Stroke is a leading cause of morbidity and mortality. It frequently occurs when anatomically specific parts of the nervous system (most often the brain, although peripheral nerves can be affected as well) are deprived of blood delivery, which deprives the sub-served tissue of glucose and oxygen.
As is the case with cardiac ischemia, current therapy emphasizes rapid restoration of blood delivery, typically through lysis or the physical removal of a vessel-obstructing blood clot. While this approach has proved useful, it has been less transformative in treating acute cerebral ischemia than it has in treating myocardial ischemia (MI). There are several reasons for this. Pain is not a major feature of stroke as it is in MI, so stroke patients tend not to present to medical attention as rapidly. Also, the time frame during which restoration of blood flow can rescue brain tissue is not as great as it is for cardiac tissue. Finally, restoration of blood flow to infarcted brain tissue can have negative consequences, such as bleeding through friable blood vessel walls and the delivery or activation of toxic molecules that can lead to a secondary reperfusion injury.
The fact that some stroke-related damage may not be directly or completely induced by a reduced or time-limited interruption in glucose or oxygen delivery has supported the hope that neuroprotection may be achieved, or neurodestruction mitigated, by interventions applied after ischemia has occurred and especially after blood flow has been restored. Relevant research along these lines has focused on cytokines, small molecules that confer signals between cells, and which represent a way through which one group of cells can influence the function of another group of cells. While many molecules function as cytokines, it is important to note many cytokines function within the context of immune and inflammation responses. Cytokines may mediate some of the damage or dysfunction observed in a variety of neurologic conditions, including stroke.
Tumor necrosis factor alpha (TNFα) is a brain and systemically generated cytokine. Many cell types synthesize it, but macrophages and microglia are particularly robust producers (Gahring et al, 1996). TNFα plays an important role in acute inflammatory responses, and it is suspected to exacerbate stroke pathology (Barone et al, 1997). Bloodstream and cerebrospinal fluid levels rise immediately following a stroke, and observational studies indicate stroke lesion size positively correlates with measured TNFα levels (Zaremba and Losy, 2001). The observational nature of these studies, though, only establishes the fact that a relationship exists between TNFα and stroke damage. It does not prove that TNFα ferments stroke pathology, nor does it provide mechanistic insight into how TNFα might function in such a capacity.
Prior studies have experimentally addressed the question of whether TNFα can itself function as a neurotoxin, and results from these studies suggest this could indeed be the case (Reimann-Philipp et al, 2001). The amount and duration of exposure in these initial experiments, though, arguably did not rigorously reflect the exposure conditions that might exist within the setting of a stroke. To better address this point, Doll et al. conducted a study that perhaps more faithfully recapitulates the effects of acute TNFα exposure on neurons (Doll et al. 2014). This study, now reported in this volume of the Journal of Neurochemistry, advances the stroke field by providing insight into how brief, likely physiologic-level TNFα exposures affect neuronal function and viability. This study further demonstrates a mechanism through which TNFα might promote neuro-demise.
The authors exposed HT22 hippocampal cells and mouse primary cortical neurons to TNFα at levels seen in blood following stroke (up to 1000 pg/ml) for 1.5 through 24 hours. Interestingly, it was found that even at 1.5 hours mitochondrial oxygen consumption rates (OCRs) were generally reduced, the amount of oxygen consumption associated with ATP production fell (which infers a reduction in ATP levels may have occurred), and there was a reduced mitochondrial membrane potential. In HT22 cells declines in these mitochondrial endpoints tended to slightly precede cell death but otherwise cell viability roughly tracked the mitochondrial changes. In primary mouse cortical neurons, the mitochondrial endpoints changed even though cell viability did not; reduced cell viability was only observed at the highest concentration at the longest duration. The fact that reduced cell viability was not observed in the absence of reduced mitochondrial function, and that reduced mitochondrial function preceded and in some cases clearly occurred in the absence of cell viability changes, is more consistent with the view that mitochondrial dysfunction in these experiments contributed to cell death, as opposed to the possibility that cell death or dying itself caused mitochondrial dysfunction.
The authors next elucidated the pathway through which TNFα produced its toxic effects. They reasoned these effects began with binding of TNFα to one of its plasma membrane receptors. By blocking each of the best characterized TNFα receptors, TNF receptor 1 (TNF-R1) and TNF receptor 2 (TNF-R2) with receptor-specific antibodies, they determined that TNFα toxicity requires TNF-R1 binding.
