Abstract
Dentin Sialophosphoprotein (DSPP) is the major noncollagenous protein of dentin and plays a significant role in dentin mineralization. Recently, animal models lacking DSPP have been developed and the DSPP KO phenotype has been characterized at the histological level. Little is known, however, about the DSPP KO dentin at nano- and meso-scale. Dentin is a hierarchical material spanning from nano- to macroscale, hence information on the effects of DSPP deficiency at the submicron scale is essential for understanding of its role in dentin biomineralization. To bridge this gap we have conducted ultrastructural studies of dentin from DSPP KO animals. Transmission electron microscopy (TEM) studies of DSPP KO dentin revealed that although the overall ultrastructural organization was similar to the WT, the mineral particles were less organized. Scanning electron microscopy in the back-scattered mode (BSS MA of the DSPP KO dentin revealed that circumpulpal dentin comprises large areas of nonmineralized matrix, with numerous spherulitic mineralized inclusions, while the mantle dentin appeared largely unaffected. Analysis of the mineral distribution in the circumpalpal dentin of the DSPP KO mice suggests a reduction in the number of mineral nucleation sites and an increase in the nucleation barrier in DSPP KO dentin. These preliminary results indicate that in addition to the reduction of mineralized and total dentin volume in DSPP KO animals significant changes in the ultrastructural organization exist. These changes are likely related to the role of DSPP in the regulation of mineral formation and organization in dentin.
Keywords: biomineralization, calcium phosphate, dentinogenesis imperfecta, transmission electron microscopy, scanning electron microscopy, microCT
Introduction
Dentin sialophosphoprotein, DSPP, the major noncollagenous dentin protein, and mutations in the dspp gene cause severe dentin anomalies e.g. dentinogenesis imperfecta and dentin dysplasia, manifested by hypomineralized thin dentin(1), resulting in mechanically compromised teeth. DSPP is cleaved upon secretion into dentin sialoprotein (DSP) and dentin phosphoprotein (DPP), which are believed to play major roles in dentin mineralization and regulation of cell differentiation and homeostasis (2-4). Analyses of teeth and alveolar bone from dspp KO mice revealed enlarged pulps and pulp exposure, widened predentin, large areas of intergloblar dentin, and apical periodontitis (5, 6).
Mineralized collagenous tissues such as dentin are organo-mineral hierarchical composite materials spanning from nano- to macro- scales. Their basic building block is a mineralized collagen fibril, which contains highly organized stacks of plate-like apatitic crystals, with the apatite c-axes co-oriented with the long axis of the collagen fibril. It has been recently shown that in the presence of phosphorylated DPP collagen fibrils mineralized in highly organized manner resembling the structural organization of mineralized collagen fibrils in bone and dentin (2). The organization of the mineralized fibrils at the nano- and mesoscales determine the functional properties of these tissues (7). The published studies focus primarily on the characterization of the DSPP KO phenotype at the histological level. To bridge this gap in knowledge we have conducted structural, compositional and mechanical analyses of the DSPP KO dentin. We believe that these studies will help better understand the function and mechanisms of action of DSPP.
Materials and Methods
Breeding pairs of DSPP KO mice were provided by Dr. Ashok Kulkarni (NIDCR) and bred in the animal facility at the Hospital for Special Surgery according to an approved protocol. The mice were euthanized at 10 days and 7 months to provide examples of young and old tissues and their heads were stored at -80°C. The heads of three 7 months old mice were defrosted in 70% ethanol and mandibular incisors were dissected and freeze dried. The heads of 6 KO and 6 WT 10 day old mice were defrosted in 70% ethanol and 1st and 2nd molars were extracted and kept in 70% ethanol.
For SEM the incisors were mounted in Epofix (EMS, Hatfield, CT) resin at the room temperature for 7 hours and polished on the Minimet polisher (Bueler, Lake Bluff, Il) using Metaldi diamond suspensions (Bueler, Lake Bluff, Il) down to 0.25 μ diamond grain size. The samples were carbon coated and observed under SEM Jeol 6335F (Jeol, Peabody, Mass) in backscattered SEM (BS SEM) mode at 10-15 KV and 8-15 mm working distance.
For TEM, freeze dried incisors were embedded in LR White hard tissue formulation (EMS, Hatfield, PA). Approximately 70 nm thick dentin sections were cut using a diamond knife onto water equilibrated with hydroxyapatite. The sections were blotted with filter paper, rinsed with DDW and air dried. The samples were examined using a JOEL 1210 TEM in bright field and selected area diffraction (SAED) modes at 100 KV. Electron tomography data acquisition was carried out using Technai 12 Spirit (FEI, Endhoven, Netherlands) and the reconstructions were performed using IMOD software (Boulder, CO).
