Abstract
Lower endogenous levels of the neuropeptide oxytocin may be an important biological predictor of social cognition impairments in schizophrenia (SZ). Prior studies have demonstrated that lower-level social cognitive processes (e.g., facial affect perception) are significantly associated with reduced plasma oxytocin levels in SZ; however, it is unclear whether higher-level social cognition, which requires inferential processes and knowledge not directly presented in the stimulus, is associated with endogenous oxytocin. The current study explored the association between endogenous oxytocin levels and lower- and higher-level social cognition in 40 individuals diagnosed with SZ and 22 demographically matched healthy controls (CN). All participants received the Social Cue Recognition Test (SCRT), which presents participants with videotaped interpersonal vignettes and subsequent true/false questions related to concrete or abstract aspects of social interactions in the vignettes. Results indicated that SZ had significantly higher plasma oxytocin concentrations than CN. SZ and CN did not differ on SCRT hits, but SZ had more false positives and lower sensitivity scores than CN. Higher plasma oxytocin levels were associated with better sensitivity scores for abstract items in CN and fewer false positives for concrete items in individuals with SZ. Findings indicate that endogenous oxytocin levels predict accurate encoding of lower-level socially relevant information in SZ.
Keywords: Oxytocin, Social Cognition, Psychosis, Interpersonal
1.0. Introduction
Although social cognition has been defined in several ways, it typically refers to the ability to perform mental processes that underlie social interactions, including interpreting, perceiving, and generating appropriate responses to the behaviors, dispositions, and intentions of others (Green et al., 2008). Individuals with schizophrenia (SZ) display impairments in multiple aspects of social cognition, and these deficits predict community-based social and vocational outcome (Horan et al., 2012; Couture et al., 2006). Impairments in social cognition have been found to load onto two distinct factors: lower-level and higher-level processes (Sergi et al., 2007). Lower-level social cognition involves evaluating and accurately encoding objective, socially relevant information from an immediately available stimulus (e.g., facial affect perception). Higher-level social cognition requires inferential processing and the ability to use knowledge not directly presented in a stimulus to make judgments about the thoughts, emotions, and intentions of others (e.g., theory of mind).
In mammals, there is evidence that the neuropeptide oxytocin plays a critical role in various aspects of social cognition and social interaction (Dantzer et al., 1990; Dickinson, 1988; Dluzen et al., 1998; Wacker and Ludwig, 2012). Relatively few studies have examined whether endogenous oxytocin levels are abnormal in people with SZ. Those studies that have been conducted have yielded inconsistent results, with the majority indicating no group differences (Rubin et al., 2014; Rubin et al., 2013; Rubin et al., 2011; Rubin et al., 2010) and some reporting lower (Goldman et al., 2008; Goldman et al., 2011) or higher endogenous oxytocin concentrations in people with SZ compared to controls (Beckmann et al., 1985). Inconsistencies in endogenous oxytocin levels among studies may reflect differences in evaluating peripheral vs. cerebrospinal fluid levels, sample-related differences in demographics (e.g., sex, age, race), the proportion of participants taking different antipsychotics, differences in disease chronicity, and the proportion of participants displaying neuroendocrine dysfunction (e.g., polydipsia). Despite these inconsistencies regarding group-level differences among studies, lower endogenous oxytocin has consistently been associated with impairments in social cognition, especially lower-level processes such as facial affect perception (Rubin et al., 2011; Goldman et al., 2008). Lower endogenous oxytocin also predicts poor social functioning and greater severity of positive and negative symptoms (Goldman et al., 2008; Keri et al., 2009; Rubin et al., 2011; Rubin et al., 2010; Strauss, in press; Walss- Bass et al., 2013). Intranasal administration of oxytocin has generally shown beneficial effects on social cognition, with many studies showing effects on lower-level social cognitive processes and several on higher-order social cognition (Averbeck et al., 2012; Davis et al., 2014; Davis et al., 2013; Feifel et al., 2010; Fischer-Shofty et al., 2013a; Fischer-Shofty et al., 2013b; Gibson et al., 2014; Pedersen et al., 2011; Woolley et al., 2014); however, not all studies have reported significant improvements on social cognition (Horta de Macedo et al., 2014; Lee et al., 2013). Furthermore, one study comparing differential effects of oxytocin on higher and lower-level social cognition demonstrated improvements specific to higher-order processes (Wooley et al., 2014). Thus, oxytocin may be critically linked to impairments in social cognition and social functioning in people with SZ; however, additional work is needed to determine whether lower- or higher-level social cognition is most highly associated with oxytocin.
