Abstract
Influenza A viruses are a significant cause of morbidity and mortality worldwide, particularly among young children and the elderly. Current vaccines induce neutralizing antibody responses directed toward highly variable viral surface proteins, resulting in limited heterosubtypic protection to new viral serotypes. By contrast, memory CD4 T cells recognize conserved viral proteins and are cross-reactive to multiple Influenza strains. In humans, Influenza-specific memory CD4 T cells were found to be the protective correlate in Influenza challenge studies, suggesting their key role in protective immunity. In mouse models, memory CD4 T cells can mediate protective responses to secondary Influenza infection independent of B cells or CD8 T cells, and can influence innate immune responses. Importantly, a newly defined, tissue-resident CD4 memory population has been demonstrated to be retained in lung tissue and promote optimal protective responses to Influenza infection. Here, we will review the generation of memory CD4 T cells following primary Influenza infection as well as mechanisms for their enhanced efficacy in protection from secondary challenge, focusing on their phenotype, localization and function in the context of both mouse models and human infection. We will also discuss the generation of memory CD4 T cells in response to Influenza vaccines and future implications for vaccinology.
1 Introduction
Infection with Influenza A viruses results in moderate to severe acute respiratory illness and is a significant cause of morbidity and mortality worldwide, particularly in children under five and adults over 65 (Thompson et al. 2006). In addition, the annual economic burden associated with Influenza infection in the United States is more than $85 billion dollars (Molinari et al. 2007). Although vaccines for Influenza are available, due to multiple factors, including variations in Influenza strains and variable induction of protective immune responses in vaccine recipients, current vaccines are not completely protective against infection with seasonal strains and are ineffective at protecting against emerging new or pandemic strains (Osterholm et al. 2012). Therefore, identifying the immune mechanisms underlying the host response to infection is a priority in the rational design of future vaccines and therapeutics for Influenza.
Current Influenza vaccines promote protective immunity to infection through the generation of neutralizing antibody responses to hemagglutinin (HA) and neuraminidase (NA) viral surface glycoproteins. Due to a combination of antigenic drift and shift, HA and NA proteins exhibit profound variations in protein sequence and antigenicity in different Influenza strains. As a result, antibody responses typically provide limited cross-protection against new viral serotypes, leading to the requirement for new vaccine formulations annually. Immunity that is cross-protective between Influenza strains expressing distinct HA and NA serotypes is termed heterosubtypic immunity. Importantly, memory T cells generated following Influenza infection have been demonstrated to mediate heterosubtypic immune responses to distinct viral strains via the targeting of conserved viral proteins (Liang et al. 1994; Epstein et al. 1997; Woodland et al. 2001). Thus, the targeted generation of virus-specific memory T cell responses by vaccines could represent a way to achieve durable, cross-protective immunity to Influenza.
Both CD4 and CD8 T cells play important roles in the adaptive immune response to Influenza. However, in contrast to CD8 T cells, which are limited to cytotoxic killing of virally-infected cells, CD4 T cells play much more diverse roles in responses to infection. Effector CD4 cells are capable of providing help necessary for both CD8 T cells and B cells to achieve their full functional potential, as well as mediating direct effector functions through cytolysis of Influenza-infected cells. Following Influenza infection, virus-specific CD4 T cells are maintained as long-lived memory populations with an enhanced capacity to protect against secondary infection, due to their ability to respond more rapidly and robustly upon antigen encounter. In addition, in contrast to naïve cells, which remain in lymphoid tissues, memory cells localize to peripheral sites, poised to respond to secondary challenge at the site of infection. In mouse models of Influenza infection, memory CD4 T cells have been shown to mediate protective responses independently of B and CD8 T cells (Teijaro et al. 2010). Additionally, CD4 memory T cell responses are the protective correlate in vivo in human Influenza challenge studies (Wilkinson et al. 2012). Furthermore, that memory CD4 T cells can be cross reactive to multiple Influenza strains (Lee et al. 2008; Richards et al. 2010) makes them an attractive target for vaccine development strategies. In this review we will discuss the general properties of memory CD4 T cells, including their generation, phenotype, localization and function in the context of Influenza infection in both mouse models and humans. We will also discuss how tissue distribution influences T cell-mediated protection and how generation of tissue-targeted memory CD4 T cells is a potentially robust strategy for promoting protection from Influenza infection.
2 Primary CD4 T cell Responses to Influenza infection
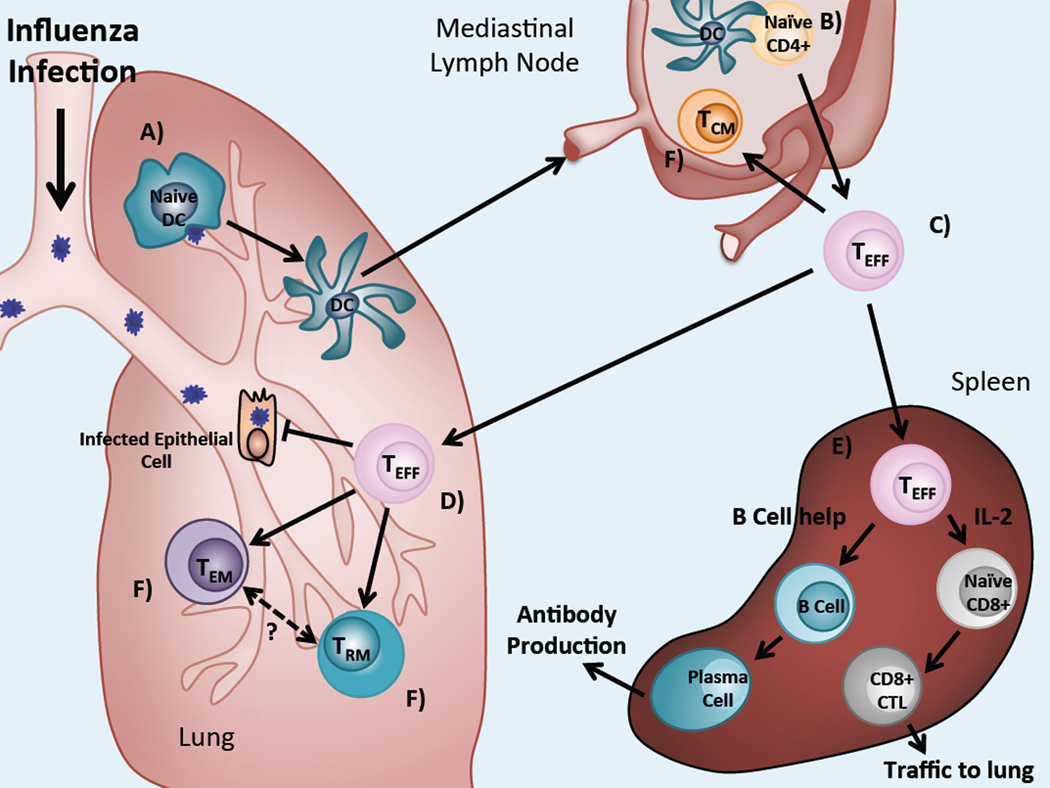
The generation of memory CD4 T cells following exposure to Influenza begins with the activation of naïve CD4 T cells. Influenza infection is confined to the lung and primary viral replication takes place within the epithelial cells of the respiratory tract expressing α (2,6) sialic acid linked membrane glycoproteins (Couceiro et al. 1993). Infected respiratory epithelial cells release inflammatory cytokines and chemokines including Tumor Necrosis Factor-α (TNF-α), Type-I Interferons (IFN), Interleukin-6 (IL-6), IFN-γ-inducible protein-10 (IP-10) and monocyte chemoattractant protein-1 (MCP-1, also known as CCL2), which in turn recruit innate immune effectors and antigen presenting cells (APCs) to the site of infection (Sanders et al. 2010). Within the infected lung, dendritic cells (DCs) take up viral particles and Influenza-derived antigens, which triggers DC activation, maturation and migration from the lung to the draining mediastinal lymph node (MLN) (Fig. 1a).
