Abstract
Binding of steroid and thyroid hormones to their cognate nuclear receptors (NRs) impacts virtually every aspect of postembryonic development, physiology and behavior, and inappropriate signaling by NRs may contribute to disease. While NRs regulate genes by direct binding to hormone response elements in the genome, their actions may depend on the activity of other transcription factors (TFs) that may or may not bind DNA. The Krüppel-like family of transcription factors (KLF) is an evolutionarily conserved class of DNA-binding proteins that influence many aspects of development and physiology. Several members of this family have been shown to play diverse roles in NR signaling. For example, KLFs 1) act as accessory transcription factors for NR actions, 2) regulate expression of NR genes, and 3) as gene products of primary NR response genes function as key players in NR-dependent transcriptional networks. In mouse models, deletion of different KLFs leads to aberrant transcriptional and physiological responses to hormones, underscoring the importance of these proteins in the regulation of hormonal signaling. Understanding the functional relationships between NRs and KLFs will yield important insights into mechanisms of NR signaling. In this review we present a conceptual framework for understanding how KLFs participate in NR signaling, and we provide examples of how these proteins function to effect hormone action.
Keywords: nuclear receptor, Krüppel-like factor, transcription
1.1 Introduction
Almost three decades after the first nuclear receptor (NR) genes were cloned, many questions remain about how these proteins activate or repress target genes to mediate the actions of hormones. In particular, the role of other transcription factors (TFs) in NR signaling is increasingly recognized as an essential but poorly understood aspect of NR function. Krüppel-like factors (KLFs) have emerged as key players in NR signaling. The KLFs are zinc-finger TFs with diverse functions in cell proliferation, differentiation and tumorigenic transformation [47]. Krüppel-like factors bind to GC/GT-rich regions in the genome and activate or repress target genes in concert with chromatin-modifying enzymes. Several KLFs have been found to function in NR signaling by different mechanisms. Some are direct NR target genes whose protein products regulate secondary response genes to mediate hormone action. Others act as accessory TFs to cooperatively activate or repress NR target genes. Still others regulate the expression of genes coding for NRs, influencing NR protein expression and therefore cellular sensitivity to hormone. Here we review the current state of knowledge of the diverse roles that KLFs play in hormone action. We focus on NR signaling in vertebrates, discuss gaps in our current knowledge of KLF functions, and propose experiments to better understand KLF/NR interactions.
1.2 Krüppel-like factors
Krüppel-like factors comprise a family of transcription factors that bind GC/GT rich sequences in the genome [47]. They have C-terminal DNA binding domains (DBDs) consisting of three Cys2-His2 zinc fingers that are highly conserved among KLFs within and among vertebrate species, and that also exhibit sequence similarity with DBDs of members of the Specificity Protein (Sp) family [33]. By contrast to the DBDs, the N-terminal regions are highly variable, which likely explain the divergent functions of KLFs despite their similar DNA-binding capabilities.
There are seventeen KLF genes in mammals that group into three major subfamilies based on similarities in their N-terminal domains (Figure 1) [49]. Krüppel-like factors 3, 8, and 12 (Group 1) typically repress gene transcription by binding to the C-terminal binding proteins (CtBPs), a family of transcriptional repressors that recruit chromatin-modifying enzymes that add repressive marks to histones [80]. Krüppel-like factors 1, 2, 4, 6 and 7 (Group 2) share acidic activation domains and thus typically act as transcriptional activators. Krüppel-like factors 9, 10, 11, 13, 14 and 16 (Group 3) share a Sin3a-interacting domain (SID), an α-helical motif that interacts with the repressor protein Sin3a [93]. These factors can repress transcription of target genes, but they are also capable of acting as transactivators [30]; whether a Group 3 KLF functions in tranrepression or transactivation may depend on the stage of cellular differentiation that it is expressed. For example, KLF9 activates the Fgfr1 promoter in proliferating myoblasts but represses it in differentiated myotubes via the same DNA binding site [48]. Krüppel-like factors 5, 15 and 17 do not group into any of the other families based on the presence of identifiable protein-protein interaction motifs, although their primary amino acid sequences place KLFs 15 and 17 closer to Group 3, and KLF5 closer to Group 1 [47, 49]. All three can act as activators or repressors of transcription [25, 52, 82](and see section 3.3), In some cases, KLFs of the same subfamily have overlapping or redundant functions and can compensate for each other if one is lost or deleted [28, 85]. Members of all three subfamilies have been found to function in NR signaling.
Figure 1. Phylogeny of human KLF proteins.
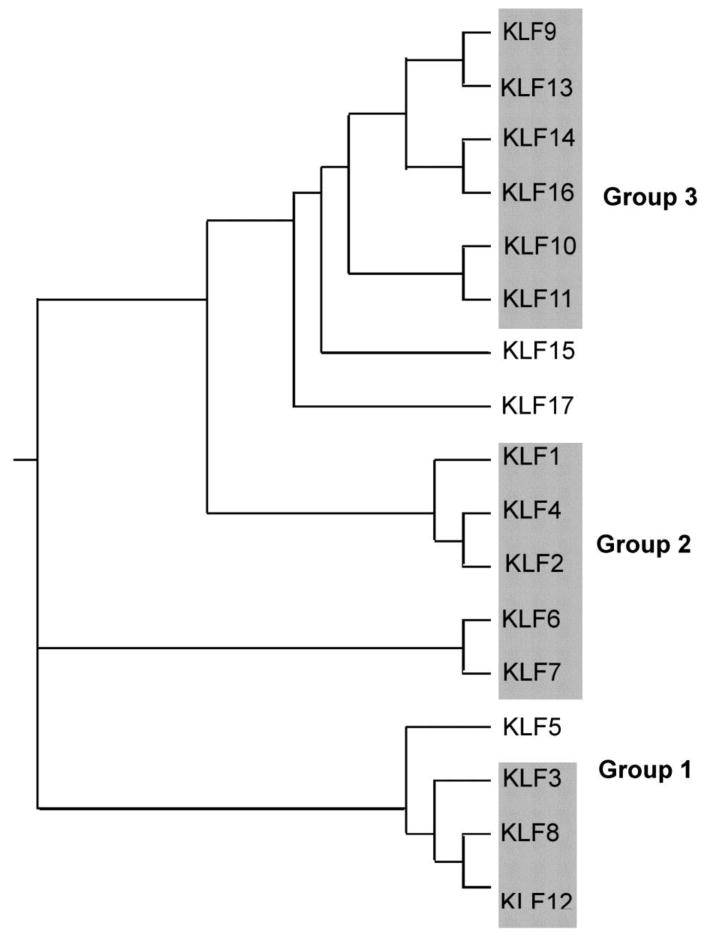
The seventeen human KLF proteins group into three major subfamilies based on structural similarities in their N-termini. The Clustal W alignment and grouping are based on [47, 49].
1.3 Nuclear receptors
Nuclear receptors comprise an ancient family of transcription factors that mediate signaling by hormones, vitamins A and D, metabolic intermediates and products, and xenobiotics; some NRs are orphan receptors for which a ligand has not yet been discovered, or that may function in a ligand-independent manner [22]. All NRs share three key functional protein domains. The ligand binding domain (LBD) located at the C-terminus binds hormone (or metabolic intermediate or xenobiotic), and also contains an activator function domain (AF-2) that binds coactivators or co-repressors in a ligand-dependent manner. The DNA binding domain (DBD) located in the center of the molecule binds to DNA, allowing for activation or repression of direct NR target genes. The N-terminus has a domain with weak, ligand-independent activation function (AF-1) [43]. Molecular phylogenetic analysis divides the human NR family into six clades that differ in size. The NRs that mediate hormone action fall into two functional groups (type I and type II) based on subcellular distribution in the unliganded state and mechanisms of action [22]. The type I receptors (e.g., steroid hormone receptors) reside in the cytoplasm in the absence of hormone where they are bound by heat shock and other proteins that facilitate folding of the receptor into a ligand-binding state (the receptor is ‘activated’). Upon ligand binding the NR and heat shock protein complex dissociate, transforming the NR into a DNA binding conformation. The NR then translocates to the nucleus where it binds to hormone response elements as a homodimer and activates or represses gene transcription. By contrast, type II receptors (e.g., thyroid – TR, and retinoid receptors - RAR/RXR) are bound to DNA in the absence of hormone, often as heterodimers. Unliganded type II receptors can function as powerful transcriptional repressors when resident in chromatin; whereas, in the liganded state they may activate or repress transcription. This switch in function is due to conformational changes that lead to changes in protein-protein interactions; unliganded receptors resident in chromatin recruit corepressors such as Nuclear Receptor Corepressor (NCoR) and Silencing Mediator of Retinoid and Thyroid receptors (SMRT) that in turn recruit histone deacetylases (HDACs). By contrast, liganded receptors recruit coactivators, some with histone acetyltransferase (HAT) activity such as CBP/p300 that catalyze acetylation of lysine residues on histone tails [14]. Type II receptors often bind DNA as heterodimers (e.g., TR-RAR or TR-RXR), which may allow for crosstalk between hormone and retinoid signaling pathways [5, 40].
In this review we focus on KLF interactions with nuclear hormone receptors because these have been the best studied, but there are some reports of KLFs influencing the expression or function of other NR types. For example, KLFs 8 and 9 were shown to regulate the expression of peroxisome proliferator activated receptor γ in differentiating adipocytes [37, 59]. In mouse liver KLF9 was found to work cooperatively with hepatocyte nuclear factor 4α on the promoter of the Deiodinase 1 gene [51].
