Abstract
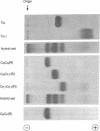
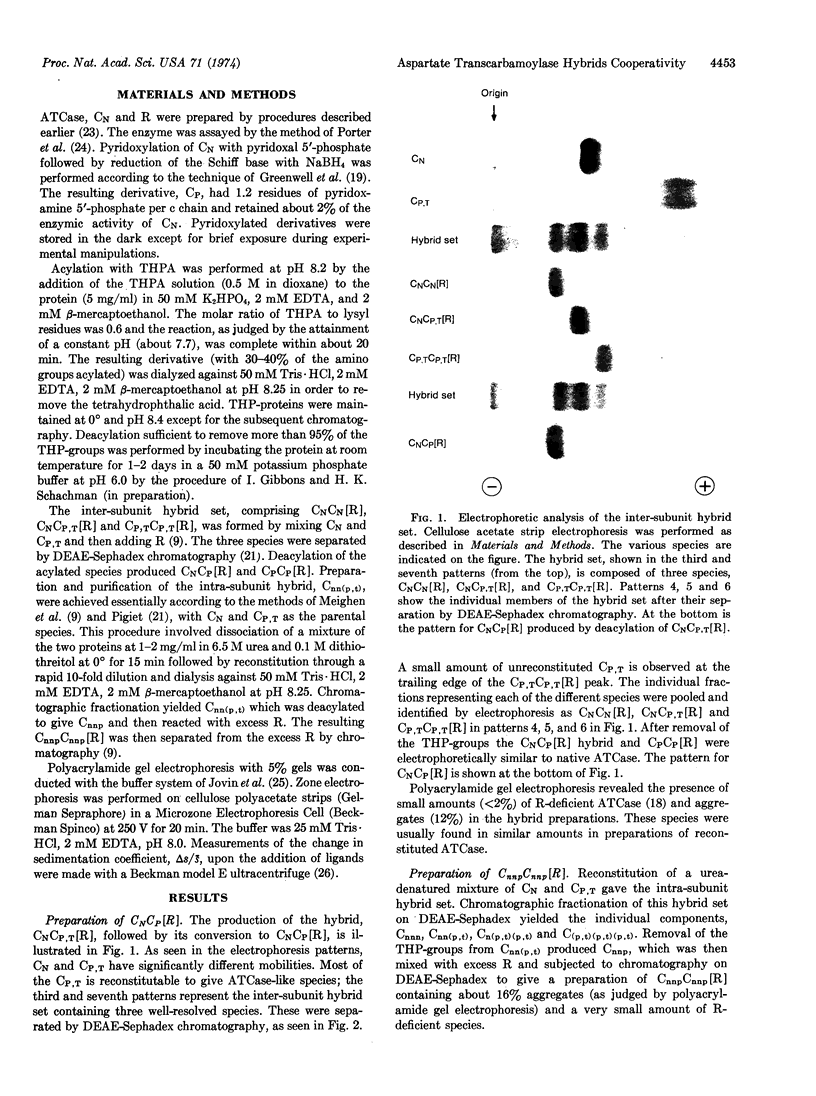
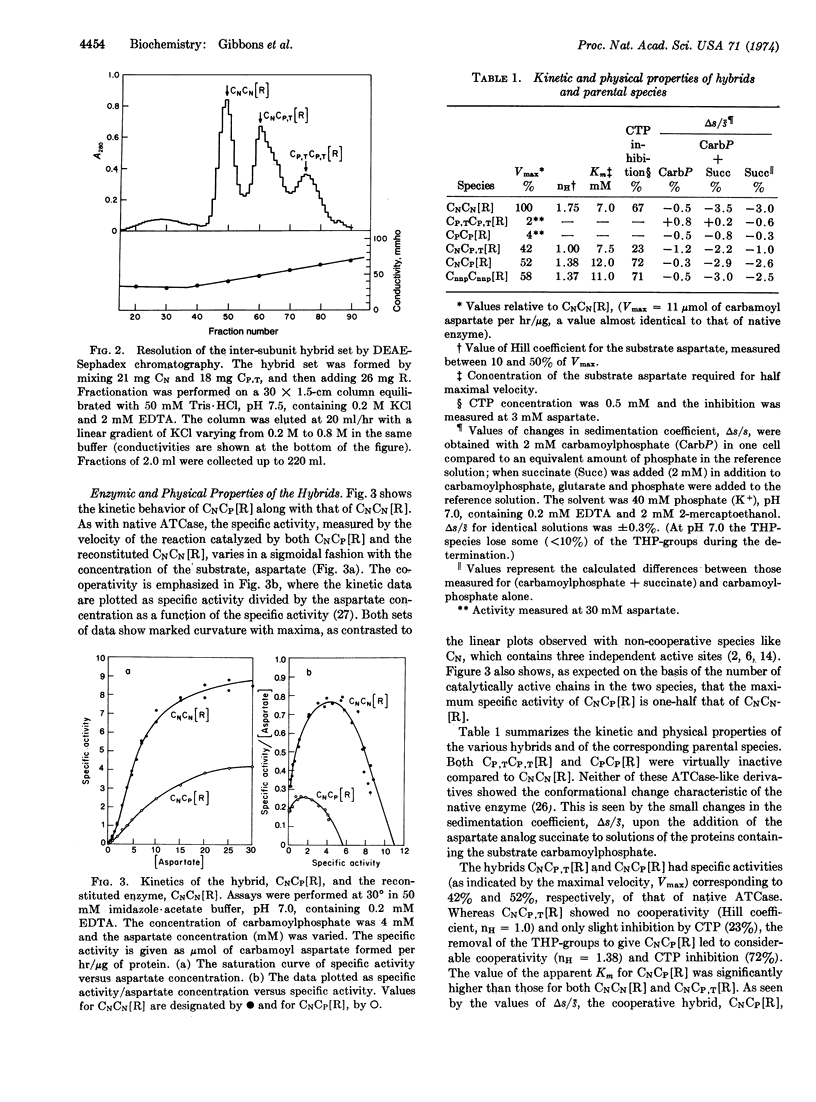
Hybrids of aspartate transcarbamoylase (EC 2.1.3.2; carbamoylphosphate: L-aspartate carbamoyltransferase) from Escherichia coli containing native (active) and pyridoxylated (inactive) catalytic polypeptide chains were constructed by a procedure involving the reversible acylation of amino groups with 3,4,5,6-tetrahydrophthalic anhydride. This technique exploited the charges contributed by the tetrahydrophthaloyl groups as a “chromatographic handle” for separating the various species. Enzyme-like molecules containing one fully active and one inactive catalytic subunit showed cooperative kinetic behavior, considerable inhibition by CTP, and a substantially increased apparent Km compared to the native enzyme. Similar properties were observed for an intrasubunit hybrid containing one inactive catalytic polypeptide chain in each subunit. The cooperative inter-and intra-subunit hybrids also exhibited conformational changes similar to those found for the native enzyme upon the addition of stereospecific ligands. These observations, taken together with data for other complexes of catalytic and regulatory subunits, illustrate the importance of the architecture of aspartate transcarbamoylase and the quaternary constraint stemming from the subunit interactions.
Keywords: allosteric enzymes, cooperativity, reversible charge modification, chromatographic handle
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bothwell M., Schachman H. K. Pathways of assembly of aspartate transcarbamoylase from catalytic and regulatory subunits. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3221–3225. doi: 10.1073/pnas.71.8.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. W., Mort J. S. A complex between the catalytic and regulatory subunits of aspartate transcarbamylase. J Biol Chem. 1973 Nov 10;248(21):7614–7616. [PubMed] [Google Scholar]
