Abstract
Plant cells grow as aggregates in suspension culture, but little is known about the dynamics of aggregation, and no routine methodology exists to measure aggregate size. In this study, we evaluate several different methods to characterize aggregate size in Taxus suspension cultures, in which aggregate diameters range from 50 μm to 2000 μm, including filtration and image analysis, and develop a novel method using a specially equipped Coulter counter system. We demonstrate the suitability of this technology to measure plant cell culture aggregates, and show that it can be reliably used to measure total biomass accumulation compared to standard methods such as dry weight. Furthermore, we demonstrate that all three methods can be used to measure an aggregate size distribution, but that the Coulter counter is more reliable and much faster, and also provides far better resolution. While absolute measurements of aggregate size differ based on the three evaluation techniques, we show that linear correlations are sufficient to account for these differences (R2 > 0.99). We then demonstrate the utility of the novel Coulter counter methodology by monitoring the dynamics of a batch process and find that the mean aggregate size increases by 55% during the exponential growth phase, but decreases during stationary phase. The results indicate that the Coulter counter method can be routinely used for advanced process characterization, particularly to study the relationship between aggregate size and secondary metabolite production, as well as a source of reliable experimental data for modeling aggregation dynamics in plant cell culture.
Introduction
Plant cell culture is an alternative production technology for complex natural products that cannot be chemically synthesized or extracted in high yields from native sources. In particular, suspension cultures consisting of undifferentiated plant cells are attractive industrially, especially compared to other types of in vitro plant systems such as differentiated cultures and transfected hairy root cultures, due to their relative similarity to other microbial cell culture systems (Kolewe et al. 2008). While a number of these suspension cell culture processes have been commercialized, most notably Taxus spp. for the production of the anti-cancer agent paclitaxel, more widespread application of this technology has thus far been limited due to some of the unique difficulties associated with plant cell culture, including low metabolite yields, biochemical and genetic instabilities, and difficulties associated with scaleup (Georgiev et al. 2009).
One unique characteristic of plant cell cultures is the tendency of plant cells to grow as aggregates. Plant cells remain connected via shared cell walls after division, and as a result, aggregates ranging from two to several hundred cells exist in culture. Aggregates can reach sizes up to several millimeters, and microenvironments within these larger aggregates are formed due to oxygen and other nutrient diffusion limitations (Naill and Roberts 2004; Hulst et al. 1989). The effect of aggregate size on metabolic activity has been studied extensively, but no definitive trend regarding aggregate size and secondary metabolite production has emerged across species and cell culture systems. Larger aggregates have been shown to have a positive effect on secondary metabolite production (Edahiro and Seki 2006; Hulst et al. 1989), a positive effect up to a critical size (Zhao et al. 2003; Madhusudhan and Ravishankar 1996), or a negative effect (Pepin et al. 1999; Bolta et al. 2003). Additionally, aggregate size has been shown to affect culture growth rates (Forni et al. 1999), rheological properties of the culture broth (Rodriguez-Monroy et al. 2004), and is the most likely cause of heterogeneity in single cell populations (Naill and Roberts 2005a; Naill and Roberts 2005b).
In order to engineer plant cell cultures by manipulating aggregate size, it is necessary to first have a practical and reliable method to measure the aggregate size distribution. Dry weight is the standard measure of biomass in plant cell culture, and the most common method to measure the aggregate size distribution is similar in nature. A crude biomass distribution is typically obtained by separating a sample of aggregates on a series of filters or sieves with different pore sizes and determining the dry weight of each resulting size fraction (i.e., McDonald et al. 2001; Zhao et al. 2003; Keβler et al. 1999; Madhusudhan and Ravishankar 1996; Mavituna and Park 1987). This method is often employed due to the straightforward nature of the procedure and materials required. However, it is both time-consuming and inefficient, and dry weight results can take greater than 24 hrs to obtain.
Other methods have been investigated to determine plant cell aggregate size, including ex situ image analysis and the focused beam reflectance method (FBRM). Image analysis techniques consist of sample dilution and plating, followed by microscopic image acquisition and subsequent analysis using a variety of software programs (Pepin et al. 1999; Rodriguez-Monroy et al. 2004). While this method can allow for better resolution than filtration, the processing steps increase the likelihood of altering the aggregate size due to breakage. Additionally, the throughput is extremely low, resulting in a long and labor intensive procedure to obtain statistically significant results, which is impractical for periodic measurements of multiple cultures. FBRM is an optical technique in which the duration of a particle’s reflectance as it passes a laser corresponds to the particle length. This method was shown to reliably measure total biomass and could also be correlated to aggregate size; however, biomass concentration and aggregate size showed significant cross interaction in FBRM measurements, such that both properties could not always be determined from a single measurement (McDonald et al. 2001).
A common method for determining particle size distributions in a range of applications, including but not limited to biological systems, is the electrical resistance pulse sizing technique, commonly known as the Coulter principle. Particles suspended in a conducting salt solution will cause a voltage pulse when passing through an aperture across which a constant current is applied, due to the displacement of electrolyte by the particle (Graham 2003). The amplitude of the voltage pulse is proportional to the volume of the particle. This technique was first applied to size bacteria over 50 years ago (Kubitschek 1958), and very quickly became a standard method to count and size yeast, bacteria, and mammalian cells (Kubitschek 1969). While several early attempts were made to apply this principle to plant cells, equipment limitations, including a maximum aperture size of 560 μm, necessitated the use of either protoplasts or cultures treated with enzymes (Kubek and Shuler 1978), and was acknowledged to be applicable only to cultures with low levels of aggregation. As electrical resistance pulse sizing technology has matured, no further research has been published to investigate the suitability of this technique to plant cell cultures.