1.4 Roles of KLFs in NR signaling
Before discussing the roles that KLFs play in NR signaling it is important to establish a conceptual framework for understanding which types of associations with NRs are most relevant for KLFs to act as mediators/integrators of NR function. Here we will discuss three ways in which KLFs can influence NR signaling (summarized in Figure 2):
Figure 2. Roles of KLFs in nuclear receptor signaling.
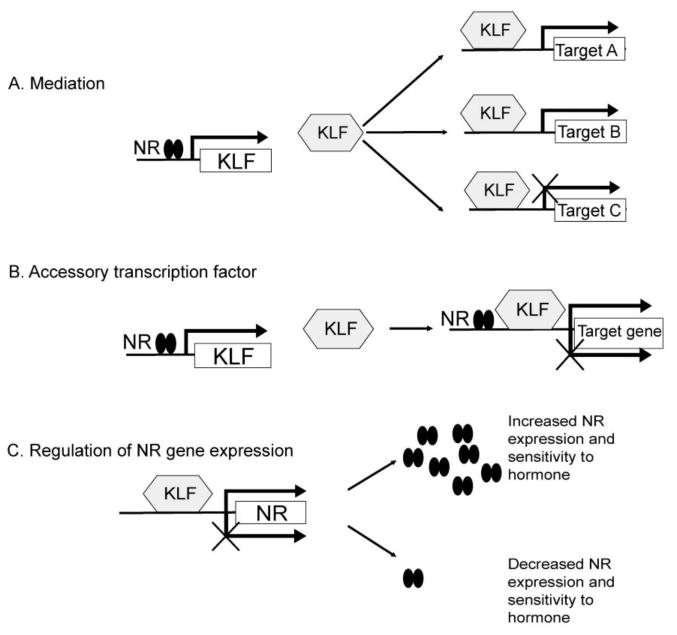
A) A KLF is a direct transcriptional target of liganded NR that activates or represses secondary response genes. B) A KLF acts as an accessory transcription factor to cooperatively activate or repress an NR target gene. C) A KLF regulates expression of the NR gene to modify cellular sensitivity to hormone.
KLF genes are direct transcriptional targets of NRs, and therefore mediate the cellular actions of NRs by regulating secondary hormone response genes (up or down-regulation). Also, KLFs may regulate transcription of genes that code for proteins that influence NR function in chromatin, which can enhance or suppress ongoing or subsequent NR signaling. Several KLF genes may be targeted by multiple NRs. Krüppel-like factor genes may be convergence points for different intracellular signaling pathways, thus serving as integrators of cellular endocrine responses.
Some KLFs act as cofactors/accessory TFs for NR function, thereby enhancing or enabling transcriptional activation or repression at NR target genes. This can involve direct DNA binding of the KLF to the NR regulated gene, or protein-protein interactions among the KLF and the NR. Modulation of NR signaling by accessory TFs is an important mechanism for imparting specificity to hormonal responses.
KLFs can regulate expression of NR genes, which would impact cellular sensitivity to hormones.
As we discuss below, there are examples for each of these mechanisms of action for KLFs on NR signaling. Furthermore, some KLFs function in multiple NR signaling pathways and/or perform different functions within a single pathway. In this review we focus on roles for KLFs in thyroid hormone, corticosteroid and sex steroid signaling via their cognate NRs.
2.1 Thyroid hormone receptors
Thyroid hormone (TH) has important roles in development, metabolism and behavior of vertebrates. The hormone is critical for normal development of the nervous system. Lack of TH during early postnatal development leads to irreversible growth arrest and mental retardation (cretinism); this condition is accompanied by histological defects in axon and dendrite development in neurons of the central nervous system (CNS) [10]. There are two thyroid hormone receptor (TR) genes, TRα and TRβ.Several isoforms are generated by alternate promoter usage or mRNA processing. The different TRs regulate overlapping and distinct sets of target genes, have different expression patterns, and therefore different physiological roles [14]. Thyroid hormone receptors are Type II NRs, meaning they can function as transcriptional repressors in the unliganded state and activators upon ligand binding (see section 1.3; for some genes such as thyroid stimulating hormone beta subunit in the pituitary and thyrotropin-releasing hormone in the hypothalamus liganded TRs repress transcription [69].
2.2 Klf9 is a direct TR target gene, and KLF9 mediates some actions of TH/TR in the central nervous system
Several KLFs may be induced by TH/TR signaling, but the molecular basis for hormone regulation and the significance for hormone action has so far been studied only for KLF9 (originally named basic transcription element binding protein - BTEB1). Thyroid hormone induces Klf9 gene expression through direct TR binding to regions upstream of the Klf9 locus in Xenopus and rodents [17]. Its transactivation in frog and mammal brain is mediated by TH response elements (T3REs) located in 5′ flanking regions [18, 21]. In addition to its strong induction by TH, Klf9 mRNA and protein are induced by corticosterone (CORT; see section 3.2) [7, 11]. Furthermore, Klf9 is synergistically activated by the two hormones. This synergistic activation is mediated by an evolutionarily conserved NR enhancer element located 5 to 6 kb upstream of tetrapod Klf9 genes that contains several hormone response elements (HREs), including the T3REs described above (the Klf9 synergy module; [4]; P. Bagamasbad, R.M. Bonett, L. Sachs and R.J. Denver, unpublished data).
Several lines of evidence support that KLF9 mediates the actions of TH on neuronal differentiation. For example, in the mouse neuroblastoma cell line Neuro2a KLF9 overexpression stimulated neurite outgrowth, similar to the action of TH on these cells [17]. Knockdown of KLF9 blocked TH-induced neurite branching in cultured rat fetal cortical neurons, supporting that KLF9 functions as an intermediary for TH action on branching morphogenesis in immature cortical neurons [13].
In the juvenile/adult brain KLF9 maintains cellular differentiation and promotes cell survival. Moore and colleagues [50] showed that KLF9 overexpression inhibited neurite outgrowth in cultured retinal ganglion cells. As KLF9 expression is low at birth and rises postnatally, this suggests that it acts to maintain a differentiated state and therefore inhibits regeneration [50]. In further support of this model, KLF9 mediated the inhibitory actions of TH on axon regeneration in cultured cerebellar Purkinje cells [3]. In addition to its inhibition of axon regeneration, KLF9 also mediates TH-dependent survival of Purkinje neurons in cerebellar culture [36].
Krüppel-like factor 9 also mediates some of the effects of TH on the development of myelinating cells in the CNS. A hallmark of developmental hypothyroidism is delayed or absent myelination [87]. Thyroid hormone induces expression of myelin-associated genes and promotes differentiation of oligodendrocytes, the primary myelinating cells in the CNS [8, 79]. KLF9 is rapidly and strongly induced by TH and forced expression of KLF9 in oligodendrocyte precursors initiated oligodendrocyte differentiation and induced expression of myelination-associated genes [19]. Conversely, siRNA knockdown of KLF9 prevented TH-induced oligodendrocyte differentiation. Myelination during development is not affected in Klf9-null mice, but they show poor recovery after demyelinating lesions [19]. Taken together, the data support that KLF9 mediates the actions of TH on neuron and oligodendrocyte differentiation.
In amphibians, KLF9 influences TH/TR signaling by regulating the expression of TRβ. In Xenopus laevis tadpoles the TRβ gene (TRβ) is directly regulated by liganded TR via a T3RE located in the promoter (i.e., the gene is autoinduced) [34] [42] [63]. Machuca and Tata [42] found that TRβ autoinduction is partially sensitive to protein synthesis inhibition, suggesting that upregulation of other proteins is required for TRβ autoinduction. The rapid kinetics of KLF9 induction by TH [21] and the presence of seven GC-boxes in the X. laevis TRβ 5′ flanking region led Bagamasbad and colleagues [6] to hypothesize that KLF9 participates in TRβ autoinduction. In support of this hypothesis they found KLF9 associated with the TRβ promoter in vivo in tadpole brain and tail, and in X. laevis tissue culture cells (XTC-2) by chromatin-immunoprecipitation (ChIP) assay. Forced expression of KLF9 in XTC-2 cells accelerated and enhanced TH-dependent induction of TRβ mRNA and promoter activity [6]. The action of KLF9 on TRβ depended on the N-terminal transactivation domains but not on the DNA binding capacity of KLF9, determined by deletion analysis and site-directed mutagenesis. Similar results were obtained in tadpole brain in vivo using electroporation-mediated gene transfer (F. Hu, J. Knoedler and R.J. Denver, unpublished data). These findings support that the rapid induction of KLF9 is necessary to support TRβ autoinduction through KLF9 acting as an accessory transcription factor to TRβ.
2.3 KLF2 functions in prenatal lung development and is negatively regulated by TH-TR
During late fetal development TH and corticosteroids cooperate to promote lung maturation and surfactant production, which is critical for postnatal survival [75]. The timing of lung development is sensitive to TH and can be disrupted by TH-mediated repression of KLF2 expression. Mice with a knock-in mutation of SMRT (a TR corepressor), SMRTmRID, that disrupts association of SMRT with TRs die shortly after birth due to a failure of Type 1 pneumocytes to differentiate. This phenotype is completely rescued by administration of the goitrogen propylthiouracil (PTU) during a narrow window in late gestation (begun at embryonic day 16.5 and withdrawn at birth), suggesting that liganded TR prevents Type 1 pneumocytes from differentiating during this period. Gene expression screening found Klf2 mRNA was decreased in SMRTmRID mice at both embryonic days 17.5 and 18.5. Chromatin immunoprecipitation followed by deep sequencing (ChIP-seq) for SMRT found that it associated with a region upstream of the Klf2 transcription start site in macrophages. Targeted ChIP assay in lung tissue culture cells found both TR and SMRT associated with this region. This DNA sequence supported TH/TR-dependent transrepression in transfection-reporter assays, suggesting that Klf2 is a negatively regulated T3 target gene. Forced expression of KLF2 upregulated several Type 1 pneumocyte marker genes in lung tissue culture cells, and KLF2 associated with their promoters in ChIP assays, suggesting that it mediates Type 1 pneumocyte differentiation. Krüppel-like factor 2 is expressed in the lungs throughout pre- and postnatal life and is required for normal lung development [89]. However, Klf2 gene expression appears to be sensitive to T3 suppression during a narrow window in late gestation, during which time it is required for Type 1 pneumocyte development. Disruption of NR signaling by a dominant-negative corepressor causes developmental defects via suppression of Klf2 during a critical prenatal period [60].
