Abstract
Pulmonary arterial hypertension (PAH) is an idiopathic cardiopulmonary disease characterized by obstruction of small pulmonary arteries by excessive proliferation and apoptosis-resistance of vascular cells, as well as inflammation, thrombosis and vasoconstriction.
Vascular obstruction increases the afterload faced by the right ventricle (RV), leading to RV failure. The proliferative, obstructive vasculopathy of PAH shares several mitochondrial abnormalities with cancer, notably a shift to aerobic glycolysis and mitochondrial fragmentation. Mitochondria in the pulmonary artery smooth muscle cell (PASMC) normally serve as oxygen sensors. In PAH, acquired mitochondrial abnormalities, including epigenetic silencing of superoxide dismutase (SOD2), disrupt oxygen sensing creating a pseudo-hypoxic environment characterized by normoxic activation of Hypoxia-Inducible Factor-1α (HIF-1α). The resulting metabolic shift to aerobic glycolysis (the Warburg phenomenon) reflects inhibition of pyruvate dehydrogenase by pyruvate dehydrogenase kinases. In addition, altered mitochondrial dynamics result in mitochondrial fragmentation. The molecular basis of this structural change includes upregulation and activation of fission mediators, notably dynamin-related protein 1 (DRP-1), and downregulation of fusion mediators, especially mitofusin-2 (MFN2). These pathogenic mitochondrial abnormalities offer new therapeutic targets. Inhibition of mitotic fission or enhancement of fusion in PAH PASMC slows cell proliferation, causes cell cycle arrest, and induces apoptosis. DRP-1 inhibition or MFN2 gene therapy can regress PAH in experimental models of PAH. This review focuses on the etiology of mitochondrial fragmentation in PAH and explores the therapeutic implications of mitochondrial dynamics in the pulmonary vasculature and RV.
Keywords: apoptosis, oxygen sensing, dynamin related protein 1 (DRP-1), mitofusin-2, cell proliferation
Introduction
Mitochondria are semiautonomous organelles that are best known for generating the energy-containing molecule adenosine triphosphate (ATP). In the lung circulation mitochondria are also oxygen sensors, using their ability to alter cytosolic redox state through production of reactive oxygen species (ROS) to regulate the many effectors (ion channels and kinases) that mediate hypoxic pulmonary vasoconstriction (HPV) [1]. Recent advances reveal additional noncanonical mitochondrial functions that recast this organelle as much more than a source of energy [2, 3]. Mitochondria exist in dynamic networks in which they rapidly divide (fission), join together (fusion) and move about the cell (trafficking). The connectivity of the mitochondrial network, the shape of individual mitochondria, and the intracellular trafficking of mitochondria reflect processes that, in aggregate, are termed mitochondrial dynamics [3].
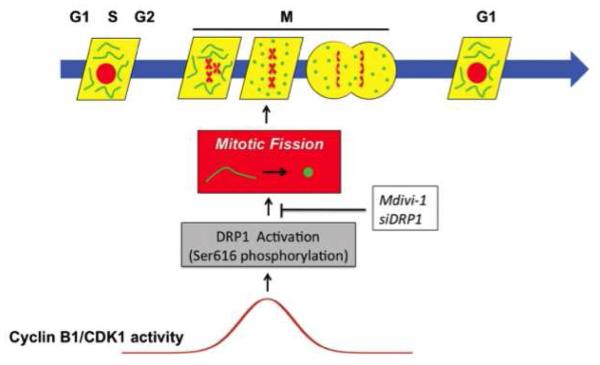
Mitochondrial fusion results in an interconnected mitochondrial network; conversely, fission results in more numerous, smaller mitochondria that are less interconnected to each other or to the endoplasmic reticulum (ER). The balance between fission and fusion is tightly regulated and can be altered by kinases that regulate the cell cycle and mitosis initiation [4, 5], phosphatases [6], cellular redox state, intracellular calcium levels, the activity of calcium-dependent kinases [7], and metabolism. Mitochondrial dynamics are relevant to the mechanisms of apoptosis, cell proliferation, and mitochondrial quality control [3] (Figure 1).
Figure 1. Schematic representation of how the cell and mitochondrial cycles interact.
Activation of the mitochondrial GTPase dynamin-related protein-1 (DRP-1) reflects regulatory kinases (cyclin B1/CDK1) that respond to signals from growth factors. In pulmonary hypertensive patients, cyclin B1 levels are elevated leading to activation of DRP-1. If fission (which is required for mitochondrial division) is inhibited, by blocking the activation of DRP-1, the cell cycle is halted, causing cells to be blocked in G2/M arrest. The intersection of the mitochondrial and cell cycles is cyclin B1/CDK1-mediated mitotic fission, and this exposes an “Achilles heel” for rapidly proliferating cells that can be therapeutically targeted. Reproduced with permission from [62].
In this review we focus on recent discoveries that demonstrate structural and functional abnormalities of the mitochondria in the pulmonary artery smooth muscle cells (PASMC) in human and experimental pulmonary arterial hypertension (PAH). These abnormalities include mitochondrial fragmentation and aerobic glycolysis and contribute to the deep phenotype of PAH [8, 9]. Both mitochondrial fragmentation and aerobic glycolysis contribute mechanistically to the apoptosis-resistant, proliferative phenotype of PASMC and the bioenergetic impairment of RV myocytes. Similar abnormalities of mitochondrial dynamics and metabolism are also found in cancer [10]. The mitochondrial-metabolic disorders in the pulmonary vasculature reflect subversion of a physiologic oxygen sensing mechanism; whilst in the RV they reflect an ischemia-induced, transcriptionally-mediated, change in metabolism. After an overview of the PAH syndrome, we summarize the contributions that impaired oxygen sensing and altered mitochondrial dynamics contribute to the syndrome. The pathophysiologic role and therapeutic implications of impaired fusion and exaggerated fission in PAH is reviewed.
Introduction to PAH
Pulmonary hypertension (PH) is defined as a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg at rest [11, 12]. To be clinically useful this broad definition requires a more granular classification system. The latest World Health Organization (WHO) nomenclature recognizes five PH groups each unified by commonalities in etiology, prognosis and therapy [13]. Group 1 PH, which includes PAH, is a devastating disease of the pulmonary arterial vasculature that causes dyspnea, exertional syncope and ultimately leads to death from right ventricular failure (RVF) [14]. PAH has a prevalence of ~20-cases/1,000,000 population, and afflicts women 4-times more than men [15-17]. PAH is defined by elevation of the resting mPAP above 25mmHg with a pulmonary vascular resistance (PVR) > 3 Wood units and a pulmonary capillary wedge pressure (PCWP) at end expiration of < 15 mmHg [18]. This increase in afterload puts strain on the RV, induces RV hypertrophy (RVH) and may ultimately result in RVF and death.
