Abstract
Immune system activation contributes to the pathogenesis of hypertension and the resulting progression of chronic kidney disease (CKD). In this regard, we recently identified a role for pro-inflammatory Th1 T lymphocyte responses in hypertensive kidney injury. As Th1 cells generate IFN-γ and TNF-α, we hypothesized that IFN-γ and TNF-α propagate renal damage during hypertension induced by activation of the renin-angiotensin system (RAS). Therefore, after confirming that mice genetically deficient of Th1 immunity were protected from kidney glomerular injury despite a preserved hypertensive response, we subjected mice lacking IFN-γ or TNF-α to our model of hypertensive CKD. IFN-deficiency had no impact on blood pressure elevation or urinary albumin excretion during chronic angiotensin II infusion. By contrast, TNF-deficient (KO) mice had blunted hypertensive responses and reduced end-organ damage in our model. As Ang II-infused TNF KO mice had exaggerated eNOS expression in the kidney and enhanced nitric oxide (NO) bioavailability, we examined the actions of TNF-α generated from renal parenchymal cells in hypertension by transplanting wild-type or TNF KO kidneys into wild-type recipients prior to the induction of hypertension. Transplant recipients lacking TNF solely in the kidney had blunted hypertensive responses to Ang II and augmented renal eNOS expression, confirming a role for kidney-derived TNF-α to promote Ang II-induced blood pressure elevation by limiting renal NO generation.
Keywords: TNF-alpha, Hypertension, Kidney, IFN-gamma, T-bet, Angiotensin II
An accumulating body of evidence indicates that activation of the adaptive immune system contributes to the pathogenesis of hypertension and the associated progression of chronic kidney disease (CKD). In the majority of CKD patients, agents that inhibit the renin angiotensin system (RAS) reduce blood pressure and urinary albumin excretion indicating that inappropriate RAS activation drives blood pressure elevation in this population.1 The key effector molecule of the RAS, angiotensin (Ang) II, activates the immune system in the setting of hypertension, leading to the infiltration of T lymphocytes into the kidney and vasculature.2, 3 Blocking this T cell accumulation limits kidney damage in hypertension, and complete deficiency of T cells blunts the chronic hypertensive response to Ang II.2, 3 Thus, T cells can potentiate blood pressure elevation and the progression of CKD in Ang II-dependent hypertension.
The precise actions of individual T cell subsets in hypertension vary according to the cytokines they secrete. For example, Th17 T cells that produce IL-17 are pro-hypertensive as animals lacking IL-17 are protected from Ang II-induced hypertension.4 By contrast, repeated transfer of T regulatory cells that produce IL-10 and TGF-β can limit Ang II-induced blood pressure elevation.5 In our own experiments, mice lacking pro-inflammatory Th1 cells that produce IFN-γ and TNF-α were protected from hypertensive damage to the kidney glomerulus despite a preserved blood pressure response to Ang II.6 However, the mechanisms through which Th1 cells instigate hypertensive renal injury and the relative contributions of IFN-γ and/or TNF-α to Th1-mediated CKD progression require elucidation. In the present studies, we therefore interrogated the actions of IFN-γ and TNF-α in Ang II-induced CKD by using mice genetically deficient of these cytokines.
Methods
Experimental animals
T-bet−/− (B6.129S6-Tbx21tm1Glm/J) and TNF-α knockout (B6129S-Tnftm1Gkl/J) mice on the C57BL/6 background were obtained from Jackson Laboratory and backcrossed to the 129/SvEv strain for 6 generations to increase susceptibility to kidney damage. Then, T-bet or TNF-α heterozygotes were intercrossed to yield the wild-type (WT) and knockout (KO) littermates for our experiments. All of the animal studies were approved by the Durham Veterans’ Affairs Medical Center Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Eight to twelve-week-old male mice were employed in the current studies.
Model of Ang II-induced Hypertensive CKD
Experimental mice (n≥9) underwent left nephrectomy followed one week later by implantation of a pressure-sensing catheter (TA11PA-C10, Data Sciences International) via the left common carotid artery as previously reported.7 After allowing 7 days for reestablishment of diurnal blood pressure variation, baseline blood pressure measurements were recorded for 3 days continuously by radiotelemetry in conscious, unrestrained animals. Next, an osmotic minipump (Alzet model 2004; DURECT) was implanted to infuse Ang II (1,000 ng·kg 1·min 1; Sigma-Aldrich) continuously for 28 days as described.7, 8
Additional Methods are available in the online Data Supplement.