3.1 Corticosteroid receptors
Corticosteroids have diverse roles in the development and physiology of vertebrates. The endocrine stress response is in part mediated by glucocorticoids (GCs;CORT in most tetrapods including rodents; cortisol in humans and bony fishes), while plasma osmolality and blood volume is regulated by mineralocorticoids (e.g., aldosterone in tetrapods). Glucocorticoids cause both rapid changes in cell physiology mediated by membrane receptors and slow (‘genomic’) actions mediated by cytosolic receptors (glucorticoid receptor - GR and mineralocorticoid receptor - MR). Several KLF genes have been found to be direct transcriptional targets of GR and therefore participate in GR-mediated physiological processes.
3.2 KLFs are regulatory targets of GR that mediate the actions of CORT on gene expression and cell proliferation
Krüppel-like factor 9 is rapidly induced by CORT in the brain of both amphibians and mammals [7, 11] and this is mediated by evolutionarily conserved GC response elements (GREs) located in the 5′ flanking regions of tetrapod Klf9 genes (one GRE is within the Klf9 synergy module discussed earlier) [7]. The physiological significance of CORT regulation of Klf9 in the CNS has -not been investigated. Klf9-null mice exhibit reduced survival of adult-born hippocampal dentate granule neurons [68]. Survival of these cells is promoted by CORT [76] so it is possible that KLF9 plays a role in this process. In human keratinocytes KLF9 is induced by CORT in phase with circadian rhythms of plasma [CORT] [77]. Knockdown of KLF9 expression in these cells abrogated the antiproliferative effects of CORT, suggesting that KLF9 is a primary mediator of CORT action. KLF9 may thus be an evolutionarily conserved mediator of CORT/GR actions in diverse tissues.
Another KLF family member, KLF11, mediates the actions of CORT on the expression of neuronal genes involved with neurotransmission. Chronically elevated CORT and altered monoaminergic neurotransmission are associated with affective disorders such as depression [32, 35, 90]. GR-dependent gene expression may link elevated CORT to altered neurotransmission through regulation of expression of biosynthetic enzymes necessary for monamine synthesis and degradation. For example, chronic stress leads to enhanced gene expression and activity of monoamine oxidase (MAO) A, an enzyme that degrades monoamines and thus reduces monoaminergic neurotransmission [24, 46]. Grunewald and colleagues [24] showed that KLF11 is a key component of this pathway. Treatment with the synthetic GC dexamethasone (DEX) increased KLF11 mRNA in human neuronal cell lines and primary rat cortical neurons. Overexpression of KLF11 led to increased MAO A mRNA expression and catalytic activity in these cells. This effect was enhanced by DEX, suggesting that KLF11 functions as an accessory transcription factor for GC induction of MAO A. In support of this, siRNA-mediated knockdown of KLF11 reduced basal and DEX-induced MAO A expression. Furthermore, KLF11 and GR could activate the MAO A promoter via GC boxes (a.k.a. Sp1 sites) and GREs located in the 5′ flanking region. Mice null for Klf11 exhibited reduced brain Mao A mRNA and MAO-A catalytic activity [24, 46]. Thus, KLF11 mediates an action of CORT on neural physiology with implications for affective behavior and mental health.
Krüppel-like factor 4 is not regulated by CORT, but its expression is required for CORT-mediated activation of genes necessary for skin barrier formation during prenatal mammalian development. The formation of the skin barrier is essential for postnatal survival. Glucocorticoids accelerate this process; skin barrier formation is a beneficial side effect of prenatal corticosteroid treatment, which is administered to accelerate lung development in anticipated premature births [2]. Krüppel-like factor 4 participates in CORT-dependent maturation of the skin barrier. Transcriptional profiling of embryonic mouse skin by DNA microarray analysis at the time of barrier formation revealed a core set of genes that were upregulated by both CORT administration and ectopic KLF4 expression, and downregulated after KLF4 knockdown. These findings suggest that KLF4 and CORT acting via the GR both participate in transcriptional regulation of these genes. Transfection-reporter assays in cultured keratinocytes showed that KLF4 activated the promoters of many of the identified target genes, many of which also contained one or more GREs [58]. These results suggest a cooperative role for KLF4 and GR in the regulation of genes necessary for establishing the skin barrier prior to birth.
3.3 KLF15 is both a mediator and a feed-forward inhibitor of GR in different tissues
In the preceding examples, the KLFs acted in a relatively straightforward manner: an NR upregulated a KLF which went on to activate or repress NR hormone target genes, or a KLF acted as an accessory transcription factor at NR target genes. However, KLF15's functions in GR signaling in the lung and skeletal muscle are more complex. Krüppel-like factor 15 is a GR target gene that both mediates the effects of CORT on target tissues, and acts as a feed-forward enhancer or inhibitor of GR signaling.
In the lung, KLF15 participates in a transcriptional network to mediate the effects of CORT on gene expression. The Klf15 gene is a direct transcriptional target of GR whose upregulation is mediated by GREs located in the 1st and 2nd introns. DNA microarray analysis showed that Klf15-null mice displayed a markedly different transcriptional response to DEX in the lungs than wild-type (WT) [66]. Loss of KLF15 led to a blunted or absent response to DEX at some genes, suggesting that KLF15 is necessary for CORT induction of these genes. By contrast, other genes that showed a temporally restricted CORT response in WT mice (initial upregulation followed by a return to baseline or suppression) showed a sustained response in Klf15-null mice, suggesting that KLF15 induction by CORT represses these genes after CORT induces them (KLF15 acts as a feed-forward inhibitor). KLF15 associated with the promoters of both classes of genes by ChIP assay, and forced expression of KLF15 resulted in upregulation of GR targets that required KLF15 to be activated and repression of GR targets that required KLF15 to be turned off. Furthermore, KLF15 overexpression resulted in increased GR occupancy at positively regulated promoters by ChIP assay and increased GR-dependent transactivation in lung tissue culture cells, suggesting that KLF15 enhances GR recruitment and transcriptional activation [66].These results support that KLF15 acts as both an accessory transcription factor of GR and as a feed-forward ‘brake’ on GR-dependent gene expression.
In skeletal muscle, the Klf15 gene is directly regulated by GR and it mediates the actions of CORT on metabolism. Chronically elevated circulating [CORT] is linked to muscle wastage in pathological conditions [67]. Maintenance of muscle mass relies on the balance between anabolism and catabolism [29]. Krüppel-like factor 15 is important for maintaining gluconeogenesis in skeletal muscle cells through its positive regulation of enzymes necessary for branched-chain amino acid catabolism [23]. Shimizu and colleagues [70] found that KLF15 acts downstream of GR to promote catabolism and shrinkage of skeletal muscle. Induction of KLF15 by liganded GR results in the upregulation of the ubiquitin proteasome components Atrogin-1 and MuRF1, leading to enhanced proteasomal degradation of cellular proteins [70]. Also, KLF15 induces the branched-chain amino acid-degrading enzyme gene BCAT2, which leads to lowered intracellular amino acid concentration, and inhibition of the activity of the pro-anabolic TF mammalian target of rapamycin (mTOR). The authors found that activated mTOR inhibits GR-dependent transactivation; inhibition of mTOR activation enhanced GR-dependent gene expression, further amplifying the physiological response to CORT. Therefore, the protein products of KLF15 target genes indirectly enhance GR transactivity by influencing intracellular nutrient levels. In skeletal muscle KLF15 mediates the physiological effects of GR by activating secondary CORT response genes, and also initiates metabolic programs that exert feedback on GR transactivity [70].
4.1 Progesterone receptors
Progesterone receptors (PR) are Type 1 NRs that bind to the ovarian steroid hormone progesterone (P4) [38]. There are two major isoforms of PR, PRA and PRB, that arise from alternative splicing [15]. The PR is critical for the initiation and maintenance of pregnancy by its mediation of the actions of P4 on differentiation of the uterine endometrium; PR knockout mice are infertile [38]. In addition, PR in the brain is critical for mediating P4 actions on sexual behavior [44].
4.2 KLFs enhance PR signaling by acting as accessory transcription factors and activating downstream genes to effect P4-dependent gene expression programs
Krüppel-like factor 9 functions in PR signaling in the uterus by acting as an accessory TF for the PR. Wang and colleagues [88] showed that KLF9 is expressed in epithelial and stromal cells of porcine pregnancy endometrium. Female mice null for Klf9 have smaller litters than wild-type mice due to failed embryo implantation [72]. Progesterone receptor levels in uterine stromal cells of Klf9-null mice are reduced during pregnancy compared to wild type animals [83]. Consistent with this finding, many PR-responsive genes that are important for normal pregnancy, including SLP1 and Hoxa10, display an aberrant response to P4 in the absence of KLF9. Many PR-responsive genes are also regulated by KLF9. In porcine endometrial epithelial cells and cultured human endometrial carcinoma cells, KLF9 overexpression was sufficient to activate the uteroferrin (UF) promoter. Cotransfection of PR with KLF9 further increased promoter activity in a P4-dependent manner, suggesting that KLF9 and liganded PR cooperatively activated this promoter [71].