It is now recognized that fixed mechanical vascular obstruction with loss of cross sectional area, rather than vasospasm, is the predominant cause of increased PVR in most patients. Indeed, only 12.6% of patients respond to potent vasodilators, like inhaled nitric oxide, with a > 20% fall in mPAP to a value < 40 mmHg while maintaining or increasing cardiac output [19]. Current PAH therapies are primarily vasodilators and do not directly address the vascular obstruction or cancer-like phenotype of vascular cells in PAH nor do most therapies target the hypertrophied RV, which is fibrotic and ischemic due, in part, to microvascular rarefaction [20]. The RV in PAH also has cancer-like metabolic changes, including a reliance on glycolytic metabolism [14]. The RV in PAH is similar to the left ventricle in patients with severe multivessel epicardial coronary artery disease and hibernating myocardium [21, 22]. Not surprisingly, with this mismatch between therapeutic targets and disease mechanisms, the annual mortality rate of PAH remains high (~15% at 1 year and 50% by 5 years) [23], despite several approved, costly therapies ($20,000-$100,000/year) [16, 24]. Thus, there is a need for a new therapeutic paradigm in PAH that addresses the fundamental abnormalities that underlie the disease in both the RV and pulmonary vasculature. In this review the contribution of mitochondrial dynamics to the etiology of PAH and value as a therapeutic target is considered.
Etiology of PAH
This focused review of mitochondrial dynamics will not address the many, well-established disease pathways that contribute to PAH. Many of these mechanisms likely intersect with the disorders of mitochondrial dynamics. Etiologic abnormalities in PAH include: elevated plasma serotonin levels [25], a decreased ratio of endothelial-derived vasodilators relative to vasoconstrictors [26-28], decreased expression/function of voltage-gated potassium channels in PASMC [29, 30], PASMC apoptosis-resistance and hyperproliferation [31]. Apoptosis resistance is due in part to de novo expression of the anti-apoptotic protein, survivin [32, 33] and activation of adventitial metalloprotease [34]. In addition, inflammation and autoimmunity play a significant role in the etiology of PAH [35]. Metabolic abnormalities in both the lung vasculature and the RV in PAH reflect a shift in metabolism from glucose oxidation in the mitochondria to the less exergonic process of uncoupled glycolysis in the cytosol [36, 37]. These metabolic changes are mediated by activation of one or more of the four isoforms of pyruvate dehydrogenase kinase (PDK), a mitochondrial enzyme that phosphorylates and inhibits pyruvate dehydrogenase (PDH) [22, 38].
In the pulmonary vasculature, the metabolic shift results from redox-mediated activation of transcription factors that control PDK expression, specifically Hypoxia-Inducible Factor-1α (HIF-1α). HIF-1α is activated as a consequence of epigenetic silencing of mitochondrial superoxide dismutase 2 (SOD2) [9]. HIF-1α activation promotes mitochondrial fragmentation [9] and enhances PASMC proliferation [38]. Hypoxia causes vasoconstriction by a hypoxia-induced change in the production in mitochondrial derived reactive oxygen species (ROS). In acute hypoxia, ROS production in resistance PASMCs is reduced leading to inhibition of oxygen sensitive ion channels, such as Kv1.5 and Kv2.1. This occurs within seconds and results in depolarization of the plasma membrane, activation of the large conductance, voltage-gated calcium channel and vasoconstriction. In chronic hypoxia HPV is suppressed [39, 40]. This reflects downregulation of Kv1.5 and other oxygen-sensitive ion channels as well as impaired function of the mitochondrial redox sensor. Chronic hypoxia activates HIF-1α, which contributes to Kv1.5 downregulation. Restoration of Kv1.5 [41] or partial deficiency of HIF-1α prevents this reduction in K+ current density [42]. Thus, chronic hypoxia reduces HPV by a HIF-1α-induced reduction in the expression O2 sensitive Kv channels and in part by depressed production of mitochondrial ROS.
PDK is transcriptionally upregulated by HIF-1α [43]. In the RV, metabolic remodeling may be initiated by ischemia, due to microvascular rarefaction and/or reduced coronary perfusion pressure, but ultimately involves activation of transcription factors, such as HIF-1α, cMyc and FOXO4 [14]. In both the pulmonary circulation and the RV the increase in PDK expression and inhibition of PDH promote reliance on uncoupled glycolysis. This glycolytic shift offers a useful biomarker in PAH, as it is detectable in both the lung and RV as increased uptake of fluorodeoxyglucose (FDG) on positron emission tomography (18FDG-PET) [36, 38]. The glycolytic switch in RV metabolism reduces RV contractility whereas in the pulmonary arteries it promotes elevated rates of cell proliferation and favors apoptosis-resistance, by inactivating the mitochondria that normally mediate apoptosis. Therapeutic reactivation of PDH increases glucose oxidation, improves RV function and regresses experimental PAH [33, 44]. Activation of PDH can be achieved directly by inhibition of PDK (by using dichloroacetate), or indirectly, by activating the Randle’s cycle. The Randle cycle is the reciprocal relationship between glucose and fatty acid oxidation (FAO). Inhibitors FAO, such as trimetazidine and ranolazine [45], increase glucose oxidation [46].
As an example of the interaction between apparently discrete mechanistic pathways, it has recently been recognized that patients with familial PAH patients, who have mutations in the bone morphogenetic protein receptor-2 (BMPR-2) [30, 47, 48], also have metabolic changes [49] consistent with those previously described in animal models of PAH and in human PAH. Thus, there may be many upstream mechanisms that trigger the observed changes in mitochondrial metabolism (and structure).
Impaired mitochondrial O2-sensing in PAH
The mitochondria in PASMC of resistance level arteries (< 200 μm) serve as vascular oxygen sensors, responding to local decreases in alveolar oxygen tension by initiating HPV [1, 50]. HPV is the lung’s autoregulatory mechanism to match perfusion to ventilation [51]. A modest decline in airway oxygen leads to localized vasoconstriction, which shunts perfusion to better-ventilated lobes within the lung. HPV is important in optimizing partial pressure of oxygen (PO2) during pneumonia and atelectasis [52]. In PAH the disease pathology is focused in these same resistance arteries and the PASMC within this vascular segment have mitochondria that behave as if they were exposed to chronic hypoxia. Specifically, the mitochondria in PAH PASMC have impaired metabolism, due to transcriptionally-mediated inhibition of mitochondrial PDH, and are fragmented, due to an imbalance of mitochondrial fission versus fusion.
According to the “Redox Theory” [53], HPV is initiated by a mitochondrial oxygen-sensor within the vascular smooth muscle cell that changes production of ROS from mitochondrial electron transport chain (ETC) complexes I and III, in proportion to alveolar PO2 [54]. With acute hypoxia there is decreased electron flux and reduced production of the diffusible second messenger hydrogen peroxide (H2O2). Although most scientists accept that the mitochondria are oxygen sensors, some report ROS increasing with hypoxia [55]. However, in the work supporting the redox theory, reviewed in [1], production of diffusible redox mediators (radicals and peroxides) in the resistance artery PASMC (as opposed to conduit artery PASMC) during physiologic hypoxia (as opposed to anoxia) is decreased. Hypoxia decreases ROS production, thereby inhibiting Kv channels [56]. This depolarizes the PASMCs leading to activation of voltage-gated, L-type calcium channels. The resulting calcium entry initiates vasoconstriction [51, 57].