Results
T-bet Deficiency Mitigates Nephrinuria during Hypertension
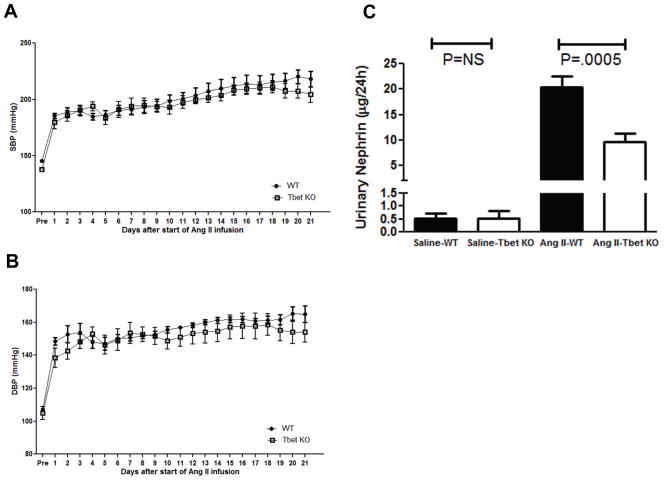
In previous studies using our Ang II infusion model of CKD, T-bet-deficient (T-bet KO) mice that are unable to mount a Th1 response excreted less urinary albumin than controls despite a similar increase in mean arterial pressure.6 To confirm that the Th1 immune response mediates damage to the kidney glomerulus without altering blood pressure elevation, we analyzed systolic and diastolic blood pressures in Ang II-infused T-bet KO mice and wild-type controls (WT) and measured urinary excretion of nephrin, a marker for the glomerular podocytes that prevent the loss of albumin in the urine. Throughout the experimental period, both the WT and T-bet KO groups sustained a robust and similar increase in systolic and diastolic blood pressures as measured by radiotelemetry (Figure 1A–B), confirming that Th1 immune responses do not influence the chronic hypertensive response to Ang II-mediated regulation of blood pressure. Following 4 weeks of Ang II infusion, both WT and T-bet KO mice had markedly increased urinary nephrin excretion compared to those infused with saline, signifying substantial loss of glomerular podocytes in the setting of Ang II-induced hypertension (Figure 1C). However, T-bet KO mice excreted 50% less nephrin than the WTs (9.6±1.7 vs. 20.4±2.2 μg/24hrs; P=0.0005). Thus, Th1 immune responses mediate damage to the kidney glomerulus during Ang II-induced hypertension through a blood pressure-independent mechanism.
Figure 1.
Th1 immune responses exaggerate urinary excretion of nephrin in Ang II-dependent hypertension. A, Systolic and B, Diastolic blood pressures measured by radiotelemetry in the experimental groups at baseline (“pre”) and during chronic Ang II infusion. Wild-type (WT), circles. T-bet KO (KO), squares. n = 7 per group. C, Urinary nephrin excretion (μg/24hrs) after 25 days of Ang II.
Deficiency of TNF-α but not IFN-γ leads to blunted hypertensive response to Ang II
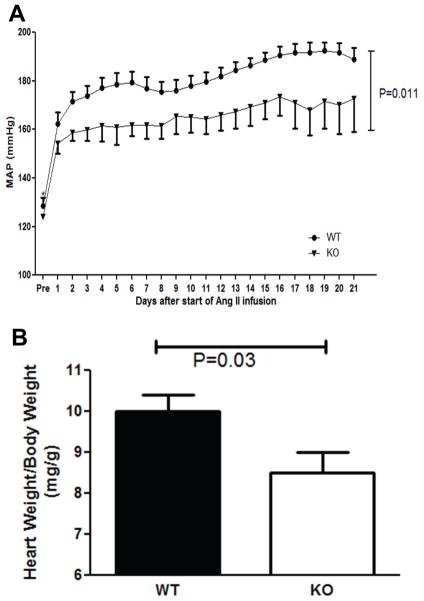
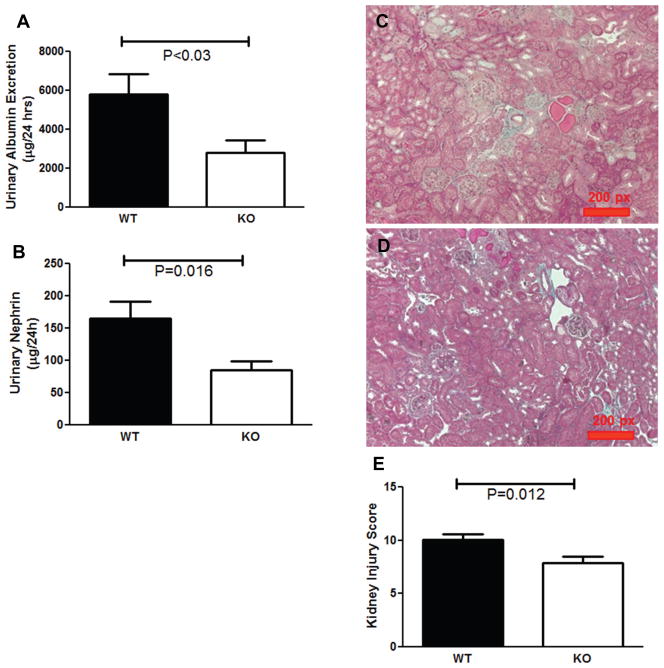
As Th1 T cells secrete both TNF-α and IFN-γ, we further dissected the contribution of Th1 immunity to hypertensive CKD by subjecting TNF-α- and IFN-γ-deficient (TNF and IFN KO) mice to our CKD model in which one native kidney is removed prior to chronic Ang II infusion, rendering the remaining kidney more susceptible to hypertensive damage. At baseline, WT and TNF KO mice had similar mean arterial blood pressures (MAPs) (128±4 vs. 124±1 mm Hg; P=NS; Figure 2A). However, chronic Ang II infusion induced significantly less blood pressure elevation in the TNF KO group than