Krüppel-like factor 9 also participates in P4/PR regulation of Wnt signaling in the uterus. The Wnt/β-catenin signaling pathway is essential for hormone-dependent uterine cell proliferation and differentiation [81]. KLF9 and PR cooperatively regulated the Wnt pathway inhibitor Dickkopf (DKK1) in cultured human endometrial stromal cells (HESCs). DKK1 expression is increased by P4 treatment. Knockdown of KLF9 enhanced DKK1 mRNA and protein expression in HESCs after P4 treatment, while knockdown of PR reduced expression of both. Combined knockdown of both PR and KLF9 did not significantly reduce DKK1 mRNA but led to an almost total loss of DKK protein; one possible explanation could be altered expression of a PR/KLF9 target regulating translation of DKK1 mRNA. Both KLF9 and PR associated with the promoter by ChIP assay, suggesting cooperative regulation wherein KLF9 may modulate the response of DKK1 to P4/PR. DNA microarray analysis of HESCs subjected to PR or combined PR/KLF9 siRNA knockdown showed that PR and KLF9 coregulated many genes related to endometrial function, further supporting a role for KLF9 as an accessory TF for the PR [53]. In tissue culture cells KLF9 enhanced P4-dependent transactivation of the PR isoform B but not A, and KLF9 was found to exist in a protein complex with PRB in the mouse uterus by co-immunoprecipitation (Co-IP). [94]. Taken together, these results suggest that KLF9 acts as an accessory transcription factor for the PR and is necessary for optimal PR regulation of uterine receptivity and pregnancy. However, little is presently known about how KLF9 and PR interact at a mechanistic level.
Other KLFs mediate PR signaling by upregulating TFs necessary for regulation of indirect P4/PR target genes. DNA microarray of P4-responsive genes in a human breast cancer cell line (T47D) by Wade and colleagues [86] identified a subset of P4-responsive genes that are enriched in binding sites for E2F transcription factors. These investigators found that E2F1 expression was increased by P4 treatment; however, this increase was partially sensitive to protein synthesis inhibition, suggesting that other factors are necessary for maximum E2F1 expression. The presence of many GC-rich regions in the E2F1 promoter led them to investigate whether KLF/Sp factors were responsible. They found that KLF15 is a direct transcriptional target of P4/PR, as determined by strong R5020 (a PR agonist) induction that was resistant to inhibition of protein synthesis [86]. KLF15 was capable of activating the E2F1 promoter, while siRNA knockdown of KLF15 reduced P4 activation of E2F1. Furthermore, ChIP assay showed direct association of KLF15 with the E2F1 promoter, supporting that E2F1 is a direct KLF15 target. This allows KLF15 to amplify the transcriptional response to P4 by cooperatively activating another TF that goes on to upregulate other genes necessary for the cellular response to P4.
5.1 Estrogen receptors
Estradiol (E2) signals by binding to estrogen receptors (ERα and ERβ). The hormone regulates the development and function of many organs, including the reproductive, skeletal and cardiovascular systems [26]. Estradiol and ERs have been the focus of intense study due to their association with reproductive behavior, osteoporosis and breast and ovarian cancer [45, 61]. E2/ER signaling is critical for postnatal mammary gland development, and dysregulation of this pathway is a major cause of proliferation, tumorigenesis and metastasis in breast cancer [95]. Several KLFs participate in E2/ER signaling in the normal reproductive system and in the context of reproductive cancer.
5.2 KLFs attenuate and interfere with ER signaling via direct and indirect mechanisms
Krüppel-like factor 9 regulates ER signaling by influencing ER expression and thus cellular sensitivity to E2. It accomplishes this by promoting ERα autorepression. Autorepression, or the downregulation of a NR by its cognate ligand, is a common phenomenon that may serve to maintain homeostasis by dampening the response to a hormonal signal [5]. ERα autorepression is observed in both breast cancer cell lines and in the mouse mammary gland in vivo [9, 27]. Velarde and colleagues [84] reported a role for KLF9 in ER autorepression in the human endometrial adenocarcinoma cell line Ishikawa. In these cells, treatment with E2 resulted in ERα recruitment to the ERα promoter and a ∼50% decrease in ERα mRNA. Knockdown of KLF9, but not the closely related KLF13, by siRNA completely eliminated the E2-dependent decrease in ERα mRNA and protein. While ChIP assay did not support KLF9 association with the ERα proximal promoter, siRNA knockdown of KLF9 eliminated the E2-dependent increase in ERα recruitment to the promoter. Forced expression of KLF9 also reduced E2-dependent ER transactivation of an E2-responsive promoter in a transfection-reporter assay. Taken together, these results identify KLF9 as a cooperative transcriptional repressor of ERα at negatively regulated promoters and as an inhibitor of ERα transactivity at positively regulated promoters [84]. However, KLF9 was not found to interact with ERα by Co-IP. It may therefore promote ERα recruitment to the ERα promoter by indirect mechanisms.
In contrast to KLF9, KLF4 inhibits ER transactivity by direct binding to the ER. Earlier reports identified a positive association between KLF4 expression and breast cancer malignancy [20, 57]. However, data mining of breast cancer gene expression by Akaogi and colleagues [1] showed that KLF4 expression is often reduced in breast carcinomas compared with normal breast tissue. In MCF7 breast cancer cells, knockdown of KLF4 enhanced E2-dependent cell proliferation. In addition, KLF4 overexpression decreased, while KLF4 knockdown increased, ER transactivation of synthetic promoters and endogenous genes. Co-immunoprecipitation experiments showed that KLF4 bound directly to the DNA-binding domain of ERα. This likely explains KLF4 inhibition of recruitment of ERα to estrogen-responsive gene promoters (determined by ChIP assay) and reduced ER transactivity (determined by transfection-reporter assays). Thus, KLF4 can have tumor suppressive effects by direct interference with ER signaling [1]. Krüppel-like factors 4 and 9 therefore are both capable of attenuating ER signaling, but do so through distinct mechanisms in different tissues.
6.1 Multiple KLFs can regulate common intracellular pathways
An emerging theme of KLF biology is that multiple KLFs can function as a ‘network’ to coregulate a common intracellular pathway. For example, KLFs 2, 4 and 5 were shown to coordinately maintain pluripotency in embryonic stem cells (ESCs). Knockdown of any single KLF was insufficient to cause differentiation of ESCs, but simultaneous knockdown of all three led to differentiation of stem cells into fibroblast-like cells [31]. ChIP-seq experiments showed that all three factors shared many genomic binding sites, suggesting redundant regulation of target genes. Krüppel-like factors 2 and 4 are part of the same KLF subfamily and share common N-terminal domains; thus, it is not surprising that they would be able to compensate for each other [47, 49]. Interestingly, KLF5, which shares little sequence similarity outside of the DBD with other KLFs [49], can also compensate for loss of KLFs 2 and 4, which suggests additional robustness in this KLF-driven network.
In many cases, the expression of multiple KLFs is affected by the same NR in the same cell, suggesting interactive or cooperative actions between different KLF family members on cell development and physiology (Figure 3). In some cases this may take the form of two or more NR-regulated KLFs acting redundantly, potentially allowing one to compensate for the loss of the other(s). This may be due to different KLFs having the same genomic targets, and thus being capable of either activating or repressing transcription of the same gene(s) (Figure 3a). In other cases, multiple KLFs may act synergistically to amplify a transcriptional response to a hormonal stimulus. This could occur by different mechanisms, including cooperative activation of the same promoter, or separate actions whereby one KLF activates a target gene and another KLF activates/represses other genes that contribute to the transcriptional response (Figure 3b). Finally, multiple KLFs may act antagonistically to sequentially activate and deactivate a particular intracellular pathway. For example, one KLF may activate a promoter while another (perhaps one whose expression is upregulated later than the first) may repress it (Figure 3c).
Figure 3. Basic structure of networks composed of multiple KLFs.
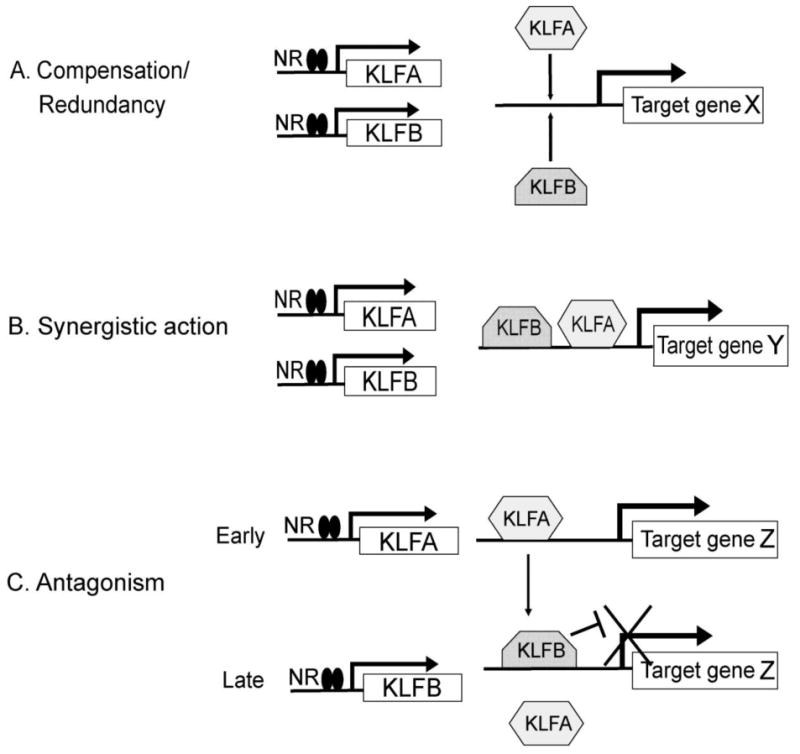
A. Two or more KLFs may both be regulatory targets of the same NR and activate or repress the same downstream gene. However, there is no additive or synergistic effect of the multiple KLFs, and any one protein can function. B. Two KLFs in the same pathway may have synergistic effects, perhaps by binding to separate response elements at the same locus (direct synergy) or by regulating other genes that contribute to the transcriptional effect (e.g. repressing the expression of a repressor). C. Two or more KLFs may have opposing or antagonistic actions. In the diagrammed example, a KLF that is upregulated early in the NR response activates a target gene while a different KLF that is upregulated later represses it, allowing a temporally restricted response to the NR.