In the current study, we extended the literature on social cognition and oxytocin by administering a well-validated measure, the Social Cue Recognition Test (SCRT: Corrigan and Green, 1993), which requires participants to watch brief video-taped vignettes of social interactions and then respond to a series of questions pertaining to concrete or abstract aspects of the interaction. Importantly, the SCRT’s use of concrete items allowed us to replicate prior associations between lower-level social cognition and endogenous oxytocin (Rubin et al., 2011; Goldman et al., 2008), and extend prior findings by also examining higher-level social cognition (abstract items). The SCRT is ideal for evaluating differential associations between oxytocin and lower- and higher-level social cognition because it evaluates these processes within a single paradigm that uses identical stimulus presentation and response formats across conditions. In line with prior studies, we hypothesized that people with SZ would have lower plasma oxytocin levels than healthy controls (CN) (Goldman et al., 2008, 2011), and that lower endogenous oxytocin would be associated with poorer SCRT performance for both concrete and abstract items.
2.0. Method
2.1. Participants
Participants included 40 individuals meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (4th ed., text rev.; DSM–IV–TR; American Psychiatric Association, 2000) criteria for schizophrenia (n = 35) or schizoaffective disorder (n = 5) (SZ) and 22 healthy controls (CN).
Individuals with SZ were recruited from the outpatient research program at the Maryland Psychiatric Research Center (MPRC) and evaluated during periods of clinical stability. Consensus diagnosis was established via a best-estimate approach based on review of medical records, multiple interviews, and subsequently confirmed using the Structured Clinical Interview for DSM-IV (SCID: First et al., 1997). All participants with SZ were prescribed antipsychotic medications at the time of testing, including either alone (clozapine, n=13; haloperidol, n=5; ziprasidone, n=3; aripiprazole, n=2; fluphenazine, n=2; olanzapine, n=2; risperidone, n=2; chlorpromazine, n=1; quetiapine, n=1; thioridazine, n=1) or in combination with another antipsychotic (clozapine and risperidone, n=5; clozapine and haloperidol, n=1; clozapine and quetiapine, n=1; haloperidol, aripiprazole, and clonazepam, n=1). All participants with SZ were assessed after a minimum period of 4 weeks of stable treatment.
CN participants were recruited through random-digit dialing and word of mouth among enrolled participants. All controls underwent a screening interview, including the SCID-I and SCID-II (Pfohl et al., 1997), and did not meet lifetime criteria for a psychotic disorder or any current Axis I disorder. Controls had no family history of SZ and did not meet DSM-IV criteria for substance use disorders. Lack of recent substance use was confirmed by urine toxicology at the time of testing. Participants were also screened for lifetime neurological disorders and were free from significant neurological conditions. As pregnancy can affect oxytocin levels, female participants completed a screen; no participants were pregnant.
SZ and CN groups did not significantly differ in age, parental education, sex, or ethnicity; SZ had lower personal education than CN (see Table 1).
Table 1.