in WT controls (166±5 vs. 183±4 mm Hg during Ang II infusion; P=0.01; Figure 2A). Consistent with their lower blood pressures, the Ang II-infused TNF KOs exhibited less cardiac hypertrophy following 4 weeks of Ang II (8.5±0.5 vs. 10.0±0.4 mg heart weight/g body weight; P=0.02; Figure 2B). To assess kidney injury, we first measured urinary albumin excretion, and found that mice lacking TNF-α had more than 50% less albuminuria than the WT mice following 4 weeks of Ang II (2812±647 vs. 5803±1036 μg/24 hr; P<0.03; Figure 3A). As seen with the T-bet KOs, urinary nephrin excretion in the Ang II-infused TNF KOs was reduced by approximately 50% versus WT controls (Figure 3B). Moreover, by blinded semi-quantitative scoring, Ang II-infused TNF KO mice had over 20% less kidney injury compared to Ang II-infused WTs (7.9±0.6 vs. 10.1±0.5 arbitrary units; P=0.012; Figure 3C–E). Consistent with the reduced levels of kidney damage, gene expression for the kidney injury marker neutrophil gelatinase-associated lipocalin (NGAL) was diminished by half in the TNF KO group compared to controls (Figure 4A). Collectively, these data suggest that TNF-α contributes to both Ang II-mediated blood pressure elevation and kidney damage in our hypertensive CKD model. By contrast, IFN KO mice mounted a blood pressure response similar to their WT controls during the Ang II infusion period, and exhibited no difference in urinary albumin excretion following 4 weeks of Ang II (Supplemental Figure S1). Thus, TNF-α rather than IFN-γ contributes to the hypertensive CKD mediated by Th1 immune responses in our T-bet KO experiments. However, the muted hypertensive response in the Ang II-infused TNF KO group coupled with the preserved blood pressure elevation in the Ang II-infused T-bet KO cohort suggests that TNF-α generated by a cell lineage other than T lymphocytes drives blood pressure elevation in our model.
Figure 2.
TNF-α potentiates Ang II-induced hypertension and cardiac hypertrophy. A, Mean arterial blood pressures measured by radiotelemetry in wild-type (WT) and TNF-α-deficient (KO) groups at baseline (“pre”) and during 3 weeks of chronic Ang II infusion. WT, circles. KO, triangles. n = 9 per group. B, Ratio of heart weight/body weight (mg/g) after 28 days of Ang II.
Figure 3.
TNF-α contributes to the progression of hypertensive CKD. A, Urinary albumin and B, nephrin excretion (μg/24hrs) in wild-type (WT) and TNF KO (KO) groups after 25 days of Ang II. C–D, Representative images of kidney sections from (C) WT and (D) TNF KO groups. E, Semi-quantitative kidney injury scores calculated per Methods after 4 weeks of Ang II.
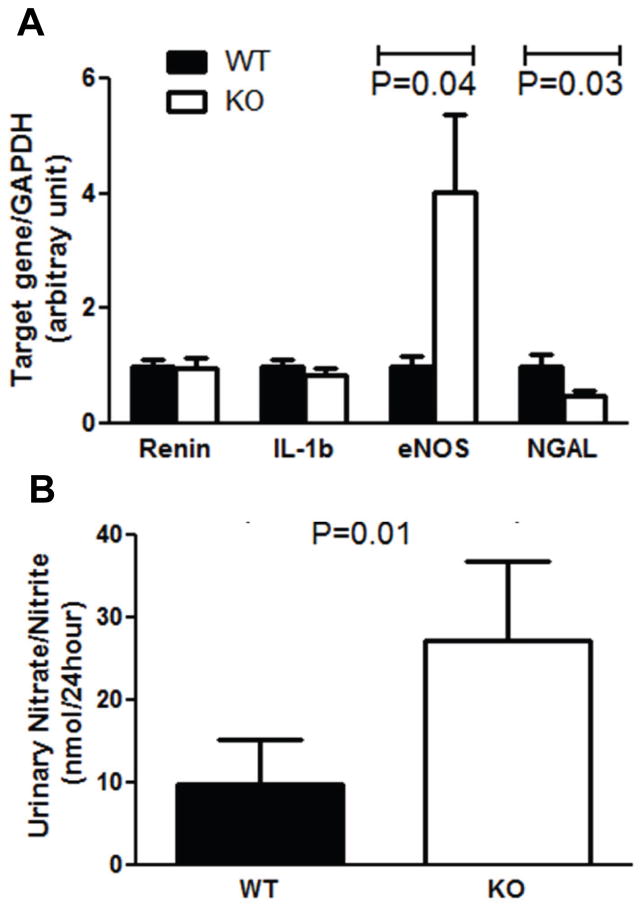
Figure 4.