6.2 Multiple KLFs participate in transcriptional networks to mediate the response of uterine cells to estrogen and progesterone
Krüppel-like factors 9 and 13 participate in a transcriptional network with bone morphogenic protein 2 (BMP2) to maintain uterine receptivity to pregnancy (Figure 4a). Bone morphogenic protein 2 is a critical regulator of uterine receptivity. Mice with targeted Bmp2 deletion in the uterus fail to decidualize (respond to P4 to prepare for embryo implantation), leading to pregnancy failure [39]. Bone morphogenic protein 2 is not expressed in predecidual uterine stromal cells but increases during decidualization. Expression of KLF9 in uterine stromal cells peaks prior to decidualization, when BMP2 is undetectable [54, 72, 83]. Krüppel-like factor 9 negatively regulates BMP2 expression; Klf9-null mice have higher Bmp2 levels in the uterus 3.5 days post-conception, and siRNA knockdown of KLF9 in HESCs increases BMP2 expression. In turn, application of BMP2 to HESCs represses KLF9 expression, suggesting feedback to control expression of these genes at different stages of the uterine cycle/pregnancy.
Figure 4. KLFs in networks and signal integration.
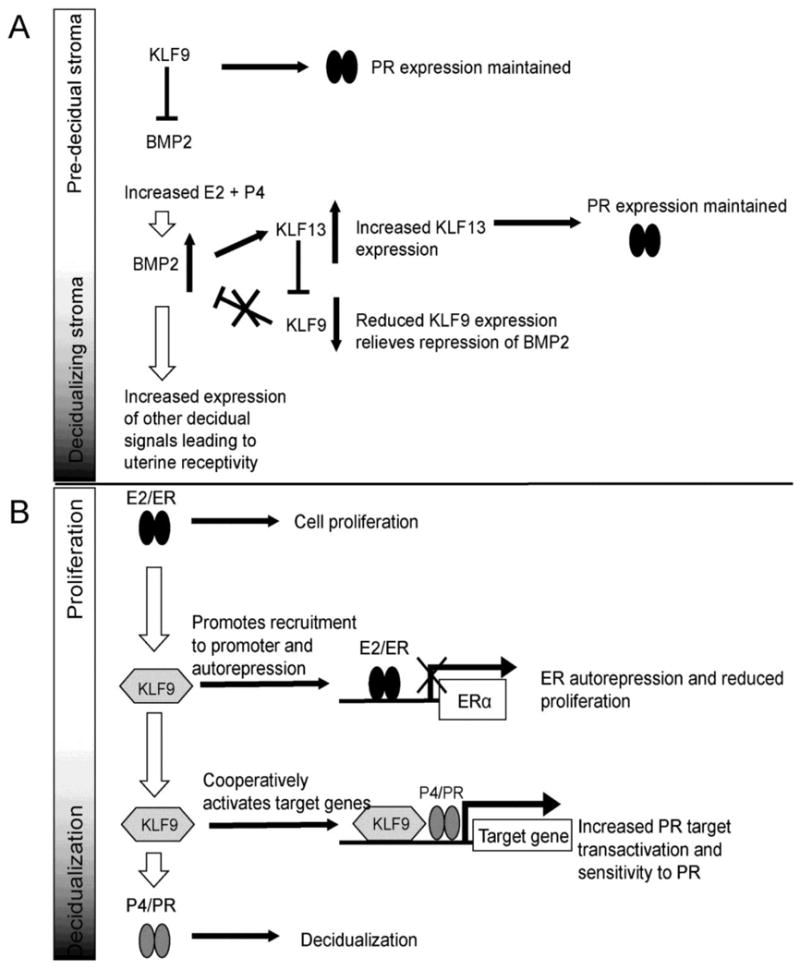
A) KLF9 and KLF913 have complementary and antagonist effects on uterine stromal cell decidualization. In the pre-implantation uterus KLF9 inhibits BMP2 expression. As P4 and E2 levels rise BMP2 expression is increased and leads to increased KLF13 expression. KLF13 repress KLF9 expression, relieving the block on BMP2 expression and further increasing the expression of decidualization signals. KLF9 upregulates PR in predecidual stroma and KLF13 upregulates PR in decidualizing stroma, which maintains cellular sensitivity to P4 across decidualization.. B) KLF9 integrates progesterone and estrogen signaling in the uterus. Estrogen leads to cell proliferation in the pre-decidual uterus. Elevated KLF9 levels promote ERα autorepression, which reduces cellular sensitivity to estrogen, thereby reducing endometrial proliferation and preparing the cell for P4 signaling. KLF9 also enhances PR transactivation, increasing the expression of P4/PR target genes and allowing decidualization to proceed. Filled arrows: direct activation or cause/effect relationship. Open arrows: sequential order of events. Abbreviations: PR, progesterone receptor; ER, estrogen receptor; BMP2, bone morphogenic protein 2; E2, estradiol; P4, progesterone.
In contrast to KLF9, KLF13 expression is low in predecidual stroma and increases as decidualization proceeds. Krüppel-like factor 13 represses KLF9 expression but activates BMP2 expression, as inferred from siRNA knockdown of KLF13 in HESCs. Increased expression of KLF13 as decidualization proceeds therefore leads to reduced KLF9 expression, in turn leading to increased BMP2 levels, and expression of BMP2-regulated genes that promote uterine receptivity. Finally, although KLF9 and KL13 have antagonistic effects on each other and BMP2 expression, both are necessary for maximal expression of PRB as demonstrated by reduced PRB expression after siRNA knockdown in HESCs, although their effects are restricted to particular stages of decidualization. Krüppel-like factor 9 promotes PRB expression only in predecidual stroma, while KLF13 does so only in decidualizing stroma. The net result is sustained uterine sensitivity to P4 even as many other molecular events are taking place [54]. Thus, two KLFs and one extracellular signaling molecule regulate each other to allow decidualization to proceed.
Despite the high degree of sequence similarity between KLF9 and KLF13 [78] and the expression of both KLFs in uterine tissues, Klf9-null mice are subfertile while Klf13-null mice do not show any fertility defects [28]. In Klf13-null mice, KLF9 protein levels are higher in the nuclear fraction of whole-uterus protein extracts at 3.5 days post-conception. This supports compensatory upregulation of KLF9 in the absence of KLF13. Unlike protein expression, Klf9 mRNA levels did not differ between the two genotypes, which suggests that the compensatory response occurs at the level of translation of Klf9 mRNA. Translation of KLF9 is inhibited by the uterine-expressed miRNA miR-200C, which is dysregulated in endometrial carcinoma and weakly induced by ovarian steroids [56]. Altered expression of this transcript may explain the compensatory upregulation of KLF9 protein in the Klf13-null mouse. Expression of KLF13 protein is higher in early pregnant uteri of Klf9-null mice compared to WT [72], but apparently it is unable to compensate to the same extent as KLF9. Taken together, these data show that KLF9 and KLF13 have both complementary and overlapping functions in the uterus, forming a robust transcriptional network to mediate the actions of P4/PR on uterine receptivity to implantation and the maintenance of pregnancy.
Krüppel-like factors 4 and 15 interact in a more straightforward manner. They exert opposite actions on a common target gene in the same cell in response to different NR signaling pathways. In epithelial cells of the uterine endometrium, E2 caused cell proliferation while E2/P4 induced differentiation [65]. In ovariectomized mice, co-treatment with E2 and P4 (co-treatment is necessary because E2 is necessary for the uterus to respond to P4 [16] repressed the expression of genes associated with cell proliferation in the uterus, including members of the Mcm complex, a multisubunit protein necessary for DNA replication during the cell cycle [55]. Down-regulation of one member of the complex, Mcm-2, by E2/P4 is partially mediated by KLF4 and KLF15.
In mice, E2 treatment enhanced while E2/P4 treatment repressed expression of Mcm-2. In addition, treatment of ovariectomized mice with E2 caused a rapid reduction in KLF15 but increased KLF4 in the uterus; conversely, P4/E2 treatment decreased KLF4 but increased KLF15. These two KLFs are therefore regulated oppositely by E2 and E2/P4, suggesting they may partially mediate the differential transcriptional responses to these hormones. The Mcm-2 promoter contains at least one predicted GC box, in addition to two predicted hormone response element half sites, suggesting that PR, ER and/or KLFs may bind to and regulate the promoter. ChIP assay from uteri of E2-treated mice showed KLF4 association with the Mcm-2 promoter; while combined treatment with E2 and P4 eliminated KLF4 association and led to KLF15 association with the Mcm-2 promoter. Occupancy of KLF4 and KLF15 at the Mcm-2 promoter therefore switches in parallel with changes in their protein expression.