Ten day old 1st and 2nd molars were analyzed by micro-computed tomography (μCT) using a Skyscan 1172 (Bruker-Skyscan, Contich, Belgium) system with a 7μm voxel size and a 40KVp beam. The CTAn 3D imaging processing software (Skyscan) was used for voume and density calculations within user-defined regions of interest (roi's). The data was averaged for 6 WT and 6 KO animals.
Results
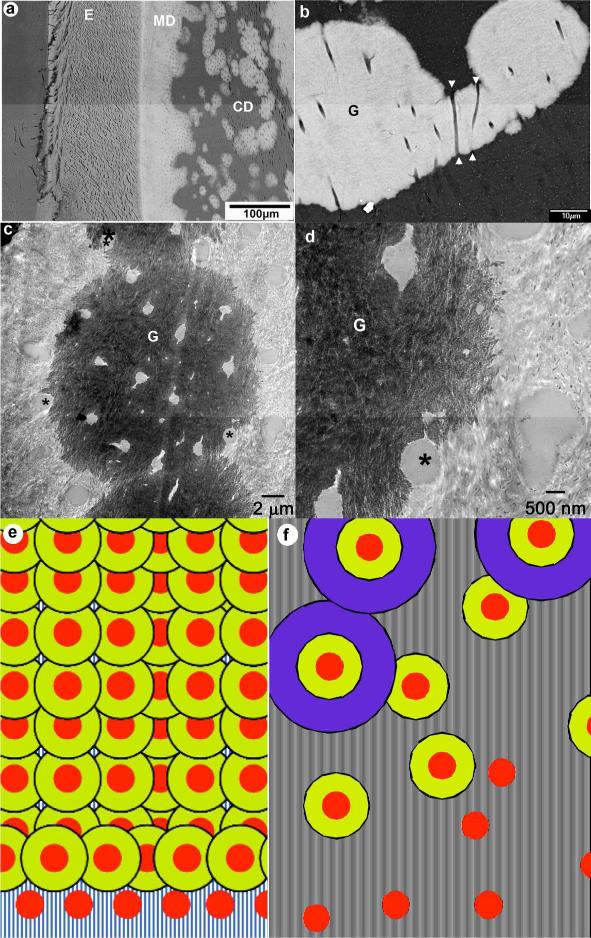
The BS SEM studies of the 7 mo incisal dentin revealed large areas of non- mineralized dentin throughout the circumpalpal dentin, while mantle dentin seemed to be unaffected (Fig. 1a). Spherulite-like mineralized inclusions were observed throughout nonmineralized areas of the dentin, providing an exaggerated globular dentin appearance. A more careful analysis of the circumpalpal dentin revealed that the individual dentinal tubules transect multiple mineralized and nonmineralized areas of circumpalpal dentin (Fig. 1b). We further investigated the relationships between the mineralized areas and dentinal tubules and noted that in the sections cut in the plane normal to the tubule axes the individual tubules at the mineralized/nonmineralized dentin boundaries were only partially mineralized (Fig. 1c,d).
Figure 1.
a) Low magnification SEM micrograph of the DSPP KO incisor. b) Intermediate magnification SEM micrograph of circumpulpal dentin showing 3 fused mineralized globules in the nonmineralized matrix. E-enamel; CD- circumpulpal dentin; G-mineralized globules; arrowheads point toward the c) TEM micrograph of a mineralized globule. d) a close up of the micrograph in c. In c and d asterisks identify tubules at the boundary between mineralized and nonmineralized dentin matrix. e) a model of normal dentin mineralization with high nucleation density and f) dentin mineralization in DSPP KO with low nucleation density. Red circles- the nucleation sites, yellow and purple concen Figure 1. a) Low magnification SEM micrograph of the DSPP KO incisor. b) Intermediate magnification SEM micrograph of circumpulpal dentin showing 3 fused mineralized globules in the nonmineralized matrix. E-enamel; CD- circumpulpal dentin; G-mineralized globules; arrowheads point toward the c) TEM micrograph of a mineralized globule. d) a close up of the micrograph in c. In c and d asterisks identify tubules at the boundary between mineralized and nonmineralized dentin matrix. e) a model of normal dentin mineralization with high nucleation density and f) dentin mineralization in DSPP KO with low nucleation density. Red circles- the nucleation sites, yellow and purple concentric rings represent progression of dentin mineralization.tric rings represent progression of dentin minerali
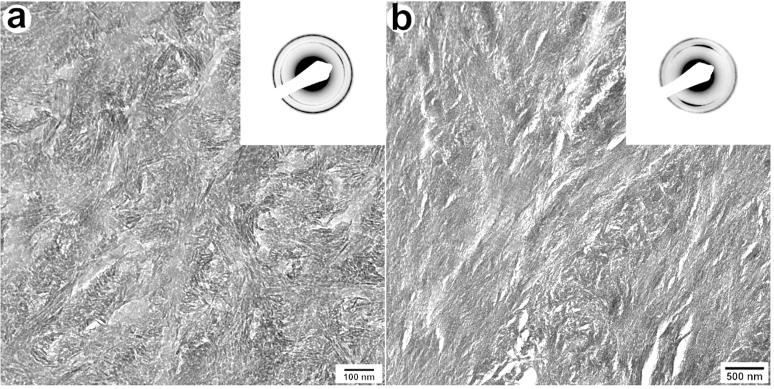
We studied the organization of apatitic crystals in the mineralized portions of the circumpulpal dentin using TEM and SAED (Fig. 2). To assess the crystal alignment we measured the angular spread of the c-axis (002) reflections taken from selected areas Ø 200 nm from 34 and 24 regions of WT and DSPP KO dentin respectively. Since the angular spread in polycrystalline materials with a high degree of crystallite alignment is narrower this method provides means to quantitatively assess the level of mineral organization(8). The angular spread in the WT was 2 times narrower (36.9°, SD±7.6) than in the DSPP KO dentin (80.2°, SD±10.33)and this difference is highly significant (p<0.005). We have also conducted electron tomography studies of the WT and DSPP KO and a much higher level of crystalline organization has been noted in the WT samples (Suppl. Fig. 2 and 3).
