Abstract
Introduction
The success of medical therapies for Peyronie's disease (PD) has not been optimal, possibly because many of them went directly to clinical application without sufficient preclinical scientific research. Previous studies revealed cellular and molecular pathways involved in the formation of the PD plaque, and in particular the role of the myofibroblast.
Aims
The current work aimed to determine under normal and fibrotic conditions what differentiates PD cells from tunica albuginea (TA) and corpora cavernosa (CC) cells, by defining their global transcriptional signatures and testing in vivo whether PD cells can generate a PD like plaque
Main Outcomes Measures
Fibroproliferative features of PD cells and identification of related key genes as novel targets to reduce plaque size
Methods
Human TA, PD, and CC cells were grown with TGFβ1 (TA+, PD+, CC+) or without it (TA−, PD−, CC−) and assayed by: a) immunofluorescence, western blot and RT/PCR for myofibroblast, smooth muscle cell and stem cell markers; b) collagen content; and c) DNA microarray analysis. The ability of PD+ cells to induce a PD like plaque in an immuno-suppressed rat model was assessed by Masson trichrome and Picrosirius Red.
Results
Upon TGFβ1stimulation, collagen levels were increased by myofibroblasts in the PD+ but not in the CC+ cells. The transcriptional signature of the PD− cells identified fibroproliferative, myogenic (myofibroblasts), inflammatory, and collagen turnover genes, that differentiate them from TA− or CC− cells, and respond to TGFβ1 with a PD+ fibrotic phenotype, by upregulation of IGF1, ACTG2, MYF5, ACTC1, PSTN, COL III, MMP3, and others. The PD+ cells injected into the TA of the rat induce a PD like plaque.
Conclusions
This suggests a novel combination therapy to eliminate a PD plaque, by targeting the identified genes to: a) improve collagenase action by stimulating endogenous MMPs specific to key collagen types, and b) counteract fibromatosis by inhibiting myofibroblast generation, proliferation and/or apoptosis.
Keywords: penis, fibrosis, fibromatosis, Dupuytren's, myofibroblast, fibroblast, smooth muscle cells, collagenase, periostin, PSTN, MYF5, IGF1, NOX4, KTN34, ACTC1, PAI-2, MMP3, ATG2
INTRODUCTION
Peyronie's disease (PD) is a relatively prevalent condition affecting about 9% of men. It may present either as a solitary or multiple fibrotic plaques within the tunica albuginea of the penis which can lead to a penile deformity [1]. This curvature impacts the self-esteem and quality of life of afflicted patients and their partners and is the main reason why most men seek treatment. One option is via surgery, but because of the possibility of penile shortening and/or erectile dysfunction, many men seek a non-surgical cure.
Current medical therapy has focused on ways to “lessen” or soften the plaque to reduce curvature [2]. The widely used intralesional injections of verapamil or interferon suffer from either a lack of conclusive evidence of its efficacy or severe side effects [1,2]. Recently, the FDA approved intralesional Clostridium histolyticum collagenase (CHC) to lessen the plaque [3,4]. However, the modest improvement in penile curvature of 16% as well as the fact that only 1 in 5 patients obtained a “straight” erection in these recent trials suggest that there is room for improvement. This may be based on basic science investigations reported primarily during the last 15 or so years [5,6].
The PD plaque forms as the result of entrapment of fibrin that extravasates as fibrinogen into the tunica albuginea following an “injury” to this tissue [7]. The fibrin induces the expression of transforming growth factor β1 (TGFβ1), which initiates the conversion of fibroblasts to myofibroblasts as well as an increase in plasminogen activator inhibitor 1 (PAI-1) that inhibits fibrinolysis, an increase in reactive oxygen species (ROS) that causes oxidative stress and an induction of the inducible nitric oxide synthase (iNOS) [8]. Unknown factors prevent the myofibroblasts from undergoing apoptosis and/or stimulate their continuous formation, so that collagen production remains unabated. Therefore, the PD plaque has been defined as a scar or as a wound that never heals [9]. In about 20% of cases the PD plaque progresses to ossification [10] that results from heterotopic osteogenesis. Therefore, successful treatment of a PD plaque would require in addition to collagenolysis, treatment modalities able to: a) inhibit collagen production by the myofibroblasts; b) induce myofibroblast apoptosis; c) inhibit oxidative stress; and/or d) prevent or reverse ossification/calcification.
Our laboratory has demonstrated via DNA microarrays, which define global transcriptional signatures, that the PD tissue is in constant turnover, i.e., collagen and other extracellular matrix (ECM) production is counteracted by their catabolism [10-13] and by endogenous mechanisms of defense one of which is the spontaneous induction of inducible nitric oxide synthase (iNOS) within the tunica albuginea. iNOS generates a sustained output of nitric oxide (NO) and cGMP that act as inhibitors of oxidative stress, collagen synthesis and myofibroblast generation [6,14], and counteract the inflammatory and pro-fibrotic processes that lead to the generation of a plaque. Collagen/ECM catabolism is triggered by the over-expression of certain metalloproteinases (MMPs) [15,16], particularly MMP 2 and MMP 9. However MMP overexpression may be overcome by either a) an increase in tissue inhibitors of MMPS (TIMPs) [17], or b) by the eventual down-regulation of key endogenous MMPs [18]; if catabolism of collagen/ECM [15] prevails over anabolism, the plaque may regress, whereas if the contrary occurs, it continues growing; c) the ossification of the PD plaque impairs collagen breakdown and may be counteracted by inhibiting intralesionally the osteogenic differentiation of either myofibroblasts or stem cells, or both [10,14,19].
The interpretation of the transcriptional signatures of the human PD plaque tissue in comparison with those for the nodules of Dupuytren's disease (that is present in 15-20% of PD patients [2]), has provided some useful information but is limited by the heterogeneity of the respective tissues [12,13]. In contrast, the availability of human cell cultures from the PD plaque and non-PD tunica albuginea, and even of smooth muscle cells derived from the corpora cavernosa [14,19,20], that can be stimulated by pro-fibrotic effectors, should allow for a more focused and controllable comparison of transcriptional gene expression and key phenotypes among these cells. This may allow for the identification of potential therapeutic targets to stimulate collagenolysis and/or inhibit plaque growth, but to our knowledge no such reports are available. The ability of the myofibroblasts and/or the stem cells present in the human PD cultures to generate a PD like plaque has not yet been tested. Even if collagen may be broken down by exogenously administered collagenase assisted by other pharmacological treatments, the persistence of proliferating and pro-fibrotic cells in the plaque may defeat the improvement of collagenolysis by inducing plaque regrowth, as shown in Dupuytren's cell cultures [21].
The aim of the current work was to identify novel therapeutic targets for PD treatment by studying the role of human PD cell cultures in collagen production, the contribution of stem cells to this process and to the generation of myofibroblasts, and the transcriptional signatures of these PD cell cultures in comparison to non-PD TA and CC cell cultures in the presence and absence of a pro-fibrotic stimulus to simulate the PD plaque milieu. The ability of the PD cells to elicit a PD like plaque in a rat model was also studied, to identify some features of fibromatosis.
MATERIALS AND METHODS
Human Tissues and Cell Cultures
Tissue sections, and tissue homogenates preserved at −80°C, derived from one of our previous studies were used. Non-PD tunica albuginea was obtained from non-PD patients (n = 4) who were undergoing penile prosthesis surgery or a penectomy because of penile cancer. The PD plaque was harvested from patients with PD (n = 8) who underwent a surgical procedure to remove the plaque [14,19,20]. All procedures were institutional review board-approved, and written informed consent was obtained.
Fibroblast primary cultures containing stem cells were obtained as previously described [14,19,20] and named TA for the ones isolated from the non-PD tunica albuginea and PD for the ones from the PD plaque. The cells were used at passages 10-20. The purity of these cultures was established by immuno-cytochemistry for the fibroblast marker vimentin, which showed 100% staining as previously described, with virtually no TA cells positive for α-smooth muscle actin (ACTA2, also ASMA). Wells with fixed cells were used for immunofluorescence, and other replicated wells for either protein homogenates for western blots, or for RNA isolation (see below)
Animal Treatments
Male Fisher 344 rats (8–11 months old, NIH/NIA colony Harlan Sprague–Dawley, Inc., San Diego, CA, USA) were treated according to the National Institutes of Health (NIH) regulations with an institutionally approved protocol. The rats (N = 5/group) were anesthetized with 2.3% isoflurane (IsoFLO, Abbott Labs, North Chicago, IL, USA) by inhalation in an induction chamber and injected in the penile tunica albuginea close to the middle of the penis with either saline or 106 PD cells in saline, as previously described for the TGFβ1 injection [22], and anesthesia was maintained with a face mask. Tacrolimus was given daily (1 mg/kg, s.c.) to avoid immuno-rejection [23] of the human PD cells. At 2 and 4 weeks after the initial injection into the tunica albuginea, the rats were anesthetized with thiopental sodium (50 mg/kg, Abbott Labs), and the penises were excised. The skin was denuded, removing the glans and adhering non-crural tissue, the penile shaft was separated from the crura, and a 3 mm transversal slice was cut around the site of the saline or PD cells injection. The tissues were postfixed or fixed overnight in 10% formalin, washed in PBS, and stored at 4°C in 70% ethanol.
