Abstract
The phenotype of the social group is related to phenotypes of individuals that form that society. We examined how honey bee colony aggressiveness relates to individual response of male drones and foraging workers. Although the natural focus in colony aggression has been on the worker caste, the sterile females engaged in colony maintenance and defense, males carry the same genes. We measured aggressiveness scores of colonies and examined components of individual aggressive behavior in workers and haploid sons of workers from the same colony. We describe for the first time, that males, although they have no stinger, do bend their abdomen (abdominal flexion) in a posture similar to stinging behavior of workers in response to electric shock. Individual worker sting response and movement rates in response to shock were significantly correlated with colony scores. In the case of drones, sons of workers from the same colonies, abdominal flexion significantly correlated but their movement rates did not correlate with colony aggressiveness. Furthermore, the number of workers responding at increasing levels of voltage exhibits a threshold-like response, whereas the drones respond in increasing proportion to shock. We conclude that there are common and caste-specific components to aggressive behavior in honey bees. We discuss implications of these results on social and behavioral regulation and genetics of aggressive response.
Keywords: Shock, Aggression, Honey bee, Sting, Abdomen Flexion, Drone
Introduction
Integration of aggressive response of individuals to a social defense is one important feature of social organisms. This defense strategy has resulted in evolution of behavioral or morphological subcastes (e.g., soldiers, guards) independently in multiple, separate social lineages. Morphologically distinct soldier castes occur in eusocial systems such as ants (Wheeler 1991), bees (Grüter et al. 2011), termites (Thorne et al. 2003; Roux and Korb 2004), aphids (Stern and Foster 1996), thrips (Chapman et al. 1999), and shrimps (Tóth and Duffy 2008). In honey bee (Apis mellifera sp.) colonies, soldier and guard tasks have age correlates and are performed by genetically distinct groups of workers (Robinson and Page 1988; Breed et al. 1990; cf see “guards” Hunt 2007).
The honey bee aggressive response at colony and individual levels relate to allocation of workers to soldier vs. forager tasks, to colony age structure (Arechavaleta-Velasco and Hunt 2004; Giray et al. 2000), and to environmental conditions influencing foraging vs. brood raising (Schneider et al. 2004; Rivera-Marchand et al. 2008). Changes in individual aggressiveness depend on individual behavioral response thresholds (Robinson 1997; Shorter and Rueppell 2012). Studies examining the link between colony and individual level response have focused on the female workers, the principal actors (Collins et al. 1984; Kolmes and Fergusson-Kolmes 1989; Giray et al. 2000; Breed et al. 2004; Hunt 2007; Shorter and Rueppell 2012). Although male honey bees (drones) do not directly participate in this response, past studies have established paternal inheritance to be highly correlated with the aggressive response phenotype of daughter colonies (Guzman-Novoa et al. 2005). The present work examines responses of workers and drones that correspond to components of aggressive behavior and how these correlate with parental colony phenotype.
The significance of studying aggressive response has prompted the development of various methods for the assaying and quantification of component behaviors (e.g., flight and chase of potential attacker, locomotor activity, stinging, etc.; Collins et al. 1984; Spangler and Sprenkle 1997; Guzman-Novoa et al. 1999; Giray et al. 2000; Guzman-Novoa et al. 2003; Breed et al 2004; Hunt 2007). At the colony level, aggression has been measured using a variety of techniques which include simulated attacks, behavior ratings, and sting counts, among others (Guzman-Novoa et al. 1999; Guzman-Novoa et al. 2003, reviewed in Rivera-Marchand et al. 2012). Findings from these methods showed that colony aggression is a highly heritable trait in honey bee populations with paternal inheritance (drone contribution) having a large effect (Collins et al. 1984; Hunt et al. 1998; Guzman-Novoa et al. 2002, 2005; Cingolani et al. 2013). This has been further corroborated in long-term selection studies. In at least one study, the aggressive response of Africanized honey bees was reduced by as much as 50 % via artificial selection (Guzman-Novoa and Page 1999). More recently, a colony-level study by Rivera-Marchand et al. (2012) showed that aggressive response was reduced in colonies of Africanized honey bees in Puerto Rico (coined gAHB, gentle Africanized honey bee, Galindo-Cardona et al. 2013).
Studies examining individual worker response showed that several variables determine the probability, degree, and timing of aggressive response. Support is strongest for genetic determinants (Kolmes and Fergusson-Kolmes 1989). Kolmes and Fergusson-Kolmes (1989) found differences in the readiness to sting in individual workers from different honey bee subspecies (see also Baldemarra et al. 1987). Age and season were also contributing factors, affecting stimulus thresholds (Baldemarra et al. 1987; Kolmes and Fergusson-Kolmes 1989). The behavioral observations from these studies were further supported by neurophysiological measures of the sting response in isolated abdominal circuits (Burrell and Smith 1994; Ogawa et al. 1995, 2011).
Results from the quantification of the individual response measures (mainly degree and frequency of stinging) used in these past studies have been highly variable. Therefore, conclusions have been constrained to differences between broad categories (e.g., subspecies, age, season) (Baldemarra et al. 1987; Kolmes and Fergusson-Kolmes 1989; Kolmes and Njehu 1990; Tel-Zur and Lensky 1995; Lenoir et al. 2006). Regardless, multiple variations of these individual-level assays have provided significant contributions. Use of one component behavior in aggressive response, sting deposited or not on a flag assay, allowed for the genomic mapping of sting associated loci (Hunt et al. 1998). Further examination of these genomic regions showed their involvement in other defensive behaviors (i.e., guarding) (Arechavaleta-Velasco and Hunt 2004). Likewise, categorization and measures of sting extension have helped in describing how this behavior is a motor pattern with both a reflex and modulatory component (Burrell and Smith 1995; Ogawa et al. 1995, 2011).
Due to the strong paternal inheritance effect on colony-level aggression, it would be significant to examine potential correlates between individual drone and worker behavior and colony phenotype (Guzman-Novoa et al. 2005). The haploid genome of males (Beye et al. 2003) in honey bees lends itself to further genomics and genetics research if the traits of interest are identifiable in drones. In addition, a comparative study of components of aggressive behavior in males and females can lead to novel insights such as on epigenetic effects on aggressive behavior (Cingolani et al. 2013). In fact, correlates, at first not obvious, across the solitary-like male honey bee and the social almost sterile worker for behavior and development provided important insights into evolution and mechanisms of learning and memory (Bhagavan et al. 1994; Ferguson et al. 2001), age-related behavioral development (Giray and Robinson 1996), and neuroendocrine regulation of behavior (Fahrbach et al. 1997). A common behavior in workers and drones in response to painful stimulus is escape (see Dinges et al. 2013), and we first targeted measurements of movement in our electric shock assay (ESA). We later made the novel observation that drones also bend their abdomen in response to painful stimulus, in a fashion similar to the sting response of the workers (Online Resources 1—photo illustration; 2—video of male abdomen flexion in ESA).