In this study, we characterize plant cell culture aggregates using a specially equipped Coulter counter, and compare results with filtration and image analysis. As a total biomass measurement can be determined from a biomass distribution, we also compare biomass measurements obtained with the Coulter counter to standard dry weight measurements. We show that the aggregate size distributions obtained are reliable and well resolved, and then apply this method to characterize the evolution of Taxus cultures over a batch period. Results demonstrate the usefulness of this method for routine cell culture monitoring, which we ultimately intend to use for a quantitative investigation into aggregation dynamics and their effect on secondary metabolism.
Materials and Methods
Cell Cultures
Taxus cuspidata cell line P991 was provided by the U.S. Plant Soil and Nutrition Laboratory in Ithaca, NY, and maintained in our laboratory. Cells were subcultured every two weeks into fresh medium, consisting of Gamborg B5 basal salts, supplemented with 20 g/L sucrose, 2.7 μM naphthalene acetic acid, and 0.1 μM benzyladenine, and adjusted to pH 5.5 prior to autoclaving. 150 mg/L citric acid, 150 mg/L ascorbic acid, and 6.0 mM glutamine were filter sterilized and added post-autoclave. Cell cultures were maintained in either 125 mL or 500 mL glass Erlenmeyer flasks with Bellco (Vineland, NJ) foam closures, and incubated in gyratory shakers in constant darkness at 23°C and 125 rpm. Every two weeks, inoculum was subcultured to fresh media at a ratio of 1:4 for a total volume of 50 mL with 2-3 mL packed cell volume in 125 mL flasks or 200 mL in 500 mL flasks. For total biomass measurements, 2 mL samples were taken with a cut-tip pipette, filtered and rinsed with ~5 mL distilled water over Miracloth© (Calbiochem, San Diego, CA) and weighed to determine fresh weight. Samples were then dried in an oven at 50°C to a constant weight and recorded as dry weight. Glucose and sucrose were measured from cell culture media samples using a blood glucose analyzer (YSI 2700 Select Biochemistry Analyzer, YSI Life Sciences, Yellow Springs, OH).
Filtration
Filters were made by sealing together nylon mesh sheeting with pore sizes of 2000 μm, 1360 μm, 1000 μm, 710 μm, 500 μm, 300 μm, and 80 μm (Small Parts, Inc., Miramar, FL) or 1680 μm polypropylene mesh sheeting with a contact adhesive (Amazing Goop All Purpose, Eclectic Products, Eugene, OR) to hollowed out polypropylene chemical containers (Sigma-Aldrich Co., product G5893, St. Louis, MO). The surface area of each filter was approximately 635 cm2. For each mesh size, after the initial filtration step (divided across several filters if necessary for a maximum amount of ~1 g biomass / filter), the biomass remaining on the filter was gently immersed in a wash solution (Gamborg B5 basal salts), and this wash was repeated at least twice. Smaller meshes generally required more washes than larger meshes, as the percent open area of the mesh decreases with decreasing pore size, causing the formation of a cell cake. The permeate from each filtration step was combined, and this process was repeated for each successively smaller mesh size. The accumulated biomass on each filter was then backflushed with wash solution onto Miracloth for subsequent dry weight measurement, or backflushed with diluent (see next section) into the Multisizer sample beaker for subsequent analysis via the Coulter counter.
Electrical Resistance Pulse Amplitude Sizing
A Multisizer 3 Coulter counter (Beckman Coulter, Inc., Brea, CA) with a 2000 μm aperture, the largest size available, was used for electrical resistance measurements. Diluent consisting of 65:35 Isoton (1% NaCl with preservatives, Beckman Coulter):Glycerol (Fisher Scientific), was vacuum filtered over a series of 3.5 μm and 2.0 μm depth filters prior to use (Whatman, Inc., Piscataway, NJ) and recycled using the same filters following each set of samples. Recycling was required for a larger scale application of this method due to the substantial quantities of diluent used for sample analysis, and we found that diluent could be reused for an indefinite period of time. Diluent resistivity and baseline noise, determined using diluent only, and 200 μm latex standard spheres (Thermo Scientific, Fremont, CA) were used to check calibration before each run. The amplitude of the voltage pulse associated with each particle passing through the aperture of the Coulter counter was converted to a particle volume based on the calibration coefficient, which is determined using the 200 μm standard beads. A -400 mA current was applied across the aperture. Data were collected and analyzed using Beckman Coulter Multisizer software, or exported and analyzed in MATLAB (Mathworks, Natick, MA). For cell culture analysis, 2 mL samples of well mixed cell culture broth were collected using a cut pipette tip, and diluted into 380 mL diluent. Samples were run for 60 seconds, at a flow rate of 5.1 mL/sec.