Binding of these two KLFs to DNA was associated with changes in chromatin structure and association of the basal transcriptional machinery, supporting that they regulate Mcm-2. Increased expression of KLF15 and binding to the Mcm-2 promoter coincided with loss of RNA polymerase II (Pol II) at the promoter. Also, P4/E2 treatment increased HDAC association and repressive chromatin marks at the Mcm-2 promoter,. Lastly, in Ishikawa cells overexpression of KLF4 enhanced E2/ER transactivation of the Mcm-2 promoter, while overexpression of KLF15 repressed promoter activity. These results suggest a mechanism by which sequential induction of different KLFs mediates the actions of E2 and P4 on gene transcription and cell proliferation [64].
7.1 Individual KLFs act as integrators of multiple NR signaling pathways
An unresolved question in NR signaling is how cells integrate responses to multiple hormonal signals. Different NRs regulate distinct target genes and have different effects on physiology, yet many cells express more than one type of NR and are therefore responsive to more than one type of hormone. The response to two different hormones in concert may require adjustments in gene expression and physiology that are effected by common NR target TFs. Because many KLFs are NR target genes and act as accessory TFs for NRs they are good candidates as integrators of NR action.
7.2 KLF9 integrates signaling by multiple NRs in the uterus and brain
Krüppel-like factor 9 integrates the uterine response to E2/ER and P4/PR in the uterus (Figure 4b). As detailed above, KLF9 acts as an accessory transcription factor for PR-B and participates in ERα autorepression. This allows it to regulate uterine responsiveness to both hormones. Loss of KLF9 results in both resistance to P4 and an aberrant proliferative response to E2. In normal pregnancy, E2 promotes cell proliferation in the uterine epithelium, while P4 blocks proliferation and promotes decidualization. Krüppel-like factor 9 protein level is not affected by E2 [84], but KLF9 is necessary for ligand-dependent ER autorepression; furthermore, it enhances uterine sensitivity to P4/PR signaling by its action as an accessory TF. Thus, KLF9 directs the context-dependent response of uterine epithelial cells to ovarian steroids by regulating both E2/ER and P4/PR signaling. This model explains why KLF9-null mice are subfertile; inefficient ERα autopression and impaired P4/PR transactivation of target genes would impair the transition of the uterus from the proliferative to the secretory phase and therefore impair decidualization and implantation. KLF9 protein levels are therefore essential for the normal physiological response to ovarian steroids; this may explain why KLF9 expression is reduced in many hormone-responsive cancers of the female reproductive tract [73, 74].
Krüppel-like factor 9 may also integrate the response to CORT and TH in the CNS. As discussed earlier (sections 2.2 and 3.2) it is upregulated by both hormones independently and synergistically (). Because Klf9 is a direct target of both GR and TR it may regulate secondary response genes common to both hormones. Synergistic upregulation of Klf9 could result in stronger activation or repression of these genes. The Klf9 gene may function as a sensitive early responder to intracellular TH and CORT concentrations, allowing for coordination of neuronal gene expression responses over a range of circulating hormone concentrations.
8. Overview and prospects for future research
Accumulating evidence supports that KLFs play diverse roles in NR signaling. Some KLF genes are directly regulated by NRs (the KLF gene is a primary hormone response gene), and thus their protein products mediate NR action by regulating downstream target genes (secondary hormone response genes). Some KLFs regulate transcription of NR genes, some of which are direct targets of the NR, such that the KLF acts within an autoregulatory loop to sustain and amplify hormone action (autoinduction; e.g., the TRβ gene during tadpole metamorphosis). Krüppel-like factors may interact with NRs and influence their function through direct protein-protein interaction [1, 51, 92]. However, to our knowledge the protein motifs required for such interactions have not been defined. Future investigation into how KLFs physically interact with NRs will be important for understanding the nature and importance of these interactions.
Krüppel-like factor actions require that they associate with discrete genomic sites where they may influence local chromatin structure; some KLFs have been shown to recruit histone modifying enzymes [12, 41, 91, 93]. The genomic regions where KLFs associate might be in close proximity to NR genomic binding sites. However, our knowledge of where KLFs associate across the genome is very limited. Chromatin immunoprecipitation sequencing (ChIPseq) has been conducted only for KLFs 2, 4 and 5 in embryonic stem cells [31], and for KLF1 in erythroid cells [62]. Interestingly, genomic sites where KLF1 associated changed across the development of erythroid progenitor cells, suggesting developmental stage-dependent KLF1 regulation of gene expression [62]. Therefore, identifying genomic sites where KLFs associate in different tissues under different physiological/developmental states will be important for understanding KLF functions generally, and how KLFs interact with NR signaling.
Acknowledgments
Preparation of this review was supported in part by grants from the National Science Foundation (IOS 0922583) and the National Institute of Neurological Disorders and Stroke (R01 NS046690) to RJD, and MCubed funding to JK.
Abbreviations
- KLF
Krüppel-like factor
- NR
nuclear receptor
- TF
Transcription factor
- T3
thyroid hormone
- TR
thyroid hormone receptor
- CORT
corticosterone
- GR
glucocorticoid receptor
- PR
progesterone receptor
- P4
progesterone
- ER
estrogen receptor
- E2
estradiol
- DBD
DNA-binding domain
- KLF/KLF
denotes the human protein/gene, while KLF/Klf denotes the rodent or frog protein/gene.
Literature Cited
- 1.Akaogi K, Nakajima Y, Ito I, Kawasaki S, Oie SH, Murayama A, et al. KLF4 suppresses estrogen-dependent breast cancer growth by inhibiting the transcriptional activity of ERalpha. Oncogene. 2009;28:2894–2902. doi: 10.1038/onc.2009.151. [DOI] [PubMed] [Google Scholar]
- 2.Aszterbaum M, Feingold KR, Menon GK, Williams ML. Glucocorticoids accelerate fetal maturation of the epidermal permeability barrier in the rat. J Clin Invest. 1993;91:2703–2708. doi: 10.1172/JCI116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avci HX, Lebrun C, Wehrle R, Doulazmi M, Chatonnet F, Morel MP, et al. Thyroid hormone triggers the developmental loss of axonal regenerative capacity via thyroid hormone receptor alpha1 and kruppel-like factor 9 in Purkinje cells. Proc Natl Acad Sci U S A. 2012;109:14206–14211. doi: 10.1073/pnas.1119853109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagamasbad P, Denver RJ. Thyroid hormone and glucocorticoids synergistically activate transcription of the basic transcription element binding protein 1 gene. Proceedings of the Endocrine Society Annual Meeting; San Franciso, CA. 2008. [Google Scholar]
- 5.Bagamasbad P, Denver RJ. Mechanisms and significance of nuclear receptor auto- and cross-regulation. Gen Comp Endocrinol. 2011;170:3–17. doi: 10.1016/j.ygcen.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagamasbad P, Howdeshell KL, Sachs LM, Demeneix BA, Denver RJ. A role for basic transcription element-binding protein 1 (BTEB1) in the autoinduction of thyroid hormone receptor beta. Journal of Biological Chemistry. 2008;283:2275–2285. doi: 10.1074/jbc.M709306200. [DOI] [PubMed] [Google Scholar]
- 7.Bagamasbad P, Ziera T, Borden SA, Bonett RM, Rozeboom AM, Seasholtz A, et al. Molecular Basis for Glucocorticoid Induction of the Kruppel-Like Factor 9 Gene in Hippocampal Neurons. Endocrinology. 2012;153:5334–5345. doi: 10.1210/en.2012-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- 9.Berkenstam A, Glaumann H, Martin M, Gustafsson JA, Norstedt G. Hormonal regulation of estrogen receptor messenger ribonucleic acid in T47Dco and MCF-7 breast cancer cells. Mol Endocrinol. 1989;3:22–28. doi: 10.1210/mend-3-1-22. [DOI] [PubMed] [Google Scholar]
- 10.Bernal J. Thyroid Hormones and Brain Development. In: Gerald L, editor. Vitamins & Hormones. Academic Press; 2005. pp. 95–122. [DOI] [PubMed] [Google Scholar]
- 11.Bonett RM, Hu F, Bagamasbad P, Denver RJ. Stressor and Glucocorticoid-Dependent Induction of the Immediate Early Gene Kruppel-Like Factor 9: Implications for Neural Development and Plasticity. Endocrinology. 2009;150:1757–1765. doi: 10.1210/en.2008-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnefond A, Lomberk G, Buttar N, Busiah K, Vaillant E, Lobbens S, et al. Disruption of a Novel Kruppel-like Transcription Factor p300-regulated Pathway for Insulin Biosynthesis Revealed by Studies of the c.-331 INS Mutation Found in Neonatal Diabetes Mellitus. Journal of Biological Chemistry. 2011;286:28414–28424. doi: 10.1074/jbc.M110.215822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cayrou C, Denver RJ, Puymirat J. Suppression of the basic transcription element-binding protein in brain neuronal cultures inhibits thyroid hormone-induced neurite branching. Endocrinology. 2002;143:2242–2249. doi: 10.1210/endo.143.6.8856. [DOI] [PubMed] [Google Scholar]
- 14.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conneely OM, Maxwell BL, Toft DO, Schrader WT, O'Malley BW. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987;149:493–501. doi: 10.1016/0006-291x(87)90395-0. [DOI] [PubMed] [Google Scholar]
