi: 10.1126/science.1101710. [DOI] [PubMed] [Google Scholar]; c Ahmed Md. M., Berry BP, Hunter TJ, Tomcik DJ, O'Doherty GA. Org. Lett. 2005;7:745. doi: 10.1021/ol050044i. [DOI] [PubMed] [Google Scholar]
- 4.Wu B, Li M, O'Doherty GA. Org. Lett. 2010;12:5466. doi: 10.1021/ol1023344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a Babu RS, O'Doherty GA. J. Am. Chem. Soc. 2003;125:12406. doi: 10.1021/ja037097k. [DOI] [PubMed] [Google Scholar]; b Babu RS, Zhou M, O'Doherty GA. J. Am. Chem. Soc. 2004;126:3428. doi: 10.1021/ja039400n. [DOI] [PubMed] [Google Scholar]; c Babu RS, O'Doherty GA. J. Carb. Chem. 2005;24:169. [Google Scholar]; d Guo H, O'Doherty GA. Angew. Chem. Int. Ed. 2007;46:5206. doi: 10.1002/anie.200701354. [DOI] [PubMed] [Google Scholar]; e Guo H, O'Doherty GA. J. Org. Chem. 2008;73:5211. doi: 10.1021/jo800691v. [DOI] [PubMed] [Google Scholar]
- 6.Shi P, Silva MC, Wang HL, Akhmedov NG, Li M, Beuning PJ, O'Doherty GA. ACS Med. Chem. Lett. 2012;3:1086. doi: 10.1021/ml300303g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajaj SO, Sharif EU, Akhmedov NG, O'Doherty GA. Chem. Sci. doi: 10.1039/C4SC00593G. DOI:10.1039/C4SC00593G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noyori R, Ohkuma T, Kitamura M. J. Am. Chem. Soc. 1987;109:5856. [Google Scholar]
- 9.Borisova SA, Guppi SR, Kim HJ, Wu B, Penn JH, Liu H-W, O'Doherty GA. Org. Lett. 2010;12:5150. doi: 10.1021/ol102144g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a Achmatowicz O, Bukowski P, Szechner B, Zwierzchowska Z, Zamojski A. Tetrahedron. 1971;27:1973. [Google Scholar]; b Li M, Scott JG, O'Doherty GA. Tetrahedron Lett. 2004;45:1005. [Google Scholar]
- 11.Aljahdali AZ, Shi P, Zhong Y, O'Doherty GA. Advances in Carbohydrate Chemistry and Biochemistry. 2013;69:55. doi: 10.1016/B978-0-12-408093-5.00004-6. [DOI] [PubMed] [Google Scholar]
- 12.Clausen MH, Madsen R. Chem.-Eur. J. 2003;9:3821. doi: 10.1002/chem.200204636. [DOI] [PubMed] [Google Scholar]
- 13.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. ICHE. 2008;29:996. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 14.Schleifer KH, Kilpper-Balz R. Int. J. Syst. Bacteriol. 1984;34:31. [Google Scholar]
- 15.Murray BE. Clin. Microbiol. Rev. 1990;3:46. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson BA, Misr R, Benson SA. Genetics. 1989;122:491. doi: 10.1093/genetics/122.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert S, Ziebandt A-K, Ohlsen K, Schafer T, Hecker M, Albrecht D, Novick R, Gotz F. Infect. Immun. 2010;78:2877. doi: 10.1128/IAI.00088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K-I, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. Lancet. 2002;359:1819. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.