Fig. 1.
Overview of the primary CD4 T cell response and memory generation following Influenza infection. (a) Following Influenza infection, naïve DCs in the lung take up virus and virally-derived particles, driving their maturation and migration to the lung-draining lymph node. (b) Mature, activated DCs activate Influenza-specific naïve CD4 T cells trafficking through the lymph nodes. (c) CD4 effector cells traffic to the lung and can also reach the spleen via the circulation. (d) Effectors in the lung secrete effector cytokines and directly lyse virally-infected epithelial cells. (e) CD4 effectors also activate B cells and CD8 T cells in lymphoid tissues, allowing them to assume effector functions. (f) Responding effectors are retained as long live memory populations at diverse sites
Mature naïve T cells express lymphoid homing receptors, including CD62L (also known as L-selectin) and CCR7, which direct their migration and entry into the secondary lymphoid tissues. Here, Influenza-specific naïve CD4 T cells interact with mature DCs bearing viral antigen and are activated (Fig. 1b). Newly activated T cells proliferate and begin to acquire effector functions including the ability to produce effector cytokines which direct other immune functions. It is well established that the cytokine environment promotes the differentiation of activated CD4 T cells into different types of effector T helper (Th) cells, with Th1, Th2, Th17, Tfh (T follicular helper) and Treg (T regulatory) subsets being the most well-characterized (O'Shea and Paul 2010). Influenza infection is generally associated with Th1-type responses dominated by IFN-γ production along with TNF-α and IL-2, as well as a robust antibody response (Gerhard et al. 1997), involving Tfh and Th2-type CD4 effector cells.
Concomitant with effector differentiation, activated T cells alter expression of homing molecules, allowing them to migrate from the lymph nodes to peripheral tissues, such as the lung in Influenza infection (Fig. 1c). These changes include downregulation of CD62L and CCR7 and upregulation of the adhesion molecule CD44 and the integrin Lymphocyte Function-Associated Antigen-1 (LFA-1, a dimer of CD11a and CD18). Peak levels of activated CD4 effector T cells in the lung are reached 10 to 15 days post Influenza infection (Fig. 1d). Here, virus-specific CD4 cells secrete effector cytokines and a subset of these cells can also direct cytolysis of virally-infected cells. Effector CD4 T cells also play roles in the activation and maturation of virus-specific B and CD8 T cells necessary for clearance of primary Influenza infection in the lung-draining lymph nodes and the spleen (Fig. 1e).
3 Generation of Memory CD4 T cells in Influenza Infection
Following resolution of primary infection, a subset of responding virus-specific CD4 T cells are retained as long-lived memory T cells which can persist for the life of the animal, conveying protective immunity upon secondary pathogen encounter (Fig. 1f). Mechanisms underlying the transition from primary effectors to memory T cells have been an area of intense study, although this process remains incompletely understood (for reviews, see (Kaech et al. 2002; Sallusto et al. 2004; Kaech and Wherry 2007)). In the context of Influenza infection, the role of antigen persistence and cytokine signaling in memory CD4 T cell generation has been specifically investigated.
During primary Influenza infection in mouse models, virus is cleared between 7 to 10 days post infection (Allan et al. 1990). However, viral antigens have been found to persist up to 28 days post infection (Jelley-Gibbs et al. 2005; Zammit et al. 2006). Importantly, the level of antigen stimulation during infection has been shown to influence the development of memory CD4 T cells. Transfer of naïve CD4 T cells into hosts infected with Influenza either during acute infection or weeks following viral clearance resulted in both transferred populations converting to memory CD4 T cells (Jelley-Gibbs et al. 2005). Interestingly, transfer of naive CD4 T cells during early phases of infection resulted in robust expansion and effector generation, with only a small fraction persisting as memory T cells, while transfer of naive CD4 T cells during later phases of infection resulted in modest expansion of intermediately activated effectors, which were more efficiently maintained as memory cells (Jelley-Gibbs et al. 2005). Moreover, the acquisition of effector function, per se, may not be necessary for the generation of memory CD4 T cells as populations of Influenza-specific CD4 T cells primed for short periods of time and lacking effector function were still able to develop into memory cells (Moulton et al. 2006). Memory CD4 T cells may require cognate TCR signals for their maintenance, as long-term functional maintenance of CD4 memory T cells requires MHC class II expression (Kassiotis et al. 2002; De Riva et al. 2007) and additional TCR stimulation (Seddon et al. 2003; Bushar et al. 2010). Together, these findings provide evidence for a key role in TCR stimulation at different phases of memory CD4 T cell development.
Another key factor required for memory CD4 T cell generation is the presence of homeostatic and survival cytokines such as IL-7 and IL-15. Naïve CD4 and CD8 T cells express IL-7R, which is required for their survival and homeostatic proliferation (Rathmell et al. 2001; Tan et al. 2001). Expression of IL-7R is downregulated following T cell activation, however, it is again expressed at high levels on memory CD4 and CD8 cells (Kaech et al. 2003; Huster et al. 2004). In CD8 T cells, IL-7, as well as IL-15, signaling contribute to memory formation. Memory CD8 precursor populations can be identified by high expression of IL-7R and low expression of the co-inhibitory receptor killer-cell lectin like receptor G1 (KLRG1) (Kaech et al. 2003; Joshi et al. 2007). Analogous memory CD4 T cell precursor markers have not yet been identified. However, IL-7 signaling does appear to be necessary for the transition from effector to memory in CD4 cells in an Influenza infection model as memory CD4 cells fail to develop in IL-7-deficient hosts (Li et al. 2003). Roles for IL-15 and continued IL-7 signaling in the survival and homeostatic proliferation CD4 memory T cells have also been described (Kondrack et al. 2003; Li et al. 2003; Lenz et al. 2004; Purton et al. 2007). Together, these findings indicate role for both TCR and cytokine signals in memory CD4 T cell persistence.
