Abstract
Background
The purpose of this study was to assess treatment patterns and examine organ utilization in the setting of single lung transplantation (SLT).
Methods
The United Network for Organ Sharing database was queried for all SLTs performed from 1987–2011. Trends in utilization of the second donor lung were assessed, both from recipient and donor perspectives. Donors were stratified into two groups: those donating both lungs and those donating only one. Independent predictors of utilizing only one donor lung were identified using multivariable logistic regression.
Results
10,361 SLTs were identified, originating from 7,232 unique donors. Of these donors, only 3,129 (43.3%) had both lungs utilized, resulting in over 200 second donor lungs going unused annually since 2005, with no significant increase in utilization over time (p=.95). Following adjustment, donor characteristics predicting the second donor lung going unused included B/AB blood groups (adjusted odds ratio [AOR]: 1.69 and 2.62, respectively, p<.001), lower body surface area (AOR 1.30, p=.02), lower donor pO2 (AOR 0.90 per 50 mmHg increase, p<.001), pulmonary infection (AOR 1.15, p=.04), extended criteria donor status (AOR 1.66, p<.001), and head trauma or anoxia cause of death (AOR 1.57 and 1.53, p<.001 and p=.001, respectively).
Conclusions
Among donors for SLT, less than half of all cases led to use of the second donor lung. While anatomic, infectious, or other pathophysiologic issues prohibit 100% utilization, more aggressive donor matching efforts may be a simple method of increasing the utilization of this scarce resource, particularly for less common blood types.
Keywords: single lung transplantation, resource utilization, donor allocation
Introduction
Organ availability and utilization remain critical factors in the ongoing realization of lung transplantation therapy in the United States. As the number of patients with end-stage lung disease listed for transplant continues to grow, the existing supply of donor lungs is becoming less able to meet this rising demand.1, 2 Single lung transplantation (SLT), wherein each donor can potentially provide organs for two lung transplant candidates, offers a unique opportunity to efficiently utilize the available donor pool among appropriately selected recipients.3 Furthermore, there are societal implications of implementing a SLT versus bilateral lung transplant (BLT) policy for certain patient populations.4, 5 Despite these cited advantages, annual rates of SLT have remained relatively stagnant for the past two decades, compared to steady growth in the rate of BLT.2
Even with this growth in BLT, less than a quarter of available lungs from multi-organ donors are ever used for transplantation.1, 6, 7 In an effort to improve donor utilization rates, the transplant community has attempted to expand the donor pool by revising donor selection guidelines and by using extended criteria donors (ECD).1, 7–10 We have noted, however, in our own practice that when procuring for a SLT, the other lung is often not taken for another SLT. In considering how to improve donor utilization, this loss of a viable SLT is critically important. We sought to determine rates of utilization among donors to SLT recipients, particularly with respect to how often both donor lungs are utilized. The purpose of this study was to assess national treatment patterns and examine donor-centric organ utilization in the setting of SLT.
Materials and Methods
Data Source
The United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research files, which include data on all organ transplant events occurring in the United States since 1987. The lung transplantation dataset used for the present study includes a prospectively maintained cohort of lung transplantations performed between 10/1987 and 12/2011. The Duke University Institutional Review Board approved this retrospective analysis (IRB#: Pro00038191).
Study Design
All patients undergoing SLT between October 1987 and December 2011 were included for analysis. Patients undergoing bilateral lung, heart-lung, and multi-visceral transplant were excluded, as were pediatric recipients less than 18 years of age. In the primary descriptive analysis, annual trends in use of SLT were assessed. To examine utilization among donors to SLT recipients, donors were stratified into two cohorts based on unique donor identifier recorded in UNOS: those individuals from whom both lungs were donated and utilized (i.e. as two separate SLTs), and those from whom only one lung was transplanted and the other was not used (single SLT). To explore potential reasons for situations wherein only one lung is utilized, donor chest x-ray and bronchoscopy data were analyzed across both donor types, and were further stratified according to the transplanted versus the contralateral lung for donors from whom only one lung was used. As this data was not routinely collected by UNOS prior to October 1999, only SLTs performed after this time were included for this particular analysis.