Image Acquisition and Analysis
For experiments comparing measurements between the Coulter counter and image analysis, 0.5 mL samples were collected from the well-mixed residual diluted samples remaining after Coulter counter analysis. Fluorescein diacetate from a stock solution of 5 mg/mL in acetone was added at a final working concentration of 20 mg/L, and cell samples were incubated for at least 15 minutes, resulting in the fluorescence of viable cells and aggregates, which could easily be distinguished from cell debris. 150 μL samples were then spread over a microscope slide using a cut pipette tip, and no coverslip was used to prevent flattening and breakage of aggregates. Images were collected on an Olympus IX71 (Center Valley, PA) inverted epifluorescent microscope using a 4X/.13 Olympus UPlanFl objective for a total magnification of 40X, with 470/40 nm excitation and 495 nm longpass emission filters (Chroma, Rockingham, VT). Between 50-100 pictures were taken and combined into a single mosaic using a moving stage and a custom script with IP Lab Software (BD Bioscience, Rockville, MD) to cover the sample area, which was generally 1-2 cm2, depending on how the sample was plated on the slide. Each image was converted to a binary image by adjusting the binary threshold manually, and both the area in pixels of each particle and ellipse fitting parameters were automatically determined using ImageJ (National Institutes of Health, Bethesda, MD). In calculating the aggregate volume, we assumed that the polar axis was the average of the major and minor axes. An equivalent spherical diameter was then calculated from each aggregate volume.
Results and Discussion
Standard Methods to Measure Aggregation
A crude biomass distribution obtained by filtering (Fig. 1a) reveals that the majority of biomass consists of aggregates between 300 μm and 1500 μm. Time course experiments monitoring changes in aggregate profiles over a batch subculture period using this technique proved ineffectual (data not shown), as the poor resolution in the distribution combined with error resulting from flask to flask variability prevented detection and quantification of subtle differences in distribution shapes. This was not unexpected as no significant changes in aggregate distribution were observed in shake flask plant cell cultures using similar techniques (Madhusudhan and Ravishankar 1996; Zhao et al. 2003). The limitations of this method, in addition to procedural inefficiency, are that resulting distributions are coarse and generally imprecise, and it has been suggested that usefulness of filtration is limited in differentiating aggregate sizes (McDonald et al. 2001, Trejo-Tapia et al. 2003). In fact, if the sieving method does not include several wash steps, particularly a gentle immersion in wash solution for a tangential flow filtration effect as opposed to simply adding wash solution over the cell cake, the resulting separation is extremely poor. Even with careful washing, it is virtually impossible to remove all of the smallest aggregates from each fraction, and the separation efficiency of the filters is still imperfect (see Validation of Aggregate Size Measurement).
Fig. 1.
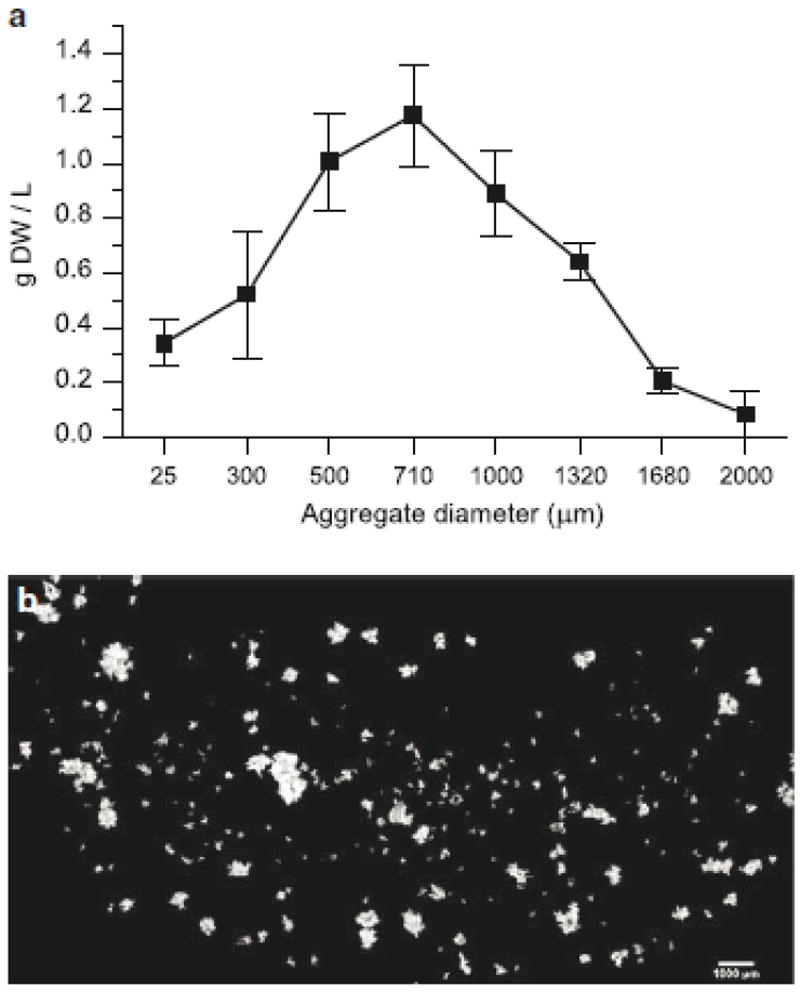
Typical size and morphology of aggregates in Taxus cuspidata suspension culture. a Distribution obtained from filtration of cultures on day 7; data points represent biomass retained on corresponding filter size, error bars represent standard deviations from three replicate flasks. b Composite image of aggregates stained with flourescein diacetate
In addition to the inherent limitations of mechanical separation, the distribution resolution is limited by the number of filters used to separate the aggregates into fractions and the amount of total biomass needed for analysis. Minimization of biomass is important so that aggregate distributions can be monitored over time by periodic sampling of the same cultures as opposed to harvestation of an entire culture to obtain a single distribution point. As the number of fractions increases, reliability is limited due to the lack of biomass in each aggregate size fraction. If the sample size is significantly increased and entire cultures are used to overcome biomass limitations, reliability is limited due to the flask to flask variability inherent in plant cell culture.