Here, we analyze the association between colony and individual by examining behavioral response of workers, worker-produced male offspring, and the parental colonies. We ask (1) whether individual response to electric shock in both workers and drones correlates with colony aggressive phenotype, and (2) whether male and worker responses to electric shock are similar. Furthermore, we introduce a behavioral assay which can quantify individual-level response using two measures: activity level across time in workers and drones and stinging behavior in workers and a similar posture, abdomen flexion, in males in response to shock.
Materials and methods
Collections
Thirteen focal source colonies from our apiary at the Gurabo Agricultural Research Station of the University of Puerto Rico in Gurabo, Puerto Rico were phenotyped for aggression via a ratings test. Ratings tests have been shown to be reliable and reproducible (Guzman-Novoa et al. 2003) assays of colony aggressive response even when comparing subspecies and hybridized races (Guzman-Novoa et al. 1999). Our specific test is a standardized version of the one presented by Giray et al. (2000) which was previously used in our specific hybrid (Rivera-Marchand et al. 2012; see Table 1). The behavioral phenotypes were scored twice with a 3-day interval between each bout. This interval assured any behavioral or transcription-level effects induced by alarm pheromone presentation on the first bout would not alter results obtained in the second bout (see Alaux and Robinson 2007; Urlacher et al. 2010).
Table 1.
Description of ratings used for phenotyping colony aggression
| Behavior | Intensity |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Sting | No stings | Attempt to sting (hit) | 1 sting | Multiple stings |
| Hang | No bees at bottom offrame | A layer of bees | Clump of bees | Clumps falling from the frame |
| Run | Very little movement | Some walking on the frame | Running on the frame | Running, dispersion from the frame |
| Fly | No flight | Few fly, undirected | Some fly to observer | Continued flight and pursuit |
Behavioral categories are in the first column, while ranks of intensity are in the first row
In each scoring bout, we used the minimum amount of smoke while opening the colony (three puffs) so as to standardize its calming effect. Once the colony was opened, we extracted one frame from which the aggressive response of the colony was rated (see Table 1). The selected categories were: run, sting, hang, and fly. As outlined in Table 1, each of the behaviors was scored on a four-point intensity scale with a score of one denoting the lowest response possible and four the highest. The sting and fly categories are ready measures of aggression as they are the principal categories involved in an aggressive response. We did not keep track of specific number of stings after the first one. This is because one sting could elicit further responses due to alarm pheromone release. Both the run and hang categories have been established by previous studies to be indicative behaviors of degree of hybridization in Africanized honey bees that correlate with colony aggression (Guzman-Novoa et al. 1999, 2003; Schneider et al. 2004).
From each of the focal source colonies phenotyped as described, 20 foragers were collected and tested via individual response assay described below (total n = 260). Following this, a portion of the each colony (~5,000 female workers, two to four frames) were isolated in small experimental units (nucleus supers) in order to induce male production by workers. Over the course of 2 months, these colonies were checked weekly, and any queen cup or cell was removed or destroyed to prevent queen rearing. During checks, we also were careful to determine if any “false queen” events were observed. This occurs when an individual worker’s ovaries develop to such an extent that they produce enough levels of queen mandibular pheromone (QMP) to be treated as “queens” by their sisters (see Cargel and Rinderer 2006). These events were undesirable since our goal was to sample as worker genotype across each colony, and a “false queen” sequesters the majority of reproductive output to its specific genotype. These queenless nucleus colonies were established merely to obtain sons of workers to contrast to the source colony that had been phenotyped for aggressiveness. The phenotype of these queenless nucleus colonies was not relevant to the study; in addition, due to extensive manipulation required to induce worker drone production, the worker phenotypes would not reflect the source colonies.
At the end of the 2-month period, resulting males were collected for individual testing. Our target drone per colony test size was 20 (similar to worker). For logistic reasons, the per colony average sample size was 14; individuals were eliminated if they did not interact with the assay (see below) or were assessed not to be reproductively mature (Dinges et al. 2013). This is a considerable portion of the drones of certain developmental stage (>10 day old flying drones) produced by drone-layer colonies (see Giray and Robinson 1996).
Assay
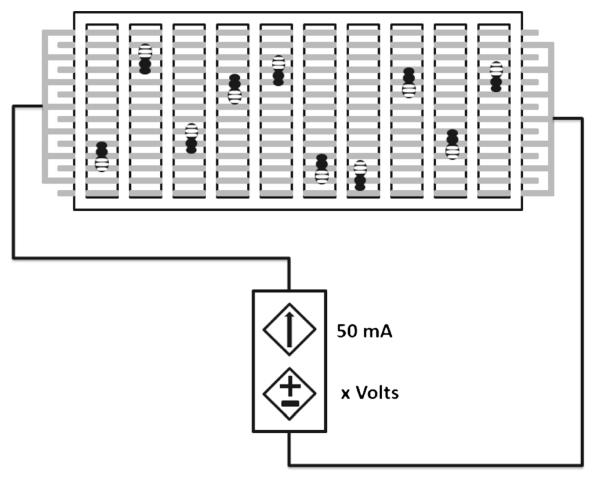
Our apparatus is similar to that described in Agarwal et al. (2011). The specific components were a steel grid, intercalated so as to create an open circuit, a poster board cut so as to provide individual lanes, and transparent Plexiglas™ sheets to enclose the space while allowing observation (see Fig. 1 and Agarwal et al. 2011 for description). Bees tested were in individualized lanes separated by 0.5-cm-thick divisions which prevented them from seeing each other. There were ten lanes allowing for ten bees to be tested at a time. Together, the components created a cassette with a layer of Plexiglas™ upon which the steel grid sat with poster board lanes on top and enclosed by another Plexiglas™ sheet as the transparent ceiling. This last sheet was always coated with petroleum jelly to prevent bees from escaping shock by walking on the ceiling. The resulting cassette was placed on a LCD monitor (DELL, Model #: E156FPc). The monitor provided a yellow background for contrast during video recordings.
Fig. 1.
Diagrammatic representation of the apparatus used in the experiment. Gray wires intercalate to create an open circuit grid, which bees close as they walk. Current was set throughout the experiment at 50 mA, while voltage was presented in the manner reported
The negative stimulus used for our presentations was electric shock. Electric shock as a negative stimulus has been widely used in both aggression and learning studies (Núñez et al. 1983; Abramson 1989; Lenoir et al. 2006; Agarwal et al. 2011). This stimulus is ideal for assaying response, since it is unlikely that honey bees encounter it in nature, making it unbiased. Having an unbiased, novel negative stimulus is of particular importance in tests of aggressive behavior as they control for possible confounding factors, such as olfactory, tactile, or visual cues that might precondition response.