Quantitative Estimations in Tissue Sections and Cell Cultures
For histochemistry, 5 μm adjacent tissue sections obtained from the human or rat tissues were used. The PD like plaque area was either stained with hematoxylin/eosin, or the expression of collagen III and I was identified in adjacent sections stained with Picrosirius Red, under polarizing filter; the collagen fibers are green/yellow (collagen III) or red/orange (collagen I) [22]. For the collagen/smooth muscle cells ratio Masson trichrome staining (Sigma Diagnostic, St. Louis, MO, USA) was applied [22]. Quantitative image analysis (QIA) was performed using the ImagePro 5.1 program (Media Cybernetics, Silver Spring, MD, USA) coupled to an Olympus BHS microscope equipped with a Spot RT color digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA). The PD like plaque in the rat tunica albuginea was estimated within the half section of the corpora cavernosa where the tunical injection was given. The plaque size was expressed as the ratio between the area that stained positive for collagen fibers (blue), divided by the total area of smooth muscle cells (red) plus the remaining lacunar spaces and the cytoplasm of nonstained cells, mainly fibroblasts (white). Five non-overlapping fields were screened. Three sections per tissue specimen from the groups of eight rats were then used to calculate the mean ± standard error of the mean (SEM).
For non-quantitative dual immunohistochemistry in cell cultures, the primary monoclonal antibody against collagen I (1/50 dilution) was from Chemicon Int, Victoria, Australia and the antibodies against ACTA2 and OCT4 [24] were as indicated below for western blots. The secondary anti-mouse IgG antibody was biotinylated (goat, 1/200, Vector Laboratories, Burlingame, CA, USA). The complex was detected using streptavidin linked to Texas Red (red, for collagen I) or fluorescein (FITC) (green, for ACTA2 or OCT4). After washing with PBS, the sections were mounted with Prolong antifade containing DAPI to mark the nuclei (Molecular Probes, Carlsbad, CA, USA). The negative controls in all cases omitted the first antibodies or they were replaced by IgG isotype. Tissue sections or cells were visualized under a Nikon Eclipse Ti microscope (Nikon Instruments Inc., Melville, NY, USA) equipped with epifluorescence and coupled to the NIS-Elements acquisition software. Images were imported to Adobe Photoshop 7.0 (San Jose, CA, USA), cropped and adjusted for brightness and contrast only, and saved as tagged image file format (TIFF) files.
Western Blot and Densitometry Analysis
Cell lysates (20–50 μg of protein) were subjected to western blot analyses by 4–15% Tris-HCl polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA, USA) in running buffer (Tris/glycine/sodium dodecyl sulfate) [34]. Proteins were transferred overnight at 4°C to nitrocellulose membranes in transfer buffer (Tris/glycine/methanol). The next day, nonspecific binding was blocked by immersion of the membranes in 5% nonfat dried milk, 0.1% (v/v) Tween 20 in tris buffered saline (TBS). After washes with TBS Tween 0.1% the membranes were incubated for 1 hour with the primary antibodies. Monoclonal antibodies were as follows: (i) ACTA2, monoclonal (1/1,000) (Calbiochem, La Jolla, CA, USA); (ii) calponin I (Calp 1) mouse monoclonal (Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA) 1:500; (iii) Oct-4, rabbit polyclonal (BioVision.Incorporated, Milpitas, CA, USA) 1:500, and (iv) glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1/10,000) (Chemicon International, Temecula, CA, USA). In negative controls, we either omitted the first antibody or used a non-immune IgG. The washed membranes were incubated for 1 hour at room temperature with 1/3,000 dilution of anti-mouse or anti-rabbit secondary antibody linked to horseradish peroxidase.. After several washes, the immunoreactive bands were visualized using the Super Signal West Pico Chemiluminescent detection system (Pierce, Rockford, IL, USA). Densitometry of the bands was done with the Scion Image software beta 4.0.2 (Scion Corp., Frederick, MD, USA).
Collagen Estimation in Fresh Tissue
As previously described [25], the tissue was homogenized in saline and hydrolyzed with 2 N NaOH for 30 min at 120°C, followed by the estimation of hydroxyproline by a modification of the Neumann and Logan reaction using Chloramine T and Ehrlich reagent, using a hydroxyproline standard curve, and measuring at 550 nm. Values were expressed as micrograms of collagen per milligram of tissue.
Global transcriptional profiles (signature)
For the multiple mRNA profile [24], RNA was isolated from 25 cm2 flasks using the Trizol method, followed by cleanup using the RNeasy Plus Micro kit (Qiagen, Valencia, CA) with quality determined by the Agilent 2100 Bioanalyzer (Santa Clara, CA). Assays were performed in a duplicate set of penile tissue RNAs by the UCLA DNA microarray core, applying the Affymetrix Human ST1.0 Gene array. Only genes that were up- or down-regulated by at least 2-fold were considered unless specifically detailed.
RT-PCR
RNA was extracted from triplicate wells of 6 well plates for each type of cultured cell using the RNeasy Plus Micro kit (Qiagen), and then 350 ng of total RNA from each sample was reverse transcribed using the Invitrogen Superscript III kit [26]. The cDNA samples were amplified by PCR beginning with 4 min of denaturation at 94°C, followed by cycles of 94°C for 30 sec, primer annealing at 58°C for 30 sec, and extension at 68°C for 1 minute, followed by a final extension at 68°C for 4 min and a 4°C hold. PCR products were electrophoresed in 1% agarose gels, stained with ethidium bromide, and photographed under ultraviolet light. The sequences of the forward/reverse PCR primers, the predicted fragment sizes, and the number of PCR cycles were as follows: (i) MYF5: Forward TGCCTCATGTGGGCCTGCAAAG; Reverse AGGACTGTTACATTCGGGCATGC (321BP, 39 cycles); (ii) ACTC1: Forward GTGCCAAGATGTGTGACGACGAGG; Reverse CCATAACTCCCTGGTGCCGCG (147 BP, 45 cycles); (iii) IGFI Forward CCAAGACCCAGAAGTATCAGCCCCC; Reverse GCAAAG- GATCCTGCGGTGGCAT (174 BP, 36 cycles); (iv) OCT4: AGCAAAACCCGGAGGAGTCCCATGTGTCTATCTACTGTGTGTCCCAGGC (956 BP, 38 cycles); (v) NANOG: TGACGCAGAAGGCCTCAGCACGCCAGAGACGGCAGCCAAGG (991 BP, 38 cycles); (vi) GAPDH: GTCGCCAGCCGAGCCACA TCTGACCTTGGCCAGGGGTGCT (521 BP, 26 cycles).
Statistics
The data are expressed as the mean ± (SEM). The normality of the data distributions was established by the Wilk–Shapiro test, and pairs of groups were compared by the t-test. Multiple comparisons among groups were analyzed by one-way analysis of variance, followed by post hoc Student–Neuman–Keuls tests. Differences were considered significant at P < 0.05.
RESULTS
Human PD cells do not differ substantially from normal TA cells in the expression of myofibroblast contractile protein ACTA2, but a pro-fibrotic stimulus triggers both ACTA2 and collagen production
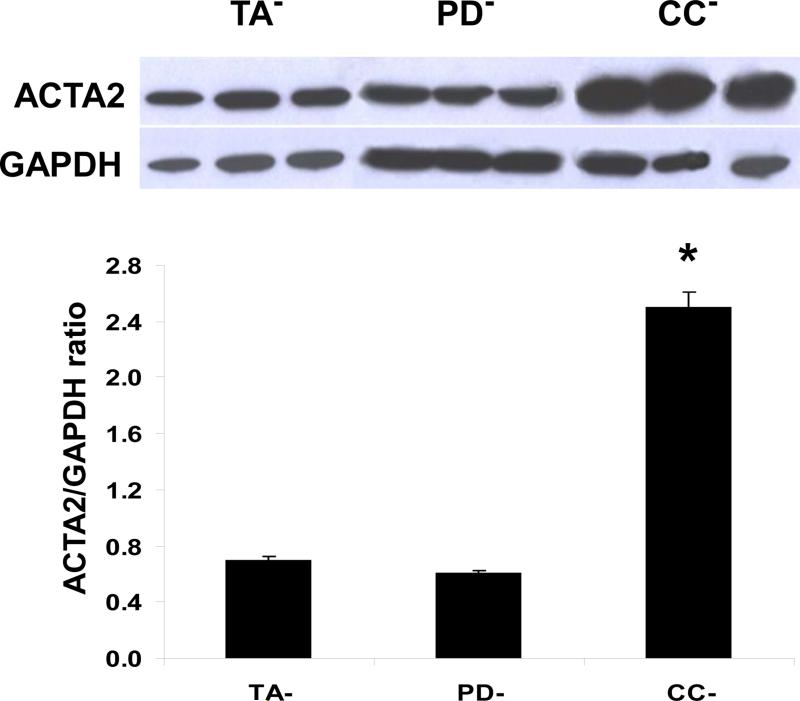
Cell cultures were grown from pools of three specimens per tissue type from human PD plaques (PD−), non-PD tunica albuginea (TA−), and corporal smooth muscle (CC−) and maintained for 8-15 passages. Figure 1 shows that in the absence of a fibrotic stimulus, the levels of α-smooth muscle actin (ACTA2, also known as ASMA), a protein expressed in both myofibroblasts and smooth muscle cells (SMC) are not different between the PD− and TA− cultures. This suggests that in a normal non-stimulated environment either myofibroblast content in the PD cultures is not higher than in the TA cultures or that the PD− cells are not yet expressing contractile proteins. The much higher ACTA2 protein levels in the CC− cultures do not represent myofibroblasts but originate from its expression by the SMC.