The typical size and morphology of Taxus cuspidata aggregates can be seen in Fig. 1b. Aggregates of more than a few cells are generally non-spherical, and the equivalent spherical diameters range from 50 μm to around 2000 μm. Although there have been reports of single cells in plant culture medium (Bolta et al. 2003), very few single cells were found in our Taxus cultures, though many aggregates of only 2-3 cells were present.
Quantifying size distributions using these images is inherently difficult due to the low numbers of large aggregates present in shake flasks and the resulting low probability of collecting these aggregates in the small sample volume used for image analysis. All studies using this technique have reported a mean aggregate size, as opposed to an aggregate distribution and the mean or expected value of the distribution. A minimum of 1000 counts was needed (Edahrio and Seki 2006) to determine the average aggregate size in strawberry suspension cultures. The counts needed to determine a reliable distribution is much higher, and will depend on the range of aggregate sizes present and the number of bins used in specifying the distribution.
Additionally, the non-spherical nature of the aggregates requires the assumption of a standard orientation of all the aggregates on the slide so that an equivalent aggregate volume can be calculated from the aggregate area, which is directly measured. We assumed that the polar axis was an average of the major and minor axes, which essentially minimizes the irregularity of each aggregate by assuming the roundest shape possible. Several reports have used a similar averaging method (Edahiro and Seki 2006; Keβler et al. 1999), while others have used the minor axis as the polar axis (Pepin et al. 1999). Calculating volume using either the major or minor axis as the polar axis will shift the equivalent spherical diameter size distribution. While the differences based on these assumptions can be accounted for using separate correlations to other measurement techniques (see below) and are irrelevant when comparing between samples, they are indicative of the difficulties in determining an “absolute” aggregate size or volume.
Electrical Resistance Pulse Amplitude Sizing
For this application, the particle volumes are binned and displayed as a volume distribution against particle diameter, where for each distribution bin, the total volume of particles is plotted versus the equivalent spherical diameter (Fig. 2). This representation is most practical, as a volume distribution is essentially a biomass distribution, and the particle diameter provides an intuitive visualization of the aggregate size profile. As with the measurement of any population distribution, a higher number of total counts will yield smoother data; however, one requirement of the method to be developed was that it would allow for multiple samples to be taken from a shake flask over a time course experiment, which limited the sample volume available. Due to this sample size limitation, a trade-off was made in which the sample size (and thus total counts) was minimized, yet still accurately and consistently characterized the size distribution.
Fig. 2.
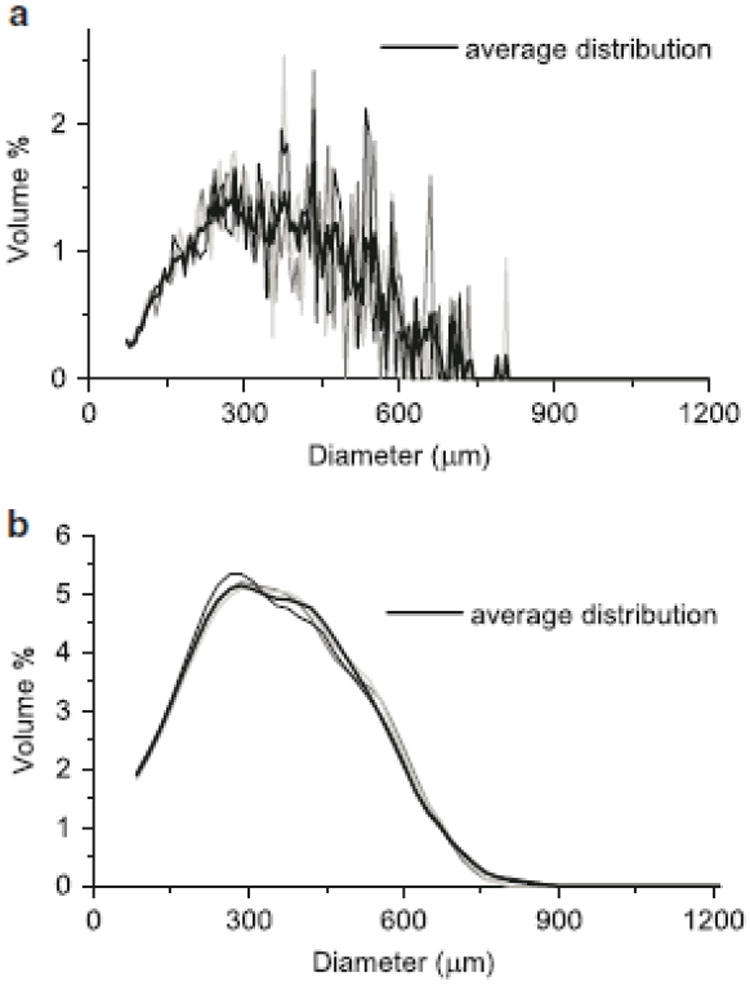
Aggregate size distributions obtained using a Coulter counter. a Resolution of 200 size bins from 70 μm to 1200 μm, comparing average of five samples to three representative individual samples. b Resolution of 50 size bins from 70 to 1200 μm, smoothed using a weighted moving average, comparing average of five samples to three representative averages of two samples
Quantifying precision in the measurement of a distribution as opposed to the measurement of a single value requires the comparison of every point along the distribution. Standard statistical comparisons generally provide a confidence level as to whether a distribution fits the shape of a known type of distribution (e.g., normal). In this case, we are interested in directly comparing the precision of measured distributions. To do this, we used a variation of a mean squared error calculation, and compared results of a single distribution measurement to the average of five distributions, the average of two distributions to the average of five distributions, and so on. Reducing the resolution of the distribution by using fewer bins in conjunction with using a moving average significantly improved precision (Fig. 2), while still maintaining a relatively fine resolution of 50 bins or size classes. Averaging two distributions compared to using only one further reduced the mean squared error by 44%. While further improvements could be gained by including additional samples in the analysis, improvements were modest (e.g., 22% reduction in error with 50% more biomass) and would require significantly more biomass, hence limiting time course studies. Therefore, two × 2 mL samples at each time point were determined to be sufficient to reliably characterize the aggregate distribution.