For each test, the whole steel grid was electrified to create an open circuit. In this manner, honey bees received the stimulus when they closed this circuit as they moved and touched the grid electrodes. Shock delivery was controlled by a button mechanism. This allowed the experimental setting to remain constant with the only changing variable being the shock during presentations.
Experimental design
Similar to Kolmes and Fergusson-Kolmes (1989), we did individual presentations of an increasing voltage scale. Unlike that previous study, we did our presentations in a stepwise, rather than continuous, fashion. In this manner, each female worker experienced 10-s presentations of shock followed by 20-s rest intervals. Shock was presented to females in 2-V increments on a scale from 0 to 26 V. Two groups of males were tested; in one, we used 20 s voltage presentations followed by 40-s rest intervals. In this first study on drones, shock for the males was presented in 4-V increments on a scale from 0 to 28 V. This was to account for the generally slower response from the drones.
In the second study on drones, we used the same presentation pattern as used for workers, i.e., a voltage scale of 0–26 V with 2-V increments, and 10-s shock presentations with 20-s intervals. These drones were collected from queen right colonies during June–August 2013 by blocking the colony entrance with a queen excluder during the hours of 2 to 5 pm in the afternoons. Collection times were set based on previous work by Galindo-Cardona et al. (2012). Response from this group was compared with the original drone and the worker response.
The variables of interest were movement rate, voltage at maximum movement, and sting response (females) or abdomen flexion (drones) during shock presentation (see Online Resources 1 (photo illustrating drone and worker during abdomen flexion) and 2 (video of drone abdomen flexion during assay)). For each individual, the voltage at which sting (foragers)/flexion (drones) occurred was measured. We classify this variable as a measure of an individual’s response voltage. We likewise measured the voltage level at which maximum movement rate was achieved for both workers and drones. This variable presented a measure of sensitivity to the negative stimulus and will be referred to as voltage sensitivity. Together, these variables provided an assessment of individual levels of degree of response and sensitivity, respectively.
Movement rate was quantified as the highest movement rate achieved in each individual trial minus the baseline (movement rate at 0 V) this is called baseline-adjusted maximum movement rate. In this manner, a baseline-adjusted rate of 0 was achieved by individuals that matched the initial movement rate. No individuals exhibited a negative maximum movement rate. Described variables were examined in male and females to generate comparisons with the aggression score of the focal source colony.
Analysis
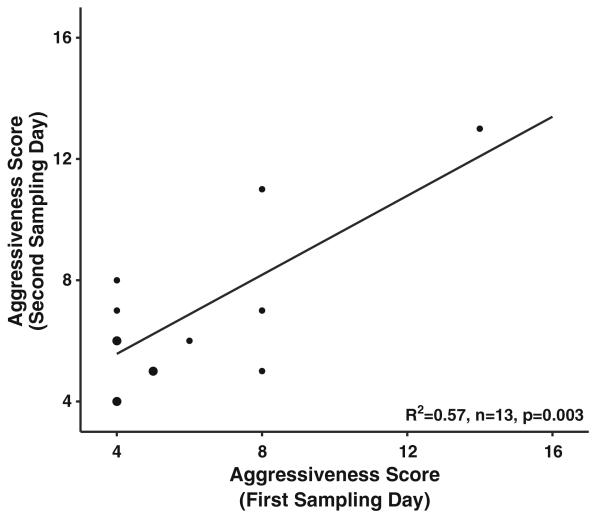
Comparisons of voltage at response (stinging or abdominal flexion) for female workers and drones were analyzed using two-sample Kolmogorov-Smirnov test of distributions. We derived a single colony aggressiveness score by regressing scores from the first day of colony ratings test against scores from the second day (Fig. 2). We used a Kendall’s Tau non-parametric rank correlation analysis for all comparisons between these fitted aggressiveness scores and individual responses. All statistical analyses were performed using the statistical software program R (R Development Core Team 2008).
Fig. 2.
Distribution of parental colony phenotypes. Correlation of colony aggressiveness scores across our sample population of 13 colonies. Line presents the fitted values derived from the regression model and used in correlative tests. Circle size is proportional to number of colonies that scored within the same range. The majority of colonies scored similarly from day 1 to day 2 of ratings test
Results
Parental colony aggressiveness scores
Colony aggressiveness scores from the first and second day of sampling were significantly correlated (simple linear regression, F(1, 11)=14.39, p=0.003). The range of the averaged scores across sample colonies was from the lowest score of 5.3 to a population maximum of 12.2 out of a possible maximum of 16. Distribution of resulting scores was right skewed with a median of 6 and a range of 6.9; this is comparable to average and range obtained for gAHB previously reported (see Rivera-Marchand et al. 2012).
Worker sting response vs. drone abdomen flexion response
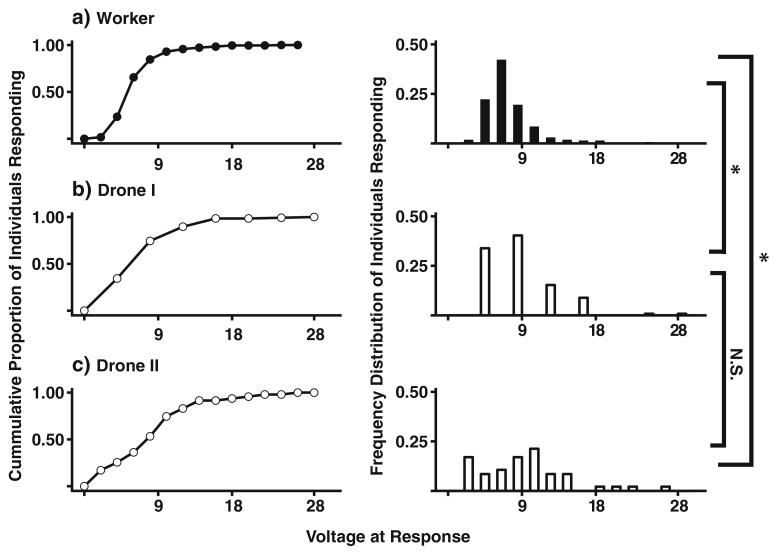
The distributions of responses of female foraging workers and the first study (worker-sired) male drones’ voltage to response differed (Kolmogorov-Smirnov two-sided test, D=0.32, p⟪ 0.001). Significant differences were similarly maintained when workers were compared to queen-produced drones from our second study (Kolmogorov-Smirnov two-sided test, D= 0.32, p⟪0.001). Conversely, distributions of voltage to response did not significantly differ between drones from the two studies (Kolmogorov-Smirnov two-sided test, D=0.21, p=0.09; Fig. 3). The shape of the cumulative distribution of voltage to response for workers was different than that of drone groups. Cumulative examination of female workers’ voltage to response appears sigmoid in shape while both drone groups’ cumulative response curves lack this. A greater number of drones than workers responded more readily at lower voltage levels (Fig. 3). Contrastingly foraging workers reached maximum response for the group well before either of the drone groups (Fig. 3a).
