Abstract
Carboxypeptidase N (CPN) is a member of the carboxypeptidase family of enzymes that cleave carboxy-terminal lysine and arginine residues from a large number of biologically active peptides and proteins. These enzymes are best known for their roles in modulating the activity of kinins, complement anaphylatoxins and coagulation proteins. Although CPN makes important contributions to acute inflammatory events, little is known about its role in autoimmune disease. In this study we used CPN−/− mice in experimental autoimmune encephalomyelitis (EAE), the animal model for multiple sclerosis. Unexpectedly, we observed several EAE disease phenotypes in CPN−/− mice compared to wild type mice. The majority of CPN−/− mice died within five to seven days after disease induction, before displaying clinical signs of disease. The remaining mice presented with either mild EAE or did not develop EAE. In addition, CPN−/− mice injected with complete or incomplete Freund's adjuvant died within the same time frame and in similar numbers as those induced for EAE. Overall, the course of EAE in CPN−/− mice was significantly delayed and attenuated compared to wild type mice. Spinal cord histopathology in CPN−/− mice revealed meningeal, but not parenchymal leukocyte infiltration, and minimal demyelination. Our results indicate that CPN plays an important role in EAE development and progression and suggests that multiple CPN ligands contribute to the disease phenotypes we observed.
Keywords: carboxypeptidase, demyelinating disease, kinins, MS/EAE
Introduction
Carboxypeptidase N (CPN) is a zinc-dependent metalloprotease and a member of one of two groups of mammalian carboxypeptidases that includes CPH/E, CPM, CPD, and CPZ. The active form of these enzymes is secreted or membrane-bound and cleaves the carboxy-terminal arginine or lysine residues from a wide array of proteins, peptides or prohormones (reviewed in (Koomen et al., 2005; Matthews et al., 2004; Skidgel, 1996; Skidgel and Erdos, 2007)). CPN substrates include kinins (bradykinin, kallidin and met-lys-bradykinin), complement anaphylatoxins (C3a and C5a), creatine kinase MM and cell surface proteins that bind plasminogen (so-called plasminogen receptors) (Matthews et al., 2004; Skidgel and Erdos, 2007). Through these cleavages, CPN broadly modulates a number of biologically important functions including inflammation, chemoattraction, leukocyte activation and trafficking, and plasmin-dependent extracellular matrix degradation. Complete CPN deficiency has not been documented, however an individual with partial CPN deficiency presented with angioedema and elevated histamine levels, supporting the importance of CPN in controlling kinin- and complement-mediated inflammation (Mathews et al., 1986; Mathews et al., 1980). The full range of CPN biological functions in human health and disease remains unknown.
The successful generation of CPN−/− mice provided the opportunity to begin careful dissection of the biological roles of this enzyme in greater detail (Mueller-Ortiz et al., 2009). Under routine husbandry, CPN−/− mice presented with no overt phenotype, however, they were hypersensitive to anaphylactic shock due to complement activation in a C5a-dependent fashion. Furthermore, C5a-induced histamine release was primarily responsible for the lethality observed in these studies (Mueller-Ortiz et al., 2009). More recently, we examined the role of CPN in experimental cerebral malaria (ECM), the animal model for cerebral malaria, the most fatal form of this parasitic disease (Darley et al., 2012). CPN−/− mice survived longer than wild type mice, but ultimately succumbed to ECM. These findings were surprising in that deletion of CPN in an infectious disease setting, where complement activation is occurring, was at least transiently protective and clearly did not exacerbate disease onset and severity. These results prompted us to examine the role of CPN in experimental autoimmune encephalomyelitis (EAE), the animal model for the human demyelinating disease, multiple sclerosis. Using MOG35-55 peptide-induced EAE, we found that CPN−/− mice presented with multiple phenotypes including lethal adjuvant-induced shock, mild EAE or the failure to develop EAE. CPN−/− mice that developed EAE generally had a monophasic course of disease followed by remission. These results combined with previous studies confirm that CPN biology in inflammatory diseases is highly complex.