A concern about the suitability of this method was whether the stirring action of the impeller in the sample beaker would break apart the aggregates and alter the size distribution. For all impeller speeds within the typical operating range, the impeller speed had no effect on the distribution measured (data not shown), implying that the aggregates are in fact insensitive to the shear effects of the impeller. This is likely due to a combination of low residence time in the sample beaker and the 40% glycerol diluent, which reduces shear forces on the aggregates as a result of its increased viscosity.
Total Biomass Correlation
The area under the volume distribution curve represents the total volume of particles analyzed from the sample. If the density of aggregates is constant with respect to aggregate size, this volume should linearly correlate to the total biomass of the sample via the biomass density. A series of dilutions was performed for culture samples, and samples from each dilution were collected for both dry weight analysis and particle sizing on the Coulter counter. As both the shape and mean of the size distribution of the same cell line varied significantly over the course of several months and subculture cycles (Fig. 3a), this dilution series was repeated to ensure that correlations developed from the distribution data are applicable over the range of aggregate sizes that may be encountered.
Fig. 3.
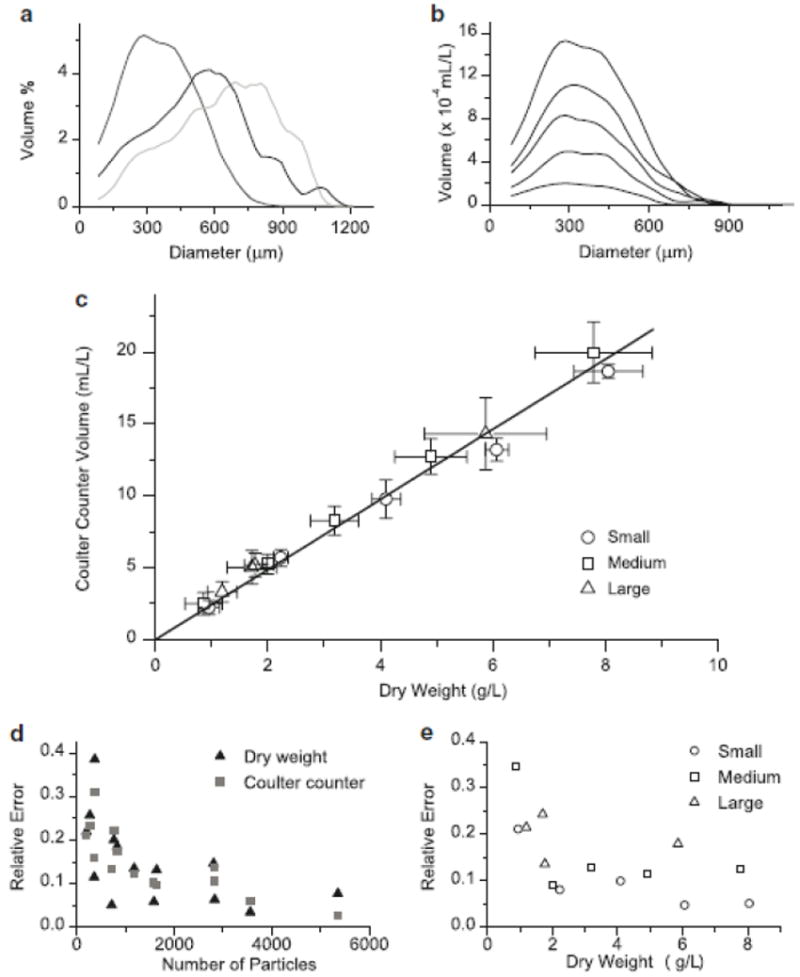
a Average aggregate size distributions from mid-exponential phase cultures of Taxus cuspidata. b Effect of biomass concentration on distribution curves from a dilution series of culture with a mean aggregate size of 365 μm. c Correlation between dry weight and total volume measured on Coulter counter for cultures with different aggregate size distributions (from Fig. 3a); Small = mean 365 μm, Medium = mean 537μm, Large = mean 625 μm, error bars represent standard deviation of 5 measurements. d Comparison of relative error (standard deviation divided by mean) for total biomass measurements comparing dry weight and Coulter counter biomass, based on total particle number as determined by the Coulter counter. e Comparison of relative error in total biomass measurements (average of Coulter counter and dry weight error) for cultures with different aggregate size profiles (Fig. 3a and Fig. 3b)
The shape of the aggregate distributions showed no significant differences upon dilution (Fig. 3b) (representative data from one dilution series), indicating that particle size measurement is not affected by biomass concentration. A concentration dependent effect on particle size measurement in these types of aperture flow systems is known as coincidence. Coincidence refers to the passing of multiple particles through the aperture at the same time, which results in larger particle measurements when uncorrected for. Coincidence is biomass dependent since the probability of two or more particles passing through the aperture simultaneously increases with increasing particle concentration. Much of the earlier work on electronically sizing cellular particles focused on developing algorithms to determine coincidence (Kubek and Shuler 1978), but the coincidence correction algorithm embedded within the Multisizer software appeared to adequately account for this effect, since no concentration dependent effect on distribution shape was observed (Fig. 3b).