Fig. 3.
Cumulative response to shock of two honey bee castes. Curves show the cumulative group response of female foraging worker sting (a) and male drone abdomen flexion (b, c) to a noxious stimulus (shock). Histograms present the frequency distribution of the response to voltage in each of the groups a, b, and c. Histogram for Worker response (a) is significantly different from both Drone I (b) and Drone II (c). Drone I group (b) represents the haploid nephews of the Worker group (a). Drone II group (c) is an additional group of males that were assessed later using the same presentation protocol as Workers (a) (see “Materials and methods” for description)
Worker and drone response vs. sensitivity
Individual voltage sensitivity (voltage at maximum movement) positively correlated with voltage to response in workers (Kendall’s Tau, n=255, τ=0.20, z=4.00, p⟪0.001). This was not the case in drones, where voltage sensitivity did not significantly correlate with voltage to response (Kendall’s Tau, n=124, τ=0.04, z=0.57, p=0.57).
Worker and drone response vs. colony phenotype
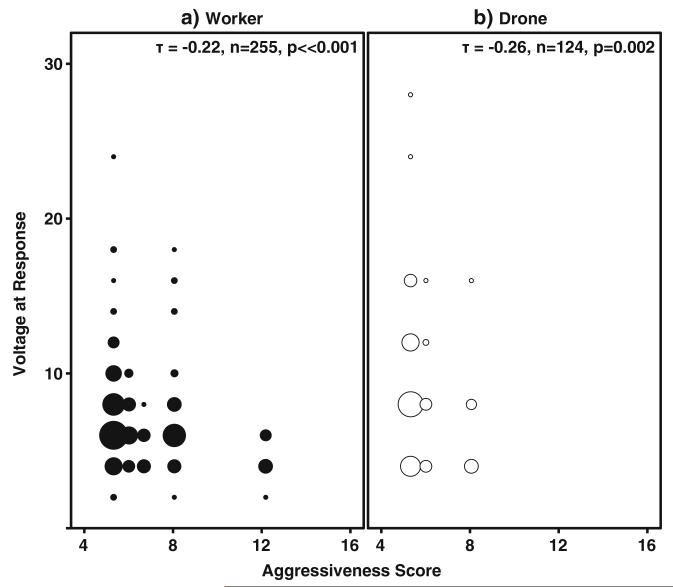
Colony aggression score was negatively correlated with female workers’ voltage to response (Kendall’s Tau, n=255, τ= −0.22, z=−4.28, p⟪0.001; Fig. 4a). A similar correlation was observed with male voltage to response (abdomen flexion) (Kendall’s Tau, n=124, τ=−0.26 z=−3.17, p=0.002; Fig. 4b).
Fig. 4.
Relationship between individual and parental colony aggressive response. Nonparametric correlation of individual response and colony phenotype in both Foragers (a) and Drones (b). Worker voltage to response decreased with colony aggression (Kendall’s Tau, n= 255, t=−0.22, p⟪0.001). Drone voltage to response also negatively correlated with colony phenotype (Kendall’s Tau, n= 124, t=−0.26, p=0.002)
Worker and drone movement vs. colony phenotype
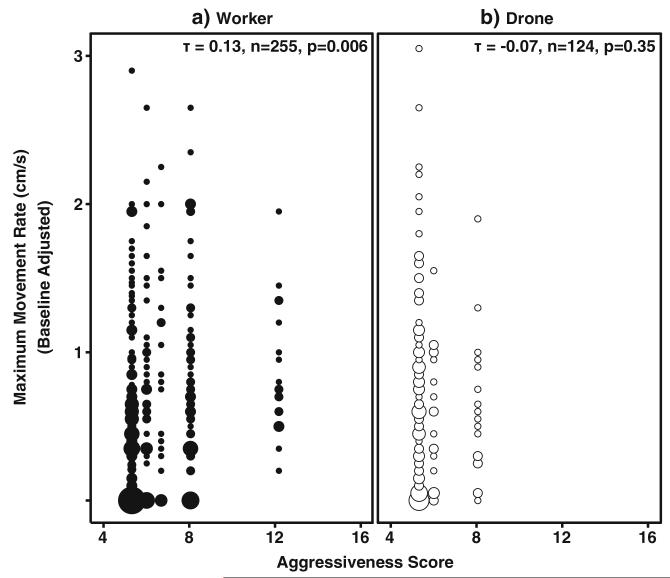
Individual voltage sensitivity did not correlate with colony aggressiveness in workers (Kendall’s Tau, n=255, τ=0.04, z= 0.82, p=0.42). Conversely, voltage sensitivity of drones positively correlated with parental colony score (Kendall’s Tau, n=124, τ=0.20, z=2.60, p=0.01). Movement rate positively correlated with colony phenotype in workers (Kendall’s Tau, n=255, τ=0.13, z=2.74, p=0.006; Fig. 5a) but not in drones (Kendall’s Tau, τ=−0.07, n=124, z=−0.94, p=0.35; Fig. 5b).
Fig. 5.
Relationship between colony phenotype and individual movement rates. Non-parametric correlation of individuals’ baseline-adjusted maximum movement rate and colony phenotype for Foragers (a) and Drones (b). Circle size corresponds to number of individual responses. Movement rates and colony phenotype correlated positively in the Workers (Kendall’s Tau, n=255, t=0.13, p=0.006). No significant correlation was observed between the movement rate of drones and parental colony phenotype
Discussion
The principal finding of this study is that individual response to electric shock of female workers and male drones correlate with colony aggressiveness behavior. However, cumulative distributions of response differed between the castes (Fig. 3), suggesting that underlying mechanisms are different. Indeed associations between the measures of response (voltage to response) and sensitivity (voltage at maximum movement) were different across the castes; in workers, both measures correlated positively while there was no such association in the drones.
Colony aggressiveness is a multicomponent response (Hunt 2007) and the variables of our individual aggressiveness assay may approximate some of these. The concordance in correlation between voltage to response and colony phenotype implies a strong hereditary component to the behavior, in agreement with previous studies demonstrating paternal effects (Guzman-Novoa et al. 2002, 2005). In contrast, the other component variables, such as adjusted maximum movement rate differed in their relation to colony phenotype which may reflect caste-specific differences. In the future, genetic studies on individual aggressive response and colony phenotype may identify aggressiveness genes that are regulated similarly and differently across castes.
Worker sting response
One important difference across castes was in the cumulative response to increasing shock voltage. In hind sight, the threshold dependence of worker group response was expected. For honey bee workers, the cost associated with stinging (vertebrates) is an abrupt reduction in lifespan (Winston 1987). Furthermore, release of alarm-associated pheromones that occurs in stinging induces a potentially costly (in terms of workforce) colony-wide aggressive response. Thus the synergy between imminent individual death and potential loss of workforce in honey bees posit a clear incentive to delay response. However, the observed response could have alternative explanations such as the cost of venom in solitary ancestors.