Materials and methods
Mice
CPN−/− mice were generated as described (Mueller-Ortiz et al., 2009). CPN1−/− mice develop normally, display no gross abnormalities, are comparable in size to wild type C57BL/6 mice and are fertile. CPN−/− mice used in these studies (male and female) were crossed more than ten times to the C57BL/6 background. Inbred C57BL/6 mice from our own colony were used as controls for all experiments. All studies were performed with approval from the UAB IACUC.
Induction of active and adoptive transfer EAE
For active EAE, wild type and CPN−/− mice were immunized with MOG peptide35-55 as previously described (Bullard et al., 2007). In some experiments, mice were immunized with PLP139-151. MOG and PLP peptides were synthesized by standard 9-fluorenyl-methoxycarbonyl chemistry and were >95% pure as determined by reversed phase-HPLC (Biosynthesis, Lewisville, TX). Onset and progression of EAE clinical signs was monitored daily (30 days) using a standard clinical scale ranging from 0 to 6 as follows: 0, asymptomatic; 1, loss of tail tone; 2, flaccid tail; 3, incomplete paralysis of one or two hind limbs; 4, complete hind limb paralysis; 5, moribund; 6, dead. Only mice with a score of at least 2 (flaccid tail) observed for 2 or more consecutive days were judged to have onset of EAE. A cumulative disease index (CDI) was calculated from the sum of the daily clinical scores observed between day 7 and day 30. For transferred EAE, spleens of wild type donors were removed two to three weeks following induction of active EAE, and prepared as previously described (Szalai et al., 2002). Transferred EAE was induced by injecting ~5×106 purified T cells into wild type or CPN−/− mice as described in the Results section. Mice were evaluated daily for 19 days using the scoring system described above.
Histopathology
Mice with actively induced EAE were sacrificed at 30 days p.i. by CO2 inhalation, and spinal columns were removed, fixed in 10% buffered-formalin, and paraffin embedded. Sections (5μm thick) from the cervical, thoracic, and lumbar spinal cord were cut and either stained with hematoxylin and eosin for overall lesion evaluation and characterization of inflammatory responses or with Luxol fast blue for evaluation of demyelination. The extent of inflammation and demyelination was scored based on lesion size (0-4) and lesions were evaluated for lymphocyte accumulation, neutrophil infiltration, demyelination, axonal degeneration, and gliosis (0-4). Tissues were evaluated without identification as to experimental group. Severity scores were calculated as the mean over all segments of the products of the intensity scores multiplied by the extent scores for each lesion characteristic (inflammation, axonal degeneration, gliosis, and demyelination). The means of the individual lesion characteristic severity scores were summed to give the overall severity score.
Isolation and flow cytometric analysis of leukocytes from spinal cords
Spinal cords were removed from control and CPN−/− mice with active EAE (15 days post-induction) after perfusion with PBS, ground through a cell strainer, washed in PBS, resuspended in 40% Percoll and layered on 70% Percoll. After centrifugation at 2000 rpm (RT, 25 min.), cells at the interface were removed and washed in PBS and stained as described. Cells obtained from spinal cords were incubated with anti-CD16/32 (24G2, FcR block) to prevent non-specific staining. Spinal cord leukocytes were stained with anti-CD4-FITC (GK1[CR1].5), anti-CD8-PE (53-6.7), anti-IFN-γ-FITC (XMG1.2) or anti-CD25-PE all from eBiosciences (San Diego, CA). Some samples were also permeabilized using the eBioscience regulatory T cell staining kit and then stained with anti-Foxp3-APC. Stained cells and forward scatter were analyzed using a FACSCalibur and the data analyzed using CellQuest software (BD Biosciences, San Jose, CA).
Statistics
Statistical significance between wild type and CPN−/− mice for active EAE experiments was calculated using the Wilcoxon signed rank test, while for disease onset and max clinical score, the student's t-test was used. Results of evaluation for inflammation and demyelination were analyzed using analysis of variance for main effects and Tukey's test for pair-wise mean comparisons.