Figure 2.
TEM micrographs and corresponding diffraction patterns of DSPP KO (a) and WT (b) dentin and the corresponding diffraction patterns. Note that the arc of (002) reflections is much narrower in the diffraction pattern in (b) suggesting much higher degreee of alignment of the crystals in the collagen fibrils of WT dentin.
Our preliminary μCT studies of molars from 10 day old mice revealed 11% (1st molars) and 16% (2nd molars) reduction average and median mineral density in DSPP KO mice (Suppl. Fig. 3). The average density of dentin was lower in the 2nd molars in both WT and KO animals. The range of dentin mineral densities within the developing crown, expressed as width of mineral distribution peak at half height (Suppl. Fig 3) was 28%-30% broader, for both 1st and 2nd molars, in the DSPP KO molars vs. WT.
Discussion
The results of this preliminary study confirm the presence of large nonmineralized areas in circumpalpal dentin in DSPP KO mice(5, 6). It is possible that the compromised mineralization is related to the cellular effects of the absence of DSPP. Such effects might include changes in the secretion patterns of other extracellular proteins by odontoblasts or changes in the mineral ion transport. Indeed the fact that the collagen matrix deposition is suppressed in these animals (5) leading to a thinner dentin layer and wider pulp chambers supports this notion. However the heterogeneous pattern of mineralization in dentin matrix cannot be explained by overall changes in the expression levels of ECM proteins. Our observations of partially mineralized dentinal tubules sectioned in the transverse plane suggest that another mechanisms is in play here. During dentinogenesis each odontoblast produces one odontoblast process, occupying a dentinal tubule, and a portion dentin matrix surrounding the tubule. Since the appositional deposition of dentin occurs along the tubule axes, in the sections cut in the plane normal to the tubule axis the matrix surrounding one tubule is secreted at the same time by one odontoblast, and is likely to have a similar composition. However our data show that the matrix on one half of the transversely sectioned dentinal tube can be mineralized while it is not mineralized on the other side (Figure 1c,d). Such situation was only observed on the boundary of mineralized globular dentin. Assuming that that the composition of the organic matrix produced by one odontoblast cell at any given time is the same, we interpret this phenomenon as an evidence of the progression of the mineralization from the center of the mineralized globule outward. We therefore propose a hypothesis of the heterogeneous mineralization of dentin in DSPP KO through a classical theory of crystal nucleation and growth. Biomineralization systems, including dentin, contain a combination of nucleators and inhibitors, which together fine tune the mineralization process (9), preventing spontaneous precipitation, and allowing an organized controlled mineralization to take place. It is known that at least one of the cleavage products of DSPP, DPP, can act as promoter of mineralization (2, 3, 10) thus we suggest that in the absence of DSPP the mineral nucleation barrier is increased globally throughout the dentin, leading to a smaller number of the nucleation events at the predentin- dentin boundary, and causing the globular appearance of dentin (Fig. 1e,f).