Fig. 3c shows a linear relationship between dry weight and total particle volume. A single correlation fits all of the data extremely well (R2 of 0.999), and can thus be used as a general correlation regardless of aggregate morphology. While the density of aggregates of different sizes may vary, the variation is less than the error introduced from sampling alone, and can therefore be neglected. Therefore, the technique presented here provides for a fast and reliable option to measure total biomass in plant cell culture systems. Finding a fast and simple method to determine biomass has long been a subject of investigation in plant cell culture process engineering research, as dry weight measurements are time consuming and require a time lag of at least one day (Ryu et al. 1990). Very few simple methods exist due to the aggregated nature of plant cells, which precludes methods such as cell counting, and the heterogeneity of the cell culture broth, which precludes measurements such as optical density. Fast methods such as packed cell volume have limited reliability, while others such as media conductivity have been shown to have limited applicability across different plant systems (Kwok et al. 1992).
Analysis of the relative error confirms that the Coulter counter is as reliable a measurement method for total biomass as dry weight measurements (Fig. 3d). In fact, when comparing these two methods, the precision of the total biomass measurement is dependent on the properties of the sample, rather than on the measurement technique used. This is due in large part to the heterogeneous nature of the broth and difficulties in obtaining a well mixed sample of aggregates, which tend to settle when not under constant agitation. When the mean aggregate size was larger or total biomass was lower, the precision of distribution measurements decreased (Fig. 3e). This could be predicted from basic statistical considerations, as samples with the same total biomass but larger aggregate size, or less total biomass with the same aggregate size, will both consist of fewer total particles. Attempts were not made to alter the sample size to account for differing biomass concentrations during subsequent cell culture monitoring for the sake of procedural consistency.
Validation of Aggregate Size Measurement
To quantitatively evaluate whether the Coulter counter could differentiate particles of different sizes, fractions of aggregates that had been filtered were analyzed. Fig. 4a shows an obvious separation of filtered fractions. The overlap of fractions is likely due in large part to inefficient filtration as discussed earlier, which is a result of inconsistent filtration of non-spherical aggregates and the aggregate breakage that inevitably occurs over the course of several wash steps. The poor efficiency of filtration, especially the presence of smaller particles in the retentate caught on the filter, is apparent on visual inspection of suspended aggregate fractions following filter backflush, as well as in the spread of data in the size distribution of each fraction (Fig. 4a). This effect is particularly noticeable in the distributions of the largest fractions, where the spread of the data due to small aggregates is apparent because of the low numbers of large aggregates. While this limited separation efficiency must be taken into account when using filtration as a means to study aggregate size effects, these results demonstrate that filtration can be a reasonably effective means to separate plant cell aggregates given the proper procedural consideration, and also demonstrate that the Coulter counter can adequately differentiate plant cell aggregates on the basis of size.
Fig. 4.
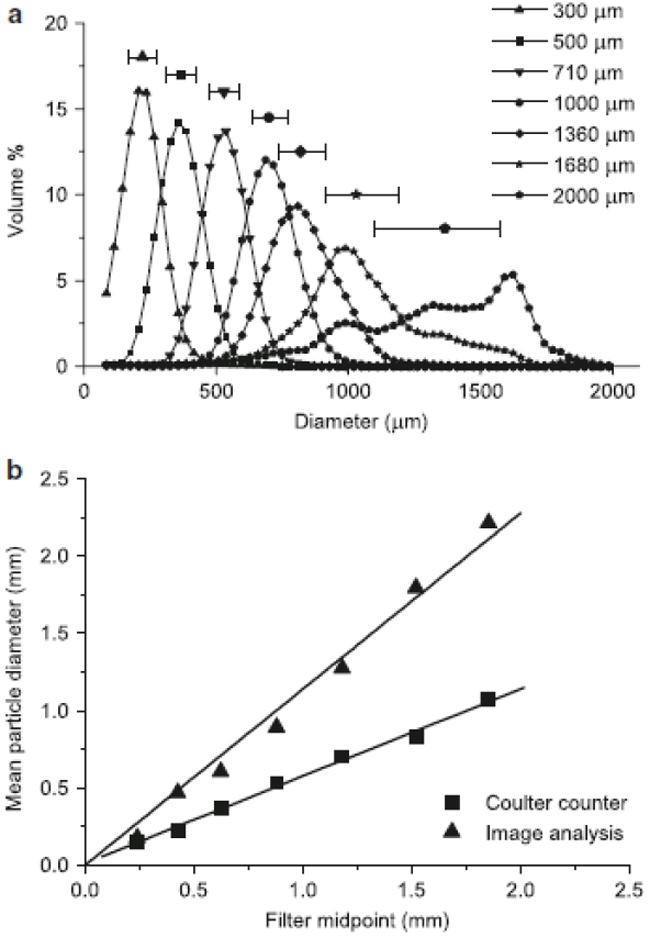
a Size distributions of aggregates separated on a series of filters and analyzed on the Coulter counter; each data point above the corresponding distribution represents the median and error bars represent median 50% of data, labels refer to filter size on which the sample was collected. b Correlation between mean size of filtered fractions determined using both the Coulter counter and image analysis against the volume weighted average of filter sizes between which aggregates were separated
It is apparent that the particle volumes measured on the Coulter counter are smaller than the corresponding filter sizes used to separate the fractions. This discrepancy is a result of using latex beads as a calibration standard, which are different than cell aggregates in a number of ways: aggregates have a heterogeneous, highly aqueous composition, resulting in some residual conductivity and less equivalent volume of electrolyte displaced; aggregates may compress slightly due to the pressure drop when crossing the aperture; and diluent may enter the aggregate via open pores or diffusion, which will also result in less displaced electrolyte and a lower measured particle volume. Regardless of the differences in absolute size measured, a linear correlation (dcoulter counter = 0.57 dfilter, R2 = 0.998) was developed using the mean of the volume distribution for each fraction versus the equivalent volume weighted midpoint of the filters used to separate the aggregates (Fig. 4b). A similar correlation was also developed for the mean particle size collected via image analysis (dimage analysis = 1.14 dfilter, R2 = .995). For this correlation, the mean of the particles as opposed to the mean of the distribution was used since only several hundred counts as opposed to several thousand were taken for each fraction. From this analysis, it appears that image analysis gives slightly larger particle sizes than the corresponding filter fractions. This is in part due to the assumption that the polar axis is the average of the major and minor axes. The polar axis may actually be smaller than either the major or minor axis, and the calculations to determine particle size could likely be tuned to reach a closer absolute agreement with the filter values.