Results
CPN−/− mice are sensitive to adjuvant-induced lethal shock on EAE induction; survivors have attenuated disease
We first analyzed and compared the EAE phenotype in CPN−/− and wild type mice to determine any differences in initiation and progression of disease. We found that of the seventeen CPN−/− mice induced for EAE, seven died between days five and seven (41% mortality) post-disease induction, before onset of clinical signs of disease (Table 1). No wild type mice died in the same post-disease induction time period. To determine if this exceptionally high mortality rate might be adjuvant-induced, we injected wild type and CPN−/− mice with complete Freund's adjuvant containing PLP139-151, a myelin-derived peptide that does not induce EAE in C57BL/6 mice or PLP139-151 and various adjuvant components (MTB or oil). We observed that 33% of the CPN−/− mice died on or before day six, while none of the wild type mice died by day sixteen post-injection, indicating that CPN−/− mice are sensitive to a delayed form of adjuvant-induced lethal shock.
Table 1.
Adjuvant-induced mortality in CPN−/− mice.
| Adjuvant/peptide components | Mortality (%) |
|---|---|
| CFA + MOG35-55 | 7/17 (41%) |
| CFA + PLP139-151 | 1/4 (25%) |
| MTB + PLP139-151 | 1/4 (25%) |
| Oil + PLP139-151 | 2/4 (50%) |
| 4/12 (33%) |
CFA, complete Freund's adjuvant
PLP, proteolipid protein
MTB, inactivated mycobacterium tuberculosis
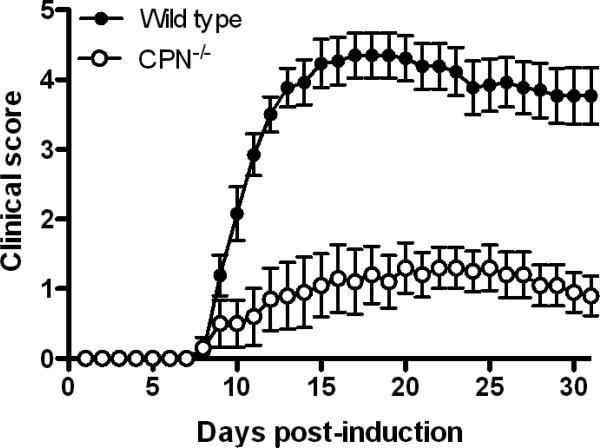
Surprisingly, the overall course of active EAE in CPN−/− mice that survived disease induction was exceedingly mild compared to wild type mice (Fig. 1 and Table 2; CDI: 24 vs. 85, p<0.01, Wilcoxon signed rank test). The maximum clinical score for CPN−/− mice was also significantly lower than for wild type mice (1.9 vs. 4.5, p<0.0001, unpaired t-test, Table 2) and none of the CPN−/− mice died after developing clinical signs of EAE. Disease onset was markedly delayed in CPN−/− mice compared to wild type mice (21.2 days vs. 10.4 days, p<0.0006, unpaired t-test). Disease incidence was also significantly attenuated in CPN−/− mice, with only 60% of mice presenting with a clinical score of 2 or more, for two consecutive days.
Fig. 1.
The clinical course of active EAE is attenuated in CPN−/− mice compared to wild type mice. Active EAE was induced with MOG35-55 peptide and clinical signs scored for 30 days as described in Materials and Methods. CPN−/− mice (n=10) have significantly reduced disease from day 10 through 30 (p<0.001, Wilcoxon rank sum test) compared to wild type mice (n=13). Results shown are the daily mean clinical score for wild type and CPN−/− mice from four experiments.
Table 2.
EAE clinical signs in wild type and CPN−/− mice.