Figure 1. The content of activated myofibroblasts in the non-stimulated PD culture (PD−) was not higher than in the non-stimulated non-PD tunica albuginea culture (TA−), as shown by the expression of ACTA2 protein.
PD− and TA− cell cultures maintained in regular DMEM/20% fetal calf serum for 8-15 passages in the absence of added TGFβ1 were essentially fibroblast cultures, as assayed with vimentin, whereas CC− cultures were essentially SMC, as assayed with calponin (24-26). Cells on monolayer in 6-well plates were homogenized from triplicate wells for each culture and subjected to quantitative western blot assay for ACTA2 expression, correcting by GAPDH expression. In the case of the CC− cultures, ACTA2 does not discriminate myofibroblasts from SMC. *p<0.05
When the PD− and CC− cell cultures were incubated in a pro-fibrotic milieu (5 ng/ml of TGFβ1) for 7 days, myofibroblast content or the expression of contractile proteins in PD+ cultures was considerably stimulated (“active myofibroblasts”), as indicated by ACTA2 expression. However TGFβ1 did not virtually alter ACTA2 expression in the stimulated CC+ cultures, confirming that the CC cells mostly contain SMC and not myofibroblasts (Figure 2 top). This was corroborated by calponin 1 expression (restricted to SMC), which acted similarly to ACTA2 in the CC+ cells and did not vary by the fibrotic stimulus (not shown). As a result of the increase in active myofibroblasts by TGFβ1, the PD+ cultures were activated to produce more collagen, paralleling the respective ACTA2 concentration in the PD− and PD+ cultures. In contrast, collagen production by the SMC with the synthesizing phenotype, was not stimulated by TGFβ1, thus also ruling out the existence of myofibroblasts that should have produced more collagen. The three stimulated cultures (PD+, TA+, CC+) also secrete collagen into the medium, as indicated by merges of triple immunocytofluorescence detection, where nuclei are shown in blue, ACTA2+ cells are in red (a low fraction of the total TA+ cells, as expected), and collagen is in green (Figure 2 bottom).
Figure 2. Fibrotic stimulation was essential for collagen synthesis by PD cultures but not for CC cultures, and this process occurred inside or adjacent to ACTA2 expressing cells, presumably activated myofibroblasts in the PD+ and TA+ cultures or synthesizing phenotype SMC in the CC+ cultures.
Top: PD− and CC− cultures were incubated with 5 ng/ml of TGFβ1 for 1 week and subjected to western blot as in Figure 1. The resulting cultures were named PD+ and CC+, respectively. Collagen production was estimated in the homogenates after hydrolysis by the hydroxyproline assay *p<0.05. Bottom: TGFβ1-stimulated cells were immunostained on 8 well removable plates for ACTA2 (FITC; green fluorescence) and collagen I (Texas red; red fluorescence) and then all nuclei were detected with DAPI (blue fluorescence). Collagen is seen adjacent to or within ACTA2+ cells. Each panel is the merge of the three images. Magnification: 200X
Some of the cells in the PD and CC cultures associated with collagen production express a key stem cell gene, OCT4
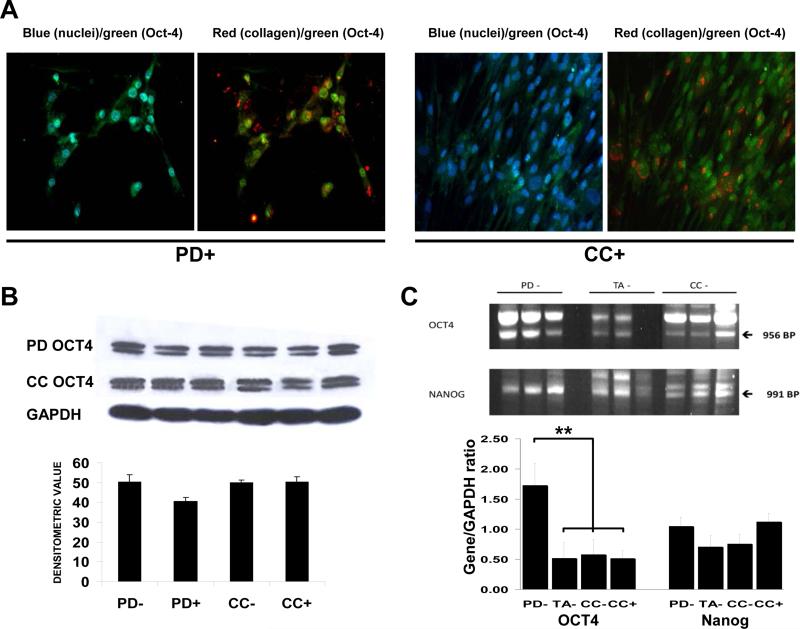
Since we have previously described the presence of cells expressing nuclear OCT4a in the non-stimulated PD− and CC− cultures and identified them as stem cells [25,53], we now determined whether these cells are also involved in collagen deposition. These cultures were now subjected to a TGFβ1 stimulus for 7 days and then were analyzed by immuno-cytofluorescence. Figure 3 top (left) shows that among the 19 PD+ cells present in the selected field, as denoted by their blue fluorescent nuclei, 7 do express green-stained OCT4 (the OCT4a isoform) in the nucleus (light blue in the merge), whereas in the CC+ cells the fraction of nuclear OCT4+ cells is much smaller i.e. 12 out of 90 in this specific field. Cells having cytoplasmic OCT4 (the OCT4b isoform) are not considered to be stem cells [35]. Collagen shown in red (right) is proportionally more abundant in the PD+ than in the CC+ cultures, and is deposited close to or around the OCT4+ green nuclei, suggesting that some collagen was produced by activated stem cells, and not just by the myofibroblasts or SMC previously identified in Figure 2.
Figure 3. The PD and CC cultures contained cells expressing the stem cell genes OCT4 and NANOG, presumably stem cells.
Top: immunofluorescence detection. TGFβ1-stimulated cells were stained as on Figure 2 bottom but FITC green fluorescence was used for detecting OCT4 instead of ACTA2. Collagen is seen adjacent to or within OCT4+ cells. Magnification: 200X. Bottom left: western blots for OCT4 identifying the 45 kDa nuclear isoform. Bottom right: RNA was isolated from triplicate cultures of cells not subjected to TGFβ1 and then subjected to RT/PCR and subsequent agarose gel electrophoresis and ethidium bromide staining. **p<0.01
However, the fibrotic stimulus does not change the number of OCT4a+ stem cells, as shown by western blot for the nuclear Oct-4, specifically the Oct-4a 45 kDa isoform. This expression was not affected by TGFβ1 as indicated by the comparison of PD− with PD+ and CC− with CC+ cultures (Figure 3 bottom). The stem cell content in PD− cultures, as judged by the transcriptional expression of Oct4a and the other key stem cell gene Nanog measured by RT/PCR, is much higher than in the TA− and CC− cell cultures.