Participant Demographic and Clinical Characteristics
| SZ (n = 40) | CN (n = 22) | Test-statistic, p-value | |
|---|---|---|---|
| Age | 43.73 (11.85) | 43.14 (9.44) | F = 0.05, p = 0.81 |
| Participant Education | 12.95 (2.08) | 15.05 (1.86) | F = 15.47, p < 0.001 |
| Parental Education | 13.46 (2.45) | 14.18 (2.42) | F = 1.03, p = 0.32 |
| % Male | 70.7% | 68.2% | χ2 = 0.04, p = 0.83 |
| Ethnicity | χ2 = 1.12, p = 0.77 | ||
| % Caucasian | 90.2% | 95.5% | |
| % African-American | 4.9% | 4.5% | |
| % Native-American | 2.4% | 0% | |
| % Bi-racial | 2.4% | 0% | |
| Plasma Oxytocin (pg/ml) | 24.46 (7.54) | 19.66 (5.86) | F = 6.69, p < 0.02 |
| Symptoms | |||
| BNSS Total | 26.08 (16.97) | -- | -- |
| BPRS Total | 38.97 (9.09) | -- | -- |
| BPRS Positive | 2.41 (1.13) | -- | -- |
| BPRS Negative | 2.25 (1.12) | -- | -- |
| BPRS Disorganized | 1.51 (0.45) | -- | -- |
| Functional Outcome | |||
| LOF Total | 18.32 (7.01) | -- | -- |
| LOF Social | 4.55 (2.57) | -- | -- |
| LOF Work | 1.79 (2.60) | -- | -- |
Note. BNSS = Brief negative Symptom Scale; BPRS = Brief Psychiatric Rating Scale; LOF = Level of Function Scale
2.2. Procedures
Participants completed a standard clinical interview that was performed by a clinical psychologist (GPS) trained to MPRC reliability standards (reliability >0.80). After this interview, participants with SZ were rated on the Brief Negative Symptom Scale (Kirkpatrick et al., 2011; Strauss et al., 2012a; Strauss et al., 2012b), Brief Psychiatric Rating Scale (Overall and Gorham, 1962), and Level of Function Scale (Hawk et al., 1975).
Plasma oxytocin levels were determined by radioimmunoassay in extracted samples using a magnetic bead kit from Phoenix Pharmaceuticals, Inc. Samples were assayed in duplicate; the average of these samples was taken as the final oxytocin concentration. Assay sensitivity was 5 pg/ml, with minimal cross reactivity with vasopressin. The coefficient of variation averaged 5–8% across the assay.
2.3. Measures
The Social Cue Recognition Test (SCRT: Corrigan and Green, 1993) was administered to evaluate social cognition. The SCRT includes 8 brief (2–3 minutes) videotaped interpersonal vignettes. Four of the videos involve interpersonal interactions that are characterized by high emotional expressivity (e.g., a verbal argument); the other 4 are characterized by low emotional expressivity (e.g., a casual conversation). After watching each video, the scenario is recapped with the participant and they are asked to complete a series of true/false questions related to concrete or abstract aspects of the vignette. Concrete cues assess lower-level social cognition and evaluate accurate encoding of objective, socially relevant information (e.g., Mark and Sally were looking over a book together). Abstract cues assess higher-level social cognition and require inferential processing including the ability to use knowledge not directly presented in the stimulus to make judgments about the thoughts, emotions, and intentions of others (e.g., Carl felt hurt because Mark and Sally would not talk to him).
Several scores are calculated for the SCRT. Hits (i.e., correct true responses) and false positives (FP) (incorrect true responses) are calculated across two conditions: abstract and concrete cues. As done in prior SCRT studies in SZ (Corrigan and Green, 1993), a measure of sensitivity (A′) was also calculated using the formula:
Sensitivity reflects the proportion of true responses that were accurately identified as such. These scores range from 0 (low) to 1 (high). People with SZ have reliably evidenced lower sensitivity than CN in prior SCRT studies (e.g., Corrigan and Green, 1993; Corrigan and Nelson, 1998; Roberts et al., 2010).
3.0. Results
3.1. Endogenous Oxytocin Levels
One-way ANOVA indicated that participants with SZ had significantly higher plasma oxytocin levels than CN, F (1, 60) = 6.69, p <0.02 (see Table 1).