| CDI | Disease Onset (days) | Disease Incidence | Max Clinical Score | Mortality (%) | |
|---|---|---|---|---|---|
| Wild Type n=13 | 85 | 10.4d | 100 | 4.5 | 23 |
| CPN−/− n=10 | 24* | 21.2d** | 60 | 1.9*** | 0 |
CDI = cumulative disease index
Significantly different than controls, p<0.01, from days 10 through 31
Significantly different than controls, p=0.0006
Significantly different than controls, p<0.0001
Spinal cord inflammation and demyelination during EAE is reduced in CPN−/− mice compared to wild type mice
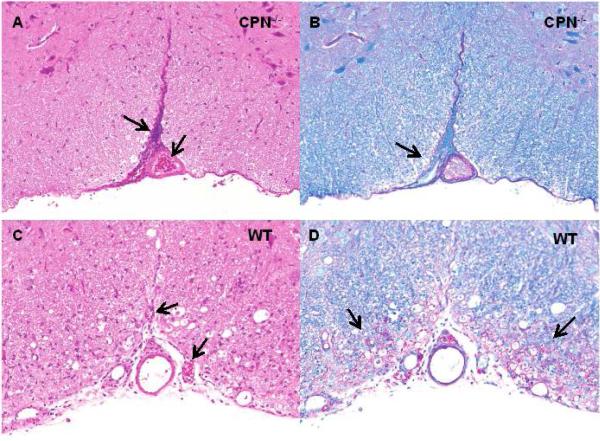
Histological evaluation of spinal cord sections taken at 30 days post-immunization revealed substantially fewer lesions and correspondingly mild inflammation in CPN−/− mice compared to wild type mice. Representative sections obtained from CPN−/− mice stained with H&E showed remarkably little parenchymal infiltration and inflammation (Fig. 2A) compared to wild type mice which had extensive mononuclear infiltrate (Fig. 2C). Similarly, sections from CPN−/− mice stained with LFBPAS (Luxol fast blue + periodic acid-Schiff) showed little demyelination and axonal degeneration throughout the spinal cord (Fig. 2B) compared to wild type mice, which had dilated myelin sheaths and numerous degenerating axons (Fig. 2D). Although the outer ventrolateral, white-matter tracts of CPN−/− mice were largely unaffected in EAE, there was a striking meningeal infiltrate composed of neutrophils, macrophages, and lesser numbers of lymphocytes, that filled the ventral fissure (Fig. 2A).
Fig. 2.
Leukocyte infiltration and demyelination in CPN−/− mice during EAE. Spinal cords from wild type and CPN−/− mice (n=4 for each group) were obtained at 30 days post-immunization, fixed in 10% buffered formalin and paraffin-embedded. Sections from the cervical, thoracic and lumbar regions (5μm) were stained with H&E (A and C) or Luxol fast blue (B and D) and scored as described in the Materials and Methods. (A, B) In representative sections from a CPN−/− mouse, infiltration is largely limited to the meninges and the ventral fissure (arrows) and there is little inflammation or demyelination. (C,D) In representative sections from a wild type mouse stained there is extensive parenchymal and meningeal infiltration combined with inflammation and a moth-eaten appearance and extensive demyelination and inflammation compared to CPN−/− mice (arrows). Original magnification 20X.
T cell infiltration in to the spinal cords of CPN−/− mice during EAE is reduced
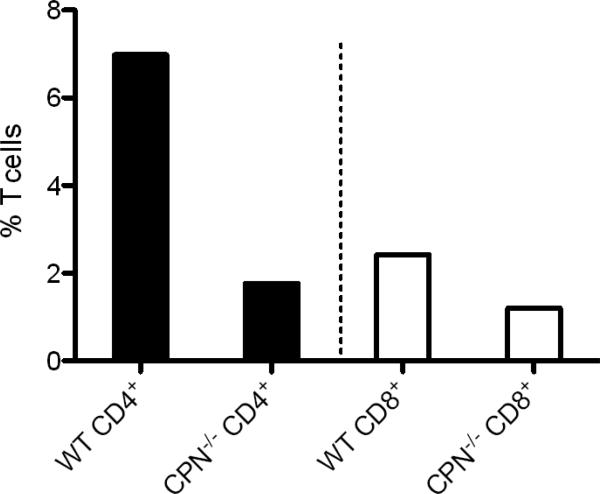
To quantitatively compare the level of T cell infiltration in wild type and CPN−/− mice with active EAE (day 15 post-immunization) we performed flow cytometry on single cell suspensions derived from spinal cords and analyzed them for expression of CD4 and CD8. Spinal cord infiltrates from CPN−/− mice had markedly reduced frequencies of CD4+ and CD8+ T cells (75% and 50% reduction, respectively) compared to wild type mice (Fig. 3). The percent of CD4+CD25+FoxP3+ Tregs infiltrating the CNS was also very low compared to wild type mice (data not shown). The low percent of T cells infiltrating into the spinal cords of CPN−/− mice parallels the minimal leukocyte infiltration we observed by histopathology (Fig. 2). Although their infiltration was reduced in CPN−/− mice, IFN-γ production by CD4+ T cells was identical to that of wild type mice, demonstrating that the absence of CPN did not compromise their ability to produce this key disease-promoting cytokine (data not shown).