TNF-α suppresses generation of nitric oxide (NO) in the kidney during hypertension. A, mRNA expression of renin, IL-1b, eNOS, and NGAL in the WT and TNF KO kidneys measured by real-time PCR after 4 weeks of Ang II. B, Total urinary excretion of NO metabolites after 25 days of Ang II.
TNF KO mice have enhanced eNOS expression and renal nitric oxide production
We therefore explored whether TNF-α produced by non-immune cell lineages could influence the hypertensive response to Ang II. We considered the hypothesis that TNF-α produced in the vasculature promotes blood pressure elevation. However, we found that WT and TNF KO mice had similar elevations in blood pressure after increasing doses of acute intravenous Ang II infusion (Supplemental Figure S2), indicative of a preserved vascular response in the TNF KO cohort.
As the thick ascending limb in the kidney nephron is a potent source of TNF-α during RAS activation,9 we turned next to the possibility that TNF-α produced by renal parenchymal cells could regulate the hypertensive response. First, we quantitated mRNA expression in the kidney of several molecules implicated in blood pressure regulation. By realtime RT-PCR, levels of neither renin nor Interleukin-1β expression were altered in kidneys from Ang II-infused TNF KO mice compared to their littermate WT controls (Figure 4A). By contrast, mRNA expression of nitric oxide synthase 3 (eNOS) was 4-fold higher in the kidneys from Ang II-infused TNF KO mice than in the WTs (4.02±1.36 vs. 1±0.17; P=0.04; Figure 4A). As eNOS catalyzes the generation of nitric oxide (NO)10, we quantitated NO bio-availability in the kidney by measuring the metabolites of NO in the urine. Despite similar levels of food and water intake (Supplemental Figure S3), the Ang II-infused TNF KO mice excreted markedly higher levels of nitrate/nitrite compared to WT controls (27.3±9.4 vs. 9.8±5.5 nmol/24hours; P=0.01; Figure 4B). These data support the notion that TNF-α potentiates the hypertensive response by suppressing NO bio-availability within the kidney.
An essential role for kidney-derived TNF-α in Ang II-dependent hypertension
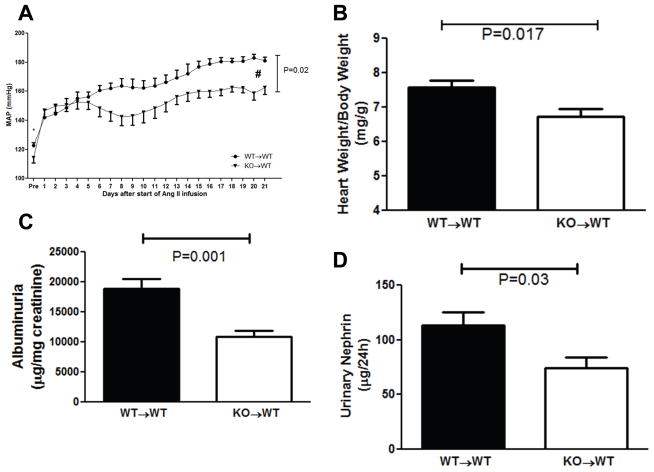
To determine whether TNF-α produced in the kidney suppresses eNOS and thereby promotes blood pressure elevation, we transplanted TNF KO or WT kidneys into genetically matched WT recipients and subjected these “KO→WT” mice lacking TNF only in the kidney or “WT→WT” transplant controls to our chronic Ang II infusion model. Even at baseline, the absence of TNF-α in the kidney alone in the KO→WT cohort was associated with an 8 mm Hg reduction in blood pressure versus WT→WT controls (122.6±2.0 vs. 114.2±3.5 mmHg; P=0.05; Figure 5A). During Ang II infusion, both groups of transplanted mice had a robust increase in blood pressure. However, just as seen in the global TNF KOs, the KO→WT mice had a blunted hypertensive response compared to the WT→WT cohort (153.2±3.5 vs. 166.3±3.4 mmHg; P=0.02). Consistent with the lower blood pressures in the Ang II-infused KO→WT mice, cardiac hypertrophy in this group was less severe than in the WT→WTs (6.7±0.2 vs. 7.6±0.2 mg/gm; P=0.017; Figure 5B). The blunted hypertensive response in the KO→WT group was associated with less damage to the kidney glomerulus as measured by albuminuria (Figure 5C) and nephrinuria (Figure 5D), whereas renal T cell infiltration (Supplemental Figure S4) and renal mRNA expression for NGAL (1.0±0.1 vs. 1.0±0.1 au; P=NS) were similar in the KO→WT and WT→WT groups. Thus, TNF-α produced in the kidney plays an essential role in mediating Ang II-induced blood pressure elevation.