Statistical Analysis
Trends in utilization of the second donor lung were evaluated across the study period using the Cochran-Armitage trend test. Continuous and categorical characteristics of donors were described with medians and interquartile range (IQR) for continuous variables and proportions (percentage) for discrete variables, and compared with Kruskal-Wallis ANOVA and Pearson’s chi-square test, respectively. Independent predictors of utilizing only one donor lung were identified using multivariable logistic regression, which included the following donor-specific variables for consideration: age, sex, ethnicity, ABO blood group, body mass index, body surface area (BSA, defined as high [>1 SD above the mean], low [>1 SD below the mean], or normal [within 1 SD of the mean]), history of tobacco use >20 pack-years, illicit drug use, presence of tattoos, diabetes mellitus, pulmonary infection (defined as a confirmed deceased donor infection from a pulmonary source), arterial partial pressure of oxygen (PaO2) on inspired oxygen of 100% prior to transplant, extended criteria donor (ECD, kidney criteria) status, and cause of death. To eliminate biases originating from the very early lung transplant experience in the United States, and to address missingness of key variables such as ECD status that were not collected up through 1993, only SLTs performed in 1994 and later were included in the predictive model.
A p-value of <.05 was considered statistically significant for all comparisons. Missing data were handled with complete case analysis, given the completeness of the variables studied. All analyses were performed using R version 3.0.2, R Foundation for Statistical Computing, Vienna, Austria.
Results
A total of 10,361 SLTs were identified during the study timeframe, originating from 7,232 unique donors. Trends in use of organs for SLT from the perspective of the recipient and donor populations are shown in Figures 1 and 2, respectively. Donor characteristics, stratified by whether one or both lungs were utilized, are shown in Table 1. While donors from whom both lungs were used tended to be older and have slightly higher rates of significant tobacco use, they also had higher paO2s, were more likely to be CMV negative and were less likely to have documented pulmonary infections or meet ECD criteria. Among cases where only one lung was donated, left-sided organs were more commonly utilized (54.3 vs. 45.7%, p=.008).
Figure 1.
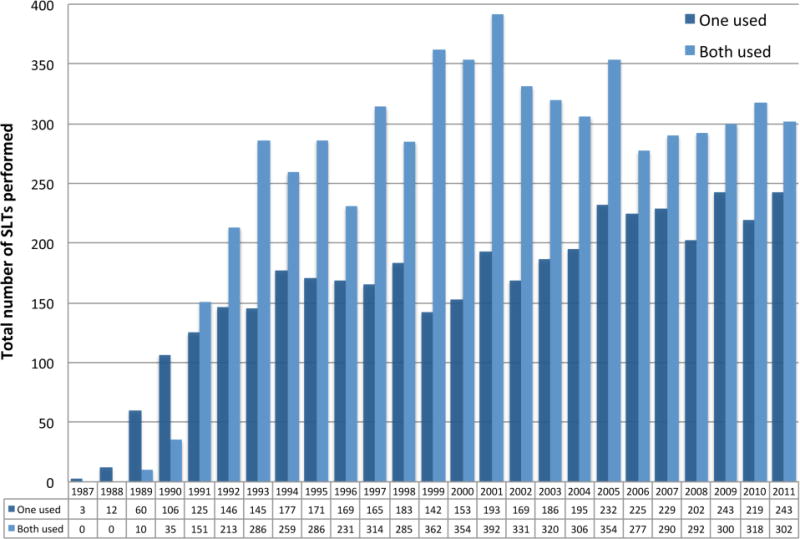
Utilization of organs for SLT annually, from lung transplant recipient perspective, 1987 to 2011.
Figure 2.
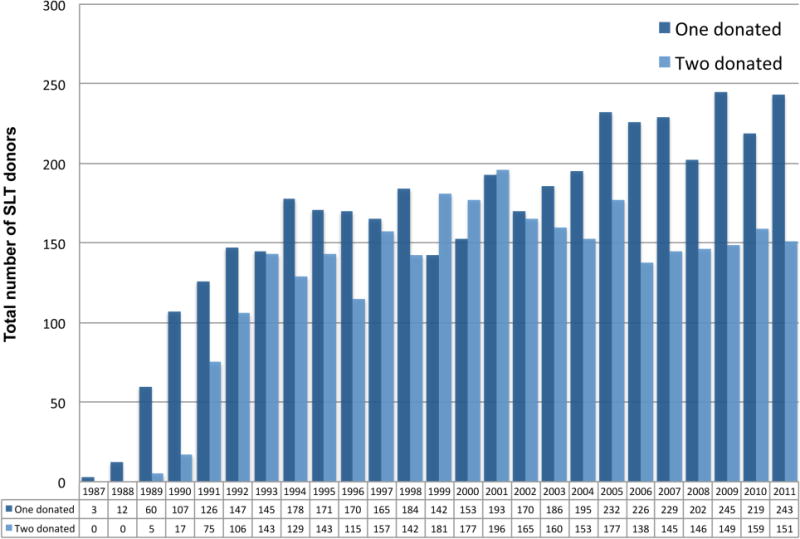
Utilization of organs for SLT annually, from organ donor perspective, 1987 to 2011.