4 Heterogeneity of Memory CD4 T cells
4.1 Circulating central and effector memory subsets
Memory CD4 T cells comprise a heterogeneous population in terms of phenotype, function and localization (Table 1). Like effector T cells, all memory CD4 T cells retain high-level expression of CD44 and human cells generally continue to express the CD45RO isoform. However, memory T cells are heterogeneous in their expression of CD62L and CCR7 which led to delineation of two memory subsets: CD62L+/CCR7+, lymphoid-homing, central memory (Tcm), and CD62L−/CCR7− effector memory (Tem) cells present in peripheral tissues (Sallusto et al. 1999; Masopust et al. 2001). The lymphoid versus peripheral tissue homing properties of these subsets were confirmed for mouse memory CD4 T cells (Reinhardt et al. 2001) and specifically in the context of Influenza infection (Ahmadzadeh and Farber 2002; Bingaman et al. 2005). Following Influenza infection, virus-specific CD4 Tem are more predominant in peripheral tissues, including the lungs, while CD4 Tcm are more predominate lymph nodes (Bingaman et al. 2005). Both populations are present in the spleen. Influenza-specific CD4 Tem and Tcm from spleen are capable of producing IL-2 and effector cytokines after stimulation while the proliferative capacity of Tcm is slightly greater than the Tem subset (Bingaman et al. 2005).
Table 1.
Phenotype of naïve, effector and memory CD4 T subsets
| Property | Naïve | Effector | Tcm | Tem | Trm |
|---|---|---|---|---|---|
| CD44 | Low | High | Intermediate | High | High |
| CD62L | High | Low | High | Low | Low |
| CCR7 | High | Low | High | Low | Low |
| CD45 Isoform (Humans Only) | CD45RA | CD45RO | CD45RO | CD45RO/CD45RA (Temra) | CD45RO |
| CD69 | Low | High | Low | Low | High |
| CD103 | – | – | – | – | –a |
| Migratory Properties | Lymphoid tissues, Circulation | Peripheral tissues | Lymphoid tissues | Peripheral tissues | Resident in peripheral tissue |
Present in CD8 Trm
4.2 Tissue Resident Memory subsets
In addition to Tem and Tcm subsets, a distinct population of non-circulating CD4 memory T cells has been identified to persist in the lung long-term after infection. These lung-resident memory CD4 T cells are analogous to populations of non-circulating memory CD8 T cells, designated tissue-resident memory T cells (Trm), that have been found to persist long-term in peripheral tissues including the brain, skin, gut and lung (Gebhardt et al. 2009; Liu et al. 2010; Masopust et al. 2010; Wakim et al. 2012; Wu et al. 2013). Phenotypically, CD4 Trm can be distinguished from circulating Tem and Tcm by upregulated CD69 and CD11a expression (Teijaro et al. 2011; Turner et al. 2013). CD8 Trm also express CD69 as well as the integrin CD103, the α chain of the αEβ7 integrin (Mueller et al. 2013), which is not significantly upregulated by CD4 Trm (Sathaliyawala et al. 2013).
In contrast to Tem and Tcm populations, which circulate throughout the peripheral and secondary lymphoid tissues, respectively, recent studies have highlighted the tissue-specific nature of the Trm subset. In mouse models of Influenza infection, a population of virus-specific CD4 Trm persists in the lungs for long periods following infection (Teijaro et al. 2011). When lung-derived CD4 Trm are transferred into secondary recipients, they preferentially recirculate back to the lungs, suggesting that this subset has an intrinsic mechanism for specific homing to and/or retention in the lung (Teijaro et al. 2011). Similar to populations described in mice, Influenza-specific CD4 and CD8 Trm populations have also been identified in human lung tissue (Lee et al. 2011; Piet et al. 2011; Sathaliyawala et al. 2013; Turner et al. 2013). Interestingly, the tissue-specific localization of Trm seems to be dependent on the site of infection. In humans, Influenza-specific CD8 T cells were only found within the lung Trm subset while memory CD8 T cells specific for the systemic virus Cytomegalovirus (CMV) were distributed in both lung and spleen (Turner et al. 2013). In another study, lung-derived CD4 Trm, but not CD4 cells derived from skin or blood responded to stimulation with Influenza virus, further supporting the site-specific localization of this population (Purwar et al. 2011).
The functional importance of lung resident CD4 Trm populations has been highlighted in studies in demonstrating the ability of this subset to provide optimal protection from reinfection with respiratory pathogens and, more specifically, from Influenza infection (Hogan et al. 2001; Teijaro et al. 2011). Functional studies of lung-derived CD4 Trm have demonstrated that these cells rapidly acquire effector function and respond vigorously to secondary stimulation with Influenza virus (Purwar et al. 2011; Turner et al. 2013). Importantly, in an Influenza infection model, lung-resident HA-specific memory CD4 T cells mediated enhanced viral clearance and survival to lethal Influenza infection when transferred to secondary recipients, whereas spleen-derived HA-specific memory CD4 T cells did not confer significant protection (Teijaro et al. 2011).