In the absence of a profibrotic stimulus the transcriptional signature of the PD− cultures differs from the TA− and the CC− cultures for some genes related to inactive myofibroblasts, fibrosis, inflammation and cell growth
RNA isolated from the three types of cultures, incubated either in the absence or the presence of TGFβ1, was assayed by DNA microarrays to identify genes suggesting: a) that PD myofibroblasts may originate from the stem cells identified above, b) a transition from an inactive to an active myofibroblast, and c) the stimulation of key fibrotic pathways important for the in vivo growth of the PD plaque. The non-stimulated PD− cultures, in comparison to both the non-stimulated TA− and CC− cultures (the PD−/TA− and PD−/CC− expression ratios), over-expressed genes (Table 1; see full names on this table and table 2, and selected citations in the discussion) for myofibroblast generation (early myogenesis), such as ACTG, MYF5 by around 20-30-fold and BMP4, ACTC1 by 4.0- to 4.3-fold. Other overexpressed genes were for proliferation/myogenesis (IGF1) by 2.7-fold and for fibrinolysis inhibition/fibrosis/inflammation (EDNRB, POSTN,TNFα, IL6, PAI-2, SerpinB-10), by 2.7- to 15.5-fold. Remarkably, the expression of the contractile protein ACTA2, a marker of both myofibroblasts and SMC was not altered. The expression of these genes in the PD− cells is thus very different from the TA− and CC− cultures that in general express those genes to about the same levels (TA−/CC− ratio close to 1), except for ACTA2 that is also expressed in SMC, establishing a cell type signature for PD− cells. Interestingly, KRT34, a keratin family member, is considerably down-regulated in the PD− and even more in the CC− cells, in comparison to the TA− cells, whereas another keratin, KRT19, was upregulated in PD− versus TA− or CC− cells. The basal PD− cell type specific signature is consistent with a proliferating but still inactive myofibroblast, i.e., with myogenic features but not yet with a contractile phenotype or a collagen over-production in an in vitro non-fibrotic environment .
Table 1.
In the absence of a fibrotic stimulus the transcriptional signature of the PD- cells differ from the ones for the TA- and CC- cells for some key genes (“Basal PD cell type specific signature”)
| GENES | RATIOS | |||
|---|---|---|---|---|
| PD-/TA- | PD-/CC- | TA-/CC- | ||
| Myogenic factor 5 | MYF5 | 29.8 | 38.5 | 1.3 |
| Smooth muscle actin γ-2 | ACTG2 | 20.0 | 0.6 | 0.1 |
| Endothelin receptor B | EDNRB | 15.5 | 0.7 | 0.1 |
| Periostin | POSTN | 7.3 | 1.7 | 0.2 |
| Keratin 19 | KRT19 | 5.6 | 0.9 | 0.2 |
| Bone morphog. prot 4 | BMP4 | 4.3 | 3.5 | 0.8 |
| TNFα-induced protein 6 | TNFα | 4.0 | 2.8 | 0.7 |
| α-cardiac muscle actin 1 | ACTC1 | 3.9 | 5.8 | 1.5 |
| Plasminogen activ. inhib. 2 | PAI-2 | 3.0 | 2.1 | 0.7 |
| Insulin-like growth factor 1 | IGF1 | 2.7 | 1.9 | 0.7 |
| Serpin peptidase inhib. 10 | SERPINB10 | 2.7 | 2.9 | 1.1 |
| Interleukin 6 | IL6 | 2.7 | 2.2 | 0.8 |
| α-Smooth muscle actin | ACTA2 | 1.3 | 0.3 | 0.2 |
| Keratin 34 | KRT34 | 0.1 | 3.0 | 29.1 |
• Total changes: PD-/TA-: 203; PD-/CC-: 647; TA-/CC-: 615
The expression of several genes in the PD−signature is modified by a fibrotic stimulus, thus defining a PD+ cell phenotype, corresponding to active myofibroblasts
The exposure of the cell cultures to TGFβ1 to stimulate the induction of mesenchymal/proliferative/fibrotic pathways in the PD cells (PD+) increased considerably the expression of many of these genes in comparison to non-TGFβ1 stimulated PD− cultures, as shown by the PD+/PD− ratios (Table 2). This occurred particularly with: a) proliferation-related genes up-regulated by 2.0 to 17.4 fold (IGF-1, EGR2, IGFBP7, IGF1R); b) genes related to myogenesis by 2.0 to 3.8 (ACTC1, ACTA2, but not MYF5 or ACTG2); c) fibrosis by 2.0 to 6.2 (FNDC1, ELN, ACTA2, EDN1, PAI-2, SERPINB10, FGF5, BMP4, TGFβR1); d) oxidative stress by 8.7 (NOX4); e) inflammation by 1.8 to 8.7 (IL11, FGF5, IL6), and f) osteogenesis/fibrosis by 5.1 (POSTN). POSTN was drastically upregulated by TGFβ1 in the cultures of the TA fibroblasts and moderately in the corporal SMC, consistent with the proclivity of the PD and TA cells, as well as the CC SMC, to undergo heterotopic ossification in men [27]. The fibrotic stimulation of the TA− cells led to overexpression of IGF-1 and IL11 in addition to PSTN, and the three genes were up-regulated in the TGFβ1-stimulated CC+ cells, where ACTC1 expression was also increased. In fact, with the exception of a few genes shown in italics all the others were upregulated (Table 2) or down regulated (BMP4, CLDN11) in the three culture types. Therefore, a common fibrotic signature is shared by these three cell cultures, but with some differences.
TABLE 2.
TGFβ1 modulates similarly key genes in the PD, TA and CC cells (“Common fibrotic signature”)
| PD+/PD- | TA+/TA- | CC+/CC- | ||
|---|---|---|---|---|
| Insulin-like growth factor 1 | IGF-1 | 17.4 | 8.1 | 4.6 |
| NADPH oxidase 4 | NOX4 | 8.7 | 5.0 | 8.9 |
| Interleukin 11 | IL11 | 8.7 | 7.0 | 2.3 |
| Early growth response 2 | EGR2 | 7.1 | 6.6 | 3.0 |
| Fibronectin type III domain 1 | FNDCI | 6.8 | 1.5 | 1.5 |
| Periostin | POSTN | 5.1 | 30.9 | 4.6 |
| Elastin | ELN | 4.4 | 2.0 | 2.4 |
| Keratin 34 | KRT34 | 4.3 | 1.1 | 2.7 |
| α-Smooth muscle actin | ACTA2 | 3.8 | 2.2 | 2.3 |
| Endothelin 1 | EDN1 | 3.2 | 2.3 | 4.0 |
| Plasminogen activ. inhib. 2 | SERPINE2 | 3.0 | 1.0 | 0.7 |
| Fibroblast growth factor 5 | FGF5 | 2.5 | 0.5 | 0.5 |
| TGFβ receptor 1 | TGFβR1 | 2.3 | 1.3 | 2.4 |
| Serpin peptidase inhib. B10 | SERPINB10 | 2.0 | 0.9 | 1.1 |
| α-cardiac muscle actin 1 | ACTC1 | 2.0 | 1.1 | 5.6 |
| IGF1 binding protein 7 | IGFBP7 | 2.0 | 3.5 | 1.3 |
| IGF1 receptor 1 | IGF1R | 2.0 | 1.2 | 1.5 |
| Plasminogen activ. inhib. 1 | SERPINE1 | 2.0 | 1.4 | 1.1 |
| Interleukin 6 | IL6 | 1.8 | 2.3 | 3.0 |
| Mitogen-activated prot. kinase 8 MAPK8 | 1.8 | 1.7 | 2.2 | |
| Smooth muscle actin γ-2 | ACTG2 | 1.2 | 0.9 | 2.1 |
| Bone morphogenic protein 4 | BMP4 | 0.4 | 0.4 | 0.4 |
| Claudin 11 | CLDN11 | 0.1 | 0.1 | 0.2 |
In italics: change only in PD cells
When the TGFβ1-stimulated PD cultures (PD+), were compared against the TGFβ1 non-stimulated tunical fibroblasts (TA−), i.e., the PD+/TA− expression ratios (Table 3), a remarkable phenotype was identified represented by selected genes among the approximately only 2% of PD+/TA− ratios that were changed by >2.0 or <0.5. This paradigm was characterized by dramatic increases (fold values in parenthesis) of: a) myofibroblast/fibrosis/calcification genes, such as PSTN (36.9), ACTG2 (24), MYF5 (17.8), ACTC1 (7.7), SERPINE2 (9.2), FNDCI (6.2), ACTA2 (ASMA) (4.6), and others; b) proliferation/apoptosis inhibition genes, IGF1 (46.7), EDNRB (13.6), EGR2 (8.4), and others; c) oxidative stress (NOX4 (3.3); and d) some for inflammation, such as IL11 and IL6 by 6- and 5-fold, and others, respectively. KRT34 remained with even lower expression in comparison with TA.
Table 3.