It appears to be the unique ability of PASMC mitochondria to modulate their production of ROS that explains the unique occurrence of HPV in resistance PAs. For example, mitochondria from renal arteries do not significantly alter ROS in response to physiologic changes in PO2 and renal arteries dilate to hypoxia [58]. Although the role of mitochondrial dynamics in HPV is not yet understood it is clear in the ductus arteriosus that mitochondrial fission is an obligatory initial step in the mechanisms that precede changes in mitochondrial ETC function and ROS signaling [59].
Although exaggerated HPV can generate severe acute PH, and contributes to conditions like high altitude pulmonary edema (HAPE) in genetically susceptible individuals who rapidly ascend to altitude [60], the relevance of oxygen sensing to understanding the mechanism of PAH is related to shared redox pathways. Prolonged exposure to environmental hypoxia suppresses the mitochondrial metabolic and redox signaling pathways that underlie the oxygen-sensing system. Specifically, chronic hypoxia activates HIF-1α, decreases mitochondrial production of H2O2 and eliminates PO2-sensitive ROS production, reducing HPV [40]. Chronic hypoxic PH primarily results from adverse vascular remodeling (i.e. medial hypertrophy of small (< 200 μm) PAs), rather than ongoing HPV [51]. In PAH PO2-independent abnormalities of mitochondrial dynamics (increased dynamin related protein 1, DRP-1, reduced mitofusin-2, MFN2), redox signaling (reduced SOD2 and activated HIF-1α), oxidative metabolism (activated PDK and inhibited PDH) and effector targets (decreased expression of O2-sensitive Kv channels) contribute to the obstructive vasculopathy. The concept that impaired oxygen sensing contributes to the etiology of PAH is a variant on the Warburg hypothesis, which proposes that cancer cells rely on glycolysis despite availability of adequate oxygen to support oxidative metabolism. In both cancer and PAH there is a failure of oxygen sensing due to changes in mitochondrial redox signaling which acutely manifests as impaired oxygen sensing and in the longer term creates the Warburg phenomenon [56].
In PAH, cyclin B CDK1 triggers mitosis and simultaneously phosphorylates DRP-1 at serine 616, thus activating mitochondrial fission [61]. Inhibition of mitotic fission arrests the cell in G2/M phase, leading to cell death [62]. It is unknown whether hypoxic fragmentation of mitochondria leads to vasoconstriction. However, in the ductus arteriosus, another oxygen-sensitive tissue, a change in PO2 results in rapid mitochondrial fission (<60 sec) and the resulting burst of ROS initiates vasoconstriction [59].
A genetic disease that was originally described in individuals from the Chuvash region of Russia provides an example of how impaired oxygen sensing can cause PH. Individuals with a loss of function mutation in von Hippel Lindau factor (vHL) [63, 64] develop PH. vHL normally ubiquitinates HIF-1α, marking it for proteasomal degradation. As would be predicted, these patients have normoxic activation of HIF-1α and develop a PH syndrome accompanied by polycythemia, all occurring despite normal arterial and alveolar oxygen levels. This syndrome, called Chuvash pulmonary hypertension, demonstrates that impaired oxygen sensing (and the resulting normoxic activation of HIF-1α □□□ HIF-2α ) is sufficient to cause PH [63].
In PAH, acquired changes in mitochondrial redox signaling create a transcriptional and proteomic fingerprint similar to that seen with sustained hypoxia. These abnormalities persist even when PAH cells are exposed to high oxygen concentrations, as occurs during cell culture, creating a state of pseudohypoxia. PAH-associated abnormalities in the pulmonary vascular oxygen-sensing pathway are summarized in Table 1. They include: (1) persistent activation of HIF-1α during normoxia [8]; (2) a transcriptional upregulation and activation of the inhibitory enzyme PDK, both in the pulmonary vasculature [44, 65], and RV [45, 66, 67], which results in a shift from oxidative metabolism to aerobic glycolysis [68]; and (3) impaired mitochondrial fusion and increased fission which results in fragmentation of the PASMC’s mitochondrial network [62, 69]. The remainder of this review focuses on the changes in mitochondrial dynamics in PAH, and summarized in Figure 2.
Table 1.
Oxygen sensing is impaired in pulmonary arterial hypertension
| Normal PASMC |
Acute Hypoxia | Chronic hypoxia | PAH | ||||
|---|---|---|---|---|---|---|---|
| PO2 | Normal | Hypoxic | Hypoxic | Normal | |||
|
Mitochondrial
respiration |
Normal | Decreased | Decreased | Decreased | |||
|
Mitochondrial
network |
Fused | ? | Fragmented | Fragmented | |||
|
SOD2
expression/activity |
Normal | ? | ? | Decreased | |||
| HIF-1α activation | (−) | (−) | (+) | (+) | |||
|
Mitochondrial-
derived H2O2 production |
Normal | Decreased | Decreased | Decreased | |||
|
Whole Cell K+
current |
Normal | Decreased | Decreased | Decreased | |||
|
O2-sensitive Whole
Cell K+ current |
Normal | Inhibited | Decreased | ? | |||
|
Kv channel
activity/expression |
Normal | Inhibited/nor mal |
Inhibited/decrea sed |
Inhibited/decrea sed |
|||
|
PASMC membrane
potential |
Normal | Depolarized | Depolarized | Depolarized | |||
|
Activity of CaL
channels |
Minimal activity | Increased | Increased | Increased | |||
| Intracellular calcium | Low (60- 100 nM) |
High (>100 nM) |
High (>100 nM) | High (>100 nM) | |||
|
Rho
kinase |
Inactive | Beginning to activate |
Active | Active | |||
| Vascular tone | Low | Acute vasoconstricti on |
High | High | |||
|
Response to acute
hypoxia |
Constricti on |
HPV | Decreased HPV | ? | |||
|
Response to ETC
Complex I inhibitor (rotenone) |
Constricti on |
No additional constriction |
No additional constriction |
? | |||
|
Vascular
remodeling |
(−) | (−) | (+) | (+++) | |||
|
Cell
proliferation/apopt osis |
(−) | (−) | (+) | (+++) |
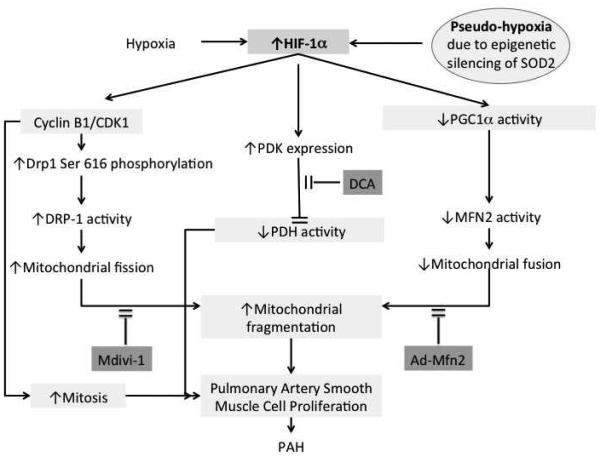
Figure 2.