Interestingly, the SEM and TEM characterization techniques employed in this study were not able to detect any observable differences in mantle dentin between the WT and DSPP KO specimens. This supports the concept that there are two different mechanisms of mineralization in the circumpuplal vs. mantle dentin and suggests that DSPP plays a lesser role in the formation of mantle dentin.
Our studies of the mineralized portions of dentin by means of SAED and electron tomography have revealed that the mineral crystals were much more organized in WT than the DSPP KO. This observation is in agreement with the results of earlier in vitro mineralization studies demonstrating that DPP, which is the major cleavage product of DSPP, controls organized mineralization of collagen fibrils (2). Alternatively, these changes might be caused by changes in the organization of collagen fibrils, leading to a considerable decrease in D-spacing of collagen from 67 to 60 nm in DSPP KO dentin (5).
The results of our microCT studies in very young animals demonstrate a significant decrease in the mineral density in the DSPP KO, confirming the results of previous reports (5, 6, 11). For the first time our microCT results quantitatively demonstrate a broader density distribution of the mineralized areas of dentin in DSPP KO, indicating heterogeneity in the degree of mineralization. These observations further support the hypothesis that DSPP cleavage products act as mineralization promoters in dentin.
In summary, our data show that the DSPP cleavage products, most likely DPP, control structural organization of mineralized collagen fibrils in dentin. Our data also suggest that the cleavage products of DSPP are promoters of mineral formation, and in their absence dentin becomes hypomineralized with substantial variations in mineral density across the mineralized areas. Finally we have noted that the mantle dentin in the DSPP KO is not affected, suggesting a difference in the mineralization mechanisms between these two dentin types.
Supplementary Material
Acknowledgements
The authors are grateful for the support from NIH, DE016703 (EB), DE04141 (AB), AR046121 (AB), which was instrumental for our ability to conduct these studies. We also thank Dr. Ashok Kulkarni (NIH, Bethesda) who has provided us with DSPP KO mice.
References
- 1.Kim JW, Simmer JP. Hereditary dentin defects. J Dent Res. 2007;86(5):392–9. doi: 10.1177/154405910708600502. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande AS, Fang P-A, Zhang X, Jayaraman T, Sfeir C, Beniash E. Primary Structure and Phosphorylation of Dentin Matrix Protein 1 (DMP1) and Dentin Phosphophoryn (DPP) Uniquely Determine Their Role in Biomineralization. Biomacromolecules. 2011;12(8):2933–45. doi: 10.1021/bm2005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George A, Veis A. Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Chemical Reviews. 2008;108(11):4670–93. doi: 10.1021/cr0782729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadlowiec J, Koch H, Zhang XY, Campbell PG, Seyedain M, Sfeir C. Phosphophoryn regulates the gene expression and differentiation of NIH3T3, MC3T3-E1, and human mesenchymal stem cells via the integrin/MAPK signaling pathway. Journal of Biological Chemistry. 2004 Dec;279(51):53323–30. doi: 10.1074/jbc.M404934200. [DOI] [PubMed] [Google Scholar]
- 5.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, et al. Dentin Sialophosphoprotein Knockout Mouse Teeth Display Widened Predentin Zone and Develop Defective Dentin Mineralization Similar to Human Dentinogenesis Imperfecta Type III. Journal of Biological Chemistry. 2003 Jul 4;278(27):24874–80. doi: 10.1074/jbc.M303908200. 2003. [DOI] [PubMed] [Google Scholar]
- 6.Gibson MP, Zhu Q, Liu Q, D'Souza RN, Feng JQ, Qin C. Loss of dentin sialophosphoprotein leads to periodontal diseases in mice. J Periodont Res. 2013 Apr;48(2):221–7. doi: 10.1111/j.1600-0765.2012.01523.x. [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner S, Wagner HD. The material bone: Structure mechanical function relations. Annual Review of Materials Science. 1998;28:271–98. [Google Scholar]
- 8.Wenk HR, Van Houtte P. Texture and anisotropy. Reports on Progress in Physics. 2004 Aug;67(8):1367–428. [Google Scholar]
- 9.Addadi L, Beniash E, Weiner S. Assembly and mineralization processes in biomineralization: strategies for forming biological composite materials. In: W J, CR R, editors. Supramolecular Organization and Materials Design. Cambridge University Press; Cambridge: 2002. pp. 1–34. [Google Scholar]
- 10.Boskey AL, Maresca M, Doty S, Sabsay B, Veis A. Concentration-dependent effects of dentin phosphoryn in the regulation of invitro hydroxyapatite formation and growth. Bone and Mineral. 1990 Oct;11(1):55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Sreenath T, Haruyama N, Honeycutt C, Terse A, Cho A, et al. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 2009 May;28(4):221–9. doi: 10.1016/j.matbio.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.