Table 1.
Baseline Characteristics of Donors to Recipients of Single-Lung Transplantation, Stratified by Whether One or Both Lungs Were Subsequently Transplanted
| Variablea | All donors (n = 7,232) |
Single donated (n = 4,103) |
Both donated (n = 3,129) |
p-value |
|---|---|---|---|---|
| Age, years | 29 (20, 43) | 28 (20, 43) | 30 (20, 44) | <0.001 |
| Sex | 0.426 | |||
| Female | 2,547 (35.2) | 1,429 (34.8) | 1,118 (35.7) | |
| Male | 4,685 (64.8) | 2,674 (65.2) | 2,011 (64.3) | |
| BMI, kg/m2 | 24 (21.5, 27) | 24 (21.6, 27.1) | 23.9 (21.5, 26.9) | 0.425 |
| BSA, m2 | 1.9 (1.7, 2) | 1.9 (1.7, 2) | 1.9 (1.7, 2) | 0.64 |
| Ethnicity | 0.003 | |||
| White | 5,104(70.6) | 2,872 (70) | 2,232 (71.3) | |
| Black | 1,126 (15.6) | 611 (14.9) | 515 (16.5) | |
| Asian | 815 (11.3) | 503 (12.3) | 312 (10) | |
| Other | 187 (2.6) | 117 (2.9) | 70 (2.2) | |
| Tobacco abuse | 4,791 (66.2) | 2,687 (65.5) | 2,104 (67.2) | <0.001 |
| Drug use | 1,722 (23.8) | 965 (23.5) | 757 (24.2) | 0.523 |
| Diabetes | 256 (3.5) | 147 (3.6) | 109 (3.5) | 0.871 |
| Tattoos | 0.009 | |||
| No | 3,384 (46.8) | 1,859 (45.3) | 1,525 (48.7) | |
| Yes | 1,136 (15.7) | 677 (16.5) | 459 (14.7) | |
| Unknown | 2,712 (37.5) | 1,567 (38.2) | 1,145 (36.6) | |
| PaO2, mm Hg | 431 (332, 497) | 409 (302.5, 480) | 455 (376, 513) | <0.001 |
| Bilirubin, g/dl | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.2) | 0.579 |
| AST, g/dl | 44 (28, 75) | 45 (29, 79) | 42 (27,70.2) | <0.001 |
| ALT, g/dl | 30 (20, 51) | 31 (20, 55) | 29 (19,46) | <0.001 |
| CMV status | <0.001 | |||
| Negative | 2,999 (41.5) | 1,646 (40.1) | 1,353 (43.2) | |
| Positive | 4,188 (57.9) | 2,421 (59) | 1,767 (56.5) | |
| Unknown | 45 (0.6) | 36 (0.9) | 9 (0.3) | |
| Cause of death | <0.001 | |||
| Anoxia | 474 (6.6) | 285 (6.9) | 189 (6) | |
| CNS Tumor | 61 (0.8) | 30 (0.7) | 31(1) | |
| CVA/stroke | 2,396 (33.1) | 1,243 (30.3) | 1,153 (36.8) | |
| Head trauma | 3,730 (51.6) | 2,186 (53.3) | 1,544 (49.3) | |
| Other | 561 (7.8) | 352 (8.6) | 209 (6.7) | |
| Unknown | 10 (0.1) | 7 (0.2) | 3(0.1) | |
| ECD donor | 489 (7.9) | 295 (8.5) | 194 (7) | 0.036 |
| Pulmonary infection | 1,573 (21.8) | 943 (23) | 630 (20.1) | 0.004 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BSA, body surface area; CMV, cytomegalovirus; CNS, central. nervous system; CVA, cerebrovascular accident; ECD, extended criteria donor; PaO2, partial pressure of arterial oxygen.