3.2. Social Cue Recognition
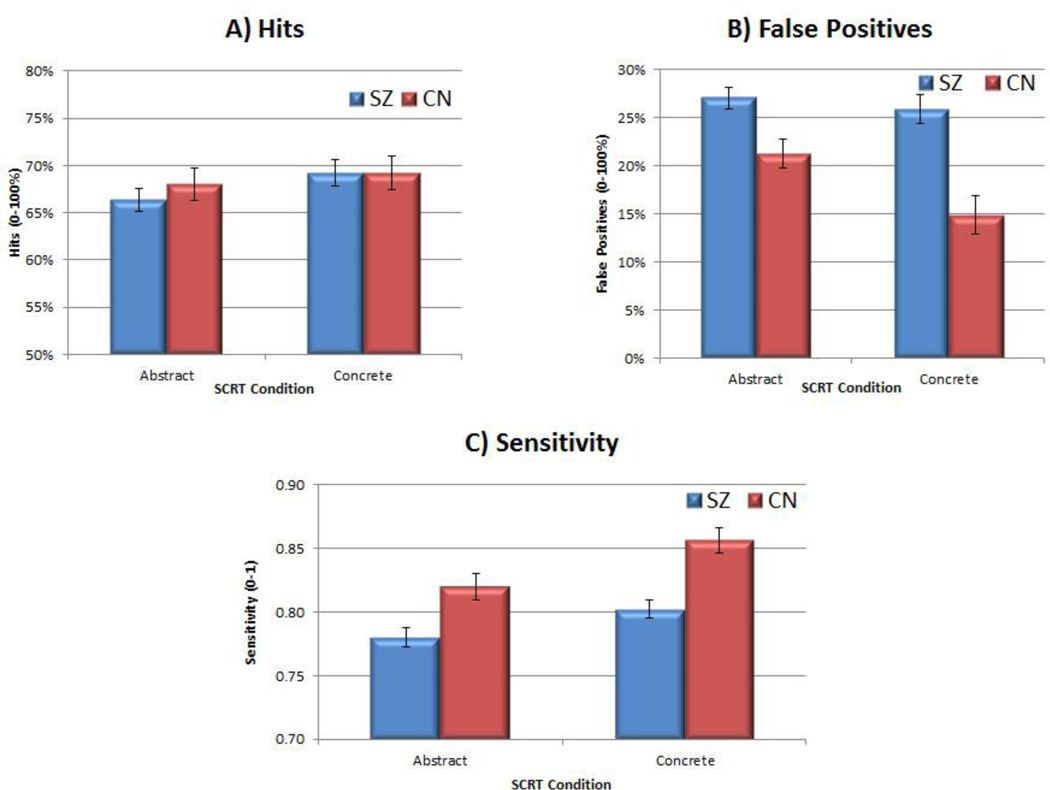
Figure 1 presents mean SCRT performance for CN and SZ participants for hits, false positives, and sensitivity in relation to abstract and concrete cues. MANOVA examining hit rate for abstract and concrete cues indicated a nonsignificant effect of Group, F (2, 59) = 0.56, p = 0.58, and the individual effects for abstract and concrete cues were also nonsignificant. MANOVA examining false positive rates revealed a significant overall Group effect, F (2, 59) = 10.41, p < 0.001, as well as significant individual effects for concrete, F (1, 60) = 19.30, p <0.001, and abstract, F (1, 60) = 9.49, p < 0.01, cues. MANOVA also revealed a significant Group effect for sensitivity (A′) scores, F (2, 59) = 9.59, p < 0.001, with significant group differences for both abstract, F (1, 60) = 9.60, p < 0.001, and concrete, F (1, 60) = 19.35, p < 0.001, cues.
Figure 1.