Fig. 3.
Spinal cord T cell infiltration is reduced in CPN−/− mice during EAE. Leukocytes isolated from spinal cords of control (n=6) and CPN−/− mice (n=6) as described in Materials and Methods were immunostained for CD4 and CD8 at day 15 post-induction. The infiltration of both T cell subsets at day 15 after disease induction was markedly reduced in CPN−/− mice compared to controls. The results shown are from two experiments using cells pooled within each group of mice.
Discussion
In this study we made the observation that CPN−/− mice induced for EAE present with one of three phenotypes: death within five to seven days post-induction without showing any clinical signs of disease (41%), development of clinically mild disease (35%), or resistance to developing EAE (24%). Mortality post-disease induction prior to the development of clinical signs of EAE is rare in our hands, occurring in less than 1% of mice induced for EAE. To our knowledge, this combination of EAE-related phenotypes has not been reported for any congenic laboratory strain of mouse or rat, or for any gene-targeted, mutant-mouse line. One of the more interesting findings in our study was the observation that CPN−/− mice injected with complete or incomplete Freund's adjuvant components containing PLP peptide (which does not induce EAE in C57BL/6 mice) had a mortality rate comparable to mice injected with adjuvant containing MOG peptide (33% vs. 41%, respectively). These results suggest that the encephalitogenic peptides themselves are not contributing to the high mortality rates, but that specific component(s) of the adjuvant are responsible. In fact, our results (Table 1) suggest that either inactivated TB or emulsion oil are sufficiently shock inducing by an undefined mechanism to lead to mortality in CPN−/− mice. The interplay of CPN with other carboxypeptidases and their substrates, may contribute to this complex set of disease phenotypes.
It has been known for sometime that CPN inactivates the complement anaphylatoxins C3a and C5a (Bokisch and Muller-Eberhard, 1970), raising the possibility that complement anaphylatoxin-mediated shock may contribute to the phenotypes we report here. In a study by Mueller-Ortiz and colleagues (Mueller-Ortiz et al., 2009), CPN−/− mice were highly susceptible to mortality on treatment with cobra venom factor (CVF, a potent complement activator), or injected with physiologically relevant levels of C5a. Conversely, CPN−/− mice crossed with C5aR−/− mice were highly resistant to CVF-mediated mortality indicating an important role for CPN in modulating C5a-mediated anaphylactic shock. However, our finding that CPN−/− mice die within a few days, as opposed to a few hours, after EAE induction, may suggest that the immune-related mechanisms leading to death in our studies are distinct from the complement anaphylatoxins, particularly with respect to the C5aR, however this remains to be determined. We and others, have shown that C5aR−/− and C5a/GFAP tg mice (the latter expressing C5a in the CNS under the control of an astrocyte-specific promoter) develop EAE with kinetics and severity identical to wild type mice, indicating a non-essential role for this receptor/ligand pair in demyelinating disease (Reiman et al., 2002; Reiman et al., 2005). In contrast, deletion of the C3aR resulted in modestly attenuated EAE (predominantly in the chronic stage of disease) while ectopic expression of C3a under the control of an astrocyte-specific promoter (C3a/GFAP tg mice) produced a severe and frequently fatal form of EAE (Boos et al., 2004). Although this might argue for a causal relationship between CPN and C3a in the phenotypes we report here, disease-induced mortality occurs at distinctly different time points between the two mutant lines of mice.