Continuous variables are expressed as median (interquartile range) and categoric variables as number (%).
Of the SLT donors (7,232 from October 1987 and December 2011), only 3,129 (43.3%) had both lungs utilized, resulting in over 200 second potential donor lungs unused every year since 2005. Importantly, though overall donor rates are increasing the percentage of unused SLT has been relatively stable. (Figure 3, p=.95). Thus, the total number of unused SLT has increased. Of the 3,129 donor pairs in this cohort, 1,742 (55.7%) were subsequently transplanted at the same recipient center, while the other 1,387 (44.3%) went to two different centers.
Figure 3.
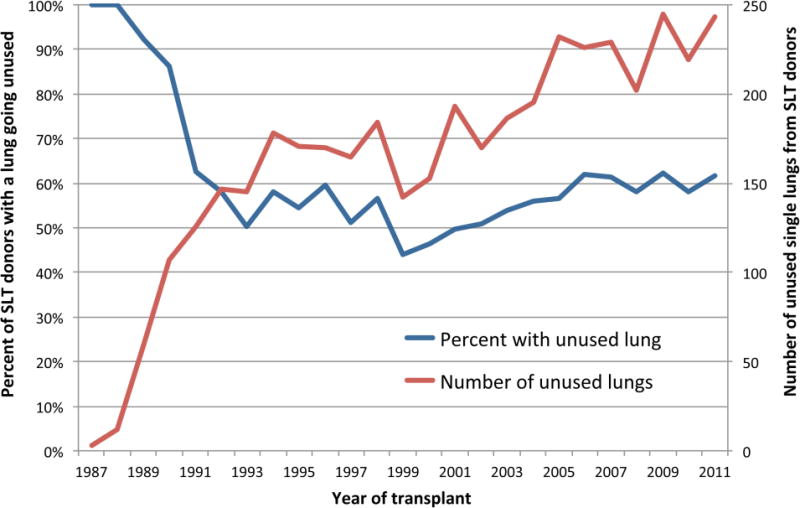
National trends in the proportion and annual number of lung transplant donors who do not donate both lungs, 1987 to 2011.
Donor chest x-ray and bronchoscopy findings for SLTs occurring after 10/1999 are shown in Table 3. Among donors from whom both lungs were used, 18.3% had abnormal chest x-ray findings and 8.5% had abnormalities on bronchoscopy. Similarly, among donors from whom only one lung was used, on the transplanted side 19.2% had abnormalities on chest x-ray and 12.8% had abnormalities on bronchoscopy. On the non-transplanted side, however, 38.7% had an abnormal chest x-ray finding, while the large majority (72.8%) did not have available bronchoscopy data regarding this non-utilized lung.
Table 3.
Chest X-ray and Bronchoscopy Findings Among Single Lung Transplantation, Stratified by Use of the Second Donor Lung
| Variable | No. (%) |
|---|---|
| When both lungs were used | (n = 3,892) |
| Chest X-ray | |
| Normal | 1,713 (44) |
| Abnormal | 713 (18.3) |
| Unknown | 1,466 (37.7) |
| Bronchoscopy | |
| None | 315 (8.1) |
| Normal | 2,144 (55.1) |
| With purulent secretions | 185 (4.8) |
| With foreign body aspiration | 17 (0.4) |
| With blood | 101 (2.6) |
| With abnormal anatomy/lesion | 26 (0.7) |
| Results unknown | 89 (2.3) |
| Unknown | 1,013 (26) |
| When one lung was used | (n = 2,511) |
| Chest X-ray | |
| Transplanted side | |
| Normal | 1,323 (52.7) |
| Abnormal | 483 (19.2) |
| Unknown | 705 (28.1) |
| Other side | |
| Normal | 834 (33.2) |
| Abnormal | 972 (38.7) |
| Unknown | 705 (28.1) |
| Bronchoscopy | |
| Transplanted side | |
| None | 181 (7.2) |
| Normal | 1,460 (58.1) |
| With purulent secretions | 207 (8.2) |
| With foreign body aspiration | 10 (0.4) |
| With blood | 74 (2.9) |
| With abnormal anatomy/lesion | 15 (0.6) |
| Unknown | 564 (22.5) |
| Other side | |
| Unknown | 1,828(72.8) |
| None | 91 (3.6) |
| Normal | 403 (16) |
| With purulent secretions | 129 (5.1) |
| With foreign body aspiration | 7 (0.3) |
| With blood | 42 (1.7) |
| With abnormal anatomy/lesion | 11 (0.4) |
After multivariable adjustment, independent donor characteristics associated with the second donor lung going unused included B and AB blood groups (adjusted odds ratio [AOR]: 1.69 and 2.62, respectively, p<.001), lower BSA (AOR 1.30, p=.02), lower donor pO2 (AOR 0.90 per 50 mmHg increase, p<.001), pulmonary infection (AOR 1.15, p=.04), ECD status (AOR 1.66, p<.001), and head trauma or anoxia cause of death (AOR 1.57 and 1.53, p<.001 and p=.001, respectively) (Table 2).