- 16.de Ziegler D, Fanchin R, de Moustier B, Bulletti C. The hormonal control of endometrial receptivity: estrogen (E2) and progesterone. J Reprod Immunol. 1998;39:149–166. doi: 10.1016/s0165-0378(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 17.Denver RJ, Ouellet L, Furling D, Kobayashi A, Fujii-Kuriyama Y, Puymirat J. Basic transcription element-binding protein (BTEB) is a thyroid hormone-regulated gene in the developing central nervous system - Evidence for a role in neurite outgrowth. Journal of Biological Chemistry. 1999;274:23128–23134. doi: 10.1074/jbc.274.33.23128. [DOI] [PubMed] [Google Scholar]
- 18.Denver RJ, Williamson KE. Identification of a Thyroid Hormone Response Element in the Mouse Kruppel-Like Factor 9 Gene to Explain Its Postnatal Expression in the Brain. Endocrinology. 2009;150:3935–3943. doi: 10.1210/en.2009-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dugas JC, Ibrahim A, Barres BA. The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Molecular and Cellular Neuroscience. 2012;50:45–57. doi: 10.1016/j.mcn.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster KW, Frost AR, McKie-Bell P, Lin CY, Engler JA, Grizzle WE, et al. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 2000;60:6488–6495. [PubMed] [Google Scholar]
- 21.Furlow JD, Kanamori A. The transcription factor basic transcription element-binding protein 1 is a direct thyroid hormone response gene in the frog Xenopus laevis. Endocrinology. 2002;143:3295–3305. doi: 10.1210/en.2002-220126. [DOI] [PubMed] [Google Scholar]
- 22.Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58:685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- 23.Gray S, Wang B, Orihuela Y, Hong EG, Fisch S, Haldar S, et al. Regulation of Gluconeogenesis by Krüppel-like Factor 15. Cell Metabolism. 2007;5:305–312. doi: 10.1016/j.cmet.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunewald M, Johnson S, Lu D, Wang Z, Lomberk G, Albert PR, et al. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J Biol Chem. 2012;287:24195–24206. doi: 10.1074/jbc.M112.373936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumireddy K, Li A, Gimotty PA, Klein-Szanto AJ, Showe LC, Katsaros D, et al. KLF17 is a negative regulator of epithelial-mesenchymal transition and metastasis in breast cancer. Nat Cell Biol. 2009;11:1297–1304. doi: 10.1038/ncb1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 27.Hatsumi T, Yamamuro Y. Downregulation of estrogen receptor gene expression by exogenous 17beta-estradiol in the mammary glands of lactating mice. Exp Biol Med (Maywood) 2006;231:311–316. doi: 10.1177/153537020623100311. [DOI] [PubMed] [Google Scholar]
- 28.Heard ME, Pabona JM, Clayberger C, Krensky AM, Simmen FA, Simmen RC. The reproductive phenotype of mice null for transcription factor Kruppel-like factor 13 suggests compensatory function of family member Kruppel-like factor 9 in the peri-implantation uterus. Biol Reprod. 2012;87:115. doi: 10.1095/biolreprod.112.102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman EP, Nader GA. Balancing muscle hypertrophy and atrophy. Nature Medicine. 2004;10:584–585. doi: 10.1038/nm0604-584. [DOI] [PubMed] [Google Scholar]
- 30.Imataka H, Sogawa K, Yasumoto K, Kikuchi Y, Sasano K, Kobayashi A, et al. Two regulatory proteins that bind to the basic transcription element (BTE), a GC box sequence in the promoter region of the rat P-4501A1 gene. EMBO J. 1992;11:3663–3671. doi: 10.1002/j.1460-2075.1992.tb05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 32.Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- 33.Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanamori A, Brown DD. The regulation of thyroid-hormone receptor beta genes by thyroid-hormone in Xenopus laevis. Journal of Biological Chemistry. 1992;267:739–745. [PubMed] [Google Scholar]
- 35.Kieran N, Ou XM, Iyo AH. Chronic social defeat downregulates the 5-HT1A receptor but not Freud-1 or NUDR in the rat prefrontal cortex. Neurosci Lett. 2010;469:380–384. doi: 10.1016/j.neulet.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebrun C, Avci HX, Wehrle R, Doulazmi M, Jaudon F, Morel MP, et al. Klf9 is necessary and sufficient for Purkinje cell survival in organotypic culture. Molecular and Cellular Neuroscience. 2013;54:9–21. doi: 10.1016/j.mcn.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Lee H, Kim HJ, Lee YJ, Lee MY, Choi H, Kim JW. Kruppel-Like Factor KLF8 Plays a Critical Role in Adipocyte Differentiation. Plos One. 2012;7 doi: 10.1371/journal.pone.0052474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee K, Jeong J, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Molecular mechanisms involved in progesterone receptor regulation of uterine function. J Steroid Biochem Mol Biol. 2006;102:41–50. doi: 10.1016/j.jsbmb.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, et al. Bmp2 Is Critical for the Murine Uterine Decidual Response. Molecular and Cellular Biology. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Privalsky ML. Heterodimers of retinoic acid receptors and thyroid hormone receptors display unique combinatorial regulatory properties. Mol Endocrinol. 2005;19:863–878. doi: 10.1210/me.2004-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lomberk G, Mathison AJ, Grzenda A, Seo S, Demars CJ, Rizvi S, et al. Sequence-specific Recruitment of Heterochromatin Protein 1 via Interaction with Kruppel-like Factor 11, a Human Transcription Factor Involved in Tumor Suppression and Metabolic Diseases. Journal of Biological Chemistry. 2012;287:13026–13039. doi: 10.1074/jbc.M112.342634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machuca I, Tata JR. Autoinduction of thyroid-hormone receptor during metamorphosis is reproduced in Xenopus XTC-2 cells. Molecular and Cellular Endocrinology. 1992;87:105–113. doi: 10.1016/0303-7207(92)90238-2. [DOI] [PubMed] [Google Scholar]
- 43.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mani SK, Blaustein JD. Neural progestin receptors and female sexual behavior. Neuroendocrinology. 2012;96:152–161. doi: 10.1159/000338668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manolagas SC, O'Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013 doi: 10.1038/nrendo.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao QQ, Ip SP, Ko KM, Tsai SH, Xian YF, Che CT. Effects of peony glycosides on mice exposed to chronic unpredictable stress: Further evidence for antidepressant-like activity. Journal of Ethnopharmacology. 2009;124:316–320. doi: 10.1016/j.jep.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 47.McConnell BB, Yang VW. Mammalian Kruppel-Like Factors in Health and Diseases. Physiological Reviews. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell DL, DiMario JX. Bimodal, reciprocal regulation of fibroblast growth factor receptor 1 promoter activity by BTEB1/KLF9 during myogenesis. Mol Biol Cell. 2010;21:2780–2787. doi: 10.1091/mbc.E10-04-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore DL, Apara A, Goldberg JL. Kruppel-like transcription factors in the nervous system: novel players in neurite outgrowth and axon regeneration. Mol Cell Neurosci. 2011;47:233–243. doi: 10.1016/j.mcn.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohguchi H, Tanaka T, Uchida A, Magoori K, Kudo H, Kim I, et al. Hepatocyte nuclear factor 4alpha contributes to thyroid hormone homeostasis by cooperatively regulating the type 1 iodothyronine deiodinase gene with GATA4 and Kruppel-like transcription factor 9. Mol Cell Biol. 2008;28:3917–3931. doi: 10.1128/MCB.02154-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oishi Y, Manabe I, Tobe K, Ohsugi M, Kubota T, Fujiu K, et al. SUMOylation of Kruppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nature Medicine. 2008;14:656–666. doi: 10.1038/nm1756. [DOI] [PubMed] [Google Scholar]
- 53.Pabona JMP, Simmen FA, Nikiforov MA, Zhuang D, Shankar K, Velarde MC, et al. Kruppel-Like Factor 9 and Progesterone Receptor Coregulation of Decidualizing Endometrial Stromal Cells: Implications for the Pathogenesis of Endometriosis. Journal of Clinical Endocrinology & Metabolism. 2012;97:E376–E392. doi: 10.1210/jc.2011-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pabona JMP, Zeng Z, Simmen FA, Simmen RCM. Simmen, Functional Differentiation of Uterine Stromal Cells Involves Cross-Regulation between Bone Morphogenetic Protein 2 and Kruppel-Like Factor (KLF) Family Members KLF9 and KLF13. Endocrinology. 2010;151:3396–3406. doi: 10.1210/en.2009-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan H, Deng Y, Pollard JW. Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proceedings of the National Academy of Sciences. 2006;103:14021–14026. doi: 10.1073/pnas.0601271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panda H, Pelakh L, Chuang TD, Luo X, Bukulmez O, Chegini N. Endometrial miR-200c is altered during transformation into cancerous states and targets the expression of ZEBs, VEGFA, FLT1, IKKbeta, KLF9, and FBLN5. Reprod Sci. 2012;19:786–796. doi: 10.1177/1933719112438448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandya AY, Talley LI, Frost AR, Fitzgerald TJ, Trivedi V, Chakravarthy M, et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res. 2004;10:2709–2719. doi: 10.1158/1078-0432.ccr-03-0484. [DOI] [PubMed] [Google Scholar]