Model showing how conditions associated with hypoxia promote hypoxia-inducible factor-1α (HIF-1α) regulation of mitochondrial fission and fusion of pulmonary artery smooth muscle cells (PASMCs). Additionally, HIF-1α regulates pyruvate dehydrogenase kinase (PDK) expression. PDK phosphorylates and deactivates pyruvate dehydrogenase (PDH) thereby to altered PASMC metabolism and contributing to increased mitochondrial fragmentation. The effect of PDK on PDH can be blocked by dichloroacetate (DCA). Mdivi-1 inhibits the effects of dynamin-related protein 1 (DRP-1) on mitochondrial fission. These effects contribute to pulmonary arterial smooth proliferative remodeling and pulmonary arterial hypertension (PAH). Increased HIF-1α decreases peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α), which results in a decrease in mitofusin-2 (MFN2) and an increase in mitochondrial fragmentation. The effects of decreased MFN2 can be reduced by administration of Adenovirus labeled with MFN2 (Ad-MFN2). HIF-1α can be increased by both hypoxia and pseudohypoxia, which can occur in the setting of epigenetic downregulation of superoxide dismutase 2 (SOD2).
Tools for Observing Mitochondrial Dynamics
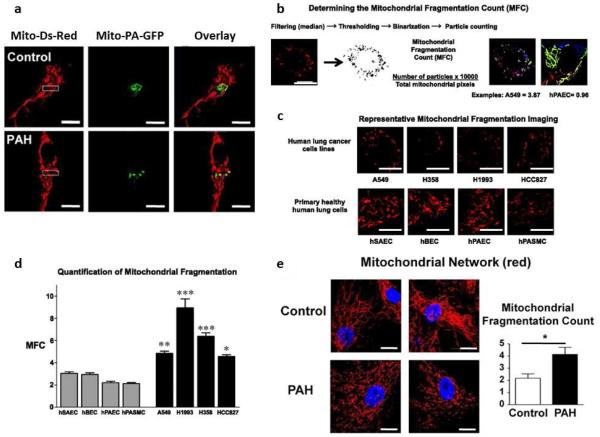
Our understanding of mitochondrial dynamics has been advanced by the ability to observe mitochondria in live cells either using potentiometric dyes or mitochondrial-targeted fluorescent proteins. Potentiometric dyes, such as tetramethylrhodamine methyl ester are targeted to the mitochondria by the organelle’s extremely negative charge (~180mV). These probes allow qualitative assessment of mitochondrial membrane potential and permit observation of morphology and mobility [31, 62]. However, to directly measure fusion, cells are transfected with plasmids encoding mitochondrial matrix-targeted-photoactivatable green fluorescent protein (mito-PA-GFP) and mitochondrial-targeted red fluorescent protein (mito-Ds-Red). Mito-Ds-Red is tonically fluorescent whereas mito-PA-GFP does not fluoresce until photoactivated. Mito-PA-GFP is selectively activated in a small sample volume containing few mitochondria (using a focused 488 nm laser). Serial observation allows measurement of the spread of the green protein within adjoined mitochondria. More fused networks have more rapid spread of the matrix GFP green signal outside the activation zone, which is quantified as the mitochondrial networking factor (MNF) [11, 43] (Figure 3). Complementing this metric, automated particle counting of discrete fluorescent mitochondria per field yields a mitochondrial fragmentation count (MFC). In states of fission, like lung cancer or PAH, MNF is reduced and MFC is elevated [11, 43]. Thus, MNF is a measure of network connectivity, while MFC reflects particle number [5] (Figure 3).
Figure 3. Fragmented mitochondria in lung cancer and in PAH.
(a) Photoactivation experiments confirm the decreased mitochondrial networking in PAH PASMCs. The mitochondrial networking factor (MNF) is lower in human PAH PASMCs and Mdivi-1 (25 μmol/L), an inhibitor of DRP-1, reverses the fragmented phenotype and increases the MNF, demonstrating that increased DRP-1 activity is the major determinant of the fragmented mitochondrial morphology in PAH. Scale bar=10 μm. Reproduced with permission from [62].
(b): Determining the Mitochondrial Fragmentation Count (MFC).
(c) Mitochondrial fragmentation in human lung cancer cells. Cells were loaded with mitochondrial red fluorescent dye tetramethylrhodamine methyl ester (TMRM) and imaged with confocal microscope to assess the mitochondrial network structure. The acquired images were background subtracted, filtered (median), thresholded, and binarized to identify mitochondrial segments using ImageJ. Continuous mitochondrial structures were counted with the particle counting subroutine, and the number was normalized to the total mitochondrial area (in pixels) to obtain the MFC for each imaged cell. Representative images of the mitochondrial imaging from cultured human lung cancer cell lines (A549, H358, H1993, HCC827) or human lung epithelial and vascular cells (hSAECs, hBECs, hPAECs, hPASMCs) shows a marked predominance of mitochondrial network fragmentation in the cancer cells. For every cell line or intervention, ≥n = 25 randomly selected cells were imaged to calculate the respective MFC values. Scale bar = 10 μm (all images).
(d) Quantification of the MFC confirms that all the lung cancer cells lines have a significantly higher level of mitochondrial network fragmentation than any of the nonmalignant human lung epithelial or vascular cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs. nonmalignant cells; ANOVA with post hoc test. Reproduced with permission from [5].
(e) Mitochondria are more fragmented in PAH vs. control PASMCs. Quantification of MFC reveals a doubling of the number of individual mitochondria in PAH vs. control PASMCs. Scale bar = 20 μm. Reproduced with permission from [62].