Table 2.
Donor Charactsristics Predictive of Only One Lung Being Used for Transplantation, Among All Single Lung Transplants Performed From 1994 to 2011
| Predictor | Odds ratio (95% CI) | p-value |
|---|---|---|
| Age per decade | 0.96 (0.90–1.02) | 0.20 |
| Male sex | 0.94 (0.80–1.10) | 0.43 |
| ABO group | ||
| O | Reference | |
| A | 1.05 (0.92–1.20) | 0.51 |
| B | 1.69 (1.36–2.10) | <0.001 |
| AB | 2.62 (1.60–4.27) | <0.001 |
| BMI | 1.01 (1 00–1.03) | 0.14 |
| BSA | ||
| ≤1 SD of the mean | Reference | |
| Low | 1.30 (1.05–1.60) | 0.02 |
| High | 1.11 (0.90–1.36) | 0.33 |
| Smoking history of > 20 years | ||
| No | Reference | |
| Yes | 0.98 (0.83–1.16) | 0.82 |
| Unknown | 1.54 (0.76–3.10) | 0.23 |
| Drug use | 1.02 (0.88–1.17) | 0.83 |
| Diabetes | 1.12 (0.82–1.52) | 0.48 |
| Ethnicity | ||
| White | Reference | |
| Black | 0.91 (0.77–1.08) | 0.28 |
| Asian | 1.21 (1 00–1.47) | 0.05 |
| Other | 1.15 (0.79–1.67) | 0.46 |
| Donor Po2 per 50 mm Hg | 0.90 (0.88–0.92) | <0.001 |
| Pulmonary infection | 1.15 (1.01–1.32) | 0.04 |
| Donor tattoos | ||
| No | Reference | |
| Yes | 1.17 (1.00–1.36) | 0.047 |
| Unknown | 2.09 (0.72–6.03) | 0.17 |
| ECD donor | 1.66 (1.28–2.15) | <0.001 |
| Cause of death | ||
| CVA/stroke | Reference | |
| Head trauma | 1.57 (1.33–1.86) | <0.001 |
| Anoxia | 1.53 (1.19–1.96) | 0.001 |
| Other | 1.39 (0.94–2.04) | 0.10 |
BSA, body surface area; CI, confidence interval; CVA, cerebrovascular accident; ECD, extended criteria donor; Po2 partial pressure of oxygen; SD, standard deviation.
Discussion
Single lung transplantation can be an effective strategy for the treatment of end-stage lung disease in appropriately selected patients, and offers potential benefit with respect to a more efficient use of scarce resources. The findings of our study, however, suggest that among organ donors providing lungs to SLT recipients, the second donor lung was not utilized in the majority of cases. After adjusting for a robust array of donor characteristics, we identified a number of predictors independently associated with such inefficiency. The surprisingly high rate of non-utilization with respect to the second lung provides for opportunities to substantially expand the available donor pool in the setting of single lung transplantation.