In addition to the complement anaphylatoxins, CPN removes the carboxy-terminal arginine residues of several kinins including bradykinin, kallidin, and met-lysbradykinin (Erdos, 1979; Erdos and Sloane, 1962). Early studies in rabbits demonstrated significant differences in the levels of bradykinin and desArg9-bradykinin during EAE (Germain et al., 1988). Cleavage of bradykinin and kallidin by CPN generates desArg9-bradykinin and desArg10-kallidin, both of which mediate chronic inflammatory responses on binding to the B1 kinin receptor (Couture et al., 2001; Regoli and Barabe, 1980). Subsequently, it was shown that bradykinin exerted anti-inflammatory effects on LPS-treated microglia and astrocytes in a B1 receptor-dependent fashion (Noda et al., 2007a; Noda et al., 2007b). More recent studies have demonstrated an important role for the B1 receptor in controlling inflammation and leukocyte infiltration into the CNS during EAE (Dutra et al., 2011; Gobel et al., 2011; Schulze-Topphoff et al., 2009). It is possible that the des-Arg form of these kinins contribute to the early phase mortality we observed after induction of EAE. However, it is currently unknown if these CPN-modified inflammatory mediators are produced in sufficient quantity and are present in the appropriate time frame to cause the mortality we observed. Could other carboxypeptidases be responsible, at least in part, for the phenotypes we observed? The carboxypeptidase thrombin activatable fibrinolysis inhibitor (a.k.a., TAFIa or carboxypeptidases U, R or B) upon activation by thrombin, trypsin, or plasmin, cleaves carboxy-terminal lysines from fibrin thus altering clot dissolution (Gils, 2008; Leurs et al., 2004; Walker and Bajzar, 2004). However, TAFI can also cleave bradykinin and C5a to their des-Arg forms (Boffa and Koschinsky, 2007; Myles et al., 2003) raising the possibility that it may contribute to the phenotype we observed, perhaps in a compensatory fashion. It remains unclear if carboxypeptidases D and E contribute to demyelinating disease, but is possible they have a physiological role in our model system that extends beyond prohormone processing.
Perhaps the most unusual finding in our study was the mild EAE observed for those CPN−/− mice that survived disease induction. Within that group of mice, only two presented with fulminant EAE comparable to that seen in wild type mice during disease onset. However, unlike most wild type mice, both of these mice went into remission during the chronic phase of EAE. In fact, CPN−/− mice had remarkably lower average clinical scores at the completion of disease monitoring compared to wild mice (0.9 vs. 3.8, p<0.0001, student's unpaired t-test). These findings match the limited T cell infiltration and spinal cord demyelination seen in CPN−/− mice during EAE. It is interesting to note that leukocytes were readily attracted to spinal cord meninges in CPN−/− mice during EAE, but rarely trafficked into the parenchyma (Fig. 2). It remains to be determined if disrupted CPN processing of kinins and/or C3a and C5a contributes to this meningeal localization. Taken together, our data indicate an important role for CPN in the development and maintenance of EAE, although the precise mechanisms are unknown. Future studies in which CPN−/− mice crossed to complement anaphylatoxin receptor-deficient or B1-deficient mice along with EAE studies in other carboxypeptidase-deficient mice will provide insight with respect to role of CPN and other carboxypeptidases in the development and progression of EAE.
Table 3.
Spinal cord T-cell infiltration in wild type and CPN−/− mice during EAE.
| CD4+ | CD8+ | IFN-γ | CD4+/CD25+/FoxP3+ | |
|---|---|---|---|---|
| Wild Type | 7.4% | 2.5% | 98.6% | 3.4% |
| CPN−/− | 1.0% | 0.68% | 96.4% | 0.4% |
Acknowledgements
This work was supported by NIH grants T32 AI07051 and NS077811 (to TNR), and AI025011 (to R.A.W.). Histologic services for this project were supported in part by the Gnotobiotic and Genetically-Engineered Mouse Core of the UAB Mucosal HIV and Immunobiology Center.
Abbreviations
- CPN
carboxypeptidase N
- CVF
cobra venom factor
- EAE
experimental autoimmune encephalomyelitis
- MOG
myelin oligodendrocyte glycoprotein
Footnotes
Conflict of interest statement
The authors declare they have no conflict of interests.