Fission and dynamin related protein 1 (DRP-1) in PAH
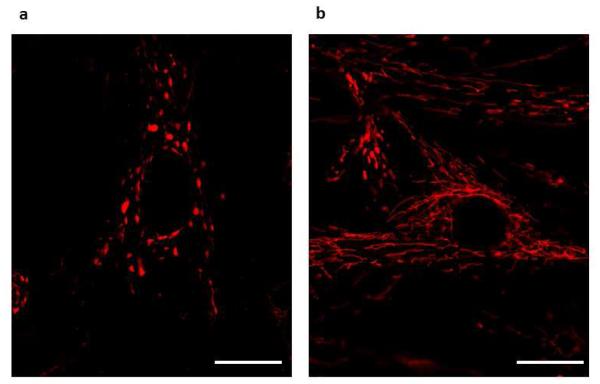
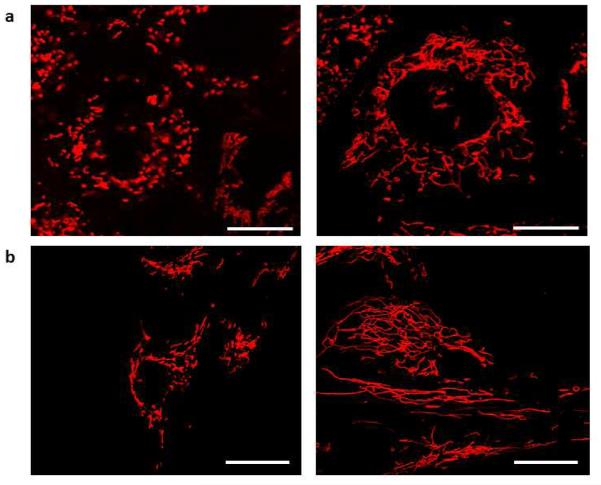
Fission is mediated by a highly conserved, large guanosine triphosphatase (GTPase), DRP-1 [6]. Inactive DRP-1 exists as a cytosolic monomer. When activated, DRP-1 translocates to the outer mitochondrial membrane where GTP hydrolysis results in DRP-1 multimerization. These DRP-1 multimers form structures resembling collars that encircle, segment and divide the mitochondrion (fission) [2, 3, 70]. Silencing DRP-1 in PAH PASMCs with siDRP-1 recovers mitochondrial network connectivity (Figure 4).
Figure 4. Silencing DRP-1 recovers mitochondrial network.
(a) Pulmonary Artery smooth muscle cells (PASMCs) derived from patients with pulmonary arterial hypertension (PAH) showing fragmented mitochondria. (b) Silencing DRP-1 with si-DRP-1 results in elongated mitochondrial network in PAH PASMCs. Cells were loaded with mitochondrial red fluorescent dye tetramethylrhodamine methyl ester (TMRM) and imaged with confocal microscope to assess the mitochondrial network structure.
DRP-1 activity is rapidly regulated by posttranslational mechanisms, the best characterized being changes in phosphorylation of serines 616 and 637, resulting in DRP-1 activation and inhibition, respectively. In both PAH and lung cancer, there are complementary pro-fission abnormalities, including phosphorylation of DRP-1 at serine 616 and dephosphorylation of DRP-1 at serine 637 [5, 62]. Phosphorylation of DRP-1 at serine 616 is mediated by the mitosis initiator, cyclin B1-CDK1, and perhaps also by the calcium-sensitive calmodulin kinase (CaM kinase).
Coordination between mitochondrial division and the cell cycle is required for normal cell division. In normal cells, a burst of DRP-1-mediated fission during M-phase allows equitable distribution of the organelles to the daughter cells [71]. This mitotic fission results from phosphorylation of DRP-1 at serine-616 by cyclin B1-CDK1 [4, 5, 62]. Upon mitotic exit, ubiquitination of DRP-1, by anaphase-promoting complex/cyclosome (APC) and its coactivator Cdh1 (APC/CCdh1), marks it for proteasomal degradation, allowing the mitochondrial network to reassemble [72]. In PAH PASMCs a CDK1 inhibitor (RO-3306) appears more effective than a CamK-inhibitor (KN93) in reducing DRP-1 phosphorylation, suggesting that cyclin B-CDK1 may be particularly important [62]. However, cytosolic Ca2+ is elevated in PAH PASMCs and increasing cytosolic Ca2+ in the normal PASMC induces fission, suggesting a potential role for CamK. Shared dependence of proliferation and DRP-1 activation on these kinases could link mitochondrial fission to the cell cycle and cytosolic Ca2+, respectively.
Several strategies can be used to inhibit DRP-1 as an experimental PAH therapy. DRP-1 can be inhibited using the small molecule GTPase inhibitor, mitochondrial division inhibitor (mdivi-1) [73]. Since DRP-1 must bind partners in the outer mitochondrial membrane to initiate fission, one can also block fission by interrupting interaction between DRP-1 and its binding partners. For example, the peptide P110 prevents DRP-1 activation and fission by blocking the protein-protein interaction between Fis1 and DRP-1 [74]. Alternatively, one can inhibit fission by reducing DRP-1 expression, using small inhibitory RNA targeting DRP-1 (siDRP-1) [71].
In PAH PASMCs [62] and cancer cells [5], mdivi-1 and siDRP-1 yield concordant anti-proliferative, pro-apoptotic effects, although this does not exclude the possibility of off-target effects of mdivi-1. Returning to the theme of impaired oxygen sensing in PAH, repeated injection of cobalt, a HIF-1α activator, produces a form of PH in normoxic rats characterized by medial hypertrophy of small PAs, RVH and reduced functional capacity [62]. The PASMC mitochondria in this model are fragmented. In vivo, mdivi-1 (50 mg/kg IP) reverses mitochondrial fragmentation and regresses experimental PAH. Mdivi-1 had anti-proliferative effects in the chronic hypoxia and cobalt model of PAH and decreased muscularization of the PAs. Interestingly, incubation of normal PASMC in cobalt for 3 hours is sufficient to activate HIF-1α and DRP-1, resulting in normoxic mitochondrial fission, creating a phenocopy of the PAH PASMC. Since the gene encoding DRP-1 lacks a hypoxia response element, HIF-1αβ1 affect on DRP-1 in PAH is assumed to be indirect. In the regression studies of mdivi-1, daily treatment in animals with established PAH (monocrotaline rat model, PH confirmed by echocardiography), and continued for 5 days improved RV function and decreased both PASMC proliferation rates, as well as muscularization of small PA. Mdivi-1 improved exercise capacity and decreased PVR as assessed by echocardiography. Neither P110 nor siDRP-1 has been studied therapeutically in vivo in experimental PAH.
Fusion and Mitofusin-2 (MFN2) in PAH
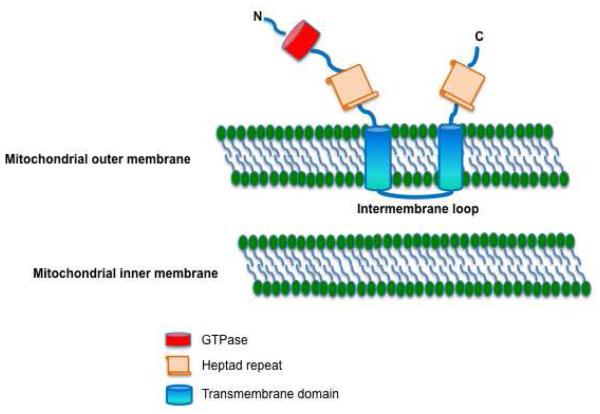
Fusion is regulated by the GTPases, mitofusin-1 (MFN1) and MFN2 located in the outer mitochondrial membrane. Mitofusins tether nearby mitochondria initiating the docking required for fusion [75]. Fusion also requires annealing of the inner mitochondrial membrane, which is mediated by the GTPase, optic atrophy-1 (OPA1). Mitofusin activity relates to 3 features: an amino-terminal GTPase domain, two cytosolic, hydrophobic heptad repeats (HR1 and HR2) and a critical 2–3-amino acid, tryptophan-containing intermembrane loop [76] (Figure 5). The heptad repeats bind each other and tether the mitochondria through a GTPase-dependent mechanism [77]. Fusion between mitochondria permits redistribution of key proteins/genes and may mitigate the accumulation of mutations in the mitochondrial DNA [77]. In addition, fusion can modify rates of cell proliferation. Indeed, MFN2 was initially referred to as hyperplasia suppressor gene when it was cloned because of its antiproliferative effect [78].