A comparison of sizes measured using all three techniques points to the difficulties in determining the absolute size of plant cell aggregates. While the Coulter counter underestimates particle size compared to the other methods, the relative sizes are consistent as evidenced by linear correlations (Fig. 4b), and the calibration coefficient to convert voltage pulses to particle sizes could easily be altered so that measurements match either filtration or microscope measurements. However, filtration is inconsistent due to irregularly shaped aggregates and aggregate breakage, and sizes obtained from microscopic image analysis are dependent on the assumptions used in calculating particle size. For continuing work, relative differences in aggregate size are of more practical value, and the Coulter counter is as accurate and reliable a method as any other to determine these relative differences, and can be utilized at a fraction of the procedural effort.
Batch culture process monitoring
To demonstrate the utility of the aggregate size measurement using the Coulter counter, we monitored the aggregate distribution over a batch subculture period, in conjunction with sucrose and glucose measurements in the culture media. Fig. 5a shows the evolution of the aggregate distribution over approximately two weeks. Total biomass accumulation was evaluated using the previously described correlation, and the resulting growth curve showed the expected pattern of exponential growth followed by a stationary phase, which corresponded to the point at which sugar was exhausted in the media (Fig. 5b). Over the course of the batch, the aggregate distributions spanned an increasing broader range of sizes, and showed a significant increase in mean aggregate size of 55% (Fig. 5c). The increase in aggregate size corresponded with the exponential growth phase of the culture, but this trend reversed once the culture reached stationary phase. This suggests that aggregate growth is associated with cell division and growth as opposed to other possible mechanisms such as agglomeration. Slight variations with respect to sugar consumption and a batch culture’s progression through the growth cycle are likely present over several subculture cycles, which are typically not controlled for as subculturing is determined by time (14 days) as opposed to process measurements (see Methods for details). It is possible that these variations contribute to the periodic size fluctuations observed (Fig. 3a), though there are most likely other contributing factors, including underlying metabolic variability as well as epigenetic and circadian variations. Understanding the phenomenological basis of these variations is a goal of continuing work.
Fig. 5.

Process monitoring of cell cultures over batch subculture period. a Aggregate distribution data obtained on a Coulter counter over successive days of the batch cycle, each curve represents the average of two 2mL samples. b Growth curve obtained from distribution data; biomass correlation (Fig. 3c) used to calculate dry weight from Coulter counter data, substrate concentration is the sum of sucrose and glucose in the culture medium. c Mean diameter of aggregates over batch cycle
Conclusions
We have evaluated several methods for characterizing plant cell aggregates in tissue suspension culture, including a novel technique utilizing electrical pulse amplitude sizing, or Coulter counter technology. The Coulter counter can quickly measure total biomass as a cumulative total of all aggregates, and provides reliable data compared to the standard method of dry weight. The Coulter counter can also measure aggregate size distributions as well as, if not more accurately, than both filtration and image analysis and can be used for a fraction of the time and procedural effort. The absolute size of aggregates is difficult to quantify, but relative measurements from all three techniques can be linearly correlated. Analysis of changes in the aggregate size distribution over a batch cycle reveals that aggregate size increases during exponential growth but decreases during stationary phase, and demonstrates the utility of this method for future studies into the advanced process characterization of plant cell tissue culture dynamics.
Acknowledgments
This work was supported by grants from the National Science Foundation (CBET 0730779) and the National Institutes of Health (GM070852). M.E.K also acknowledges support from a National Research Service Award T32 GM08515 from the National Institutes of Health and the National Science Foundation-sponsored Institute for Cellular Engineering IGERT program DGE-0654128.