In organisms that use venom as a form of defense, delays to deploy are well documented and metabolically justified (Morgenstern and King 2013). The high energetic cost of venom production (see Morgenstern and King 2013 for a general review) could impose selection on use, and if so, would lead one to expect a threshold pattern to be widespread across Aculeata. It would not be a large evolutionary leap for an ancestral threshold response to then experience more acute selection in the social hymenoptera so as to increase recruitment efficiency and synchronization, possibly facilitated by signaling mechanisms (e.g., alarm pheromones). Therefore, one would expect that within social hymenoptera, the delay in response could be mediated to accommodate the best interest of the colony (discussed in Haight and Tschinkel 2003 and references therein), while solitary female hymenoptera would do so in the best economic interest of the individual (Morgenstern and King 2013).
Comparative examination across social and solitary models can thus provide further insight into the behavior. Predictions could be tested for social hymenoptera examining how alarm signaling mechanisms, such as alarm pheromone in honey bees can impact components of the response. In this case, the signal would work to reduce the point of threshold for the group in social hymenoptera for a quick response.
In contrast, our results of male group voltage to response show a cumulative curve that increases proportionately with shock level until saturation. This finding is in agreement with the hypothesized cost of stinging because drones have little to no cost to response; indeed it might be beneficial to not delay response if there is a mimicry-based defense (discussed below). More individual drones responded at lower voltages than females, and fewer new individuals responded at higher voltages (Fig. 3). Further comparative studies examining social and solitary models or how signaling may affect response can test the cost of sting vs. cost of mimicry hypotheses.
Our results on workers further suggest both interindividual and intraspecies variation in the sting response. Previous studies on interindividual response threshold differences to aversive stimuli using the sting extension response (SER) paradigm (Vergoz et al. 2007) is providing insight to organization of labor in honey bee colonies (see Roussel et al. 2009; Tedjakumala and Giurfa 2013). Recently, in a study by colleagues examining the same threshold a similar pattern was observed in workers from a separate subspecies of bees commonly found in Turkey (Apis mellifera anatoliaca; Abramson, Giray, and colleagues unpublished results). However, thresholds reported here are lower than those of British (Apis mellifera mellifera) and Italian (Apis mellifera lingustica) subspecies (Kolmes and Fergusson-Kolmes 1989). Taking into consideration these past studies and that our local honey bee, though gentle, is an Africanized hybrid, these findings suggest that greater level ecological factors can select differences in response thresholds.
Drone abdomen flexion
Our measurement for drone response was voltage at abdomen flexion (for descriptive materials, see Online Resources 1 (photo illustration of abdomen flexion) and 2 (video of drone bee responding to electric shock)). Abdomen flexion in male Hymenoptera is described as a behavioral “bluff” mechanism through which they potentially enhance their sexual automimicry (Starr 1981; Evans and West-Eberhard 1971). Observational accounts describe species in which eversion of the aedeagus (male insect reproductive organ) is common and some species even conscript modified abdominal structures used in territorial bouts, all to enhance the display (Evans and West-Eberhard 1971). Studies that have analyzed this or other defensive behaviors in male hymenoptera are scarce.
In wasps, males exhibit behavior and conscript morphology to mimic ovipositor/sting apparatus, but this behavioral and physiological response has not been studied at depth (Evans and West-Eberhard 1971). However, two studies suggest complex interactions may modulate both display and behavior. In bumble bees, a tradeoff between sexual color automimicry to resemble stinging females and thermal requirements of darker color in cold and lighter color in warm places has been described, suggesting an interaction of selective pressures (Stiles 1979). In another representative study, behavioral differences across males in the wasp genus Polistes were quantified (Starr 1981). The author describes that in these primitively eusocial wasps, male contribution to nest defense varies with degree of sexual color dimorphism. The author further concluded that the more similarly colored the sexes, the greater the frequency of participation of males on nest-threat behavioral displays (Starr 1981).
In honey bees, studies on drones have found that they can perceive social distress cues (Vetter and Visscher 1997), but examinations of possible behavioral response to these cues have been lacking. Alternately, the described behavior could be a pain-induced reflex to the negative stimulus (shock). However, cumulative response curves and correlations for components of aggressive response with colony phenotype differ across drones and workers. It can be speculated that mimicry or pain reflex hypotheses may apply to our observations, but this needs further examination.
Independent of the evolutionary explanation, the observed differences between worker and drone response suggest mechanistic differences in the neural control of the behavior. Indeed, recent results from a study that analyzes learning and sting response of workers under exposure to varying concentrations of ethanol suggest that a control mechanism modulates worker sting behavior (Abramson, Giray, and colleagues unpublished results; see also Burrell and Smith 1995).
Movement rate and response across castes
For workers, a positive correlation between voltage sensitivity and voltage to response indicates that detection of stimulus is predictive of response. The pattern differs for drones. Voltage sensitivity of drones did not predict their voltage to response. This result suggests a disassociation between voltage sensitivity and voltage to response in the male caste. When considered with results from the cumulative response analysis of the groups (Fig. 3 and above), our findings further support mechanistic differences in these component behaviors across the castes. We therefore conclude that in spite of reaction to aversive stimulus, workers seem to delay response until a specific threshold, probably through modulatory regulation (Núñez et al. 1983; Burrell and Smith 1995; discussed in Fu et al. 2013), while male response occurs synchronously with increased voltage presentation.
Colony aggressiveness and worker behavior
The observed negative correlation between worker voltage to response and colony phenotype establishes that more aggressive colonies are generally composed of individuals with lower levels of tolerance to the aggravating stimulus (Fig. 4a). The aggressive response of the colony to attack or manipulation is a concerted, pheromone-mediated effort of the component workers. When taken together with previous studies, our findings suggest that colony-level selection on aggressive response modifies individual response thresholds (Guzman-Novoa et al. 1999).
In workers, movement rate positively correlated with colony aggressiveness. Therefore, more aggressive colonies had individuals that reached higher possible movement rates. Results of activity measures match previously reported observations. The rate bees run and disperse on the comb is a widely used variable in the rating test employed here, and other ratings tests as a component group behavior when scoring colony aggression (Guzman-Novoa et al. 1999, 2003; Schneider et al. 2004). The variable has been positively correlated with other group measures of colony aggression. Hence, it is interesting that individual level activity also matches with colony phenotype in workers.
Surprisingly, voltage sensitivity did not correlate with colony phenotype. However, when examined together with the positive correlation between voltage to response and voltage sensitivity, the individual response is seen to have stages, such that individual workers respond to the stimuli first by increasing their movement rate, and they reach the maximum rate (voltage sensitivity) only before the sting response.