Figure 5.
Ang II mediates blood pressure elevation through TNF-α generated in the kidney. A, Mean arterial blood pressures measured by radiotelemetry in wild-type recipients of TNF KO kidneys (KO→WT, triangles) or wild-type kidneys (WT→WT, circles) at baseline (“pre”) and during 3 weeks of Ang II infusion. * P=0.04 for baseline, #P=0.02 over Ang II infusion period, n 6 per group. B, Ratio of heart weight/body weight (mg/g) after 4 weeks of Ang II. C–D, Urinary excretion of (C) albumin (μg/mg creatinine) and (D) nephrin (μg/24hrs) following 4 weeks of Ang II.
Renal TNF-α suppresses eNOS expression and nitric oxide production in the kidney
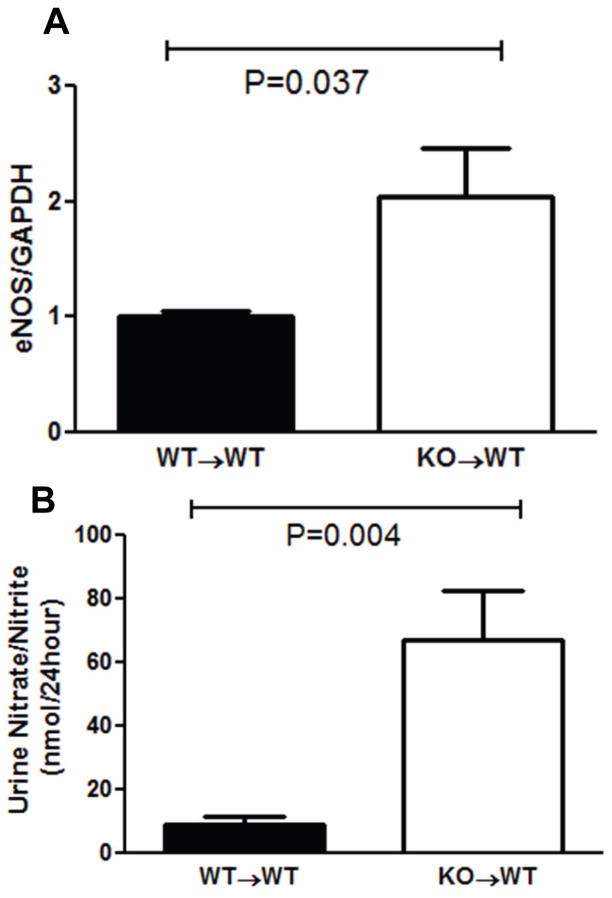
Next, we tested whether TNF-α produced by kidney cells directly regulates renal eNOS expression. Just as seen in the global TNF KOs, mRNA expression of eNOS was dramatically higher in the kidneys from Ang II-infused KO→WT mice than in WT→WT controls (2.04±0.43 vs. 1±0.05; P=0.037; Figure 6A). In turn, following 4 weeks of Ang II, KO→WT mice lacking TNF-α only in the kidney excreted 8-fold higher levels of urinary nitrate/nitrite compared to the WT→WTs (66.9±15.6 vs. 8.6±2.6 nmol/24hours; P=0.004; Figure 6B). These data suggest that kidney-derived TNF-α potentiates the chronic hypertensive response to Ang II by suppressing local eNOS expression and thereby diminishing local generation of NO.
Figure 6.
Renal generation of nitric oxide (NO) in kidney transplantation groups. A, mRNA expression of eNOS measured by real-time PCR in the transplanted kidney after 4 weeks of Ang II. B, Urinary excretion of NO in the transplant groups at day 25 of Ang II.
Discussion
The current study pinpoints with increased precision how effectors of the Th1 immune response contribute to blood pressure elevation and hypertensive CKD. T-bet is a Th1 cell-specific transcriptional factor that controls expression of the hallmark Th1 cytokines, IFN-γ and TNF-α.11 Accordingly, T-bet-deficient mice are unable to mount a Th1 immune response as illustrated in several models of autoimmunity.12, 13 In our model of hypertensive CKD, T-bet KO mice have a hypertensive response that is nearly identical to wild-type controls. In spite of this preserved hypertensive response to Ang II, T-bet deficiency limits the urinary excretion of nephrin, consistent with reduced glomerular podocyte loss in the absence of a Th1 immune response.6
Among the Th1 effectors, IFN-γ signaling has the capacity to regulate glomerular podocyte function in immune-mediated models of renal injury.14 Nevertheless, we were unable to detect differences in blood pressure or albuminuria between wild-type and IFN-γ KO mice during Ang II-dependent hypertension in the present studies. Thus, consistent with reports from other groups, our data do not implicate IFN-γ in potentiating blood pressure elevation or Th1-induced damage to the kidney glomerulus.3, 15
By contrast, we found that mice lacking TNF-α had a blunted hypertensive response and protection from target organ damage in our hypertensive CKD model. The finding that TNF-α promotes blood pressure elevation is consistent with observations from several in vivo models of hypertension both in the presence or absence of renal injury, suggesting that the effects of TNF-α to raise blood pressure during RAS activation do not depend on TNF-induced kidney damage.3, 16, 17 Conversely, as TNF-blockade2, 18 or T-bet deficiency6 can reduce kidney injury in hypertension without alterations in blood pressure, TNF-α appears to potentiate hypertensive renal damage independently of blood pressure.