- Cohlberg J. A., Pigiet V. P., Jr, Schachman H. K. Structure and arrangement of the regulatory subunits in aspartate transcarbamylase. Biochemistry. 1972 Aug 29;11(18):3396–3411. doi: 10.1021/bi00768a013. [DOI] [PubMed] [Google Scholar]
- Evans D. R., Warren S. G., Edwards B. F., McMurray C. H., Bethge P. H., Wiley D. C., Lipscomb W. N. Aqueous central cavity in aspartate transcarbamylase from Escherichia coli. Science. 1973 Feb 16;179(4074):683–685. doi: 10.1126/science.179.4074.683. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. II. Evidence for different conformational states of the protein in the presence and absence of specific ligands. Biochemistry. 1968 Feb;7(2):538–552. doi: 10.1021/bi00842a600. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Greenwell P., Jewett S. L., Stark G. R. Aspartate transcarbamylase from Escherichia coli. The use of pyridoxal 5'-phosphate as a probe in the active site. J Biol Chem. 1973 Sep 10;248(17):5994–6001. [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Stark G. R. Aspartate transcarbamylase. A study of possible roles for the sulfhydryl group at the active site. J Biol Chem. 1973 Dec 10;248(23):8003–8014. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Meighen E. A., Pigiet V., Schachman H. K. Hybridization of native and chemically modified enzymes. 3. The catalytic subunits of aspartate transcarbamylase. Proc Natl Acad Sci U S A. 1970 Jan;65(1):234–241. doi: 10.1073/pnas.65.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Richards K. E., Williams R. C. Electron microscopy of aspartate transcarbamylase and its catalytic subunit. Biochemistry. 1972 Aug 29;11(18):3393–3395. doi: 10.1021/bi00768a012. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P., Weber K. Subunit structure of aspartate transcarbamylase from Escherichia coli. J Biol Chem. 1971 Mar 25;246(6):1644–1657. [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]
- Yang Y. R., Syvanen J. M., Nagel G. M., Schachman H. K. Aspartate transcarbamoylase molecules lacking one regulatory subunit. Proc Natl Acad Sci U S A. 1974 Mar;71(3):918–922. doi: 10.1073/pnas.71.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]