Figure 5.
Schematic diagram of MFN2 showing anchoring of MFN2 on mitochondrial outer membrane and its different domains.
MFN2 expression is down regulated in PAH [31]. Transfection of normal PASMC with siRNA targeting MFN2 recapitulates a PAH phenotype, leading to a fragmented mitochondrial network and increasing rates of PASMC proliferation. The downregulation of MFN2 in PAH appears to be acquired, since it is found not only in human PAH, but also in rodent models of PAH, created in genetically normal female rats [31]. There are several possible mechanisms for acquired downregulation of MFN2. First, proliferative stimuli that are upregulated in PAH, such as platelet derived growth factor (PDGF) [79] and endothelin 1 (ET-1) [28], can reduce MFN2 expression in systemic arterial SMC [78]. Second, PGC-1α, which is a transcriptional co-activator of MFN2, is down regulated in PAH [69]. PGC-1α also stimulates the activity of the MFN2 promoter through estrogen-related receptor-alpha (ERR-α)-binding elements. ERR-α is itself a transcriptional coactivator of the MFN2 promoter [80]. In light of the 4:1 female:male gender ratio in PAH the possibility of a sex hormone link to the regulation of MFN2 is intriguing.
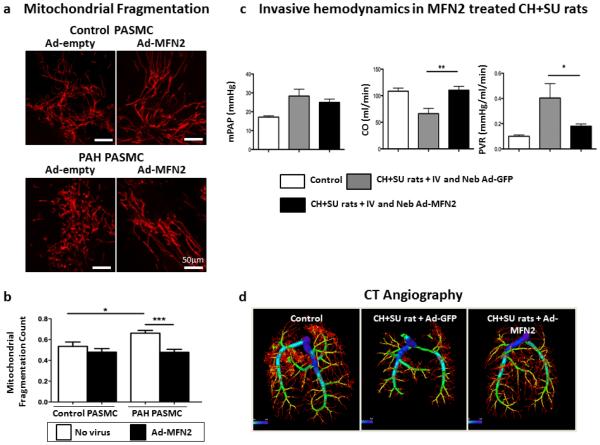
In keeping with the hypothesis that PAH is a disease resulting in part from impaired oxygen sensing, hypoxia (5% O2) reduces MFN2 expression in normal PASMC by ~20% and contributes to the mitochondrial fragmentation seen in these hypoxic cells [31]. Adenoviral overexpression of MFN2, using Ad-MFN2, recovers mitochondrial networks in MFN2−/− mouse embryonic fibroblast cells (MEFs) and PAH PASMCs (Figure 6). This same vector increases MFN2 expression, reduces proliferation, and restores normal rates of apoptosis in PAH PASMC. Infection of PASMCs cultured from patients with PAH with Ad-MFN2 restores fusion of the mitochondrial network, as evident by a decrease in the MFC (Figure 7 a&b).
Figure 6. MFN2 regresses PAH and decreases mitochondrial fragmentation in MFN2 −/− MEFs and PAH PASMCs.
(a) MFN2 knockout (KO) MEFs showing fragmented mitochondria (left); MFN2 KO MEFs infected with an adenovirus containing MFN2 cDNA (Ad-MFN2) showing fused mitochondria (right).
(b) PASMCs derived from patients with PAH showing fragmented mitochondria (left); PAH PASMCs infected with Ad-MFN2 showing elongated mitochondrial network (right). Cells were loaded with mitochondrial red fluorescent dye tetramethylrhodamine methyl ester (TMRM) and imaged with confocal microscope to assess the mitochondrial network structure. Scale bar=5 μm in (A); scale bar=7.5 μm in (B).
Figure 7. Mitochondrial Fragmentation in PAH can be treated with Ad-MFN2.
(a) Increased mitochondrial fragmentation is observed in human PAH PASMC compared with control in cells loaded with the mitochondrial targeted dye tetramethylrhodamine methyl ester. Mitochondria fragmentation is reversible when cells are transfected with Ad-MFN2. Mitochondria are labeled in red.
(b) MFN2 overexpression reduces the mitochondrial fragmentation count. Total of 20–25 cells studied in each group.
(c) Ad-MFN2 significantly increased cardiac output (CO) and reduced pulmonary vascular resistance (PVR) in CH+SU5416 rats.
(d) Representative computed tomography (CT) angiogram. Note that compared with control, CH+SU5416 rats have decreased percentage of small vessels (< 0.1 μm) and treatment with Ad-MFN2 significantly increased the percentage of small vessels.
Reproduced with permission from [31].
In early preclinical studies the replication-deficient vector Ad-MFN2 was evaluated as gene therapy for PAH. The vector was administered intravenously and/or via tracheal nebulization to rats with experimental PAH, both the monocrotaline model and a rat model involving a combination of chronic hypoxia plus the VEGF receptor antagonists, SU5416 (CH+SU5416). In this series of studies, PAH was confirmed in the animals by echocardiographic measurement of pulmonary artery acceleration time (PAAT). Gene therapy was performed in anesthetized rats by administration of 0.1 ml of Ad-MFN2 or Ad-GFP virus (in saline, 2×109 plaque-forming units) in nebulized form and with intravenous administration through the internal jugular vein. Exercise capacity and hemodynamics were measured 2–3 weeks after therapy. Ad-MFN2 therapy improved functional capacity and reduced PVR in both CH+SU5416 and monocrotaline rats with PAH (Figure 7c). In addition, Ad-MFN2 gene therapy resulted in positive vascular remodeling, evident as increased numbers of small PAs, as quantified using computed tomography pulmonary angiography (Figure 7d), suggesting the feasibility of regressing PAH with therapies that promote a fused mitochondrial network.
It remains controversial whether the anti-proliferative effects of MFN2 require fusion since deletion of MFN2’s GTPase domain, including the p21 Ras signature motif but not its mitochondrial-targeting domain, abolishes MFN2-induced growth arrest [78]. Non-fusogenic mutants of MFN2 may induce cell-cycle arrest and inhibit proliferation by inhibition of ERK/MAPK signaling [78]. While it has been demonstrated that the mutant cytosolic form of MFN2 is antiproliferative [81], it is unknown whether compensatory changes in other fission/fusion proteins (such as MFN1) may occur in the N-terminal MFN2 mutants that might permit ongoing mitochondrial fusion. The concordant effects of DRP-1 inhibition and MFN2 augmentation on proliferation and fusion suggest that achieving network fusion, regardless the means, is important to the anti-proliferative, pro-apoptotic effects of both strategies [31, 62].