References
- Boffa MB, Koschinsky ML. Curiouser and curiouser: recent advances in measurement of thrombin-activatable fibrinolysis inhibitor (TAFI) and in understanding its molecular genetics, gene regulation, and biological roles. Clinical biochemistry. 2007;40:431–42. doi: 10.1016/j.clinbiochem.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Bokisch VA, Muller-Eberhard HJ. Anaphylatoxin inactivator of human plasma: its isolation and characterization as a carboxypeptidase. The Journal of clinical investigation. 1970;49:2427–36. doi: 10.1172/JCI106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos L, Campbell IL, Ames R, Wetsel RA, Barnum SR. Deletion of the complement anaphylatoxin C3a receptor attenuates, whereas ectopic expression of C3a in the brain exacerbates, experimental autoimmune encephalomyelitis. J Immunol. 2004;173:4708–14. doi: 10.4049/jimmunol.173.7.4708. [DOI] [PubMed] [Google Scholar]
- Bullard DC, Hu X, Schoeb TR, Collins RG, Beaudet AL, Barnum SR. Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:851–7. doi: 10.4049/jimmunol.178.2.851. [DOI] [PubMed] [Google Scholar]
- Couture R, Harrisson M, Vianna RM, Cloutier F. Kinin receptors in pain and inflammation. European journal of pharmacology. 2001;429:161–76. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- Darley MM, Ramos TN, Wetsel RA, Barnum SR. Deletion of carboxypeptidase N delays onset of experimental cerebral malaria. Parasite immunology. 2012;34:444–7. doi: 10.1111/j.1365-3024.2012.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra RC, Leite DF, Bento AF, Manjavachi MN, Patricio ES, Figueiredo CP, Pesquero JB, Calixto JB. The role of kinin receptors in preventing neuroinflammation and its clinical severity during experimental autoimmune encephalomyelitis in mice. PloS one. 2011;6:e27875. doi: 10.1371/journal.pone.0027875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdos EG. In: Bradykinin, Kallidin and Kallikrein. Erdos EG, editor. Vol. 25. Springer; Heidelberg: 1979. pp. 427–487. [Google Scholar]
- Erdos EG, Sloane EM. An enzyme in human blood plasma that inactivates bradykinin and kallidins. Biochemical pharmacology. 1962;11:585–92. doi: 10.1016/0006-2952(62)90119-3. [DOI] [PubMed] [Google Scholar]
- Germain L, Barabe J, Galeano C. Increased blood concentration of des-Arg9-bradykinin in experimental allergic encephalomyelitis. Journal of the neurological sciences. 1988;83:211–7. doi: 10.1016/0022-510x(88)90069-x. [DOI] [PubMed] [Google Scholar]
- Gils A. Which carboxypeptidase determines the antifibrinolytic potential? Journal of thrombosis and haemostasis : JTH. 2008;6:846–7. doi: 10.1111/j.1538-7836.2008.02927.x. [DOI] [PubMed] [Google Scholar]
- Gobel K, Pankratz S, Schneider-Hohendorf T, Bittner S, Schuhmann MK, Langer HF, Stoll G, Wiendl H, Kleinschnitz C, Meuth SG. Blockade of the kinin receptor B1 protects from autoimmune CNS disease by reducing leukocyte trafficking. Journal of autoimmunity. 2011;36:106–14. doi: 10.1016/j.jaut.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Koomen JM, Li D, Xiao LC, Liu TC, Coombes KR, Abbruzzese J, Kobayashi R. Direct tandem mass spectrometry reveals limitations in protein profiling experiments for plasma biomarker discovery. Journal of proteome research. 2005;4:972–81. doi: 10.1021/pr050046x. [DOI] [PubMed] [Google Scholar]
- Leurs J, Nerme V, Sim Y, Hendriks D. Carboxypeptidase U (TAFIa) prevents lysis from proceeding into the propagation phase through a threshold-dependent mechanism. Journal of thrombosis and haemostasis : JTH. 2004;2:416–23. doi: 10.1111/j.1538-7836.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- Mathews KP, Curd JG, Hugli TE. Decreased synthesis of serum carboxypeptidase N (SCPN) in familial SCPN deficiency. Journal of clinical immunology. 1986;6:87–91. doi: 10.1007/BF00915368. [DOI] [PubMed] [Google Scholar]