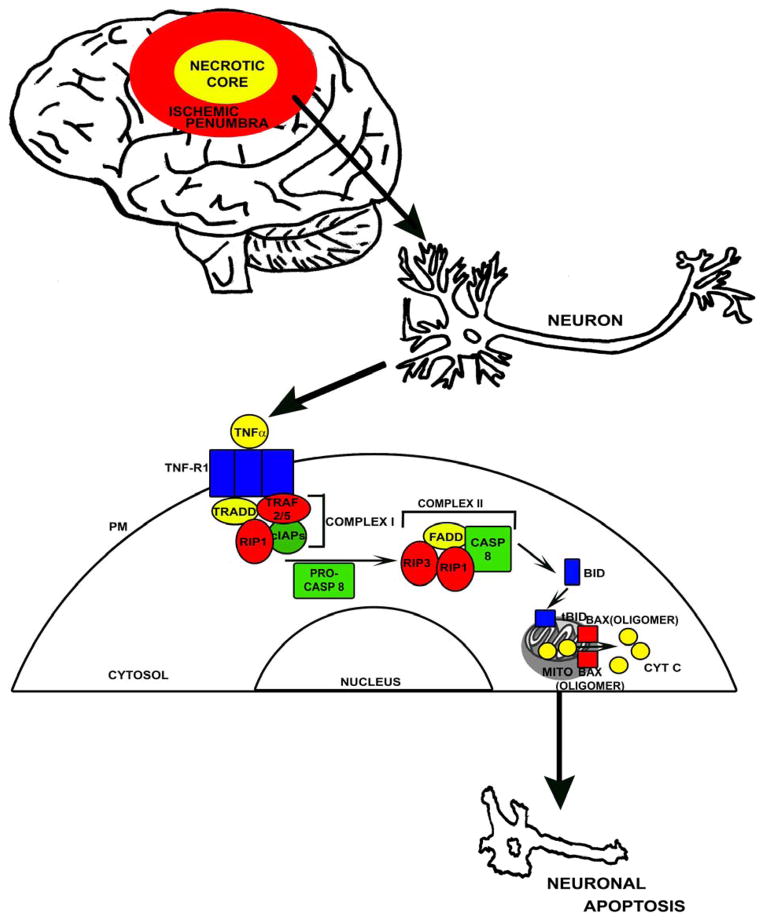
It is known that when TNFα binds to TNF-R1, TNF-R1 forms a trimer on the plasma membrane surface. This trimer binds a cytosolic complex called complex I (not to be confused with complex I of the respiratory chain) (Figure 1). Complex I consists of several proteins including TNF receptor associated death domain (TRADD), cellular inhibitor of apoptosis proteins (cIAPs), receptor interacting protein 1 (RIP1), and TNF receptor associated factors 2 and 5 (TRAF2/5) (Vandenabeele et al. 2010). Complex I, in turn, recruits additional proteins to form a new complex, complex II, that contains Fas associated protein with a death domain (FADD), RIP1, receptor interacting protein 3 (RIP3), and procaspase 8. Within complex II cleavage of inactive procaspase 8 can occur, giving rise to active caspase 8.
Figure 1. Putative TNFα-mediated apoptosis signalling pathway.
Within the ischemic penumbra of a stroke, increasing TNFα levels may activate TNF-R1 by causing it to form a trimer on the plasma membrane (PM). An intracellular protein complex then forms consisting of TRADD, TRAF2/5, RIP1, and cIAPs (also referred to as complex I). Complex I recruits additional proteins (FADD, RIP3, and procaspase 8). If the inactive procaspase 8 (PRO-CASP8) is cleaved to active caspase 8 (CASP 8) by this new complex (complex II), it can process BID to its active truncated form (tBid). tBID causes BAX to oligomerize and form pores in the outer membranes of mitochondria (MITO), which in conjunction with depolarization of the mitochondrial membrane potential facilitates the release of cytochrome c (CYT C) to the cytosol. In the cytosol, cytochrome c can initiate terminal apoptotic cascades.
Caspase 8 can initiate apoptotic signaling through different pathways. In their culture models, Doll et al. found caspase 8 activation indeed occurred in cells treated with TNFα. Caspase 8 can cleave the apoptosis-related protein BH3 interacting-domain death agonist (BID), thereby activating it to truncated BID (tBID). tBID then causes Bcl-2-associated X protein (BAX) to oligomerize and form pores within the mitochondrial outer membrane. In conjunction with depolarization of the mitochondrial membrane potential, outer membrane permeabilization should facilitate the subsequent release of cytochrome c to the cytosol.
In the Doll et al. study some of these steps are inferred. For example, BID and BAX changes were assumed to occur because activation of two other caspase 8-activated caspase enzymes that mediate apoptosis, caspase 3 and caspase 7, was not observed. Also, the role of cytochrome c in the death of the cultured cells was made through association, as opposed to being mechanistically demonstrated. Nevertheless, these results do define a plausible series of events through which an inflammatory cytokine, TNFα, whose concentrations increase in the setting of stroke, could independently initiate apoptotic neuronal cell death. In this case, TNFα binds TNF-R1, which activates caspase 8. Caspase 8 sets in motion events that ultimately induce mitochondrial dysfunction and specific consequences of mitochondrial dysfunction (decreased respiration, depolarization, reduced ATP production, and the release of cytochrome c). Cytochrome c release, in turn, leads to apoptotic cell death.