The PD+/TA- DNA transcriptional signature indicates a fibrotic/inflammatory/oxidative proliferative/early myogenic/osteogenic phenotype (“Representative PD signature”)
| For abbreviations see Tables 1 and 2 |
|---|
| • Total from 28,880: >2.0: 347; <0.5: 271 |
| • Fibrosis/calcification/fibrinolysis: POSTN (36.9); SERPINE2 (9.2); FNDC1 (6.2); ELN (4.9); ACTA2 (4.6); TGFBR1 (2.9); BMP6 (2.8); PLAT (0.1) |
| • Collagens/MMPs: COL III (6.1); MMP3 (5.3); COL XII (2.6); COL I (2.1); see Table 4 |
| • Myogenesis: ACTG2 (23.4); MYF5 (17.8); ACTC1 (7.7) |
| • Cell proliferation/apoptosis: IGF1 (46.7); EDNRB (13.6); EGR2 (8.4); SERPINEB10 (5.5); IGFR1 (2.7); IGFBP7 (2.6); IGFBP6 (0.4) |
| • Oxidative stress: NOX4 (3.3) |
| • Inflammation/cytokines: IL 11 (6.1); IL6 (4.9); TNFAIP6 (4.7); FGF10 (4.5) |
| • Unknown: KRT19 (4.4); KRT34 (0.5) |
PD+/TA- ratios in parenthesis
In terms of collagenolysis, the endogenous metalloproteinases (MMPs) (−2 and −3), the tissue inhibitors of MMPs (TIMPs) (−3 and −1), and some collagen types (3, 14, 5, 41, 12, and 6) were up-regulated (Tables 3 and in more detail, Table 4). This PD+/TA− comparison in cell culture is assumed to predict the one that would occur in vivo between the growing PD plaque versus the non-PD (“normal”) TA. It is consistent with a fibrotic/inflammatory/proliferative/early myogenic/oxidative process, produced in part by myofibroblast generation, replication, and activity. In fact the stimulation of ACTA2 expression suggests that the inactive myofibroblasts in PD− cultures are now in the PD+ cultures actively expressing contractile proteins
Table 4.
Transcriptional changes in collagenolysis-related genes in PD+ cells as compared to TA- cells
| GENE | LEVEL | CHANGE |
|---|---|---|
| • MMP-3 | 650 | 5.3 |
| • MMP-2 | 9,283 | 1.7 |
| • MMP-14 | 1,248 | 1.1 |
| • MMP-1 | 475 | 1.0 |
| • TIMP-3 | 11,182 | 1.4 |
| • TIMP-1 | 16,436 | 1.2 |
| • TIMP-2 | 5,552 | 0.9 |
| • COL 3A1 | 2,452 | 6.1 |
| • COL 14A1 | 392 | 3.7 |
| • COL 5A2 | 1,275 | 2.7 |
| • COL 4A1 | 667 | 2.7 |
| • COL 5A1 | 663 | 2.6 |
| • COL 1A1 | 12,047 | 2.1 |
| • COL 12A1 | 6,715 | 2.6 |
| • COL 6A3 | 4,237 | 2.0 |
| • COL 1A2 | 9,080 | 1.9 |
Some of the changes in the global transcriptional profile differences were confirmed by RT/PCR for MYF5, ACTC1 in the PD−, TA− and CC− cells, and for IGF1 in the PD+ cells in comparison to the TA− and PD+ cells (Fig. 4)
Figure 4. RT/PCR confirmed the differences in mRNA expression of some selected genes detected by DNA microarrays in TGFβ1-stimulated PD+ cell cultures versus the TA+ and CC+ cell cultures.
RNA was isolated from triplicate cultures of TGFβ1-stimulated cells and then subjected to RT/PCR for myofibroblast-related MYF5, ACTC1, and IGF1 and subsequent agarose gel electrophoresis and ethidium bromide staining. **p<0.01
The human PD cells subjected in vitro to a fibrotic stimulus are able to elicit a PD like plaque in the tunica albuginea of immunosuppressed rats
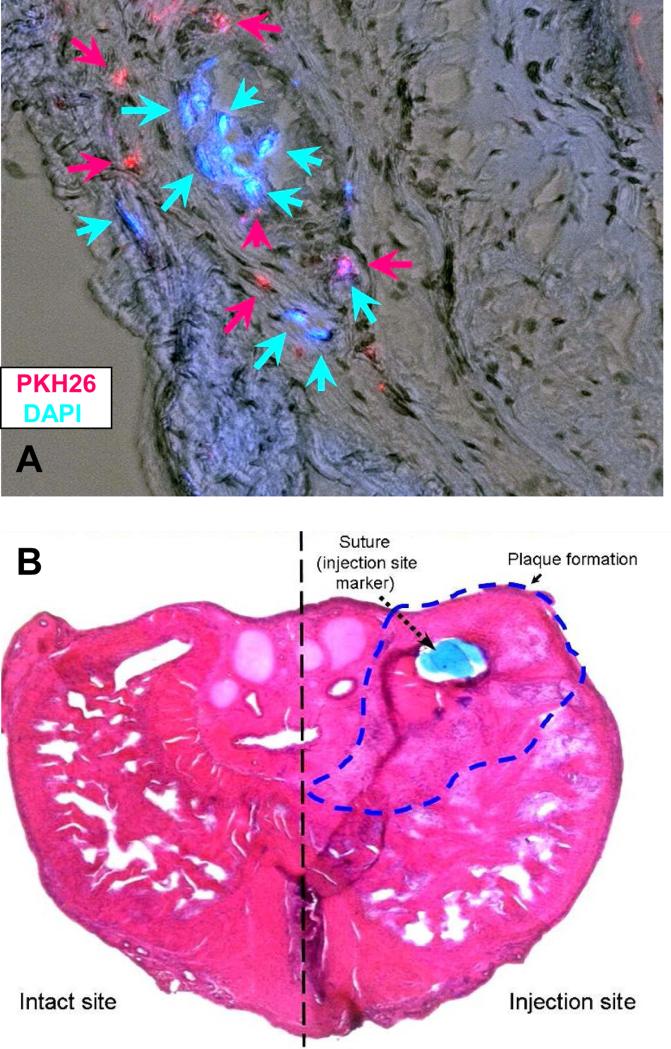
We assumed that the PD cells in addition to stimulate fibrosis have a highly proliferative phenotype, since we had shown previously that stem cells were present in the PD plaque and the PD cell cultures, and that these PD cells, but not the TA cells, had the ability to display anchorage independence [19] and overexpress IGF-1 and other cell growth-related genes. Therefore, we tested whether the PD cultures derived from a non-ossified plaque that were incubated with TGFβ1, i.e., the PD+ cells, can survive when implanted into the tunica albuginea of rats and also induce the formation of a PD like plaque. A mixture of cells labeled either with DAPI (blue fluorescence nuclei) or PKH26 (red fluorescence membrane) were implanted in rats immunosuppressed with tacrolimus. Figure 5 top shows that after 2 weeks there are still cells that can be identified in the area where a PD like plaque was formed in the region marked by a non-absorbable nylon suture tag, and that the plaque has the expected appearance around the site of injection (bottom). Virtually no plaque was induced by the saline injection also identified by the suture (not shown, but see Figure 6).
Figure 5. Human PD cells implanted into the immunosuppressed tunica albuginea of the rat survived and induced a PD like plaque.
Top: TGFβ1-stimulated PD+ cells were labeled with either DAPI (nuclei; blue fluorescence) or PKH26 (membranes; red fluorescence), mixed and injected into the tunica albuginea of tacrolimus-injected rats. Animals were sacrificed at 2 weeks and cryosections around the site of injection were stained with hematoxylin as counterstain for all nuclei. A merge of the visible light (hematoxylin) and fluorescence light (DAPI and PKH26) is shown. Magnification: 100X. Bottom: A series of fields from the sections stained with hematoxylin were assembled together to show the whole cross section of the penis. The nylon suture marking the site of injection is shown, and the dashed line delineates the PD like plaque area. Magnification: 40X .
Figure 6. Collagen III was expressed in the PD like plaque generated in the rat tunica albuginea by the human PD cells, but the plaque regressed with time.
Top: Picrosirius Red staining visualized under polarized light showing green/yellow (collagen III) or red/orange (collagen I). Magnification: 40X. Bottom: estimation of total collagen per total area in the PD like plaque region determined by Masson trichrome as an alternative representation of the PD like plaque. *p<0.05
Moreover, collagen III deposition, a landmark of the human and rat PD plaques [16-18], was detected in the 2-weeks induced PD like plaque of Figure 7 by applying Picrosirius Red that stains it greenish with polarized light, whereas collagen I is reddish orange (Figure 6 top). The PD like plaque was at 2 weeks significantly larger and with increased collagen content than the counterpart normal TA injected with saline in Masson-trichrome-stained sections. However, in contrast to the TGFβ1-induced PD like plaque [6,14], the PD plaque induced by PD+ cells- did became spontaneously smaller at 4 weeks post implantation (bottom).
DISCUSSION
To our knowledge this is the first characterization in PD and in penile tissues in general of the global transcriptional profiles (and implicitly the phenotypes) of myofibroblasts versus those of fibroblasts and SMC whose features are partially shared by myofibroblasts. In addition, there is a wider impact because of the human origin of these cell cultures and also because of the potential extrapolation of these results to fibrosis of smooth muscle tissues (mainly in the vasculature). In the arteries and corpora cavernosa both fibroblasts and myofibroblasts may contribute to the fibrotic process.