References
- Bolta Z, Baricevic D, Raspor P. Biomass segregation in sage cell suspension culture. Biotechnol Lett. 2003;25:61–65. doi: 10.1023/a:1021790418429. [DOI] [PubMed] [Google Scholar]
- Edahiro J, Seki M. Phenylpropanoid metabolite supports cell aggregate formation in strawberry cell suspension culture. J Biosci Bioeng. 2006;102:8–13. doi: 10.1263/jbb.102.8. [DOI] [PubMed] [Google Scholar]
- Forni C, Frattarelli A, Damiano C. Different size, shape and growth behavior of cells in suspension cultures of strawberry (Fragaria × ananassa Duch.) Plant Biosyst. 1999;133:205–212. [Google Scholar]
- Georgiev MI, Weber J, Maciuk A. Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl Microbiol Biot. 2009;83:809–823. doi: 10.1007/s00253-009-2049-x. [DOI] [PubMed] [Google Scholar]
- Graham MD. The Coulter principle: Foundation of an industry. J Assoc Lab Autom. 2003;8:72–81. [Google Scholar]
- Hulst AC, Meyer MT, Breteler H, Tramper J. Effect of aggregate size in cell cultures of Tagetes petula on thiophene production and cell growth. Appl Microbiol Biotechnol. 1989;30:18–25. [Google Scholar]
- Keβler M, ten Hoopen HJG, Furusaki S. The effect of the aggregate size on the production of ajmalicine and tryptamine in Catharanthus roseus suspension culture. Enzyme Microb Tech. 1999;24:308–315. [Google Scholar]
- Kolewe ME, Gaurav V, Roberts SC. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol Pharm. 2008;5:243–256. doi: 10.1021/mp7001494. [DOI] [PubMed] [Google Scholar]
- Kubek DJ, Shuler ML. Electronic measurement of plant cell number and size in suspension culture. J Exp Bot. 1978;29:511–523. [Google Scholar]
- Kubitschek HE. Electronic counting and sizing of bacteria. Nature. 1958;182:234–235. doi: 10.1038/182234a0. [DOI] [PubMed] [Google Scholar]
- Kubitschek HE. Counting and sizing micro-organisms with the Coulter counter. In: Norris R, Ribbons DW, editors. Methods in Microbiology. Vol. 1. Academic Press; NY: 1969. pp. 593–610. [Google Scholar]
- Kwok KH, Tsoulpha P, Doran PM. Limitations associated with conductivity measurement for monitoring growth in plant-tissue culture. Plant Cell Tiss Org Cult. 1992;29:93–99. [Google Scholar]
- Madhusudhan R, Ravishankar GA. Gradient of anthocyanin in cell aggregates of Daucus carota in suspension cultures. Biotechnol Lett. 1996;18:1253–1256. [Google Scholar]
- Mavituna F, Park JM. Size distribution of plant cell aggregates in batch culture. Chem Eng J. 1987;35:B9–B14. [Google Scholar]
- McDonald KA, Jackman AP, Hurst S. Characterization of plant suspension cultures using the focused beam reflectance technique. Biotechnol Lett. 2001;23:317–324. [Google Scholar]
- Naill MC, Roberts SC. Preparation of single cells from aggregated Taxus suspension cultures for population analysis. Biotechnol Bioeng. 2004;86:817–826. doi: 10.1002/bit.20083. [DOI] [PubMed] [Google Scholar]
- Naill MC, Roberts SC. Cell cycle analysis of Taxus suspension cultures at the single cell level as an indicator of culture heterogeneity. Biotechnol Bioeng. 2005a;90:491–500. doi: 10.1002/bit.20446. [DOI] [PubMed] [Google Scholar]
- Naill MC, Roberts SC. Flow cytometric identification of paclitaxel-accumulating subpopulations. Biotechnol Prog. 2005b;21:978–983. doi: 10.1021/bp049544l. [DOI] [PubMed] [Google Scholar]
- Pepin MF, Smith MAL, Reid JF. Application of imaging tools to plant cell culture: Relationship between plant cell aggregation and flavonoid production. In Vitro Cell Dev-Pl. 1999;35:290–295. [Google Scholar]
- Rodriguez-Monroy M, Trejo-Espino JL, Jimenez-Aparicio A, Morante ML, Villarreal ML, Trejo-Tapia G. Evaluation of morphological properties of Solanum chrysotrichum cell cultures in a shake flask and fermentor and rheological properties of broths. Food Technol Biotech. 2004;42:153–158. [Google Scholar]
- Ryu DDY, Lee SO, Romani RJ. Determination of growth rate for plant cell cultures: Comparative studies. Biotechnol Bioeng. 1990;35:305–311. doi: 10.1002/bit.260350312. [DOI] [PubMed] [Google Scholar]
- Trejo-Tapia G, Hernandez-Trujillo R, Trejo-Espino JL, Jimenez-Aparacio A, Rodriguez-Monroy M. Analysis of morphological characteristics of Solanum chrysotrichum cell suspension cultures. World J Microb Biot. 2003;19:929–932. [Google Scholar]
- Zhao D, Huang Y, Jin Z, Qu W, Lu D. Effect of aggregate size in cell cultures of Saussurea medusa on cell growth and jaceosidin production. Plant Cell Rep. 2003;21:1129–1133. doi: 10.1007/s00299-003-0631-8. [DOI] [PubMed] [Google Scholar]