TNF-α could potentially regulate blood pressure by acting in several cardiovascular control centers, including the immune system, the vasculature, the sympathetic nervous system, and the kidney. Within the immune system, TNF-α can control the expression of other cytokines such as IL-1 through the activation of NF-κB,19 and our preliminary studies point to a role for IL-1 receptor signaling in the pathogenesis of hypertension.20 Nevertheless, renal levels of IL-1 were similar between our WT and TNF KO groups, and the persistence of a full chronic hypertensive response in our T-bet-deficient animals suggests that TNF-α produced by Th1 T lymphocytes is not a major contributor to Ang II-induced blood pressure elevation.
Within the vasculature, TNF-α can act at the level of the vascular smooth muscle cell (VSMC) or the endothelium. In VSMCs, for example, TNF activates NADH leading to oxidative stress.21 Accordingly, TNF-α drives blood vessel remodeling in the airway during pulmonary inflammation,22 and TNF blockade relieves aortic stiffness detected in human patients with inflammatory arthropathies.23 Within the vascular endothelium, TNF-α induces adhesion molecules including ICAM-1 and VCAM-1 via an NF-κB-dependent pathway.24, 25 These pleiotropic effects of TNF-α in the vasculature might be expected to enhance susceptibility to a hypertensive stimulus. Nevertheless, TNF-deficiency had no impact on the acute in vivo vasopressor response in our experiments.
Within the nervous system, TNF-α can similarly potentiate sympathetic outflow by activating the NF-κB signaling pathway. Through this mechanism TNF-α generates reactive oxygen species in the paraventricular nucleus, which can have profound effects on the sensitivity to Ang II-induced blood pressure elevation.26, 27 In this regard, our kidney transplant model will admittedly underrepresent a contribution of the sympathetic nervous system (SNS) as the transplanted kidneys are denervated.8 Nevertheless, our transplanted wild-type animals mounted a robust blood pressure elevation in our hypertensive CKD model that was similar to our non-transplanted wild-type cohort, suggesting that the effects of the renal nerve on the hypertensive response in our particular model may be limited.
In the absence of measurable effects of immune-, vascular-, or SNS-derived TNF-α on the hypertensive response in our model, we focused the remainder of our experiments on a possible role of TNF-α derived specifically from the kidney in mediating Ang II-induced blood pressure elevation. We found that transplanted mice lacking TNF-α solely in the kidney had a markedly blunted chronic hypertensive response. Thus, TNF-α generated by kidney parenchymal cells contributes to the pathogenesis of hypertension in the setting of RAS activation.
TNF-α could act within several cell lineages in the kidney to modulate blood pressure.28 In juxtaglomerular cells, TNF-α regulates transcription of renin, which would impact the hypertensive response via RAS activation.29 However, in the current experiments renin expression in the kidney was not different between the Ang II-infused WT and TNF KO groups, which may reflect renin suppression in both groups by exogenous Ang II administration. Similarly, the Majid group has clearly demonstrated that TNF-α can provoke renal vasoconstriction by activating TNFR1 in the renal vasculature,30 but in our experiments the induction of an acute pressor response by Ang II did not require TNF-α.
We therefore focused on the possible actions of TNF-α in the renal epithelium, particularly as the thick ascending limb is a potent source of TNF-α in the setting of RAS activation 9 and TNF-α has been reported to have both suppressive and stimulatory effects on activity of the NKCC2 sodium co-transporter in this nephron segment 10, 31. Garvin and colleagues have reported that in the thick ascending limb, TNF-α negatively regulates expression of eNOS via the Rho/Rho kinase signaling pathway.10 Consistent with this notion, we find that deficiency of TNF-α in the kidney permits exaggerated renal eNOS expression leading to enhanced bioavailability of NO in the kidney. NO, in turn, acts to suppress blood pressure elevation as evidenced by the hypertensive phenotype of L-NAME-treated or eNOS-deficient animals.32, 33
Perspectives
Judging by the broad efficacy of angiotensin receptor blockers in treating large numbers of hypertensive patients with CKD, inappropriate activation of the RAS makes a critical contribution to blood pressure elevation and progressive renal injury. The current studies indicate that, in the setting of RAS activation, TNF-α produced by pro-inflammatory T-bet-expressing Th1 lymphocytes mediates kidney damage without impacting blood pressure whereas TNF-α produced by kidney parenchymal cells raises blood pressure, likely through the suppression of local nitric oxide generation. Given the availability of TNF antagonists for the treatment of human inflammatory diseases, pinpointing the precise cell lineages through which TNF mediates pathological cardiovascular effects will be paramount, particularly as global, untargeted TNF blockade at higher doses may be detrimental in patients with cardiac dysfunction 34. By elucidating cell-specific actions of TNF in the pathogenesis of hypertension and CKD, the current studies should aid the design of more targeted therapeutics to confront these highly prevalent cardiovascular diseases.