Colony aggressiveness and drone behavior
For drones, our initial expectation was that individual behavior would correlate with colony phenotype due to strong genetic evidence that paternal contribution is a significant determinant of defensive behaviors of honey bee colonies and workers (Arechavaleta-Velasco and Hunt 2004; Guzman-Novoa et al. 2005). Thus, we hypothesized that worker-sired drones which sample the genetic structure of the representative worker population would likewise present similar behavioral correlates. Our results do not completely sustain this hypothesis. Only one of the examined component behaviors, voltage at response (abdomen flexion), mirrors the workers from the source colony.
Our analysis shows that drone response (abdomen flexion) correlated negatively with colony aggression (Fig. 4b). In this manner, more aggressive colonies yield more readily responding (lower tolerance) drones. This result validated our initial hypothesis that drone behavior would correlate strongly with colony and individual worker behavior due to allelic effects. The implication is that colony aggressive behavior is reflected principally in drone voltage to response which may be indicative of a strong genetic component to the behavior.
In contrast, different from workers, movement rates of drones did not correlate with colony phenotype (Fig. 5b). Therefore, the movement rate of drones, unlike abdomen flexion, may not be genetically associated with colony defensive behavior. In fact, increasing voltage resulted in less activity and this reduction did correlate positively with colony aggressive behavior. The positive association between reduced activity and increased colony aggressiveness suggests that more aggressive colonies produce individual drones that reach their maximum movement rate at higher voltages (i.e., are less sensitive). There is no clear connection between drone movement rate and colony aggressive response, and the movement rate under painful stimulus may need to be investigated only for the individual male.
We therefore conclude that correlation of colony-level defense to component, individual behaviors is caste-specific. Drones serve as the vehicle for the ready dispersion of aggressive traits throughout a population, and they exhibit a correlated response, abdomen flexion. The functional and evolutionary significance of drone abdomen flexion is unknown at this time. Nevertheless, mechanistic differences (i.e., gene expression differences) in aggressive response of workers and drones and their progeny will be informative on sociogenomic correlates of aggression (Robinson et al. 2005; Whitfield et al. 2002; Hunt et al. 2007; Cingolani et al. 2013).
Supplementary Material
Acknowledgments
Research was conducted with funds from a USDA-NIFA award (2009-05291) to TG, the NSF-REU program (1156810) to YRC, and the RISE Graduate Fellowship Program (R25GM061151-11) to AA. We like to thank Dr. Gene E. Robinson for his suggestions during project development. We also thank Dr. Jose L. Agosto, Manuel Giannoni-Guzmán, and the BIOL6990-Behavioral Plasticity Seminar course members for taking the time to provide revisions and critiques. We appreciate and thank Dr. Alberto Galindo for his insight and assistance, Dr. Charles I Abramson for his constructive criticism and help with the language and exposition of the manuscript, and Dr. María Eglée Pérez for statistical revision and assistance. Further thanks to Gabriel Diaz our apiary technician whose assistance was essential for project coordination.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00265-014-1689-8) contains supplementary material, which is available to authorized users.
Contributor Information
Arian Avalos, Department of Biology, University of Puerto Rico, Rio Piedras, P.O. Box 23360, San Juan 00931, Puerto Rico.
Yoselyn Rodríguez-Cruz, Department of Biology, University of Puerto Rico, Rio Piedras, P.O. Box 23360, San Juan 00931, Puerto Rico.
Tugrul Giray, Department of Biology, University of Puerto Rico, Rio Piedras, P.O. Box 23360, San Juan 00931, Puerto Rico.
References
- Abramson CI. Aversive conditioning in honeybees (Apis mellifera) J Comp Psychol. 1989;100:108–116. [Google Scholar]
- Agarwal C, Giannoni Guzmán M, Morales-Matos C, Del Valle Díaz RA, Abarmson CI, Giray TG. Dopamine and octopamine influence avoidance learning of honey bees in a place preference assay. PLoS One. 2011;6:e25371. doi: 10.1371/journal.pone.0025371. doi:10.1371/journal.pone.0025371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux C, Robinson GE. Alarm pheromone induces immediate-early gene expression and slow behavioral response in the honey bees. J Chem Ecol. 2007;33:1346–1350. doi: 10.1007/s10886-007-9301-6. doi:10.1007/s10886-007-9301-6. [DOI] [PubMed] [Google Scholar]
- Arechavaleta-Velasco ME, Hunt GJ. Binary trait loci that influence honey bee (Hymenoptera: Apidae) guarding behavior. Ann Entomol Soc Am. 2004;97:177–183. doi:10.1603/0013-8746(2004)097[0177:BTLTIH]2.0.CO;2. [Google Scholar]
- Baldemarra N, Díaz H, Sequeda A, Núñez J, Maldonado H. Behavioral and pharmacological analysis of the stinging response in Africanized and Italian bees. In: Mercer A, Menzel R, editors. Neurobiology and behavior of the honeybee. 1st edn. Springer-Verlag; Berlin Heidelberg: 1987. pp. 121–128. [Google Scholar]
- Beye M, Hasselmann M, Fondrk MK, Page RE, Jr, Omholt SW. The gene csd is the primary signal for sexual development in the honeybee that encodes an SR-Type protein. Cell. 2003;114:419–429. doi: 10.1016/s0092-8674(03)00606-8. doi: 10.1016/S0092-8674(03)00606-8. [DOI] [PubMed] [Google Scholar]
- Bhagavan S, Benatar S, Cobey S, Smith BH. Effect of genotype but not age or caste on olfactory learning performance in the honey bee, Apis mellifera. Anim Behav. 1994;48:1357–1369. doi:10.1006/anbe.1994.1372. [Google Scholar]
- Breed MD, Robinson GE, Page RE., Jr Division of labor during honey bee colony defense. Behav Ecol Sociobiol. 1990;27:395–401. doi: 10.1007/BF00164065. [Google Scholar]
- Breed MD, Guzman-Novoa E, Hunt GJ. Defensive behavior of honey bees: organization, genetics, and comparisons with other bees. Annu Rev Entomol. 2004;49:271–298. doi: 10.1146/annurev.ento.49.061802.123155. doi:10.1146/annurev.ento.49.061802.123155. [DOI] [PubMed] [Google Scholar]