In terms of the PD cells, we have shown that: a) in the absence of a profibrotic stimulus, they contain proliferative myofibroblasts which have myogenic features but are only in the initial stage of expressing the contractile protein ACTA2 or synthesizing collagen; b) upon pro-fibroticTGFβ1 that mimicks the active PD plaque milieu, myofibroblast differentiation yields active myofibroblasts that acquire the full contractile/synthesizing phenotype by over-expressing ACTA2 and producing high levels of collagen; this is in contrast to the CC cells that are relatively non-reactive to TGFβ1; c) stem cells present in the PD− cell cultures contribute to some basal collagen synthesis but are not responsive to TGFβ1, suggesting that they are not a significant factor for collagen deposition in the PD plaque;
We have also found out that: d) the transcriptional signature of the non-fibrotic PD− cells identifies a cell specific PD− phenotype characterized by an upregulation of mRNAs for proliferation/apoptosis, myogenesis (myofibroblasts), and fibrosis/osteogenesis, particularly ACTG2 [28]; MYF5 [29], POSTN [19,30,31], BMP4 [32], ACTC1 [33], IGF1 [34], EDNRB [35], and inflammation as well as collagen turnover (see below); e) a gene of unknown function, KRT34 [36], is expressed at high level in TA− cells, but not in the PD− or CC− cells; we propose that it should be a marker to differentiate fibroblasts from SMC or PD− cells.
Upon a fibrotic stimulation like in the PD plaque, our results showed that: f) the PD+ cells acquire the PD signature that may also characterize these cells within the plaque itself; g) in the PD+/TA− comparison, the most representative genes for the activated myofibroblasts are again ACTG2, MYF5, ACTC1, and POSTN, and possibly IGF1 and EDNRB; and h) upregulation of POSTN or periostin, a process occurring in the PD+, TA+ and CC+ cells may indicate, in addition to a fibrotic progression phenotype, the proclivity of the PD plaque, but as well as other penile tissues, to undergo calcification and ossification [37]; i) the human PD+ cells can induce a PD like plaque when injected into the tunica albuginea of immunosuppressed rats, thus suggesting that they can proliferate extensively and then affect the normal tunical tissue to produce excessive collagen, thus acting like in a true myofibroblast fibromatosis, such as Dupuytren's disease [38].
In the present study, tissue specimens were obtained from surgery and therefore the derived cell cultures correspond to the stable phase of the disease. It is possible that our findings do not apply to the acute phase, where the procurement of specimens is difficult or impossible. The decision of not using cell cultures at very early passages was dictated by the needs to reach purity and collect sufficient cells for multiple assays and future studies and the fact that we have not observed changes in proliferation rate, morphology, or marker expression when compared to previous studies conducted at earlier passages [16,19,20]. The reported chromosomal instabilities in the PD cells increasing with passage [39] may be of concern in terms of the potential tumorigenicity already reported for these cultures [45], but we did not observe at this stage any overexpression of directly tumor related genes other than epithelial mesenchymal transition (EMT) genes. In addition, chromosome instabilities are not directly related to the myofibroblast, pro-fibrotic and inflammatory genes on which we have focused, but further studies may be performed at earlier passages. Even with these caveats, our results confirm the well-known pro-fibrotic role of the myofibroblasts in the PD plaque, particularly in relation to collagen deposition, but are novel in showing that the cultures obtained from a non-ossified PD plaque have a very distinctive phenotype that can be considered as a true transcriptional signature.
In a previous work we defined some restricted transcriptional signatures for PD plaques and compared them with the ones for the fibrotic nodules of Dupuytren's disease [13]. The DNA microarrays for human probes that were used earlier [11-13] had a much more limited range in terms of the over 30,000 number of RNA sequences that can be currently detected and had shown considerable upregulation in the PD tissue of some genes now found in the PD+ cells such as ACTA2, PSTN, EGRP, and others not overexpressed in these cells, such as MMP2 and MMP9 for collagenolysis; MCP-1 (monocyte chemotactic protein 1) for inflammation; OSF-1 and OSF-2 (osteoblast-specific factors OSF-1 and OSF-2) for osteogenesis. Remarkably, PAI-1 was not substantially upregulated in the PD+ cells in contrast to what occurs with PAI-1 protein in the human PD plaque, thus suggesting that it may originate from other cell types in the plaque. The reason for other discrepancies is unknown but may reflect the confounding effects of different stages of PD progression and the presence of other cells in the tissue in vivo, such as normal fibroblasts. However, we postulate that the upregulation of these key genes in the PD+ cells representing similar families to the ones upregulated in the plaque, is the key factor in the plaque induction and progression and that the most relevant mechanistic, diagnostic, and therapeutic targeting information on PD pathophysiology can be gained form the study of the PD+ cells rather than of the heterogeneous and variable plaque tissues.
These findings need to be expanded by determining their reproducibility in separate PD cultures from patients at various stages of the disease, but so far they are meaningful and consistent with the known pathophysiology of PD, and with other studies using DNA microarrays on myofibroblasts of different localizations. Some showed the TGFβ1-mediated overexpression of the components of the IGF1 axis, among them IGFBP3, and the reactive oxygen species (ROS) producer reduced NOX4 [40] which occurs in the development of benign prostatic hyperplasia and prostate cancer, characterized by extensive stromal remodeling, in particular fibroblast-to-myofibroblast differentiation [41]. These changes are also found in the upregulation of the p38 MAPK pathway in Dupuytren's disease [42] and of MMP-1 (collagenase-1), MMP-3 (stromelysin-1), MMP-9 (gelatinase-B) and MMP-10 (stromelysin-2) in cardiac myofibroblasts after infarction [43].
The stem cells in the human PD cultures, previously reported by our group [19] and confirmed in the current results, may be responsible, at least in part, for the generation of myofibroblasts and for the in vivo induction of the PD like plaque that we have observed after their implantation in the rat tunica albuginea. The role of the stem cell in the PD cultures as supportive of plaque growth can also be speculated from our previously reported anchorage-independence of PD− cells in soft agar [19], an indicator of indefinite replication shared with tumor cells. Myofibroblast generation in the PD plaque may be inhibited by targeting stem cells in the tunica albuginea or by interfering with early myogenic differentiation of fibroblasts by blocking ACTG2, MYF5 or ACTC1. Therapeutically, specific shRNAs [26], endogenous microRNAs that are inhibitors of these genes [24], or pharmacological blockers if available, may be a good strategy because of the easy local administration into the PD plaque.
The recent report that stem cells implanted into the tunica albuginea of the TGFβ1 rat model of PD increased MMP and reduced TIMP expression in this tissue [44] does not contradict our hypothesis that unknown factors within the milieu of the PD plaque stimulate endogenous stem cells to undergo myofibroblast and/or osteoblast differentiation, thus contributing to fibrosis and ossification, respectively, within the tunica albuginea. Although this increase in the MMP/TIMP ratio is beneficial, no quantitative assessment of the PD−like plaque size or collagen content (despite some qualitative suggestion) was reported that would conclusively indicate that fibrosis was reduced. Even if plaque reduction would have been proven, a massive implantation of adipose tissue-derived stem cells may modify the milieu avoiding its effects on the endogenous tunical stem cells.
Our results are in agreement with studies showing that these PD cells display increased cell growth and S-phase on flow cytometry, stabilization and inactivation of p53, consistent morphologic transformation, and induction of subcutaneous nodules at 2-3 months following injection into immunodeficient mice [45], suggesting that these cells are biologically transformed. The most relevant of our findings illustrating the ability of PD cells to proliferate, and specifically their stem cells, to drive plaque growth, and implicitly to undergo uncontrolled replication outside the tunica albuginea, is the upregulation of EDNRB, EGR2, and SerpinB10, key genes for proliferation and apoptosis not just in normal but also malignant growth, as well as in stem cells [35]. EGR2 is also as an important profibrotic effector [46]. This poses the question of whether PD is just a fibrotic reaction in a wound that does not heal, or the PD cells have also the potential to trigger a fibromatosis, as postulated previously by Mulhall et al and our group [19,45]. This process consists on the formation of benign but infiltrative soft tissue tumors triggered by the proliferation of well-differentiated fibroblasts, that often show aggressive clinical behavior and frequent local recurrence [38,47]. The ability of PD+ cells to induce a PD like plaque supports this speculation, although higher cell loads may be needed to show a more prolonged growth and collagen deposition. This is a topic that requires further investigation, particularly because of the relationship of ATCG2 mutations with ossification and certain cancers [47] and may again suggest a therapeutic target to induce myofibroblast apoptosis by regulating these PD overexpressed genes.