To our knowledge, this is the first study to examine utilization on a national scale among donors who already met criteria for transplantation to a SLT recipient somewhere in the UNOS zone, and identifies critical factors for underutilization with respect to the second lung. Existing studies have demonstrated historically low acceptance rates of 15–25% for lungs from multi-organ donors overall, representing the lowest donor utilization rate of any major solid organ transplant.3, 10, 11 Extensive research over the past decade has focused on methods to potentially expand the donor pool for all lung transplants, by modifying selection criteria, using extended criteria donors, and optimizing donor-recipient selection methods. All of these efforts, however, have focused on broadening selection methods to allow for previously inappropriate donors to become marginal or acceptable donors.1, 7–9, 12, 13 Our results compliment these findings and are equally important, as even among potential lung donors who are deemed acceptable for SLT for at least one lung, the second lung is used less than half of the time. While in some cases this may be due to clinical reasons isolated to the other lung, it is likely that much of this underutilization is due to the logistics of finding two separate recipients, potentially at two different centers for transplantation within a narrow window of opportunity.
Perhaps most surprisingly, we found that more than 200 donor lungs from candidates deemed to be acceptable donors subsequently go unused each year. Based on our analysis of available chest x-ray and bronchoscopic findings among the non-utilized lungs, even the most conservative approximation would indicate that only half of unused single lungs are lost due to anatomic or medical concerns. Accordingly, we suggest that a substantial number of second donor lungs may be lost for logistical or recipient issues rather than for reasons of organ quality. It is well established that long-term survival for individual patients is improved with a BLT compared to SLT strategy. However, attempts have been made to determine how a SLT policy for eligible patients would impact societal outcomes and resources. Nearly all of these analyses and simulations comparing SLT versus BLT are predicated on the assumption that a strategy of SLT results in two lung transplants per donor compared to only one for BLTs.14–16 Our results suggest that this assumption is unfounded, and future studies must account for underutilization of the second lung in order to more equitably compare SLT to BLT policies from a societal perspective.
In our attempts to further characterize reasons for non-utilization, certain factors, such as lower donor PaO2, ECD status and cause of death were perhaps not surprisingly associated with use of only one potential lung. Other factors, such as B/AB blood type and lower BSA, suggest there are underlying logistical issues as well, with difficulties finding appropriate SLT matches pertaining to specific, less common donor characteristics. Lungs from type O donors are not limited by ABO incompatibility issues, leading to fewer barriers to finding appropriate matches compared to donors with other blood types. Similarly, while allograft size is typically not a concern for BLTs from a functional standpoint, there may be concerns regarding undersizing and post-transplantation lung function for patients undergoing SLT, which would explain the finding that donors with lower BSAs are associated with lower rates of utilization. We also observed that left sided organs were more commonly utilized compared to lungs from the right side, which is likely multifactorial but may be due to higher risk of aspiration in the right donor lung. While anatomic, infectious, and other pathophysiologic issues will always prohibit 100% utilization of the second lung, more aggressive and focused donor matching efforts may be a simple method of significantly increasing the utilization of this scarce resource. As logistics are likely a driving force in under-utilization of the second donor lung, the increasing attention on centralized organ removal may offer potential means of allowing for more efficient use of both lungs. Furthermore, it must be considered whether UNOS should consider prioritizing BLTs if both lungs are usable in the allocation scheme, or instead require organ procurement organizations to more aggressively place both lungs for SLT, even placing the second lung outside of the LAS system.
While the overall results of this study are compelling, this is nonetheless a retrospective study subject to inherent limitations and treatment-level biases. Perhaps most importantly, we lacked data on the specific reasons for organ refusal in cases where the second lung was not utilized. Likewise, while we attempted to investigate potential explanations based on donor chest x-ray and bronchoscopy findings, this approach was limited by a lack of granularity and substantial missing data, particularly among the unused lungs. Recognizing this, we apply a conservative estimate of approximately 50% would be discarded due to medical reasons. Lastly, our results only apply to the population of potential recipients for whom a single lung transplant is considered a reasonable treatment approach, and our study does not address the ongoing resource shortage for patients requiring bilateral transplants. Despite these limitations, we nonetheless provide a robust analysis of organ donation for SLT in the United States, and highlight the current inadequacies of organ utilization within this patient population.
In conclusion, we report that rates of non-utilization of the second donor lung are unacceptably high among cases of single lung transplantation. As this has important implications for the utilization of this scarce resource, the current UNOS system needs to prospectively identify specific reasons for non-utilization of the second lung. This information will further inform how we can optimize SLT donor allocation by revising current policies and allocation systems.