- 58.Patel S, Xi ZF, Seo EY, McGaughey D, Segre JA. Klf4 and corticosteroids activate an overlapping set of transcriptional targets to accelerate in utero epidermal barrier acquisition. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18668–18673. doi: 10.1073/pnas.0608658103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pei H, Yao Y, Yang Y, Liao K, Wu JR. Kruppel-like factor KLF9 regulates PPAR gamma transactivation at the middle stage of adipogenesis. Cell Death and Differentiation. 2011;18:315–327. doi: 10.1038/cdd.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pei LM, Leblanc M, Barish G, Atkins A, Nofsinger R, Whyte J, et al. Thyroid hormone receptor repression is linked to type I pneumocyte-associated respiratory distress syndrome. Nature Medicine. 2011;17:1466–U1172. doi: 10.1038/nm.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfaff D, Waters E, Khan Q, Zhang X, Numan M. Minireview: estrogen receptor-initiated mechanisms causal to mammalian reproductive behaviors. Endocrinology. 2011;152:1209–1217. doi: 10.1210/en.2010-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pilon AM, Ajay SS, Kumar SA, Steiner LA, Cherukuri PF, Wincovitch S, et al. Genome-wide ChIP-Seq reveals a dramatic shift in the binding of the transcription factor erythroid Kruppel-like factor during erythrocyte differentiation. Blood. 2011;118:E139–E148. doi: 10.1182/blood-2011-05-355107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ranjan M, Wong JM, Shi YB. Transcriptional repression of Xenopus TR-beta gene is mediated by a thyroid-hormone response element located near the start site. Journal of Biological Chemistry. 1994;269:24699–24705. [PubMed] [Google Scholar]
- 64.Ray S, Pollard JW. KLF15 negatively regulates estrogen-induced epithelial cell proliferation by inhibition of DNA replication licensing. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1334–E1343. doi: 10.1073/pnas.1118515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. Epithelial-stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med. 2010;28:27–35. doi: 10.1055/s-0029-1242990. [DOI] [PubMed] [Google Scholar]
- 66.Sasse SK, Mailloux CM, Barczak AJ, Wang Q, Altonsy MO, Jain MK, et al. The Glucocorticoid Receptor and KLF15 Regulate Gene Expression Dynamics and Integrate Signals through Feed-Forward Circuitry. Molecular and Cellular Biology. 2013;33:2104–2115. doi: 10.1128/MCB.01474-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. Journal of Endocrinology. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 68.Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, et al. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci. 2009;29:9875–9887. doi: 10.1523/JNEUROSCI.2260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shibusawa N, Hollenberg AN, Wondisford FE. Thyroid hormone receptor DNA binding is required for both positive and negative gene regulation. Journal of Biological Chemistry. 2003;278:732–738. doi: 10.1074/jbc.M207264200. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda S, et al. Crosstalk between Glucocorticoid Receptor and Nutritional Sensor mTOR in Skeletal Muscle. Cell Metabolism. 2011;13:170–182. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Simmen RC, Chung TE, Imataka H, Michel FJ, Badinga L, Simmen FA. Trans-activation functions of the Sp-related nuclear factor, basic transcription element-binding protein, and progesterone receptor in endometrial epithelial cells. Endocrinology. 1999;140:2517–2525. doi: 10.1210/endo.140.6.6625. [DOI] [PubMed] [Google Scholar]
- 72.Simmen RCM, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L, et al. Subfertility, Uterine Hypoplasia, and Partial Progesterone Resistance in Mice Lacking the Krüppel-like Factor 9/Basic Transcription Element-binding Protein-1 (Bteb1) Gene. Journal of Biological Chemistry. 2004;279:29286–29294. doi: 10.1074/jbc.M403139200. [DOI] [PubMed] [Google Scholar]
- 73.Simmen RCM, Pabona JMP, Velarde MC, Simmons C, Rahal O, Simmen FA. The emerging role of Kruppel-like factors in endocrine-responsive cancers of female reproductive tissues. Journal of Endocrinology. 2010;204:223–231. doi: 10.1677/JOE-09-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simmons CD, Pabona JMP, Heard ME, Friedman TM, Spataro MT, Godley AL, et al. Kruppel-Like Factor 9 Loss-of-Expression in Human Endometrial Carcinoma Links Altered Expression of Growth-Regulatory Genes with Aberrant Proliferative Response to Estrogen. Biology of Reproduction. 2011;85:378–385. doi: 10.1095/biolreprod.110.090654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith BT, Sabry K. Glucocorticoid-thyroid synergism in lung maturation: a mechanism involving epithelial-mesenchymal interaction. Proc Natl Acad Sci U S A. 1983;80:1951–1954. doi: 10.1073/pnas.80.7.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spanswick SC, Epp JR, Sutherland RJ. Time-course of hippocampal granule cell degeneration and changes in adult neurogenesis after adrenalectomy in rats. Neuroscience. 2011;190:166–176. doi: 10.1016/j.neuroscience.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 77.Sporl F, Korge S, Jurchott K, Wunderskirchner M, Schellenberg K, Heins S, et al. Kruppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10903–10908. doi: 10.1073/pnas.1118641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: Bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 79.Tosic M, Torch S, Comte V, Dolivo M, Honegger P, Matthieu JM. Triiodothyronine has diverse and multiple stimulating effects on expression of the major myelin protein genes. J Neurochem. 1992;59:1770–1777. doi: 10.1111/j.1471-4159.1992.tb11009.x. [DOI] [PubMed] [Google Scholar]
- 80.Turner J, Crossley M. Cloning and characterization of mCtBP2, a co-repressor that associates with basic Kruppel-like factor and other mammalian transcriptional regulators. EMBO J. 1998;17:5129–5140. doi: 10.1093/emboj/17.17.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Horst PH, Wang YY, van der Zee M, Burger CW, Blok LJ. Interaction between sex hormones and WNT/beta-catenin signal transduction in endometrial physiology and disease. Molecular and Cellular Endocrinology. 2012;358:176–184. doi: 10.1016/j.mce.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 82.van Vliet J, Crofts LA, Quinlan KG, Czolij R, Perkins AC, Crossley M. Human KLF17 is a new member of the Sp/KLF family of transcription factors. Genomics. 2006;87:474–482. doi: 10.1016/j.ygeno.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 83.Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RCM. Null Mutation of Krüppel-Like Factor9/Basic Transcription Element Binding Protein-1 Alters Peri-Implantation Uterine Development in Mice. Biology of Reproduction. 2005;73:472–481. doi: 10.1095/biolreprod.105.041855. [DOI] [PubMed] [Google Scholar]
- 84.Velarde MC, Zeng Z, McQuown JR, Simmen FA, Simmen RCM. Kruppel-like factor 9 is a negative regulator of ligand-dependent estrogen receptor alpha signaling in Ishikawa endometrial adenocarcinoma cells. Molecular Endocrinology. 2007;21:2988–3001. doi: 10.1210/me.2007-0242. [DOI] [PubMed] [Google Scholar]
- 85.Veldman MB, Bemben MA, Thompson RC, Goldman D. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev Biol. 2007;312:596–612. doi: 10.1016/j.ydbio.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 86.Wade HE, Kobayashi S, Eaton ML, Jansen MS, Lobenhofer EK, Lupien M, et al. Multimodal Regulation of E2F1 Gene Expression by Progestins. Molecular and Cellular Biology. 2010;30:1866–1877. doi: 10.1128/MCB.01060-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walters SN, Morell P. Effects of altered thyroid states on myelinogenesis. Journal of Neurochemistry. 1981;36:1792–1801. doi: 10.1111/j.1471-4159.1981.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Michel FJ, Wing A, Simmen FA, Simmen RC. Cell-type expression, immunolocalization, and deoxyribonucleic acid-binding activity of basic transcription element binding transcription factor, an Sp-related family member, in porcine endometrium of pregnancy. Biol Reprod. 1997;57:707–714. doi: 10.1095/biolreprod57.4.707. [DOI] [PubMed] [Google Scholar]
- 89.Wani MA, Wert SE, Lingrel JB. Lung Kruppel-like Factor, a Zinc Finger Transcription Factor, Is Essential for Normal Lung Development. Journal of Biological Chemistry. 1999;274:21180–21185. doi: 10.1074/jbc.274.30.21180. [DOI] [PubMed] [Google Scholar]
- 90.Weber B, Lewicka S, Deuschle M, Colla M, Vecsei P, Heuser I. Increased diurnal plasma concentrations of cortisone in depressed patients. J Clin Endocrinol Metab. 2000;85:1133–1136. doi: 10.1210/jcem.85.3.6469. [DOI] [PubMed] [Google Scholar]
- 91.Xiong Y, Khanna S, Grzenda AL, Sarmento OF, Svingen PA, Lomberk GA, et al. Polycomb Antagonizes p300/CREB-binding Protein-associated Factor to Silence FOXP3 in a Kruppel-like Factor-dependent Manner. Journal of Biological Chemistry. 2012;287:34372–34385. doi: 10.1074/jbc.M111.325332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang DY, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RCM. Direct interaction of the Kruppel-like family (KLF) member, BTEB1, and PR mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology. 2002;143:62–73. doi: 10.1210/endo.143.1.8590. [DOI] [PubMed] [Google Scholar]
- 93.Zhang JS, Moncrieffe MC, Kaczynski J, Ellenrieder V, Prendergast FG, Urrutia R. A conserved alpha-helical motif mediates the interaction of Sp1-like transcriptional repressors with the corepressor mSin3A. Mol Cell Biol. 2001;21:5041–5049. doi: 10.1128/MCB.21.15.5041-5049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang XL, Zhang DY, Michel FJ, Blum JL, Simmen FA, Simmen RCM. Selective interactions of Kruppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. Journal of Biological Chemistry. 2003;278:21474–21482. doi: 10.1074/jbc.M212098200. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Z, Qiao JX, Shetty A, Wu G, Huang Y, Davidson NE, et al. Regulation of estrogen receptor signaling in breast carcinogenesis and breast cancer therapy. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1376-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