- Burrell BD, Smith BH. Age- but not caste-related regulation of abdominal mechanisms underlying the sting reflex of the honey bee, Apis mellifera. J Comp Physiol A. 1994;174:581–592. doi:10.1007/BF00217379. [Google Scholar]
- Burrell BD, Smith BH. Modulation of the honey bee (Apis mellifera) sting response by octopamine. J Insect Physiol. 1995;41:671–680. doi:10.1016/0022-1910(95)00022-M. [Google Scholar]
- Cargel RA, Rinderer TE. Queen cell acceptance in laying worker colonies of Russian and Italian honey bees. Am Bee J. 2006;146:698–700. [Google Scholar]
- Chapman TW, Crespi BJ, Kranz BD, Schwarz MP. High related-ness and inbreeding at the origin of eusociality in gall-inducing thrips. Proc Natl Acad Sci. 1999;97:1648–1650. doi: 10.1073/pnas.020510097. doi:10.1073/pnas.020510097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P, Cao X, Khetani RS, Chen CC, Coon M, Sammak A, Bollig-Fischer A, Land S, Huang Y, Hudson ME, Garfinkel MD, Zhong S, Robinson GE, Ruden DM. Intronic Non-CG DNA hydroxymethylation and alternative mRNA splicing in honey bees. BMC Genomics. 2013;14:666. doi: 10.1186/1471-2164-14-666. doi:10.1186/1471-2164-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AM, Rinderer TE, Harbo JR, Brown MA. Heritabilities and correlations for several characters in the honey bee. J Hered. 1984;75:135–140. [Google Scholar]
- Dinges CW, Avalos A, Abramson CI, Craig DPA, Austin ZM, Varnon CA, Dal FN, Giray T, Wells H. Aversive conditioning in honey bees (Apis mellifera anatoliaca): a comparison of drones and workers. J Exp Biol. 2013;216:4124–4134. doi: 10.1242/jeb.090100. doi:10.1242/jeb.090100. [DOI] [PubMed] [Google Scholar]
- Evans HE, West-Eberhard MJ. The wasps. University of Michigan Press; Michigan: 1971. [Google Scholar]
- Fahrbach SE, Giray T, Farris SM, Robinson GE. Expansion of the neuropil of the mushroom bodies in male honey bees is coincident with initiation of flight. Neurosci Lett. 1997;236:135–138. doi: 10.1016/s0304-3940(97)00772-6. doi:10.1016/S0304-3940(97)00772-6. [DOI] [PubMed] [Google Scholar]
- Ferguson HJ, Cobey S, Smith BH. Sensitivity to change in rewards is heritable in the honeybee, Apis mellifera. Anim Behav. 2001;61:527–534. doi:10.1006/anbe.2000.1635. [Google Scholar]
- Fu Y, Chen Y, Yao T, Li P, Ma Y, Wang J. Effects of morphine on associative memory and locomotor activity in the honey bee (Apis mellifera) Neurosci Bull. 2013;29:270–278. doi: 10.1007/s12264-013-1308-0. doi:10.1007/s12264-013-1308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Cardona A, Monmany AC, Moreno-Jackson R, Rivera-Rivera C, Huertas-Dones C, Caicedo-Quiroga L, Giray T. Landscape analysis of drone congregation areas of the honey bee, Apis mellifera. J Insect Sci. 2012;12:1–15. doi: 10.1673/031.012.12201. doi:10.1673/031.012.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Cardona A, Rivera-Marchand B, Acevedo J, Giray T. Genetic structure of the gentle Africanized honey bee population (gAHB) in Puerto Rico. BMC Genet. 2013;14:65. doi: 10.1186/1471-2156-14-65. doi:10.1186/1471-2156-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giray T, Robinson GE. Common endocrine and genetic mechanisms of behavioral development in male and worker honey bees and the evolution of division of labor. P Natl Acad Sci. 1996;93:11718–11722. doi: 10.1073/pnas.93.21.11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giray T, Guzman-Novoa E, Aron CW, Zelinsky B, Fahrbach SE, Robinson GE. Genetic variation in worker temporal polyethism and colony defensiveness in the honey bee, Apis mellifera. Behav Ecol. 2000;11:44–55. [Google Scholar]
- Grüter C, Memezes C, Imperatriz-Fonseca VL, Ratnieks FLW. A morphologically specialized soldier caste improves colony defense in a neotropical eusocial bee. P Natl Acad Sci. 2011;109:1182–1186. doi: 10.1073/pnas.1113398109. doi: 10.1073/pnas.1113398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Novoa E, Page RE., Jr Selective breeding of honey bees (Hymenoptera: Apidae) in Africanized areas. J Econ Entomol. 1999;92:521–525. [Google Scholar]
- Guzman-Novoa E, Page RE, Jr, Spangler HG, Erickson EH. A comparison of two assay to test the defensive behavior of honey bees (Apis mellifera) J Apic Res. 1999;38:205–209. [Google Scholar]
- Guzman-Novoa E, Hunt GJ, Uribe JL, Smith C, Arechavaleta-Velasco ME. Confirmation of QTL effects and evidence of genetic dominance of honeybee defensive behavior: results of colony and individual behavioral assay. Behav Genet. 2002;32:95–102. doi: 10.1023/a:1015245605670. doi:10.1023/A:1015245605670. [DOI] [PubMed] [Google Scholar]
- Guzman-Novoa E, Prieto-Merlos D, Uribe-Rubio JL, Hunt GJ. Relative reliability of four field assays to test defensive behavior of honey bees (Apis mellifera) J Apic Res. 2003;42:42–46. [Google Scholar]
- Guzman-Novoa E, Hunt GJ, Page RE, Jr, Uribe-Rubio JL, Prietos-Merlos D, Becerra-Guzman F. Paternal effects on the defensive behavior of honey bees. J Hered. 2005;96:376–380. doi: 10.1093/jhered/esi038. doi:10.1093/jhered/esi038. [DOI] [PubMed] [Google Scholar]
- Haight KL, Tschinkel WR. Patterns of venom synthesis and use in the fire ant, Solenopsis invicta. Toxicon. 2003;42:673–682. doi: 10.1016/j.toxicon.2003.09.005. doi:10.1016/j.toxicon.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hunt GJ. Flight and fight: a comparative view of the neurophysiology and genetics of honey bee defensive behavior. J Insect Physiol. 2007;53:399–410. doi: 10.1016/j.jinsphys.2007.01.010. doi:10.1016/j.jinsphys.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GJ, Guzman-Novoa E, Fondrk MK, Page RE., Jr Quantitative trait loci for honey bee stinging behavior and body size. Genetics. 1998;148:1203–1213. doi: 10.1093/genetics/148.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GJ, Amdam GV, Schlipalius D, Emore C, Sardesai N, Williams CE, Rueppell O, Guzmán-Novoa E, Arechavaleta-Velasco M, Chandra S, Fondrk MK, Beye M, Page RE., Jr Behavioral genomics of honeybee foraging and nest defense. Naturwissenschaften. 2007;94:247–267. doi: 10.1007/s00114-006-0183-1. doi:10.1007/s00114-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmes SA, Fergusson-Kolmes LA. Stinging behavior and residual value of worker honey bees (Apis mellifera) J N Y Entomol Soc. 1989;97:218–231. [Google Scholar]