Social Cue Recognition Test Performance in Control and Schizophrenia Groups
Note. Error bars reflect standard error. SZ = schizophrenia; CN = Control
3.3. Correlations
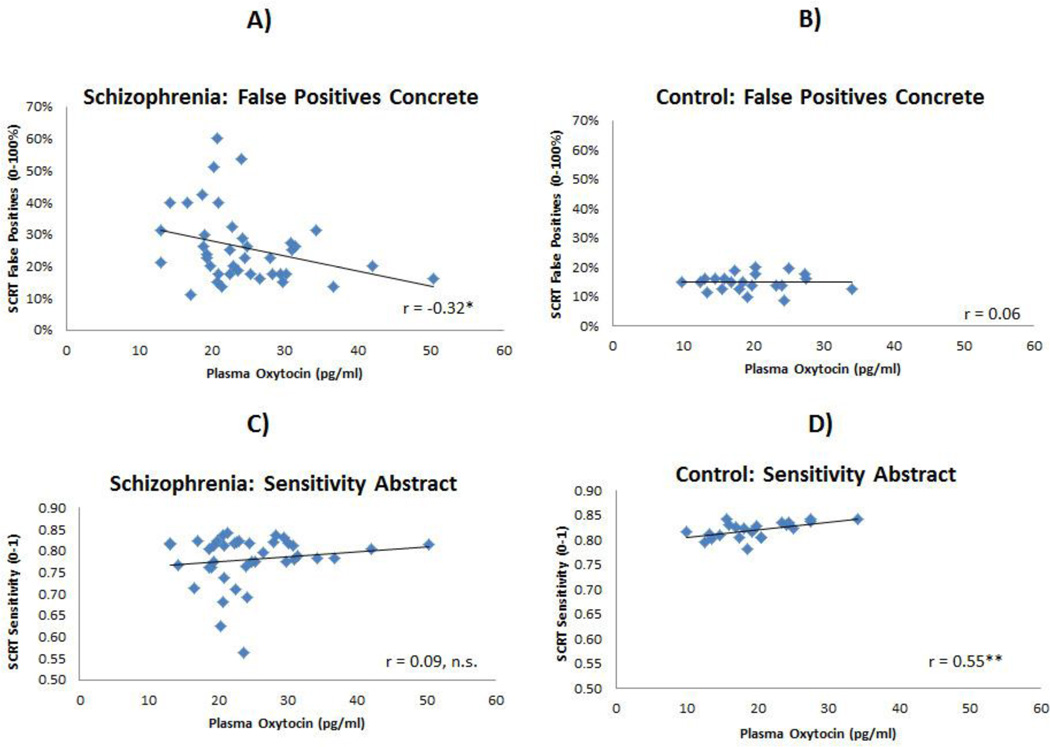
Spearman correlations indicated that higher plasma oxytocin levels were associated with higher (better) sensitivity scores for abstract cues in CN and a higher rate of false positives for concrete cues in individuals with SZ (see Table 2)1. There were no differential associations between oxytocin and any of these variables in male or female CN or SZ participants; however, the current samples were likely underpowered to adequately look at sex differences. The correlation between oxytocin and chlorpromazine equivalent dosage (Woods, 2003) was nonsignificant. Correlations between plasma oxytocin levels and measures of symptoms and functional outcome for this sample are reported in Strauss et al. (in press).
Table 2.
Correlations between Social Cognition and Plasma Oxytocin Levels
| Schizophrenia (n = 40) | |
| False positives | |
| Abstract | −0.23 |
| Concrete | −0.32* |
| Hits | |
| Abstract | −0.13 |
| Concrete | −0.14 |
| Sensitivity | |
| Abstract | 0.09 |
| Concrete | 0.24 |
| Control (n = 22) | |
| False positives | |
| Abstract | −0.42 |
| Concrete | 0.06 |
| Hits | |
| Abstract | 0.29 |
| Concrete | 0.14 |
| Sensitivity | |
| Abstract | 0.55** |
| Concrete | 0.15 |
Note.
p < 0.05.
p< 0.01
4.0. Discussion
Results were partially consistent with hypotheses. Although we hypothesized a significant correlation between oxytocin and both SCRT conditions in SZ, a significant association was only observed between endogenous oxytocin levels and concrete cues. The significant correlation observed in the SZ group is consistent with past studies reporting an association between endogenous oxytocin and lower-level social cognition in SZ using measures of facial affect perception (Rubin et al., 2011; Goldman et al., 2008). Interestingly, the pattern of correlations differed between groups. In CN, lower endogenous oxytocin was associated with poorer performance on abstract cues, which require higher-level social cognition. The reason for differential patterns of correlations between groups in unclear; however, these findings offer an interesting possibility that endogenous oxytocin may be selectively associated with lower- and not higher-level social cognition in SZ. Future studies using multiple measures are needed to test this possibility fully.