5 Mechanisms for Tissue Trafficking and Retention
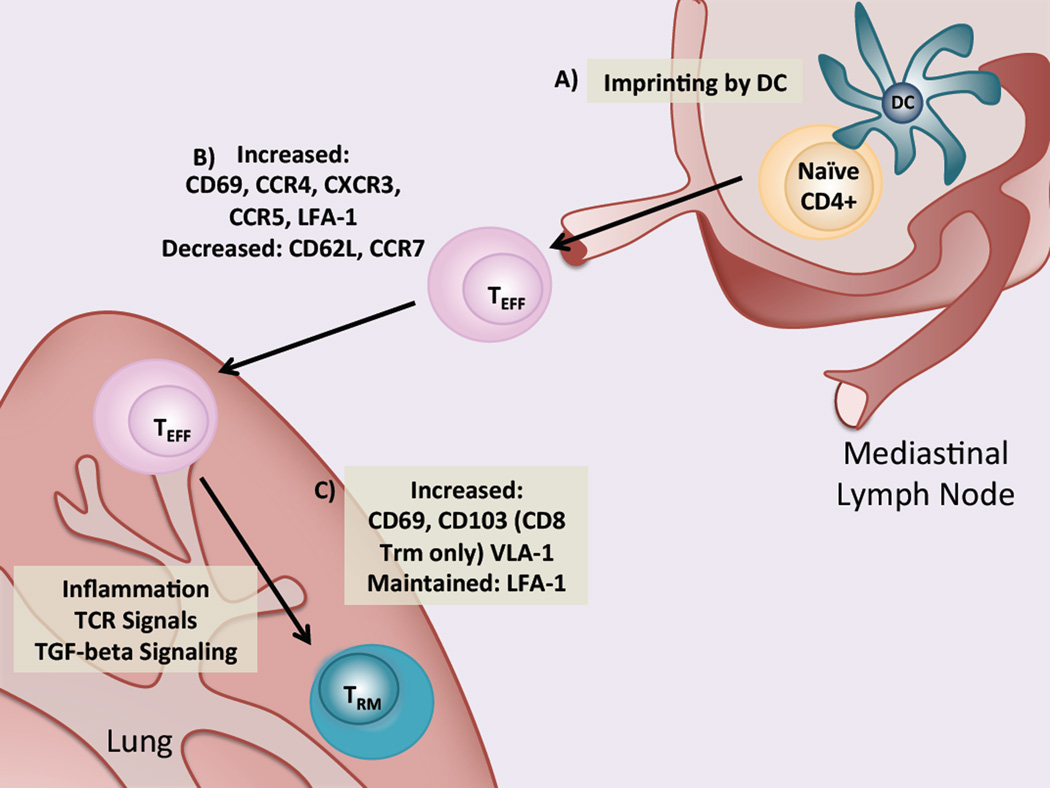
An important question with potential applications in vaccine design is how the homing and retention of activated effector and memory T cell populations occurs. Currently, the mechanisms mediating lung trafficking and retention in activated and memory T cells are incompletely understood. However, the process can be delineated into three parts: imprinting, homing and retention, as schematically outlined in Fig. 2.
Fig. 2.
DC imprinting, tissue homing and tissue retention in effector and memory CD4 T cells. (a) Following Influenza infection, naïve CD4 T cells are activated in the lung-draining lymph node by lung-derived DCs which also imprint homing receptor expression allowing T cells to traffic to the lung. (b) Newly activated effectors reduce expression of CD62L and CCR7, allowing egress from the lymph node. In addition, lung homing molecules including CCR4, CXCR3, CCR5 and LFA-1 are upregulated, allowing cells to traffic to the lung by following a chemokine gradient. Integrins, such as LFA-1, facilitate tissue entry. (c) Activated effectors remain in the lung via expression of integrins, including LFA-1 and VLA-1. Factors such as inflammation and TCR signaling may trigger expression of CD69 is increased, though it is not clear. Expression of CD103 by CD8 Trm is triggered by TGF-β signaling
Imprinting
Imprinting is a process by which DCs in the tissue-draining lymph nodes induce the expression of chemokine receptors and integrins and drive tissue-specific homing of activated T cells (Fig. 2a). Imprinting of skin and gut tissue-homing CD4 T cells has been demonstrated in several studies (Mora et al. 2003; Dudda et al. 2004; Mora et al. 2005). Recently, T cell migration to the lung during Influenza infection was shown to be mediated by CCR4 expression driven by lung DCs (Mikhak et al. 2013). CCR4 binds the chemokine ligands MCP-1 and RANTES (also known as CCL5) which are expressed by infected epithelial cells in the lung during Influenza infection (Matsukura et al. 1998; Julkunen et al. 2000) and MIP-1α (also known as CCL3), which is also expressed in Influenza infection and necessary for normal viral clearance (Cook et al. 1995; Sprenger et al. 1996). Importantly, lung DC-imprinted T cells protected against Influenza more effectively than gut or skin DC-imprinted T cells. However, imprinting in the draining lymph node is likely not the only factor responsible for T cell tissue homing as another recent study found that intranasal immunization with Salmonella typhimurium-derived antigens resulted in the generation of gut-homing T cells (Ruane et al. 2013). Other factors, such as inflammation or the presence of various pathogen-associated molecular patterns (PAMPs) during DC activation, may also influence this process.
Homing
In the context of infection, tissue homing refers to the trafficking of antigen-specific T cells to inflamed peripheral tissues. This process depends on expression of chemokine receptors and integrins by T cells, which promote their chemokine-directed migration and transmigration into inflamed tissues, respectively. The expression of tissue-homing molecules likely depends on the site and type of infection, and is driven, at least in part, by DC imprinting as described. During Influenza infection, several molecules have been implicated in the trafficking of activated T cells to the lung (Fig. 2b). After initial infection, downregulation of CD62L and CCR7 allows activated T cells to exit the lymph nodes and traffic to the peripheral tissues. Both CD4 and CD8 T cells express the integrin LFA-1, which binds to Intracellular Adhesion Molecule-1 (ICAM-1) present on endothelial cells and allows their transmigration into inflamed tissues (Makgoba et al. 1988). Expression of LFA-1 has been shown to be important in the migration and retention of effector CD8 T cells to the lung during Influenza infection (Thatte et al. 2003) and may also function similarly in CD4 T cells. The chemokine receptor CXCR3 has been shown to be important in the migration of CD4 T cells to the lung in primary Influenza infection (Wareing et al. 2004; Kohlmeier et al. 2009). One of the ligands for CXCR3, IP-10, is notably upregulated during Influenza infection (Sanders et al. 2010). The chemokine receptor, CCR5, which also binds RANTES and MIP-1α, was shown to facilitate the accelerated recruitment of memory CD8 T cells to the lung airways following secondary challenge (Kohlmeier et al. 2008). Specific molecules that are responsible for tissue-specific homing of memory CD4 cells have not been elucidated. However, lung-resident memory CD4 T cells preferentially home back to the lung after adoptive transfer (Teijaro et al. 2011), suggesting that there are likely specific factors that mediate this process.
Retention
Following the resolution of infection, maintenance of a pathogen-specific memory T cell population at the site of pathogen encounter may represent an effective strategy for protection against secondary challenge (Fig. 2c). As with homing, tissue retention of T cells seems to be regulated by the expression of various chemokine receptors and integrins. Expression of LFA-1 and the α1β1 integrin Very Late Antigen-1 (VLA-1, a dimer of CD49a and CD29), which binds collagen, were shown to contribute to retention of memory CD8 T cells in the lung following Influenza infection (Thatte et al. 2003; Ray et al. 2004), although their role in memory CD4 T cell retention is unclear. CD4 Trm were recently described to express high levels of the LFA-1 subunit CD11a (Teijaro et al. 2011; Turner et al. 2013), suggesting that integrins may also mediate memory CD4 T cell retention in the lung.