- Kolmes SA, Njehu N. Effect of queen mandibular pheromones on Apis mellifera worker stinging behavior (Hymenoptera: Apidae) J N Y Entomol Soc. 1990;98:495–498. [Google Scholar]
- Lenoir JC, Laloi D, Dechaume-Moncharmont FX, Solignac M, Pham MH. Intra-colonial variation of the sting extension response in the honey bee Apis mellifera. Insect Soc. 2006;53:80–85. doi:10.1007/s00040-005-0838-5. [Google Scholar]
- Morgenstern D, King GF. The venom optimization hypothesis revisited. Toxicon. 2013;63:120–128. doi: 10.1016/j.toxicon.2012.11.022. doi:10.1016/j.toxicon.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Núñez JA, Maldonado H, Miralto A, Balderrama N. The stinging response of the honeybee: effects of morphine naloxone and some opioid peptides. Pharmacol Biochem Behav. 1983;19:921–924. doi: 10.1016/0091-3057(83)90391-x. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Kawakami Z, Yamaguchi T. Motor pattern of the stinging response in the honeybee Apis mellifera. J Exp Biol. 1995;189:39–47. doi: 10.1242/jeb.198.1.39. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Kawakami Z, Yamaguchi T. Proprioceptors involved in stinging response of the honeybee Apis mellifera. J Insect Physiol. 2011;57:1358–1367. doi: 10.1016/j.jinsphys.2011.07.003. doi:10.1016/j.jinsphys.2011.07.003. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna Austria: 2008. ISBN 3-900051-07-0. URL http://www.R-project.org. [Google Scholar]
- Rivera-Marchand B, Giray T, Guzman-Novoa E. The cost of defense in social insects: insights from the honey bee. Entomol Exp Appl. 2008;129:1–10. doi:10.1111/j.1570-7458.2008.00747.x. [Google Scholar]
- Rivera-Marchand B, Oskay D, Giray T. Gentle Africanized bees on an oceanic island. Evol Appl. 2012;5:745–756. doi: 10.1111/j.1752-4571.2012.00252.x. doi:10.1111/j.1752-4571.2012.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GE. Modulation of alarm pheromone perception in the honey bee: evidence for division of labor based on hormonally regulated response thresholds. J Comp Physiol. 1997;160:613–619. [Google Scholar]
- Robinson GE, Page RE., Jr Genetic determination of guarding and undertaking in honey-bee colonies. Nature. 1988;333:356–358. [Google Scholar]
- Robinson GE, Grozinger CM, Whitfield CW. Sociogenomics: social life in molecular terms. Nat Rev. 2005;6:257–270. doi: 10.1038/nrg1575. doi:10.1038/nrg1575. [DOI] [PubMed] [Google Scholar]
- Roussel E, Carcaud J, Sandoz JC, Giurfa M. Reappraising social insect behavior through aversive responsiveness and learning. PLoS One. 2009;4:e4197. doi: 10.1371/journal.pone.0004197. doi:10.1371/journal.pone.0004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux EA, Korb J. Evolution of eusociality and the soldier caste in termites: a validation of the intrinsic benefit hypothesis. J Evol Biol. 2004;17:869–875. doi: 10.1111/j.1420-9101.2004.00727.x. doi:10.1111/j.1420-9101.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Schneider SS, DeGrandi-Hoffman G, Smith DR. The African honey bee: factors contributing to a successful biological invasion. Annu Rev Entomol. 2004;49:351–376. doi: 10.1146/annurev.ento.49.061802.123359. doi:10.1146/annurev.ento.49.061802.123359. [DOI] [PubMed] [Google Scholar]
- Shorter JR, Rueppell O. A review on self-destructive defense behaviors in social insects. Insect Soc. 2012;59:1–10. doi:10.1007/s00040-011-0210-x. [Google Scholar]
- Spangler HG, Sprenkle DJ. An instrument for quantifying honey bee defensiveness. Appl Acoust. 1997;50:325–332. [Google Scholar]
- Starr CK. Defensive tactics of social wasps. University of Georgia; Athens: 1981. Dissertation. [Google Scholar]
- Stern DL, Foster WA. The evolution of soldiers in aphids. Biol Rev. 1996;71:27–79. doi: 10.1111/j.1469-185x.1996.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Stiles EW. Evolution of color pattern and pubescence characteristics in male bumblebees: automimicry vs. thermoregulation. Evolution. 1979;33:941–957. doi: 10.1111/j.1558-5646.1979.tb04748.x. [DOI] [PubMed] [Google Scholar]
- Tedjakumala SR, Giurfa M. Rules and mechanisms of punishment learning in honey bees: the aversive conditioning of the sting extension response. J Exp Biol. 2013;216:2985–2997. doi: 10.1242/jeb.086629. doi:10.1242/jeb.086629. [DOI] [PubMed] [Google Scholar]
- Tel-Zur D, Lensky Y. Bioassay and apparatus for measuring the stinging response of an isolated worker honey-bee (Apis mellifera L. var lingustica Spin.) Comp Biochem Physiol A. 1995;110:281–288. [Google Scholar]
- Thorne BL, Breisch NL, Muscedere ML. Evolution of eusociality and the soldier caste in termites: influence of intraspecific competition and accelerated inheritance. P Natl Acad Sci. 2003;100:12808–12813. doi: 10.1073/pnas.2133530100. doi:10.1073/pnas.2133530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth E, Duffy JE. Influence of sociality on allometric growth and morphological differentiation in sponge dwelling alpheid shrimp. Biol J Linn Soc. 2008;94:527–540. doi:10.1111/j.1095-8312.2008.01013.x. [Google Scholar]
- Urlacher E, Francés B, Giurfa M, Devaud JM. An alarm pheromone modulates appetitive olfactory learning in the honeybee (Apis mellifera) Front Behav Neurosci. 2010;4:157. doi: 10.3389/fnbeh.2010.00157. doi:10.3389/fnbeh.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergoz V, Roussel E, Sandoz JC, Giurfa M. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS One. 2007;3:e288. doi: 10.1371/journal.pone.0000288. doi:10.1371/journal.pone.0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter RS, Visscher PK. Influence of age on antennal response of male honey bees Apis mellifera to queen mandibular pheromone and alarm pheromone component. J Chem Ecol. 1997;23:1867–1880. [Google Scholar]
- Wheeler DE. The developmental basis of worker caste polymorphism in ants. Am Nat. 1991;138:1218–1238. [Google Scholar]
- Whitfield CW, Band MR, Bonaldo MF, Kumar CG, Liu L, Pardinas JR, Robertson HM, Soares MB, Robinson GE. Annotated expressed sequence tags and cDNA microarrays for studies of brain and behavior in the honey bee. Genome Res. 2002;12:555–566. doi: 10.1101/gr.5302. doi:10.1101/gr.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston ML. Harvard University Press; Cambridge, MA: 1987. The biology of the honey bee. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.