Overall, our results add to a growing literature suggesting that oxytocin may play a vital role in social cognition in SZ. However, the findings should be viewed in light of certain limitations, including relatively small sample sizes per group, use of only one measure of higher- and lower-level social cognition, and inclusion of only chronic outpatients. All participants with SZ were also treated with antipsychotics. Drugs that block dopamine receptors may increase plasma oxytocin levels (Kiss et al., 2010). It is therefore possible that the elevated oxytocin values in the SZ group are due to the use of antipsychotics in the SZ but not the CN group; however, chlorpromazine equivalent dosage was not significantly associated with plasma oxytocin, potentially suggesting minimal influence of antipsychotics. Furthermore, different assay methods may contribute to inconsistent findings regarding whether endogenous oxytocin levels are abnormal in people with SZ. The current study used a radioimmunoassay with extraction; however, enzyme immunoassay without extraction may offer better sensitivity and specificity for oxytocin (Carter et al., 2007). It is possible that different assay methods may produce different results. It is also not clear whether peripheral oxytocin levels reflect brain oxytocin levels in the relevant neural substrates for this task, so caution is needed when trying to extrapolate from peripheral levels of oxytocin to brain oxytocin mechanisms. Finally, the current study did not measure oxytocin receptor function. Higher oxytocin levels in the SZ group could represent a compensatory response to a lower sensitivity of the oxytocin receptor to circulating levels of the hormone. Future studies assessing receptor function may be valuable in disentangling the meaning of the elevated plasma oxytocin levels. Despite these limitations, oxytocin may represent a novel therapeutic target in SZ given recent evidence for significant improvements in social cognition and social outcome following single or repeated intranasal administration (Averbeck et al., 2012; Davis et al., 2014; Davis et al., 2013; Feifel et al., 2010; Fischer-Shofty et al., 2013a; Fischer-Shofty et al., 2013b; Gibson et al., 2014; Pedersen et al., 2011; Woolley et al., 2014).
Figure 2.
Scatterplots Depicting Highlighted Correlations between Oxytocin and Social Cognition in Schizophrenia and Control Groups
Note. * p < 0.05; **p < 0.01
Acknowledgments
The authors would like to thank the participants who completed the study, as well as staff at the Maryland Psychiatric Research Center who contributed to data collection. We are especially thankful to Dana Brady for processing oxytocin levels and members of Dr. Strauss’ team who conducted subject recruitment and testing: Lauren Catalano, Adam Culbreth, Bern Lee, Jamie Adams, Travis White, Tehreem Galani, and Carol Vidal.
Role of Funding Source.
Research supported in part by US National Institutes of Mental Health Grant P50-MH082999 (WT Carpenter) and a Department of Veterans Affairs Mental Illness Research Education Clinical Centers VISN 5 pilot grant (GP Strauss)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
When plasma oxytocin levels were log transformed, correlations remained significant for SZ false positives concrete items (r = −0.31, p < 0.05) and CN sensitivity abstract items (r = 0.53, p < 0.02).
Conflict of Interest
Authors have no conflicts of interest relevant to the current study.
Contributors
Gregory Strauss, William Keller, Robert Buchanan, James Koenig, and James Gold designed the study. Statistical analyses and writing of the first draft of the manuscript were performed by Gregory Strauss. James Koenig and his lab conducted oxytocin radioimmunoassays. All authors contributed to and approved the final manuscript.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., text rev.) Washington, DC: Author; 2000. [Google Scholar]
- Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol. Med. 2012;42(2):259–266. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann H, Lang RE, Gattaz WF. Vasopressin--oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocr. 1985;10(2):187–191. doi: 10.1016/0306-4530(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Davis MC, Green MF, Lee J, Horan WP, Senturk D, Clarke AD, Marder SR. Oxytocin-augmented social cognitive skills training in schizophrenia. Neuropsychopharmacol. 2014;39(9):2070–2077. doi: 10.1038/npp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF, Marder SR. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr. Res. 2013;147(2–3):393–397. doi: 10.1016/j.schres.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol. Psychiatry. 