These results are consistent with those from prior studies that found TNFα adversely impacts mitochondria. Cardiac myocytes exposed to TNFα show reduced activity of complex III of the electron transport chain, as well as increased reactive oxygen species production (Suematsu et al. 2003). TNFα may also uncouple mitochondrial respiration from ATP production, and lead to mitochondrial DNA damage in certain model systems (Kastl et al. 2014, Suematsu et al. 2003).
This study has translational implications. Brain damage in stroke is currently understood to include an inner zone of necrotic cell death, which defines the area of the most severe ischemia and which is rapidly destroyed. Surrounding this zone is the ischemic “penumbra”, in which neuron death proceeds via apoptosis and which is somewhat delayed from the period of acute ischemia. Since rescue of the necrotic core may not be possible, most stroke neuroprotection development programs have focused on the preservation of the penumbra. Efforts at interrupting activation or perpetuation of apoptotic cascades have tried to minimize oxidative stress and glutamate excitotoxicity, but all approaches tested to date have failed to improve stroke outcomes in human clinical trials. Demonstrating that TNFα, a cytokine whose levels rise during stroke, can induce apoptosis through a specific pathway suggests a number of plausible pharmacologic targets that may be worth pursuing.
Of course, cultured cells cannot recreate the complexity of the human brain. To this end it is important to note that while the Doll et al. study shows TNFα can induce the apoptotic death of neurons, it ultimately does not resolve the question of whether increased TNFα levels in stroke truly extend the amount of brain damage that results. It is still possible that TNFα primarily acts as a biomarker of stroke, as opposed to a mediator of stroke damage. To confirm or refute these contingencies will require clinical trials, or at the very least further experiments in animal stroke models. Indeed, there are already studies that claim interfering with TNFα signaling reduces stroke volume in some experimental stroke models (Lavine et al 1998; Meistrell et al 1997). The study of Doll et al. may now help refine this line of investigation, and certainly the identification of new potential therapeutic targets should be enthusiastically welcomed.
Acknowledgments
The authors are supported by the University of Kansas Alzheimer’s Disease Center (P30AG035982), the Frank and Evangeline Thompson Alzheimer’s Treatment Program Fund, the Hugh and Betty Libby Foundation, the Greater Kansas City Automobile Dealers Association, the Gene and Marge Sweeney Chair, the University of Kansas Alzheimer’s Disease Center Pilot Program, the University of Kansas Landon Center on Aging, the University of Kansas Frontiers Heartland Institute for Clinical and Translational Research, and the University of Kansas Medical Center’s Biomedical Research Training Program.
Abbreviations used
- BAX
Bcl-2-associated X protein
- BID
BH3 interacting-domain death agonist
- cIAP
cellular inhibitor of apoptosis protein
- FADD
Fas associated protein with a death domain
- MI
myocardial infarction
- OCR
oxygen consumption rate
- RIP1
receptor interacting protein 1
- RIP3
receptor interacting protein 3
- tBID
truncated BID
- TNFα
tumor necrosis factor alpha
- TNF-R1
tumor necrosis factor receptor 1
- TNF-R2
tumor necrosis factor receptor 2
- TRADD
TNF receptor associated death domain
- TRAF2/5
TNF receptor associated factors 2 and 5
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
References
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;6:1233–44. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- Doll D, Rellick S, Barr T, Ren X, Simpkins J. Rapid Mitochondrial Dysfunciton Mediates TNF-a Induce Neurotoxicity. J Neurochem. 2014 doi: 10.1111/jnc.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahring LC, Carlson NG, Kulmar RA, Rogers SW. Neuronal expression of tumor necrosis factor alpha in the murine brain. Neuroimmunomodulation. 1996;5:289–303. doi: 10.1159/000097283. [DOI] [PubMed] [Google Scholar]
- Kastl L, Sauer SW, Ruppert T, Beissbarth T, Becker MS, Suss D, Krammer PH, Gulow K. TNF-alpha mediates mitochondrial uncoupling and enhances ROS-dependent cell migration via NF-kappaB activation in liver cells. FEBS letters. 2014;588:175–183. doi: 10.1016/j.febslet.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Lavine SD, Hofman FM, Zlokovic BV. Circulating antibody against tumor necrosis factor-alpha protects rat brain from reperfusion injury. J Cereb Blood Flow Metab. 1998;18:52–58. doi: 10.1097/00004647-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Meistrell ME, 3rd, Botchkina GI, Wang H, Di Santo E, Cockroft KM, Bloom O, Vishnubhakat JM, Ghezzi P, Tracey KJ. Tumor necrosis factor is a brain damaging cytokine in cerebral ischemia. Shock. 1997;8:341–348. [PubMed] [Google Scholar]
- Reimann-Philipp U, Ovase R, Weigel PH, Grammas P. Mechanisms of cell death in primary cortical neurons and PC12 cells. J Neurosci Res. 2001;6:654–60. doi: 10.1002/jnr.1119. [DOI] [PubMed] [Google Scholar]
- Suematsu N, Tsutsui H, Wen J, et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature reviews Molecular cell biology. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Zaremba J, Losy L. Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001;5:288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]