Supplementary Material
Figure S1. IFN-γ-deficient mice have preserved hypertensive and albuminuric responses to chronic Ang II infusion. A, Systolic blood pressures measured by tailcuff in wild-type (WT, circles), and IFN-γ-deficient (KO, squares) cohorts at baseline (“pre”) and during 3 weeks of Ang II infusion. n = 9 per group. B, Urinary albumin excretion (μg/mg creatinine) in the experimental groups after 25 days of Ang II.
Figure S2. TNF-α-deficiency does not impact the acute pressor response to Ang II. Changes in mean arterial blood pressure in mice under anesthesia injected with increasing doses of Ang II or a single dose of epinephrine. N ≥ 6 per group.
Figure S3. TNF WT and KO mice ingest similar levels of food and water in hypertension model. Uni-nephrectomized mice were placed into metabolic cages and provided a gel food as only source of water and nutrients for 1 week prior to and 2 weeks after initiation of chronic Ang II infusion. During period when blood pressures in Ang II-infused TNF WT and KO groups diverged, food and water ingestion (“food intake”) was virtually identical in the 2 groups. N ≥ 7 per group.
Figure S4. Renal T cell infiltration in TNF transplant groups during Ang II-dependent hypertension. Following 4 weeks of Ang II-induced hypertension, kidney sections from TNF transplant groups were stained with anti-CD3. Numbers of CD3+ T cells surrounding the renal vessels were scored in blinded fashion. On top, representative images with T cells stained brown. On bottom, proportion of renal vessels from the groups surrounded by 0 to 9, 10 to 29, or ≥30 CD3 T cells, respectively.
Novelty and Significance.
What is New?
The mechanisms through which Th1 immune responses direct kidney damage in hypertension during RAS activation are not established. The in vivo contribution of TNF-α in the kidney to angiotensin II-dependent blood pressure elevation requires elucidation.
What is Relevant?
Understanding the precise actions of Th1 cytokines in kidney injury and blood pressure elevation is critical as antagonists of cytokine signaling are now in clinical use.
Summary
The Th1 immune response mediates damage to the kidney in hypertension through the actions of TNF-α. By contrast, TNF-α produced by renal parenchymal cells potentiates blood pressure elevation by suppressing nitric oxide generation during RAS activation.
Acknowledgments
The authors acknowledge administrative support from Ms. Gay Gaster.
Sources of Funding
This work was supported by funding from (1) National Institutes of Health Grant DK087893-01, (2) the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, Grant BX000893-01A2 and IK2BX002240, (3) the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research, and (4) a Grant-in-Aid and Postdoctoral Fellowship from the American Heart Association (12POST11910012).
Footnotes
Disclosures
None.
References
- 1.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 2.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin ii-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin ii-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin ii-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, Sparks MA, Yan M, Howell DN, Gomez JA, Spurney RF, Coffman TM, Crowley SD. A novel role for type 1 angiotensin receptors on t lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110:1604–1617. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim H-S, Smithies O, Le TH, Coffman TM. Angiotensin ii causes hypertension and cardiac hypertrophy through its receptors in the kidney. PNAS. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreri NR, Escalante BA, Zhao Y, An SJ, McGiff JC. Angiotensin ii induces tnf production by the thick ascending limb: Functional implications. Am J Physiol. 1998;274:F148–155. doi: 10.1152/ajprenal.1998.274.1.F148. [DOI] [PubMed] [Google Scholar]
- 10.Ramseyer VD, Hong NJ, Garvin JL. Tumor necrosis factor alpha decreases nitric oxide synthase type 3 expression primarily via rho/rho kinase in the thick ascending limb. Hypertension. 2012;59:1145–1150. doi: 10.1161/HYPERTENSIONAHA.111.189761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of t-bet in th1 lineage commitment and ifn-gamma production in cd4 and cd8 t cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 12.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor t-bet controls regulatory t cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of t-bet, but not stat1, prevents the development of experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han GD, Koike H, Nakatsue T, Suzuki K, Yoneyama H, Narumi S, Kobayashi N, Mundel P, Shimizu F, Kawachi H. Ifn-inducible protein-10 has a differential role in podocyte during thy 1.1 glomerulonephritis. J Am Soc Nephrol. 2003;14:3111–3126. doi: 10.1097/01.asn.0000097371.64671.65. [DOI] [PubMed] [Google Scholar]