2010;68(7):678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Brune M, Ebert A, Shefet D, Levkovitz Y, Shamay-Tsoory SG. Improving social perception in schizophrenia: the role of oxytocin. Schizophr. Res. 2013a;146(1–3):357–362. doi: 10.1016/j.schres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Shamay-Tsoory SG, Levkovitz Y. Characterization of the effects of oxytocin on fear recognition in patients with schizophrenia and in healthy controls. Front. Neurosci. 2013b;7:127. doi: 10.3389/fnins.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, Pedersen CA. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophr Res. 2014;156(2–3):261–265. doi: 10.1016/j.schres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Goldman M, Marlow-O'Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr. Res. 2008;98(1–3):247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacol. (Berl) 2011;216(1):101–110. doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- Hawk AB, Carpenter WT, Strauss JS. Diagnostic criteria and five-year outcome in schizophrenia. A report from the International Pilot Study of schizophrenia. Arch. Gen. Psychiatry. 1975;32(3):343–347. doi: 10.1001/archpsyc.1975.01760210077005. [DOI] [PubMed] [Google Scholar]
- Horta de Macedo LR, Zuardi AW, Machado-de-Sousa JP, Chagas MH, Hallak JE. Oxytocin does not improve performance of patients with schizophrenia and healthy volunteers in a facial emotion matching task. Psychiatry Res. 2014 doi: 10.1016/j.psychres.2014.07.082. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I, Kelemen O. Sharing secrets: oxytocin and trust in schizophrenia. Soc. Neurosci. 2009;4(4):287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, Marder SR. The brief negative symptom scale: psychometric properties. Schizophr. Bull. 2011;37(2):300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Bundzikova J, Pirnik Z, Mikkelsen JD. Different antipsychotics elicit different effects on magnocellular oxytocinergic and vasopressinergic neurons as revealed by Fos immunohistochemistry. J. Neurosci. Res. 2010;88(3):677–685. doi: 10.1002/jnr.22226. [DOI] [PubMed] [Google Scholar]
- Lee MR, Wehring HJ, McMahon RP, Linthicum J, Cascella N, Liu F, Bellack A, Buchanan RW, Strauss GP, Contoreggi C, Kelly DL. Effects of adjunctive intranasal oxytocin on olfactory identification and clinical symptoms in schizophrenia: results from a randomized double blind placebo controlled pilot study. Schizophr. Res. 2013;145(1–3):110–115. doi: 10.1016/j.schres.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol. Rep. 1962;10:799–812. [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr. Res. 2011;132(1):50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Pfohl BM, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality, 1 ed. American Psychiatric Publishing, Inc.; 1997. [Google Scholar]
- Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Drogos LL, Hill SK, Ruocco AC, Keedy SK, Reilly JL, Keshavan MS, Pearlson GD, Tamminga CA, Gershon ES, Sweeney JA. Reduced Levels of Vasopressin and Reduced Behavioral Modulation of Oxytocin in Psychotic Disorders. Schizophr. Bull. 2014 doi: 10.1093/schbul/sbu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Bishop JR, Pournajafi-Nazarloo H, Harris MS, Hill SK, Reilly JL, Sweeney JA. Peripheral vasopressin but not oxytocin relates to severity of acute psychosis in women with acutely-ill untreated first-episode psychosis. Schizophr. Res. 2013;146(1–3):138–143. doi: 10.1016/j.schres.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr. Res. 2011;130(1–3):266–270. doi: 10.1016/j.schres.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr. Res. 2010;124(1–3):13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Hong LE, Gold JM, Buchanan RW, McMahon RP, Keller WR, Fischer BA, Catalano LT, Culbreth AJ, Carpenter WT, Kirkpatrick B. Factor structure of the Brief Negative Symptom Scale. Schizophr. Res. 2012a;142(1–3):96–98. doi: 10.1016/j.schres.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, Catalano LT, Culbreth AJ, Carpenter WT, Kirkpatrick B. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr. Res. 2012b;142(1–3):88–92. doi: 10.1016/j.schres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Koenig JI, Gold JM, Ossenfort KL, Buchanan RW. Plasma oxytocin levels predict olfactory identification and negative symptoms in individuals with schizophrenia. Schizophr. Res. doi: 10.1016/j.schres.2014.12.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walss-Bass C, Fernandes JM, Roberts DL, Service H, Velligan D. Differential correlations between plasma oxytocin and social cognitive capacity and bias in schizophrenia. Schizophr. Res. 2013;147(2–3):387–392. doi: 10.1016/j.schres.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Chuang B, Lam O, Lai W, O'Donovan A, Rankin KP, Mathalon DH, Vinogradov S. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocr. 2014;47:116–125. doi: 10.1016/j.psyneuen.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]