- Mathews KP, Pan PM, Gardner NJ, Hugli TE. Familial carboxypeptidase N deficiency. Annals of internal medicine. 1980;93:443–5. doi: 10.7326/0003-4819-93-3-443. [DOI] [PubMed] [Google Scholar]
- Matthews KW, Mueller-Ortiz SL, Wetsel RA. Carboxypeptidase N: a pleiotropic regulator of inflammation. Molecular immunology. 2004;40:785–93. doi: 10.1016/j.molimm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Mueller-Ortiz SL, Wang D, Morales JE, Li L, Chang JY, Wetsel RA. Targeted disruption of the gene encoding the murine small subunit of carboxypeptidase N (CPN1) causes susceptibility to C5a anaphylatoxin-mediated shock. J Immunol. 2009;182:6533–9. doi: 10.4049/jimmunol.0804207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles T, Nishimura T, Yun TH, Nagashima M, Morser J, Patterson AJ, Pearl RG, Leung LL. Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. The Journal of biological chemistry. 2003;278:51059–67. doi: 10.1074/jbc.M306977200. [DOI] [PubMed] [Google Scholar]
- Noda M, Kariura Y, Pannasch U, Nishikawa K, Wang L, Seike T, Ifuku M, Kosai Y, Wang B, Nolte C, Aoki S, Kettenmann H, Wada K. Neuroprotective role of bradykinin because of the attenuation of pro-inflammatory cytokine release from activated microglia. Journal of neurochemistry. 2007a;101:397–410. doi: 10.1111/j.1471-4159.2006.04339.x. [DOI] [PubMed] [Google Scholar]
- Noda M, Sasaki K, Ifuku M, Wada K. Multifunctional effects of bradykinin on glial cells in relation to potential anti-inflammatory effects. Neurochemistry international. 2007b;51:185–91. doi: 10.1016/j.neuint.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacological reviews. 1980;32:1–46. [PubMed] [Google Scholar]
- Reiman R, Gerard C, Campbell IL, Barnum SR. Disruption of the C5a receptor gene fails to protect against experimental allergic encephalomyelitis. European journal of immunology. 2002;32:1157–63. doi: 10.1002/1521-4141(200204)32:4<1157::AID-IMMU1157>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Reiman R, Torres AC, Martin BK, Ting JP, Campbell IL, Barnum SR. Expression of C5a in the brain does not exacerbate experimental autoimmune encephalomyelitis. Neuroscience letters. 2005 doi: 10.1016/j.neulet.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Schulze-Topphoff U, Prat A, Prozorovski T, Siffrin V, Paterka M, Herz J, Bendix I, Ifergan I, Schadock I, Mori MA, Van Horssen J, Schroter F, Smorodchenko A, Han MH, Bader M, Steinman L, Aktas O, Zipp F. Activation of kinin receptor B1 limits encephalitogenic T lymphocyte recruitment to the central nervous system. Nature medicine. 2009;15:788–93. doi: 10.1038/nm.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel RA. In: Structure and function of mammalian zinc carboxypeptidases. Hooper NM, editor. Taylor and Francis Ltd.; London: 1996. pp. 241–283. [Google Scholar]
- Skidgel RA, Erdos EG. Structure and function of human plasma carboxypeptidase N, the anaphylatoxin inactivator. Int Immunopharmacol. 2007;7:1888–99. doi: 10.1016/j.intimp.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–7. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- Walker JB, Bajzar L. The intrinsic threshold of the fibrinolytic system is modulated by basic carboxypeptidases, but the magnitude of the antifibrinolytic effect of activated thrombin-activable fibrinolysis inhibitor is masked by its instability. The Journal of biological chemistry. 2004;279:27896–904. doi: 10.1074/jbc.M401027200. [DOI] [PubMed] [Google Scholar]