Recently, the Trm-specific markers, CD69 and CD103, were shown to contribute to Trm maintenance in tissues. During T cell activation, CD69 suppresses the expression of sphingosine 1-phosphate receptor type 1 (S1PR1), preventing lymph node egress (Shiow et al. 2006). Interestingly, a recent study found that CD8 Trm cells do not express S1PR1 and that forced expression of S1PR1 prevented the establishment of Trm cells (Skon et al. 2013). As CD69 suppresses S1PR1, these results suggest that increased CD69 expression on Trm cells may represent a non-canonical role for this molecule in retention of activated T cells in peripheral tissues. For CD103, a role in tissue homing has been previously demonstrated via its ability to bind to E-cadherin on epithelial cells (Cepek et al. 1994). Expression of CD103 by lung memory CD8 T cells was shown to be induced by TGF-β during Influenza infection (Yu et al. 2013). Importantly, CD103-deficient CD8 T cells are inefficiently retained in the lungs following Influenza infection, suggesting an important role for CD103 in the establishment of lung-resident CD8 Trm populations (Lee et al. 2011). Although CD4 Trm do not express CD103 at high levels (Sathaliyawala et al. 2013), expression of other integrins, such as LFA-1, may function in the tissue-retention of this subset.
6 Memory CD4 T cell Function in Influenza
While CD4-mediated B cell and CD8 T cell help has been demonstrated to be important in generating optimal primary responses to Influenza infection, in the absence of both B and CD8 T cells CD4 T cells alone are not sufficient, nor are they required, for viral clearance. (Graham and Braciale 1997; Mozdzanowska et al. 2000; Gerhard 2001). In contrast, memory CD4 cells can direct viral clearance in the absence of B cells or CD8 T cells (Teijaro et al. 2010; McKinstry et al. 2012). However, protection is enhanced by the presence of naïve B cells or CD8 T cells and maximized when both are present (McKinstry et al. 2012).
Like primary effector CD4 T cells, memory CD4 T cells have the potential to play diverse roles in coordinating secondary responses including providing CD8 and B cell help as well as exerting independent effector functions. However, two key factors in the superior protective capacity of memory versus naive CD4 T cells are their localization and enhanced function. In contrast to naive cells, memory CD4 T cells are comprised of subsets localized to both the lymphoid (Tcm) and peripheral tissues (Tem and Trm). In the lymphoid tissues, memory CD4 cells are able to enhance B cell responses, and may enhance CD8 T responses. Localization to sites of pathogen encounter, combined with reduced requirements for activation allow memory CD4 T cells to rapidly respond to secondary infection. In addition, memory cells are able to secrete an expanded array of cytokines, which supports inflammatory responses and innate immune cell recruitment to the lung. Finally, memory CD4 T cells possess cytolytic activity, allowing them to directly kill virally-infected cells in the lung. Memory CD4 T cell responses in secondary Influenza infection are described in the following sections and are summarized in Table 2.
Table 2.
Mechanisms of protection against Influenza infection mediated by primary effector and memory CD4 T cells
| Factor | Primary Effector | Memory Effector |
|---|---|---|
| Response to Antigen Stimulation | Delayed | Immediate |
| Need for APC Costimulation | Required | Not Required |
| Location of Activation | Draining Lymph Nodes (MLN) | Local (Lung) |
| Effector Cytokine Secretion (IFN-γ, etc.) | ++ | +++ |
| Innate Immune Cell Help | − | +++ |
| B Cell Helpa | ++ | +++ |
| CD8 T Cell Helpa | +++ | Possibly |
| Cytolytic Ability | ++ | ++ |
Transferred CD4 memory are able to protect from Influenza infection independent of these factors, but the presence of B and/or CD8 T cells results in enhanced protection while protection mediated by naïve CD4 T cells requires either B cells or CD8 T cells
6.1 Helper Functions in Lymphoid Tissues
The generation of neutralizing antibodies directed toward the viral surface glycoproteins HA and NA during Influenza infection is a major mechanism of protection in both the primary and secondary responses to infection as well the mechanism by which current Influenza vaccines provide protective immunity. It is well-established that CD4 T cell help is essential for the production of class-switched antibodies by antigen-specific B cells and the generation of plasma cells (Crotty 2011). The CD4 T cells that provide help to B cells are referred to as T follicular helper (Tfh) cells. Although it has been established that Tfh cells are important in primary antibody responses it is less clear whether there is a distinct set of memory Tfh cells and whether they play a role in the response to heterosubtypic Influenza infection. Memory CD4 T cells can enable B cells to expand and class switch more rapidly than do naïve CD4 T cells (MacLeod et al. 2011). In addition, a circulating population of memory CD4 T cells expressing CXCR5 in human blood was shown to induce antibody production from naïve B cells (Morita et al. 2011). It has also been demonstrated that the presence of such Tfh-like populations in the blood after Influenza vaccination in humans correlates with the generation of virus-specific antibody responses (Bentebibel et al. 2013; Spensieri et al. 2013). To address whether a specific memory Tfh population exists, a recent study demonstrated that the adoptive transfer of CXCR5-positive antigen-specific memory CD4 T cell populations promoted the enhanced formation of germinal center B cells following infection with LCMV, providing evidence for the existence of a distinct Tfh-committed CD4 memory T cell population (Hale et al. 2013). Taken together, these findings suggest that memory CD4 cells play an important role in providing signals for B cell responses and that this may be mediated by a specific Tfh-committed memory population poised to reacquire their lineage-specific effector functions and promote strain-specific antibody production upon heterosubtypic infection.
A role for CD4 T cell help in the generation of pathogen-specific CD8 memory T cells that can respond upon secondary infection is well established (Shedlock and Shen 2003; Sun and Bevan 2003). The need for CD4 help in the generation of CD8 memory has also been demonstrated during the primary response to Influenza infection (Belz et al. 2002), however, specific roles for memory CD4 T cells in the generation, maintenance or reactivation of CD8 memory have not been established. Memory CD4 T cells are able to mediate protection against lethal Influenza infection in the absence of CD8 T cells, and transfer of memory CD4 T cells does not promote recruitment of CD8 T cells into the lung after infection (Teijaro et al. 2010; Teijaro et al. 2011). However, viral clearance is enhanced in B cell-deficient mice receiving memory CD4 T cells when naïve CD8 T cells are present compared to CD8-depleting conditions (McKinstry et al. 2012). Similarly, reconstitution of Severe combined immunodeficiency (SCID) hosts, which lack both B and T cells, with either naïve CD8 T cells, memory CD4 T cells or a combination of both demonstrated that co-transfer provided greater protection than either population alone (McKinstry et al. 2012), suggesting that memory CD4 T cells may play some role in helping naïve CD8 T cells.