Another important derivation of our findings is the confirmation at the mRNA level of the collagen III/I protein increase in the PD plaque [22], which in our current results goes from 0.20 in the TA− cells to 0.60 in the PD+ cells, and of the considerable over-expression of MMP-2. The latter has a Km of 8.5 μM for type I collagen, whereas collagen III is the preferential target of MMP-1 (Km: 1.4 μM) (the lower the Km, the more active), that is relatively unaffected. The Km for MMP-2 and −3 for the other collagens are too high, and therefore much less efficacious. This endogenous over expression of MMP-3 and MMP-2 and the potential lack of transcriptional stimulation of MMPs that would target the collagens that are overexpressed (III, XIV, IV, V, VI, XII) in the PD+ cells, suggests that the role of MMPs in the endogenous defense mechanism against fibrosis in the PD plaque is relatively inefficient. How to activate endogenous MMPs that would target the overexpressed collagen types in the PD+/TA− comparison deserves intensive research, particularly in the context of the fibrotic induction of TIMP3.
The previously postulated role of genes involved in osteogenesis, such as OSF-1 and OSF-2 in the PD plaque [11-13], and PSTN in the PD+ cells [27], has now been confirmed. This agrees with the differentiation into myofibroblasts of stem cells in the PD cultures and their conversion into osteoblasts in either normal or osteogenic medium, as previously shown. PSTN [30,31,37,38] is perhaps the most potentially interesting target for therapy, as a member of the matri-cellular protein family that regulates cell functions and cell–matrix interactions and is involved in the structure and organization of the extracellular matrix. PSTN, like OPN and related genes, plays a critical role in collagen assembly, particularly during wound healing. It binds to type I collagen and fibronectin and is a key regulator of collagen cross-linking by interacting with BMP-1 to increase its deposition in the fibronectin matrix. PSTN mRNA is among the most strongly up-regulated transcripts after tissue injury in bone, heart and muscle, suggesting that periostin does not reflect bone remodeling but rather ossification processes. PSTN, perhaps together with ACTG2, may be therefore a potential target in an antifibrotic therapy and to prevent ectopic fibro-osseous tissue responsible for heterotopic ossification, perhaps together with ACTG2 [28].
In summary, our results suggest that a successful medical therapy of PD has to induce an efficient and focused collagen breakdown in the plaque, but using: 1) an approach based on tailoring the selection of the pharmacological MMPs/collagenases from their respective Kms on at least some of the key the collagens overexpressed by the PD cells; 2) a combination therapy based on stimulating selectively the desired MMPs endogenous expression in the PD plaque; and 3) targeting the myofibroblast fibromatosis with agents inhibiting the stem cells and/or myofibroblasts uncontrolled replication, and/or the stem cell differentiation into myofibroblasts, and//or the induction of their programmed cell death. Only by the combined targeting of collagenolysis and PD cell elimination, it may be possible to counteract the strong positive turnover of the PD plaque [12,13], reduce or eliminate it, inhibit its progression to hardening and ectopic ossification, and finally to avoid the PD tissue fibromatosis manifesting itself in a more aggressive way that leaves surgery as the only available option. Translationally, the novel strategies simply need to complement the ongoing basic collagenolytic procedure. For instance, approach #2 above could potentially move rapidly to the clinic based on current systemic therapies, such as exploiting the antifibrotic action of PDE5 inhibitors or other clinically available agents. Approaches #1 and #3, by involving a local application may be more innovative, even if this would require preliminary preclinical testing e.g. targeting either the type of collagen with investigative new MMPs and/or the selective gene with available protein and transcriptional inhibitors.
Acknowledgments
This work was supported by grant NIH R21DK070003-01A1 to NGC.
Abbreviations
- CC
corpora cavernosa cell cultures
- CC−
CC cell cultures not subjected to fibrotic stimulus
- CC+
CC cell cultures subjected to fibrotic stimulus
- PD
Peyronie's disease; also, PD cell cultures
- PD−
PD cell cultures not subjected to fibrotic stimulus
- PD+
PD cell cultures subjected to fibrotic stimulus
- TA
tunica albuginea, also TA cell cultures
- TA−
TA cell cultures not subjected to fibrotic stimulus
- TA+
TA cell cultures subjected to fibrotic stimulus
- SMC
smooth muscle cells
REFERENCES
- 1.Gur S, Limin M, Hellstrom WJ. Current status and new developments in Peyronie's disease: medical, minimally invasive and surgical treatment options. Expert Opin Pharmacother. 2011 Apr;12(6):931–44. doi: 10.1517/14656566.2011.544252. Ibid. [DOI] [PubMed] [Google Scholar]
- 2.Ralph D, Gonzalez-Cadavid N, Mirone V, Perovic S, Sohn M, Usta M, Levine L. The management of Peyronie's disease: evidence-based 2010 guidelines. J Sex Med. 2010 Jul;7(7):2359–74. doi: 10.1111/j.1743-6109.2010.01850.x. [DOI] [PubMed] [Google Scholar]
- 3.Gelbard M, Lipshultz LI, Tursi J, Smith T, Kaufman G, Levine LA. Phase 2b study of the clinical efficacy and safety of collagenase Clostridium histolyticum in patients with Peyronie disease. J Urol. 2012 Jun;187(6):2268–74. doi: 10.1016/j.juro.2012.01.032. Erratum in: J Urol. 2012 Aug;188(2):678.
- 4.Gelbard M, Goldstein I, Hellstrom WJ, McMahon CG, Smith T, Tursi J, Jones N, Kaufman GJ, Carson CC., 3rd Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013 Jul;190(1):199–207. doi: 10.1016/j.juro.2013.01.087. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Cadavid NF, Rajfer J. Treatment of Peyronie's disease with PDE5 inhibitors: an antifibrotic strategy. Nat Rev Urol. 2010 Apr;7(4):215–21. doi: 10.1038/nrurol.2010.24. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Cadavid NF, Rajfer J. Experimental models of Peyronie's disease. Implications for new therapies. J Sex Med. 2009 Feb;6(2):303–13. doi: 10.1111/j.1743-6109.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 7.Devine CJ, Jr, Somers KD, Jordan SG, Schlossberg SM. Proposal: trauma as the cause of the Peyronie's lesion. J Urol. 1997 Jan;157(1):285–90. doi: 10.1016/s0022-5347(01)65361-8. [DOI] [PubMed] [Google Scholar]
- 8.Davila HH, Ferrini MG, Rajfer J, Gonzalez-Cadavid NF. Fibrin as an inducer of fibrosis in the tunica albuginea of the rat: a new animal model of Peyronie's disease. BJU Int. 2003 Jun;91(9):830–8. doi: 10.1046/j.1464-410x.2003.04224.x. [DOI] [PubMed] [Google Scholar]
- 9.Ehrlich HP. Scar contracture: cellular and connective tissue aspects in Peyronie's disease. J Urol. 1997 Jan;157(1):316–9. doi: 10.1016/s0022-5347(01)65368-0. [DOI] [PubMed] [Google Scholar]
- 10.Levine L, Rybak J, Corder C, Farrel MR. Peyronie's Disease Plaque Calcification— Prevalence, Time to Identification, and Development of a New Grading Classification. J Sex Med. 2013;10:3121–3128. doi: 10.1111/jsm.12334. [DOI] [PubMed] [Google Scholar]
- 11.Magee TR, Qian A, Rajfer J, Sander FC, Levine LA, Gonzalez-Cadavid NF. Gene expression profiles in the Peyronie's disease plaque. Urology. 2002 Mar;59(3):451–7. doi: 10.1016/s0090-4295(01)01578-3. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Cadavid NF, Magee TR, Ferrini M, Qian A, Vernet D, Rajfer J. Gene expression in Peyronie's disease. Int J Impot Res. 2002 Oct;14(5):361–74. doi: 10.1038/sj.ijir.3900873. [DOI] [PubMed] [Google Scholar]
- 13.Qian A, Meals RA, Rajfer J, Gonzalez-Cadavid NF. Comparison of gene expression profiles between Peyronie's disease and Dupuytren's contracture. Urology. 2004 Aug;64(2):399–404. doi: 10.1016/j.urology.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Vernet D, Ferrini MG, Valente EG, Magee TR, Bou-Gharios G, Rajfer J, Gonzalez-Cadavid NF. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie's fibrotic plaque and in its rat model. Nitric Oxide. 2002 Dec;7(4):262–76. doi: 10.1016/s1089-8603(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 15.Fields GB. Interstitial collagen catabolism. J Biol Chem. 2013 Mar 29;288(13):8785–93. doi: 10.1074/jbc.R113.451211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013 Jun 1;304(11):L709–21. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS. 2012;103:209–79. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuno Y, Iyoda M, Shibata T, Hirai Y, Akizawa T. Sildenafil, a phosphodiesterase type 5 inhibitor, attenuates diabetic nephropathy in non-insulin-dependent Otsuka Long-Evans Tokushima Fatty rats. Br J Pharmacol. 2011 Mar;162(6):1389–400. doi: 10.1111/j.1476-5381.2010.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vernet D, Nolazco G, Cantini L, Magee TR, Qian A, Rajfer J, Gonzalez-Cadavid NF. Evidence that osteogenic progenitor cells in the human tunica albuginea may originate from stem cells: implications for peyronie disease. Biol Reprod. 2005 Dec;73(6):1199–210. doi: 10.1095/biolreprod.105.041038. [DOI] [PubMed] [Google Scholar]
