Abstract
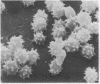
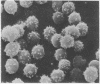
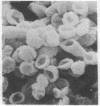
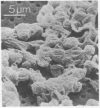
We propose that membranes whose proteins and polar lipids are distributed asymmetrically in the two halves of the membrane bilayer can act as bilayer couples, i.e., the two halves can respond differently to a perturbation. This hypothesis is applied to the interactions of amphipathic drugs with human erythrocytes. It is proposed that anionic drugs intercalate mainly into the lipid in the exterior half of the bilayer, expand that layer relative to the cytoplasmic half, and thereby induce the cell to crenate, while permeable cationic drugs do the opposite and cause the cell to form cup-shapes. This differential distribution of the drugs is attributed to interactions with the phosphatidylserine that is concentrated in the cytoplasmic half of the membrane. Impermeable amphipathic drugs intercalate only into the exterior half of the bilayer, and therefore are crenators of the intact cell. Several predictions of this hypothesis have been confirmed experimentally with erythrocytes and erythrocyte ghosts. The bilayer couple hypothesis may contribute to the explanation of many membrane-mediated phenomena in cell biology.
Keywords: membrane asymmetry, cell morphology, amphipathic drugs, anesthetics
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher M. S. Membrane structure: some general principles. Science. 1973 Aug 17;181(4100):622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Phosphatidyl-ethanolamine: differential labelling in intact cells and cell ghosts of human erythrocytes by a membrane-impermeable reagent. J Mol Biol. 1972 Nov 28;71(3):523–528. doi: 10.1016/s0022-2836(72)80020-2. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Transformation and restoration of biconcave shape of human erythrocytes induced by amphiphilic agents and changes of ionic environment. Biochim Biophys Acta. 1968 Dec 10;163(4):494–500. doi: 10.1016/0005-2736(68)90078-3. [DOI] [PubMed] [Google Scholar]
- Frazier D. T., Narahashi T., Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):45–51. [PubMed] [Google Scholar]
- Kwant W. O., Seeman P. The membrane concentration of a local anesthetic (chlorpromazine). Biochim Biophys Acta. 1969;183(3):530–543. doi: 10.1016/0005-2736(69)90167-9. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Frazier T., Yamada M. The site of action and active form of local anesthetics. I. Theory and pH experiments with tertiary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):32–44. [PubMed] [Google Scholar]
- Roth S., Seeman P. The membrane concentrations of neutral and positive anesthetics (alcohols, chlorpromazine, morphine) fit the Meyer-Overton rule of anesthesia; negative narcotics do not. Biochim Biophys Acta. 1972 Jan 17;255(1):207–219. doi: 10.1016/0005-2736(72)90023-5. [DOI] [PubMed] [Google Scholar]
- Seeman P., Kwant W. O. Membrane expansion of the erythrocyte by both the neutral and ionized forms of chlorpromazine. Biochim Biophys Acta. 1969;183(3):512–519. doi: 10.1016/0005-2736(69)90165-5. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Singer S. J. Molecular biology of cellular membranes with applications to immunology. Adv Immunol. 1974;19(0):1–66. doi: 10.1016/s0065-2776(08)60251-5. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Zwaal R. F., Roelofsen B., Comfurius P., Kastelijn D., van Deenen L. L. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta. 1973 Oct 11;323(2):178–193. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Colley C. M. Localization of red cell membrane constituents. Biochim Biophys Acta. 1973 Sep 10;300(2):159–182. doi: 10.1016/0304-4157(73)90003-8. [DOI] [PubMed] [Google Scholar]