Acknowledgments
This work was supported by the NIH funded Cardiothoracic Surgery Trials Network; 5U01HL088953-05 (M.G.H.).
Abbreviations
- UNOS
United Network for Organ Sharing
- SLT
single lung transplantation
- BLT
bilateral lung transplantation
- ECD
extended criteria donor
- BSA
body surface area
- AOR
adjusted odds ratio
Footnotes
Disclosure: The authors have nothing to disclose.
References
- 1.Van Raemdonck D, Neyrinck A, Verleden GM, et al. Lung donor selection and management. Proceedings of the American Thoracic Society. 2009;6:28–38. doi: 10.1513/pats.200808-098GO. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report–2013; focus theme: age. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:965–78. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Kotloff RM, Thabut G. Lung transplantation. American journal of respiratory and critical care medicine. 2011;184:159–71. doi: 10.1164/rccm.201101-0134CI. [DOI] [PubMed] [Google Scholar]
- 4.Thabut G, Christie JD, Ravaud P, et al. Survival after bilateral versus single lung transplantation for patients with chronic obstructive pulmonary disease: a retrospective analysis of registry data. Lancet. 2008;371:744–51. doi: 10.1016/S0140-6736(08)60344-X. [DOI] [PubMed] [Google Scholar]
- 5.Thabut G, Christie JD, Ravaud P, et al. Survival after bilateral versus single-lung transplantation for idiopathic pulmonary fibrosis. Annals of internal medicine. 2009;151:767–74. doi: 10.7326/0003-4819-151-11-200912010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hornby K, Ross H, Keshavjee S, Rao V, Shemie SD. Non-utilization of hearts and lungs after consent for donation: a Canadian multicentre study. Canadian journal of anaesthesia = Journal canadien d’anesthésie. 2006;53:831–7. doi: 10.1007/BF03022801. [DOI] [PubMed] [Google Scholar]
- 7.Sommer W, Kühn C, Tudorache I, et al. Extended criteria donor lungs and clinical outcome: results of an alternative allocation algorithm. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:1065–72. doi: 10.1016/j.healun.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 8.de Perrot M, Snell GI, Babcock WD, et al. Strategies to optimize the use of currently available lung donors. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2004;23:1127–34. doi: 10.1016/j.healun.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Reyes KG, Mason DP, Thuita L, et al. Guidelines for donor lung selection: time for revision? The Annals of thoracic surgery. 2010;89:1756–64. doi: 10.1016/j.athoracsur.2010.02.056. discussion 64–5. [DOI] [PubMed] [Google Scholar]
- 10.Ware LB, Wang Y, Fang X, et al. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet. 2002;360:619–20. doi: 10.1016/s0140-6736(02)09774-x. [DOI] [PubMed] [Google Scholar]
- 11.Arcasoy SM, Kotloff RM. Lung transplantation. The New England journal of medicine. 1999;340:1081–91. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 12.Pêgo-Fernandes PM, Samano MN, Fiorelli aI, et al. Recommendations for the use of extended criteria donors in lung transplantation. Transplantation proceedings. 2011;43:216–9. doi: 10.1016/j.transproceed.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 13.Venkateswaran RV, Patchell VB, Wilson IC, et al. Early donor management increases the retrieval rate of lungs for transplantation. The Annals of thoracic surgery. 2008;85:278–86. doi: 10.1016/j.athoracsur.2007.07.092. discussion 86. [DOI] [PubMed] [Google Scholar]
- 14.Gabbay E, Williams TJ, Griffiths aP, et al. Maximizing the utilization of donor organs offered for lung transplantation. American journal of respiratory and critical care medicine. 1999;160:265–71. doi: 10.1164/ajrccm.160.1.9811017. [DOI] [PubMed] [Google Scholar]
- 15.Munson JC, Christie JD, Halpern SD. The societal impact of single versus bilateral lung transplantation for chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2011;184:1282–8. doi: 10.1164/rccm.201104-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Rogers Ca, Bonser RS, Banner NR, Demiris N, Sharples LD. Assessing the benefit of accepting a single lung offer now compared with waiting for a subsequent double lung offer. Transplantation. 2011;91:921–6. doi: 10.1097/TP.0b013e31821060b5. [DOI] [PubMed] [Google Scholar]