- 15.Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Muller DN. Interferon-gamma signaling inhibition ameliorates angiotensin ii-induced cardiac damage. Hypertension. 2012;60:1430–1436. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 16.Sriramula S, Haque M, Majid DSA, Francis J. Involvement of tumor necrosis factor-{alpha} in angiotensin ii-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2010;56:643–649. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. Tnf-alpha inhibition reduces renal injury in doca-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner NA, Mughal RS, Warburton P, O’Regan DJ, Ball SG, Porter KE. Mechanism of tnfalpha-induced il-1alpha, il-1beta and il-6 expression in human cardiac fibroblasts: Effects of statins and thiazolidinediones. Cardiovascular research. 2007;76:81–90. doi: 10.1016/j.cardiores.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Patel MB, Griffiths R, Spurney RF, Crowley SD. Activation of the interleukin-1 receptor contributes to the pathogenesis of angiotensin ii-dependent hypertension by regulating sodium excretion. Hypertension. 2012;60:A169. [Google Scholar]
- 21.De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, Griendling KK. Tumour necrosis factor alpha activates a p22phox-based nadh oxidase in vascular smooth muscle. Biochem J. 1998;329 (Pt 3):653–657. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. Tnf-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: A controlled study. Hypertension. 2010;55:333–338. doi: 10.1161/HYPERTENSIONAHA.109.143982. [DOI] [PubMed] [Google Scholar]
- 24.Dhawan S, Singh S, Aggarwal BB. Induction of endothelial cell surface adhesion molecules by tumor necrosis factor is blocked by protein tyrosine phosphatase inhibitors: Role of the nuclear transcription factor nf-kappa b. Eur J Immunol. 1997;27:2172–2179. doi: 10.1002/eji.1830270909. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Z, Gengaro P, Wang W, Wang XQ, Li C, Faubel S, Rivard C, Schrier RW. Role of nf-kappab and pi 3-kinase/akt in tnf-alpha-induced cytotoxicity in microvascular endothelial cells. Am J Physiol Renal Physiol. 2008;295:F932–941. doi: 10.1152/ajprenal.00066.2008. [DOI] [PubMed] [Google Scholar]
- 26.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. Tnf-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 27.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin ii-induced hypertension is modulated by nuclear factor-kappabin the paraventricular nucleus. Hypertension. 2012;59:113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramseyer VD, Garvin JL. Tumor necrosis factor-alpha: Regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 2013;304:F1231–1242. doi: 10.1152/ajprenal.00557.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todorov V, Muller M, Schweda F, Kurtz A. Tumor necrosis factor-alpha inhibits renin gene expression. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1046–1051. doi: 10.1152/ajpregu.00142.2002. [DOI] [PubMed] [Google Scholar]
- 30.Castillo A, Islam MT, Prieto MC, Majid DS. Tumor necrosis factor-alpha receptor type 1, not type 2, mediates its acute responses in the kidney. Am J Physiol Renal Physiol. 2012;302:F1650–1657. doi: 10.1152/ajprenal.00426.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battula S, Hao S, Pedraza PL, Stier CT, Ferreri NR. Tumor necrosis factor-α is an endogenous inhibitor of na+-k+-2cl− cotransporter (nkcc2) isoform a in the thick ascending limb. American Journal of Physiology - Renal Physiology. 2011;301:F94–F100. doi: 10.1152/ajprenal.00650.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90:278–281. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT Investigators ftA. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: Results of the anti-tnf therapy against congestive heart failure (attach) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. IFN-γ-deficient mice have preserved hypertensive and albuminuric responses to chronic Ang II infusion. A, Systolic blood pressures measured by tailcuff in wild-type (WT, circles), and IFN-γ-deficient (KO, squares) cohorts at baseline (“pre”) and during 3 weeks of Ang II infusion. n = 9 per group. B, Urinary albumin excretion (μg/mg creatinine) in the experimental groups after 25 days of Ang II.
Figure S2. TNF-α-deficiency does not impact the acute pressor response to Ang II. Changes in mean arterial blood pressure in mice under anesthesia injected with increasing doses of Ang II or a single dose of epinephrine. N ≥ 6 per group.
Figure S3. TNF WT and KO mice ingest similar levels of food and water in hypertension model. Uni-nephrectomized mice were placed into metabolic cages and provided a gel food as only source of water and nutrients for 1 week prior to and 2 weeks after initiation of chronic Ang II infusion. During period when blood pressures in Ang II-infused TNF WT and KO groups diverged, food and water ingestion (“food intake”) was virtually identical in the 2 groups. N ≥ 7 per group.
Figure S4. Renal T cell infiltration in TNF transplant groups during Ang II-dependent hypertension. Following 4 weeks of Ang II-induced hypertension, kidney sections from TNF transplant groups were stained with anti-CD3. Numbers of CD3+ T cells surrounding the renal vessels were scored in blinded fashion. On top, representative images with T cells stained brown. On bottom, proportion of renal vessels from the groups surrounded by 0 to 9, 10 to 29, or ≥30 CD3 T cells, respectively.