- 20.Vernet D, Magee T, Qian A, Nolazco G, Rajfer J, Gonzalez-Cadavid N. Phosphodiesterase type 5 is not upregulated by tadalafil in cultures of human penile cells. J Sex Med. 2006 Jan;3(1):84–94. doi: 10.1111/j.1743-6109.2005.00197.x. discussion 94-5. [DOI] [PubMed] [Google Scholar]
- 21.Syed F, Thomas AN, Singh S, Kolluru V, Emeigh Hart SG, Bayat A. In vitro study of novel collagenase (XIAFLEX®) on Dupuytren's disease fibroblasts displays unique drug related properties. PLoS One. 2012;7(2):e31430. doi: 10.1371/journal.pone.0031430. doi: 10.1371/journal.pone.0031430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrini MG, Kovanecz I, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Effects of long-term vardenafil treatment on the development of fibrotic plaques in a rat model of Peyronie's disease. BJU Int. 2006 Mar;97(3):625–33. doi: 10.1111/j.1464-410X.2006.05955.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang JS, Kovanecz I, Vernet D, Nolazco G, Kopchok GE, Chow SL, White RA, Gonzalez-Cadavid NF. Effects of sildenafil and/or muscle derived stem cells on myocardial infarction. J Transl Med. 2012 Aug 7;10:159. doi: 10.1186/1479-5876-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovanecz I, Gelfand R, Masouminia M, Gharib S, Segura D, Vernet D, Rajfer J, Li DK, Liao CY, Kannan K, Gonzalez-Cadavid NF. Chronic High Dose Intraperitoneal Bisphenol A (BPA) Induces Substantial Histological and Gene Expression Alterations in Rat Penile Tissue Without Impairing Erectile Function. J Sex Med. 2013 Dec;10(12):2952–66. doi: 10.1111/jsm.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davila HH, Magee TR, Vernet D, Rajfer J, Gonzalez-Cadavid NF. Gene transfer of inducible nitric oxide synthase complementary DNA regresses the fibrotic plaque in an animal model of Peyronie's disease. Biol Reprod. 2004 Nov;71(5):1568–77. doi: 10.1095/biolreprod.104.030833. [DOI] [PubMed] [Google Scholar]
- 26.Magee TR, Kovanecz I, Davila HH, Ferrini MG, Cantini L, Vernet D, Zuniga FI, Rajfer J, Gonzalez-Cadavid NF. Antisense and short hairpin RNA (shRNA) constructs targeting PIN (Protein Inhibitor of NOS) ameliorate aging-related erectile dysfunction in the rat. J Sex Med. 2007 May;4(3):633–43. doi: 10.1111/j.1743-6109.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RC, Jane A, Leopold JA, Loscalzo J. Vascular Calcification: Pathobiological Mechanisms and Clinical Implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 28.Untergasser G, Gander R, Lilg C, Lepperdinger G, Plas E, Berger P. Profiling molecular targets of TGF-beta1 in prostate fibroblast-to myofibroblast transdifferentiation. Mech Ageing Dev. 2005 Jan;126(1):59–69. doi: 10.1016/j.mad.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci. 2013 Nov;70(21):4117–30. doi: 10.1007/s00018-013-1330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott CG, Wang J, Guo X, Xu SW, Eastwood M, Guan J, Leask A, Conway SJ, Hamilton DW. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci. 2012 Jan 1;125(Pt 1):121–32. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vi L, Feng L, Zhu RD, Wu Y, Satish L, Gan BS, O'Gorman DB. Periostin differentially induces proliferation, contraction and apoptosis of primary Dupuytren's disease and adjacent palmar fascia cells. Exp Cell Res. 2009 Dec 10;315(20):3574–86. doi: 10.1016/j.yexcr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pegorier S, Campbell GA, Kay AB, Lloyd CM. Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-beta1 in normal human lung fibroblasts (NHLF). Respir Res. 2010 Jun 23;11:85. doi: 10.1186/1465-9921-11-85. doi: 10.1186/1465-9921-11-85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frade AF, Teixeira PC, Ianni BM, et al. Polymorphism in the alpha cardiac muscle actin 1 gene is associated to susceptibility to chronic inflammatory cardiomyopathy. PLoS One. 2013 Dec 19;8(12):e83446. doi: 10.1371/journal.pone.0083446. doi: 10.1371/journal.pone.0083446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013 Sep;280(17):4294–314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 35.Kohan DE. Endothelin, hypertension and chronic kidney disease: new insights. Curr Opin Nephrol Hypertens. 2010 Mar;19(2):134–9. doi: 10.1097/MNH.0b013e328335f91f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang TH, Huang HD, Ong WK, Fu YJ, Lee OK, Chien S, Ho JH. The effects of actin cytoskeleton perturbation on keratin intermediate filament formation in mesenchymal stem/stromal cells. Biomaterials. 2014 Apr;35(13):3934–44. doi: 10.1016/j.biomaterials.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Merle P. Garnero The multiple facets of periostin in bone metabolism. Osteoporos Int. 2012;23:1199–1212. doi: 10.1007/s00198-011-1892-7. [DOI] [PubMed] [Google Scholar]
- 38.Vi L, Feng L, Zhu RD, Wu Y, Satish L, Gan BS, O'Gorman DB. Periostin differentially induces proliferation, contraction and apoptosis of primary Dupuytren's disease and adjacent palmar fascia cells. Exp Cell Res. 2009 Dec 10;315(20):3574–86. doi: 10.1016/j.yexcr.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulhall JP, Nicholson B, Pierpaoli S, Lubrano T, Shankey TV. Chromosomal instability is demonstrated by fibroblasts derived from the tunica of men with Peyronie's disease. Int J Impot Res. 2004 Jun;16(3):288–93. doi: 10.1038/sj.ijir.3901170. [DOI] [PubMed] [Google Scholar]
- 40.Sampson N, Koziel R, Zenzmaier C, Bubendorf L, Plas E, Jansen-Dürr P, Berger P. ROS signaling by NOX4 drives fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma. Mol Endocrinol. 2011 Mar;25(3):503–15. doi: 10.1210/me.2010-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampson N, Zenzmaier C, Heitz M, Hermann M, Plas E, Schäfer G, Klocker H, Berger P. Stromal insulin-like growth factor binding protein 3 (IGFBP3) is elevated in the diseased human prostate and promotes ex vivo fibroblast-to-myofibroblast differentiation. Endocrinology. 2013 Aug;154(8):2586–99. doi: 10.1210/en.2012-2259. [DOI] [PubMed] [Google Scholar]
- 42.Ratkaj I, Bujak M, Jurišić D, Baus Lončar M, Bendelja K, Pavelić K, Kraljević Pavelić S. Microarray analysis of Dupuytren's disease cells: the profibrogenic role of the TGF-β inducible p38 MAPK pathway. Cell Physiol Biochem. 2012;30(4):927–42. doi: 10.1159/000341470. [DOI] [PubMed] [Google Scholar]
- 43.Turner NA, Warburton P, O'Regan DJ, Ball SG, Porter KE. Modulatory effect of interleukin-1α on expression of structural matrix proteins, MMPs and TIMPs in human cardiac myofibroblasts: role of p38 MAP kinase. Matrix Biol. 2010 Sep;29(7):613–20. doi: 10.1016/j.matbio.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gokce A, Abd Elmageed ZY, Lasker GF, Bouljihad M, Kim H, Trost LW, Kadowitz PJ, Abdel-Mageed AB, Sikka SC, Hellstrom WJ. Adipose tissue-derived stem cell therapy for prevention and treatment of erectile dysfunction in a rat model of Peyronie's disease. Andrology. 2014 Mar;2(2):244–51. doi: 10.1111/j.2047-2927.2013.00181.x. [DOI] [PubMed] [Google Scholar]
- 45.Mulhall JP(1), Martin DJ, Lubrano T, Moser M, Kwon E, Wojcik E, Shankey TV. Peyronie's disease fibroblasts demonstrate tumorigenicity in the severe combined immunodeficient (SCID) mouse model. Int J Impot Res. 2004 Apr;16(2):99–104. doi: 10.1038/sj.ijir.3901183. [DOI] [PubMed] [Google Scholar]
- 46.Fang F. The early growth response gene Egr2 (Alias Krox20) is a novel transcriptional target of transforming growth factor-β that is up-regulated in systemic sclerosis and mediates profibrotic responses. Am J Pathol. 2011 May;178(5):2077–90. doi: 10.1016/j.ajpath.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fisher C. Myofibroblastic malignancies. Adv Anat Pathol. 2004 Jul;11(4):190–201. doi: 10.1097/01.pap.0000131773.16130.aa. [DOI] [PubMed] [Google Scholar]