6.2 Independent Effector Mechanisms in the Lung
6.2.1 Cytokine Production
Memory CD4 T cells, in contrast to naïve CD4 T cells, are known to rapidly produce multiple inflammatory cytokines and chemokines in response to TCR stimulation, resulting in the recruitment of macrophages, NK and other innate effector cells. These cells, in turn, produce additional inflammatory cytokines which further support the inflammatory response. In response to secondary Influenza challenge, primed mice or mice receiving memory CD4 cells upregulate factors including IFN-γ, TNF-α, IL-6, IL-12, CXCL9 and CXCL10 compared to naïve (Strutt et al. 2010). This enhanced production of cytokines and chemokines correlates with early viral control during secondary infection (Strutt et al. 2010).
The most well-characterized memory CD4 response in Influenza infection is production of the Th1-type cytokine IFN-γ. In contrast to naïve CD4 T cells, which slowly begin to upregulate IFN- γ production driven by T-bet days after TCR stimulation, the production of IFN-γ by memory CD4 T cells occurs rapidly via an NF-kB-mediated transcriptional program (Lai et al. 2011). In primary Influenza infection, IFN-γ is not required for protection from infection and the development of CTL or antibody responses were not reduced in IFN-γ knockout mice compared to wild-type (Graham et al. 1993). However, memory CD4 T cell-mediated protection to Influenza challenge is dependent on IFN-γ as neutralization of IFN-γ production abrogates protection (Bot et al. 1998; Teijaro et al. 2010; McKinstry et al. 2012). In addition, in human Influenza challenge studies, early responses to virus infection were characterized by CD4-mediated production of IFN-γ (Wilkinson et al. 2012). Taken together, these results demonstrate the importance of memory CD4-mediated production of IFN-γ in secondary responses to Influenza.
Mechanisms for IFN-γ-mediated protection during infection are not completely understood. IFN-γ has been shown to be involved in the activation and recruitment of innate immune populations such as macrophages and natural killer (NK) cells. In one study, administration of IFN-γ at early stages of Influenza infection stimulated NK cell proliferation, function and number in the lungs and protected infected mice from death in a NK cell-dependent manner (Weiss et al. 2010).
Interleukin-10 (IL-10) is an anti-inflammatory cytokine broadly produced by both innate and adaptive immune cell types (Saraiva and O'Garra 2010). During acute Influenza infection, IL-10 is produced in the lungs by virus-specific CD4 and CD8 effector T cells (Sun et al. 2009). Expression of IL-10 in this context seems to be important in limiting inflammation and lung pathology. Interestingly, mice deficient for IL-10 display increased survival after challenge with lethal doses of Influenza compared to wild-type mice (McKinstry et al. 2009). Another study has suggested that increased protection to Influenza infection in IL-10-deficient mice is the result of enhanced virus-specific antibody production (Sun et al. 2010). Memory CD4 cells, although able to enhance viral clearance, do not protect from lung pathology associated with infection (Teijaro et al. 2010). Interestingly, studies have demonstrated that CD4 memory T cells produce less IL-10 than do primary effectors (Dong et al. 2007; McKinstry et al. 2007). The decreased IL-10 response may contribute to enhanced viral clearance after secondary infection while failing to limit pulmonary pathology.
A role for IL-17 in Influenza infection is less clearly defined. It is typically associated with allergic-type responses and the promotion of inflammation (Korn et al. 2009). Overall, most sources agree that IL-17 likely contributes to immune pathology and mice deficient in the IL-17 receptor display delayed weight loss and reduced total protein and lactate dehydrogenase activity in bronchoalveolar lavage (BAL) samples compared to wild-type mice suggesting reduced pathology (Crowe et al. 2009). However, IL-17 has previously been shown to play roles in the immune response to several different lung infections (Ye et al. 2001a; Umemura et al. 2007; Murdock et al. 2013). The transfer of in vitro generated Th17-polarized TCR-transgenic CD4 memory cells protected mice from lethal Influenza infection as effectively as Th1-polarized memory cells (McKinstry et al. 2012), suggesting that Th17 responses may contribute to positive outcome in Influenza infection. Furthermore, IL-17-secreting, Influenza-specific CD4 memory T cells have been described in human lung tissue (Sathaliyawala et al. 2013). Although no mechanism for this protection has been established, IL-17 promotes neutrophil recruitment in the lung (Ye et al. 2001b), which could contribute to protection.
6.2.2 Perforin-Dependent Cytolytic Activity
The cytotoxic role of CD8 T cells in Influenza infections is well established (Topham et al. 1997), consistent with its role in many different infection systems. There is increasing evidence that effector and memory CD4 T cells can also mediate cytotoxic responses in Influenza infection. A subset of Influenza-specific effector CD4 T cells with cytolytic activity was identified some time ago (Graham et al. 1994) and later found to be mediated by a perforin-dependent mechanism (Appay et al. 2002). In vitro primed and in vivo generated CD4 effectors were able to kill Influenza-specific peptide-coated targets in cytolytic assays and were capable of protecting against lethal Influenza infection (Brown et al. 2006; Brown et al. 2012). Cytolytic memory CD4 T cells may also mediate protective immunity to Influenza infection as perforin-deficient memory CD4 T cells exhibited reduced protective capacity during infection (McKinstry et al. 2012). The mechanism by which this protection occurs has not yet been elucidated, however, recent studies have demonstrated that the development of CD4 T cells with cytotoxic potential was dependent on type I interferon signaling and IL-2 mediated induction of the transcription factors T-bet and Blimp-1 (Hua et al. 2013).
7 Implications for Vaccines
As discussed above, Influenza-specific memory CD4 T cells are capable of mediating protective immune responses to secondary viral challenge (Teijaro et al. 2010; Wilkinson et al. 2012). Furthermore, it is well established that memory CD4 T cells protective against secondary challenge are generated and retained in the host long-term following primary infection (Teijaro et al. 2011; Turner et al. 2013), and are capable of mediating protective heterosubtypic responses to multiple viral strains (Teijaro et al. 2010). By contrast, current vaccines induce neutralizing antibody responses that are only protective to seasonal strains and, therefore, do not provide lasting protection against Influenza infection. Thus, targeting the generation of memory CD4 T cells, and in particular, Influenza-specific CD4 Trm in the lung, has the potential to provide durable, long-lasting cross-strain protection against Influenza virus.
There is evidence that vaccine type and route of administration affect memory CD4 T cell generation in response to Influenza vaccination. In studies comparing inactivated trivalent Influenza vaccine (TIV), given intramuscularly, and live attenuated Influenza vaccine (LAIV), administered intranasally, the percentage of virus-specific CD4 and CD8 T cells secreting IFN-γ increased significantly after LAIV, but not TIV, immunization in children five to nine years of age (He et al. 2006). Protection against Influenza infection was also found to be superior following immunization with LAIV as compared to TIV (Belshe et al. 2007). A later study comparing combinations of TIV and LAIV prime and booster vaccinations demonstrated that only vaccine combinations containing LAIV induced Influenza-specific, IFN-γ-producing CD4 memory (Hoft et al. 2011). Thus, LAIV appears to induce stronger virus-specific CD4 responses and is better able to generate CD4 memory than the current inactivated vaccine formulation. Significantly, these findings suggest that both vaccine localization and ability of virus to replicate within the host may be important for the design of Influenza vaccines able to promote development of memory CD4 T cells.
