Abstract
Bacteriorhodopsin is a rhodopsin-like protein found in the cell membrane of Halobacterium halobium. It shows an absorption maximum at 570 nm and, in the light, undergoes cyclic spectral changes which include a relatively long-lived complex absorbing maximally at 412 nm. Excitation profiles have been obtained with several laser frequencies for two vibrations in the resonance Raman spectrum of bacteriorhodopsin. The results show that the Schiff base retinylidene lysine linkage is protonated in the 570 nm complex and that in the 412 nm complex it is unprotonated. The 412 nm complex must be present at appreciable concentrations when bacteriorhodopsin is exposed to high-energy argon ion laser light of the Raman spectrophotometer at room temperature. We conclude that the observed C=N stretch at 1622 cm-1 in the room temperature spectra, which in an earlier study by Mendelsohn was interpreted as evidence for an unprotonated linkage in bacteriorhodopsin, results from the presence of the 412 nm complex.
Keywords: light energy transduction, halobacteria, purple membrane
Full text
PDF
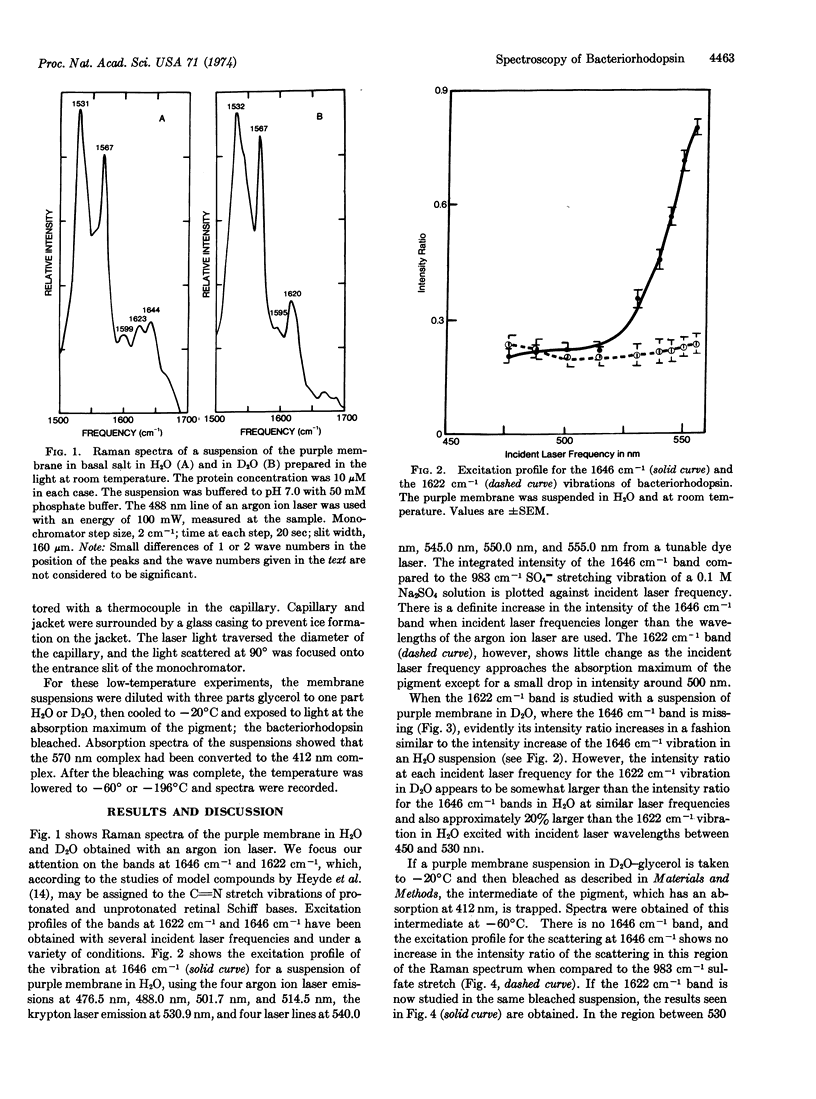
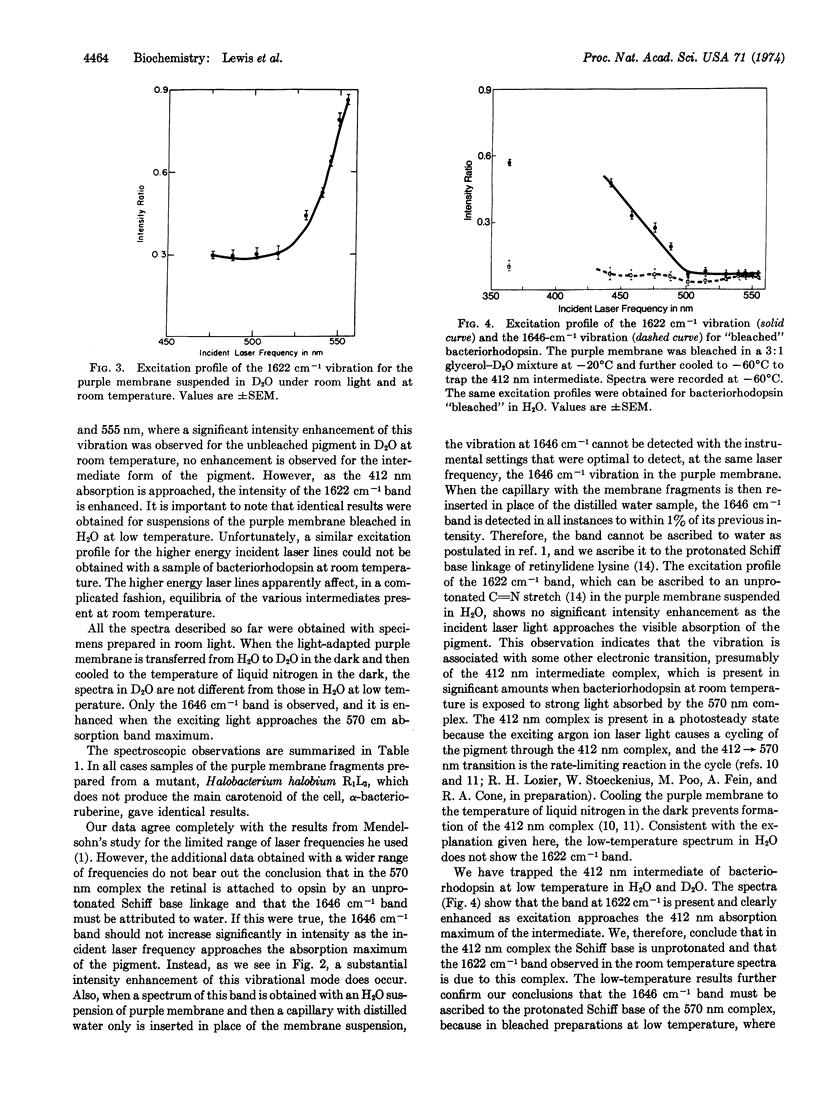
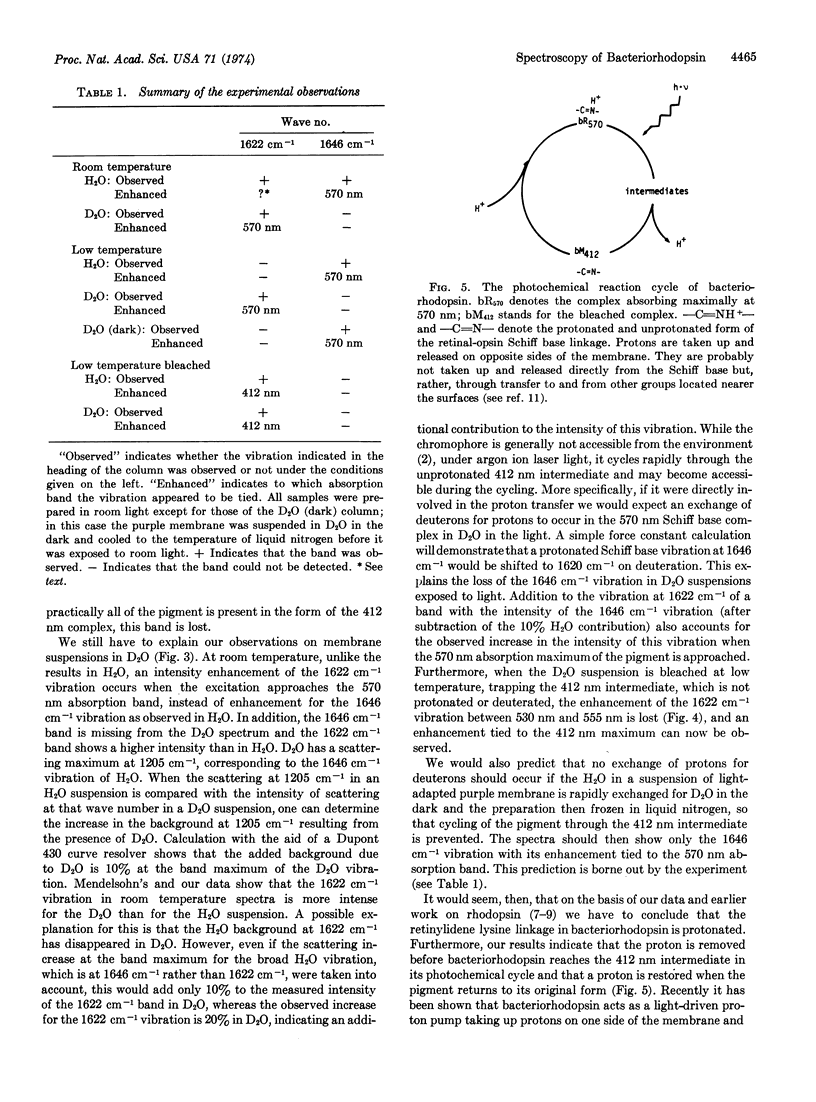

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyde M. E., Gill D., Kilponen R. G., Rimai L. Raman spectra of Schiff bases of retinal (models of visual photoreceptors). J Am Chem Soc. 1971 Dec 15;93(25):6776–6780. doi: 10.1021/ja00754a012. [DOI] [PubMed] [Google Scholar]
- Mendelsohn R. Resonance Raman spectroscopy of the photoreceptor-like pigment of Halobacterium halobium. Nature. 1973 May 4;243(5401):22–24. doi: 10.1038/243022a0. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Racker E., Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974 Jan 25;249(2):662–663. [PubMed] [Google Scholar]
- Toeckenius W., Kunau W. H. Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J Cell Biol. 1968 Aug;38(2):337–357. doi: 10.1083/jcb.38.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]