Mitochondrial Abnormalities in the Right Ventricle
RVF is the final common pathway for mortality in PAH. RV ischemia, resulting both from decreased perfusion pressure and capillary rarefaction [10], may contribute to RV dysfunction and provoke transcriptional changes that lead to abnormal metabolic pathways. Mitochondrial dysfunction is an important determinant of the metabolic shifts observed in the failing left ventricle [82]. Likewise in RVH, whether induced by PAH or pressure overload (in the pulmonary artery banding model), there are mitochondrial metabolic abnormalities, including an increase in uncoupled glycolysis [21, 22], disorders of FAO [45], and de novo induction of glutaminolysis [83]. Of note, not all forms of RVH are the same. Even when RV afterload and mass are similar some PAH patients (and most rats with PA banding-induced RVH) develop an adaptive form of RVH that is concentric and is associated with preserved RV function [84]. In contrast, many PAH patients (and most monocrotaline rats) develop maladaptive RVH, characterized by dilatation, fibrosis and RVF. Adaptive RVH is associated with greater functional capacity and longevity. In contrast, maladaptive RVH portends poor prognosis and is marked by impairment of angiogenesis and greater activation of the adrenergic signaling pathways, as well as more profound disorders of the mitochondrial metabolism [14].
The mitochondria in the RV are arranged in highly structured units associated with the Z-bands that would seem to preclude substantial mobility, suggesting that the role of fission and fusion in cardiac myocytes may be different than in vascular cells. Based on studies in mice with cardiac deletion of mitofusins or DRP-1, it appears that mitochondrial dynamics are important in the heart, through effects on ROS production and bioenergetics. It is less clear what role mitochondrial dynamics may play in RVH. Germ line deletion of DRP-1 or a tandem deletion of MFN1 and MFN2 is lethal to the embryo [85-88]. However, an inducible, cardiac-specific DRP-1 knockout model demonstrates that, loss of DRP-1 in the heart, even after development is harmful. Global cardiac loss of DRP-1 expression led to death of most animals within 4 months. The hearts of these animals displayed reduced ejection fraction and hypertrophy, as well as fibrosis. Loss of DRP-1 increased ROS production and sensitized these mice to cardiac damage during ischemia reperfusion injury [89]. In contrast, cardiac knockdown of a single mitofusin isoform seems to protect against ischemia-reperfusion injury and resulted in only mild mitochondrial dysfunction, LVH, and reduction in LV function [90]. However, the impact of PAH or RVH in DRP-1 or MFN2 knockdown mice has not been studied.
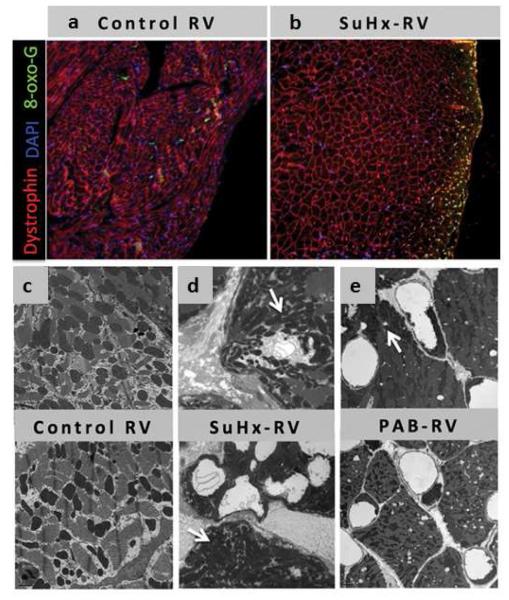
It has recently been reported that downregulation of PGC-1α expression in the failing RV decreases mitochondrial gene expression with a resultant decrease in target genes encoding proteins required for fatty acid metabolism [91]. Electron microscopy reveals decreased number of mitochondria in the failing RV with substantial abnormalities in size and shape [91] (Figure 8). The relevance of these findings in the RV requires validation.
Figure 8.
(a) & (b): Immunofluorescence shows increased 7,8-dihydro-8-oxoguanine (green) in CH+SU5416-RVD compared to control tissue.
(c), (d) & (e): Electron microscopy demonstrates abnormal ultrastructure of mitochondrial when comparing normal RV myocardium (C) with RV dysfunction from CH+SU5416 animals and RV from pulmonary artery banding (PAB) animals.
Reproduced with permission from [91].
Conclusions
There is an emerging appreciation that mitochondrial dynamics are impaired in PAH and that the increase in fission and impairment of fusion offer new therapeutic targets that address the proliferative, apoptosis-resistant phenotype seen in PAH vasculature. These findings suggest that strategies to inhibit mitochondrial fission and promote fusion might also be beneficial to the RV. The impact of modulating fission and fusion in the RV and PA in PAH merits further study.
Acknowledgements
supported by NIH-RO1-HL071115, 1RC1HL099462-01 (S.A) and the American Heart Association (AHA), CIHR Vascular Network (A.D).
Footnotes
Disclosure: Patent for use of PDK inhibitors in cancer (not commercialized).