Though current vaccines do not target the development of tissue-homing T cells, strategies that promote this population are under investigation. In one study, intranasal, but not parenteral, delivery of a cancer vaccine was able to inhibit mucosal tumor growth. Importantly, only the intranasal vaccine was able to elicit a CD8 T cell expressing both CD49a, and CD103, molecules important in lung homing and retention, respectively. These results demonstrate a link between the route of vaccination, T cell tissue homing and the development of protective immune responses (Sandoval et al. 2013). The ability of a vaccine to establish a protective Trm population has also recently been demonstrated. In a herpes simplex virus type 2 model, parenteral vaccination with viral antigens was coupled with topical chemokine application to recruit virus-specific T cells to the genital mucosa. This ‘prime and pull’ strategy resulted in the establishment of a virus-specific Trm population capable of providing protective immunity (Shin and Iwasaki 2012). The development of vaccines capable of eliciting Trm population for Influenza will likely be the key to the development of lasting, heterosubtypic protection against infection.
8 Conclusion
In contrast to their naïve counterparts, memory CD4 T cells are capable of mediating protective responses to Influenza challenge independent of B cells or CD8 T cells. One factor driving the heightened protective capacity of memory CD4 T cells is their peripheral tissue localization, facilitating their rapid response to reinfection in situ. Importantly, a newly defined, tissue-resident CD4 memory population has been demonstrated to be optimally protective against Influenza challenge. Memory CD4 T cells also exhibit enhanced effector functions compared to naïve CD4 T cells including the ability to better promote B cell responses, as well as enhanced cytokine recruitment and activation of innate effector cells. Furthermore, that memory CD4 T cells can mediate heterosubtypic immune responses to multiple Influenza strains makes them an attractive target in vaccine development strategies. Recent studies have demonstrated that route of vaccine administration and the use of live-attenuated, rather than inactivated virus significantly impact the generation of memory CD4 T cells and protective capacity of vaccines following administration. Significantly, these findings may be important for the design of Influenza vaccines able to elicit protective memory CD4 T cells.
Acknowledgements
D.L.F. is supported by NIH grants AI106697, AI100119, and AI083022.
REFERENCES
- 1.Ahmadzadeh M, Farber DL. Functional plasticity of an antigen-specific memory CD4 T cell population. Proc Natl Acad Sci U S A. 2002;99(18):11802–11807. doi: 10.1073/pnas.192263099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144(10):3980–3986. [PubMed] [Google Scholar]
- 3.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168(11):5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 4.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 5.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus- specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76(23):12388–12393. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, Xu H, Pascual V, Banchereau J, Garcia-Sastre A, Palucka AK, Ramilo O, Ueno H. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5(176):176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bingaman AW, Patke DS, Mane VR, Ahmadzadeh M, Ndejembi M, Bartlett ST, Farber DL. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur J Immunol. 2005;35:3173–3186. doi: 10.1002/eji.200526004. [DOI] [PubMed] [Google Scholar]
- 8.Bot A, Bot S, Bona CA. Protective role of gamma interferon during the recall response to influenza virus. J Virol. 1998;72(8):6637–6645. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177(5):2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86(12):6792–6803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proc Natl Acad Sci U S A. 2010;107(2):827–831. doi: 10.1073/pnas.0908126107. doi:0908126107 [pii] 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E- cadherin and the alpha E beta 7 integrin. Nature. 1994;372(6502):190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 13.Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269(5230):1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 14.Couceiro JN, Paulson JC, Baum LG. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993;29(2):155–165. doi: 10.1016/0168-1702(93)90056-s. [DOI] [PubMed] [Google Scholar]
- 15.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 16.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183(8):5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Riva A, Bourgeois C, Kassiotis G, Stockinger B. Noncognate interaction with MHC class II molecules is essential for maintenance of T cell metabolism to establish optimal memory CD4 T cell function. J Immunol. 2007;178(9):5488–5495. doi: 10.4049/jimmunol.178.9.5488. [DOI] [PubMed] [Google Scholar]
- 18.Dong J, Ivascu C, Chang HD, Wu P, Angeli R, Maggi L, Eckhardt F, Tykocinski L, Haefliger C, Mowes B, Sieper J, Radbruch A, Annunziato F, Thiel A. IL-10 is excluded from the functional cytokine memory of human CD4+ memory T lymphocytes. J Immunol. 2007;179(4):2389–2396. doi: 10.4049/jimmunol.179.4.2389. [DOI] [PubMed] [Google Scholar]
- 19.Dudda JC, Simon JC, Martin S. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J Immunol. 2004;172(2):857–863. doi: 10.4049/jimmunol.172.2.857. [DOI] [PubMed] [Google Scholar]
- 20.Epstein SL, Lo CY, Misplon JA, Lawson CM, Hendrickson BA, Max EE, Subbarao K. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain- deficient mice. J Immunol. 1997;158(3):1222–1230. [PubMed] [Google Scholar]
- 21.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. doi:ni.1718 [pii] 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 22.Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 23.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 24.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186(12):2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham MB, Braciale VL, Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180(4):1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178(5):1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38(4):805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80(23):11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, Gerber MA, Bernstein DI, Newman F, Graham I, Anderson EL, Belshe RB. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis. 2011;204(6):845–853. doi: 10.1093/infdis/jir436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193(8):981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua L, Yao S, Pham D, Jiang L, Wright J, Sawant D, Dent AL, Braciale TJ, Kaplan MH, Sun J. Cytokine-dependent induction of CD4+ T cells with cytotoxic potential during influenza virus infection. J Virol. 2013;87(21):11884–11893. doi: 10.1128/JVI.01461-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101(15):5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202(5):697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julkunen I, Melen K, Nyqvist M, Pirhonen J, Sareneva T, Matikainen S. Inflammatory responses in influenza A virus infection. Vaccine. 2000;19(Suppl 1):S32–S37. doi: 10.1016/s0264-410x(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 36.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long- lived memory cells. Nat Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 37.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27(3):393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 39.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3(3):244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 40.Kohlmeier JE, Cookenham T, Miller SC, Roberts AD, Christensen JP, Thomsen AR, Woodland DL. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J Immunol. 2009;183(7):4378–4384. doi: 10.4049/jimmunol.0902022. doi:jimmunol.0902022 [pii] 10.4049/jimmunol.0902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29(1):101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198(12):1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 44.Lai W, Yu M, Huang MN, Okoye F, Keegan AD, Farber DL. Transcriptional control of rapid recall by memory CD4 T cells. J Immunol. 2011;187(1):133–140. doi: 10.4049/jimmunol.1002742. doi:jimmunol.1002742 [pii] 10.4049/jimmunol.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee LY, Ha DL, Simmons C, de Jong MD, Chau NV, Schumacher R, Peng YC, McMichael AJ, Farrar JJ, Smith GL, Townsend AR, Askonas BA, Rowland-Jones S, Dong T. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008 doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol. 2011;85(9):4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci U S A. 2004;101(25):9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198(12):1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152(4):1653–1661. [PubMed] [Google Scholar]
- 50.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16(2):224–227. doi: 10.1038/nm.2078. doi:nm.2078 [pii] 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacLeod MK, David A, McKee AS, Crawford F, Kappler JW, Marrack P. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J Immunol. 2011;186(5):2889–2896. doi: 10.4049/jimmunol.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makgoba MW, Sanders ME, Ginther Luce GE, Dustin ML, Springer TA, Clark EA, Mannoni P, Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- 53.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. doi: 10.1084/jem.20090858. doi:jem.20090858 [pii] 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 55.Matsukura S, Kokubu F, Kubo H, Tomita T, Tokunaga H, Kadokura M, Yamamoto T, Kuroiwa Y, Ohno T, Suzaki H, Adachi M. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am J Respir Cell Mol Biol. 1998;18(2):255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- 56.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204(9):2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182(12):7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry KK, Strutt TM, Kuang Y, Brown DM, Sell S, Dutton RW, Swain SL. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J Clin Invest. 2012;122(8):2847–2856. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhak Z, Strassner JP, Luster AD. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J Exp Med. 2013;210(9):1855–1869. doi: 10.1084/jem.20130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424(6944):88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201(2):303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. doi:S1074-7613(10)00491-7 [pii] 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton VR, Bushar ND, Leeser DB, Patke DS, Farber DL. Divergent generation of heterogeneous memory CD4 T cells. J Immunol. 2006;177(2):869–876. doi: 10.4049/jimmunol.177.2.869. doi:177/2/869 [pii] [DOI] [PubMed] [Google Scholar]
- Mozdzanowska K, Maiese K, Gerhard W. Th cell-deficient mice control influenza virus infection more effectively than Th- and B cell-deficient mice: evidence for a Th-independent contribution by B cells to virus clearance. J Immunol. 2000;164(5):2635–2643. doi: 10.4049/jimmunol.164.5.2635. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. IL-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and IFN-gamma production. Infect Immun. 2013 doi: 10.1128/IAI.01477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- Piet B, de Bree GJ, Smids-Dierdorp BS, van der Loos CM, Remmerswaal EB, von der Thusen JH, van Haarst JM, Eerenberg JP, Ten Brinke A, van der Bij W, Timens W, van Lier RA, Jonkers RE. CD8+ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest. 2011 doi: 10.1172/JCI44675. doi:44675 [pii] 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204(4):951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6(1):e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167(12):6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20(2):167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410(6824):101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Richards KA, Topham D, Chaves FA, Sant AJ. Cutting edge: CD4 T cells generated from encounter with seasonal influenza viruses and vaccines have broad protein specificity and can directly recognize naturally generated epitopes derived from the live pandemic H1N1 virus. J Immunol. 2010;185(9):4998–5002. doi: 10.4049/jimmunol.1001395. [DOI] [PubMed] [Google Scholar]
- Ruane D, Brane L, Reis BS, Cheong C, Poles J, Do Y, Zhu H, Velinzon K, Choi JH, Studt N, Mayer L, Lavelle EC, Steinman RM, Mucida D, Mehandru S. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J Exp Med. 2013;210(9):1871–1888. doi: 10.1084/jem.20122762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions [see comments] Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sanders CJ, Doherty PC, Thomas PG. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res. 2010 doi: 10.1007/s00441-010-1043-z. [DOI] [PubMed] [Google Scholar]
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, Bouguin C, Merillon N, Dransart E, Tran T, Quintin-Colonna F, Autret G, Thiebaud M, Suleman M, Riffault S, Wu TC, Launay O, Danel C, Taieb J, Richardson J, Zitvogel L, Fridman WH, Johannes L, Tartour E. Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med. 2013;5(172):172ra120. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38(1):187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4(7):680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300(5617):337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491(7424):463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8(+) T cells. Nat Immunol. 2013;14(12):1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spensieri F, Borgogni E, Zedda L, Bardelli M, Buricchi F, Volpini G, Fragapane E, Tavarini S, Finco O, Rappuoli R, Del Giudice G, Galli G, Castellino F. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc Natl Acad Sci U S A. 2013;110(35):14330–14335. doi: 10.1073/pnas.1311998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger H, Meyer RG, Kaufmann A, Bussfeld D, Rischkowsky E, Gemsa D. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J Exp Med. 1996;184(3):1191–1196. doi: 10.1084/jem.184.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med. 2010;16(5):558–564. doi: 10.1038/nm.2142. 551p following 564. doi:nm.2142 [pii] 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15(3):277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300(5617):339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Torres L, Metzger DW. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J Virol. 2010;84(10):5007–5014. doi: 10.1128/JVI.02408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98(15):8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84(18):9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatte J, Dabak V, Williams MB, Braciale TJ, Ley K. LFA-1 is required for retention of effector CD8 T cells in mouse lungs. Blood. 2003;101(12):4916–4922. doi: 10.1182/blood-2002-10-3159. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194(Suppl 2):S82–S91. doi: 10.1086/507558. [DOI] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159(11):5197–5200. [PubMed] [Google Scholar]
- Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178(6):3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, Bevan MJ. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. 2012;189(7):3462–3471. doi: 10.4049/jimmunol.1201305. doi:jimmunol.1201305 [pii] 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol. 2004;76(4):886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- Weiss ID, Wald O, Wald H, Beider K, Abraham M, Galun E, Nagler A, Peled A. IFN-gamma treatment at early stages of influenza virus infection protects mice from death in a NK cell-dependent manner. J Interferon Cytokine Res. 2010;30(6):439–449. doi: 10.1089/jir.2009.0084. [DOI] [PubMed] [Google Scholar]
- Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, Staples KJ, Dong T, Douek DC, McMichael AJ, Xu XN. Preexisting influenza-specific CD4(+) T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18(2):274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- Woodland DL, Hogan RJ, Zhong W. Cellular immunity and memory to respiratory virus infections. Immunol Res. 2001;24(1):53–67. doi: 10.1385/IR:24:1:53. [DOI] [PubMed] [Google Scholar]
- Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2013 doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001a;25(3):335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001b;194(4):519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, Anguiano E, Pourpe S, Goller K, Pascual V, Banchereau J, Merad M, Palucka K. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-beta. Immunity. 2013;38(4):818–830. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24(4):439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]