References
- 1.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C, Archer SL. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012;26:2175–2186. doi: 10.1096/fj.11-196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cribbs JT, Strack S. Functional characterization of phosphorylation sites in dynamin-related protein 1. Methods in enzymology. 2009;457:231–253. doi: 10.1016/S0076-6879(09)05013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H, Matsushita M. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 9.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archer SL, Fang YH, Ryan JJ, Piao L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ. 2013;3:144–152. doi: 10.4103/2045-8932.109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 12.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 13.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res. 2014;115:176–188. doi: 10.1161/CIRCRESAHA.113.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatton N, Ryan JJ. Sex differences in response to pulmonary arterial hypertension therapy: is what's good for the goose, good for the gander? Chest. 2014;145:1184–1186. doi: 10.1378/chest.13-3061. [DOI] [PubMed] [Google Scholar]
- 16.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, Pepke-Zaba J, Sheares KK, Corris PA, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 17.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 19.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Herve P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 20.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 21.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl) 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, Michelakis ED. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 2013;91:1315–1327. doi: 10.1007/s00109-013-1059-4. [DOI] [PubMed] [Google Scholar]
- 23.Thenappan T, Ryan JJ, Archer SL. Evolving epidemiology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:707–709. doi: 10.1164/rccm.201207-1266ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35:1079–1087. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- 26.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 27.Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, Fishman MC, Zapol WM. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- 28.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 29.Reeve HL, Michelakis E, Nelson DP, Weir EK, Archer SL. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol . 2001;1985;90:2249–2256. doi: 10.1152/jappl.2001.90.6.2249. [DOI] [PubMed] [Google Scholar]
- 30.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr., Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 31.Ryan JJ, Marsboom G, Fang YH, Toth PT, Morrow E, Luo N, Piao L, Hong Z, Ericson K, Zhang HJ, et al. PGC1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187:865–878. doi: 10.1164/rccm.201209-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 34.Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest. 2000;105:21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huertas A, Perros F, Tu L, Cohen-Kaminsky S, Montani D, Dorfmuller P, Guignabert C, Humbert M. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. Circulation. 2014;129:1332–1340. doi: 10.1161/CIRCULATIONAHA.113.004555. [DOI] [PubMed] [Google Scholar]
- 36.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, Takahashi T, Nawata J, Ido T, Watanabe J, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol. 2005;45:1849–1855. doi: 10.1016/j.jacc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 37.Hagan G, Southwood M, Treacy C, Ross RM, Soon E, Coulson J, Sheares K, Screaton N, Pepke-Zaba J, Morrell NW, et al. (18)FDG PET imaging can quantify increased cellular metabolism in pulmonary arterial hypertension: A proof-of-principle study. Pulm Circ. 2011;1:448–455. doi: 10.4103/2045-8932.93543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, et al. Lung (1)(8)F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:670–679. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMurtry IF, Morris KG, Petrun MD. Blunted hypoxic vasoconstriction in lungs from short-term high-altitude rats. Am J Physiol. 1980;238:H849–857. doi: 10.1152/ajpheart.1980.238.6.H849. [DOI] [PubMed] [Google Scholar]
- 40.Reeve HL, Michelakis E, Nelson DP, Weir EK, Archer SL. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol . 2001;1985;90:2249–2256. doi: 10.1152/jappl.2001.90.6.2249. [DOI] [PubMed] [Google Scholar]
- 41.Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 42.Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L202–208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 43.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 45.Fang YH, Piao L, Hong Z, Toth PT, Marsboom G, Bache-Wiig P, Rehman J, Archer SL. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle's cycle. J Mol Med (Berl) 2012;90:31–43. doi: 10.1007/s00109-011-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutendra G, Michelakis ED. Pulmonary arterial hypertension: challenges in translational research and a vision for change. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005428. 208sr205. [DOI] [PubMed] [Google Scholar]
- 47.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, Kriett JM, Yung G, Rubin LJ, Yuan JX. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol . 2003;85:L740, 754. doi: 10.1152/ajplung.00284.2002. [DOI] [PubMed] [Google Scholar]
- 49.Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, Tada Y, Tanabe N, Tatsumi K, Hemnes AR, West JD. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ. 2012;2:201–213. doi: 10.4103/2045-8932.97606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 51.Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J. 1995;9:183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- 52.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;92:367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Archer SL, Will JA, Weir EK. Redox status in the control of pulmonary vascular tone. Herz. 1986;11:127–141. [PubMed] [Google Scholar]
- 54.Archer SL, Nelson DP, Weir EK. Simultaneous measurement of O2 radicals and pulmonary vascular reactivity in rat lung. J Appl Physiol . 1989;1985;67:1903–1911. doi: 10.1152/jappl.1989.67.5.1903. [DOI] [PubMed] [Google Scholar]
- 55.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 56.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 57.Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992;262:C882–890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- 58.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 59.Hong Z, Kutty S, Toth PT, Marsboom G, Hammel JM, Chamberlain C, Ryan JJ, Zhang HJ, Sharp WW, Morrow E, et al. Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ Res. 2013;112:802–815. doi: 10.1161/CIRCRESAHA.111.300285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maggiorini M. Prevention and treatment of high-altitude pulmonary edema. Prog Cardiovasc Dis. 2010;52:500–506. doi: 10.1016/j.pcad.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Wolin MS. Novel role for the regulation of mitochondrial fission by hypoxia inducible factor-1alpha in the control of smooth muscle remodeling and progression of pulmonary hypertension. Circ Res. 2012;110:1395–1397. doi: 10.1161/CIRCRESAHA.112.270801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, Yu QC, Solomides CC, Morrisey EE, Khurana TS, et al. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest. 2010;120:827–839. doi: 10.1172/JCI36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 65.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet SN, Haromy A, Harry G, Moudgil R, McMurtry MS, et al. An abnormal mitochondrial-HIF-1-Kv channel pathway disrupts oxygen-sensing and triggers pulmonary arterial hypertension (PAH) in fawn-hooded rats: similarities to human PAH. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 66.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med. 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piao L, Sidhu VK, Fang YH, Ryan JJ, Parikh KS, Hong Z, Toth PT, Morrow E, Kutty S, Lopaschuk GD, et al. FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: therapeutic benefits of dichloroacetate. J Mol Med (Berl) 2013;91:333–346. doi: 10.1007/s00109-012-0982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 69.Ryan JJ, Marsboom G, Fang YH, Toth PT, Morrow E, Luo N, Piao L, Hong Z, Ericson K, Zhang HJ, et al. PGC1alpha-Mediated Mitofusin-2 Deficiency in Female Rats and Humans With Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201209-1687OC. DOI 10.1164/rccm.201209-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu PP, Patterson A, Stadler J, Seeburg DP, Sheng M, Blackstone C. Intra- and intermolecular domain interactions of the C-terminal GTPase effector domain of the multimeric dynamin-like GTPase Drp1. J Biol Chem. 2004;279:35967–35974. doi: 10.1074/jbc.M404105200. [DOI] [PubMed] [Google Scholar]
- 71.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 72.Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 73.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem. 2005;280:25060–25070. doi: 10.1074/jbc.M501599200. [DOI] [PubMed] [Google Scholar]
- 76.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 77.Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci. 2003;116:2763–2774. doi: 10.1242/jcs.00479. [DOI] [PubMed] [Google Scholar]
- 78.Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, et al. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol. 2004;6:872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- 79.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes. 2006;55:1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 81.Chen KH, Dasgupta A, Ding J, Indig FE, Ghosh P, Longo DL. Role of mitofusin 2 (Mfn2) in controlling cellular proliferation. FASEB J. 2014;28:382–394. doi: 10.1096/fj.13-230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 83.Piao L, Fang YH, Parikh K, Ryan JJ, Toth PT, Archer SL. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl) 2013;91:1185–1197. doi: 10.1007/s00109-013-1064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rich S, Pogoriler J, Husain AN, Toth PT, Gomberg-Maitland M, Archer SL. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest. 2010;138:1234–1239. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Liu Y, Dorn GW., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ngoh GA, Papanicolaou KN, Walsh K. Loss of mitofusin 2 promotes endoplasmic reticulum stress. J Biol Chem. 2012;287:20321–20332. doi: 10.1074/jbc.M112.359174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC, Walsh K. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res. 2012;111:1012–1026. doi: 10.1161/CIRCRESAHA.112.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, et al. Endogenous Drp1 Mediates Mitochondrial Autophagy and Protects the Heart Against Energy Stress. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.116.303356. DOI 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 90.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassell B, Natarajan R, dos Remedios CG, Drake JI, Farkas L, Kraskauskas D, Wijesinghe DS, et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail. 2013;6:136–144. doi: 10.1161/CIRCHEARTFAILURE.111.966127. [DOI] [PMC free article] [PubMed] [Google Scholar]