Abstract
Cyclic GMP is the signal transducer of a family of transmembrane, particulate guanylyl cyclase (GC) receptors with key roles in physiology and disease. GC-G, the last member of the membrane GCs identified in mammals, is an orphan receptor and its regulation and function have remained largely unknown. In this issue of The EMBO Journal, Chao et al (2015) show that the GC-G/cGMP pathway, which is expressed in a specific cluster of olfactory neurons of neonatal mice, functions as a cold-induced thermosensor, which triggers protective maternal care.
See also: Y-C Chao et al (February 2015)
Besides soluble guanylyl cyclase (sGC, the receptor for nitric oxide), seven transmembrane guanylyl cyclase (GC) receptors synthesize the second-messenger cyclic GMP (cGMP) (Kuhn, 2003). At difference to sGC, which is expressed in many types of cells, these particulate (p)GCs (GC-A to GC-G) have distinct cellular expression patterns and functions (Fig1). They all share a basic structural topology, including an extracellular domain, a short transmembrane region, and a large intracellular part ending with the catalytic, cGMP-synthesizing protein region (Fig1). Although the presence of the extracellular domain suggested that endogenous hormones activate pGCs, only GC-A through GC-D are receptors in this classical sense, since peptide ligands have been ascribed for their activation. GC-A mediates the blood pressure-regulating effects of cardiac atrial and B-type natriuretic peptides (Kuhn, 2003). GC-B, the specific receptor for C-type natriuretic peptide, is essential for long bone growth. GC-C, densely expressed in the brush border membrane of intestinal epithelia, mediates paracrine effects of guanylin and uroguanylin on secretion in the crypts and epithelial differentiation. Retinal GC-E and GC-F, colocalized within the same photoreceptor cells, are involved in phototransduction. Finally, GC-D and GC-G are pseudogenes in the human and have been mostly studied in rodents. GC-D is confined to the necklace olfactory neuroepithelium and has chemosensory roles (Leinders-Zufall et al, 2007). In contrast, GC-G has a broad tissue expression pattern including the lung, kidney, intestine, skeletal muscle and sperm, raising the possibility of a humoral regulation. However, until now, a specific endogenous ligand of GC-G has not been discovered. Studies in mice with genetic ablation of GC-G indicated that this cGMP-producing orphan receptor enhances sperm motility and protects the kidneys from ischemic injury (Huang et al, 2006; Lin et al, 2008). Ultimately, the regulation and function of GC-G have remained largely unknown.
Figure 1. Activators, general structure, main tissue expression sites and main regulatory functions of transmembrane, particulate guanylyl cyclases (pGCs).
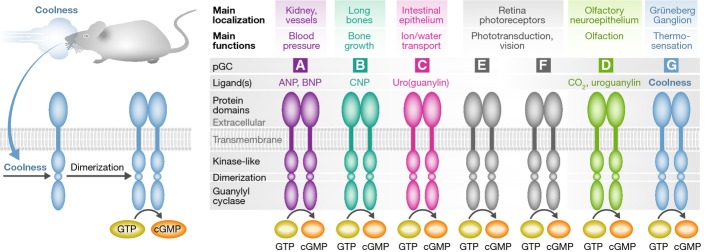
Left side: GC-G is expressed in a specific cluster of olfactory neurons of neonatal mice, the Grüneberg Ganglion (GG). Cold-ambient temperatures induce GC-G dimerization and thereby stimulate cGMP production. Activation of the GG neurons by a coolness-induced GC-G/cGMP/CNG/calcium cascade triggers ultrasound calls which alert the mother to take care of her pups.
While human and mouse genetics consistently have unveiled various physiological roles of members of the pGC family, overall the mode of ligand-dependent as well as ligand-independent activation of these transmembrane enzymes leading to intracellular cGMP synthesis remains enigmatic. The intracellular region of pGCs consists of a juxtamembranous protein kinase–homology domain, an amphipathic α-helical or hinge region, and the C-terminal cyclase catalytic domain (Fig1) (reviewed by Potter, 2011). The hinge region is involved in higher order oligomerization. Hence, although pGCs contain a single cyclase site per polypeptide chain, receptor dimerization is essential for the activation of this cGMP-synthesizing domain (Potter, 2011). The crystal structures of the extracellular domain of GC-A, in complex with atrial natriuretic peptide, or in absence of the ligand, suggested that hormone binding induces a rotation of the juxtamembrane domains, which is transmitted across the transmembrane helices and induces a similar rotation in each of the dimerized intracellular GC catalytic domains (Ogawa et al, 2004). Presumably, this activates the GC catalytic activity and synthesis of cGMP by GC-A (which was used as model system) and also by the other hormone-responsive pGCs. But this model of course is not applicable to the ligand-independent pGCs, GC-E, F and G.
In this issue of The EMBO Journal, Chao et al (2015) describe a novel mechanism of activation for the orphan GC-G and reveal the function of this receptor as cold-activated thermosensor in a specific cluster of olfactory neurons called the Grüneberg Ganglion (GG) (Fig1). The GG, found in some mammals including humans, is localized in the anterior region of the nose and is separated from the nasal cavity by a keratinized epithelium. This epithelial layer is permeable to water-soluble compounds, which suggested that the GG neurons are stimulated by specific external odors. The GG neurons project their axons to a unique domain of interconnected glomeruli in the caudal olfactory bulb and may have chemosensory functions. Indeed, recent studies showed that GG neurons are activated by “alarm” pheromones (e.g. 2-sec-butyl-4,5-dihydrothiazole) released by conspecific animals under stress conditions (Brechbuhl et al, 2013). In mice, the GG neurons are also activated by cold-ambient temperatures (Mamasuew et al, 2008). Therefore, the GG seems to operate as a dual sensory organ, which detects odorous compounds and thermal stimuli. Importantly, the molecular identity of the thermosensory process was less clear.
Previous studies had already revealed that most GG neurons express all signaling components for a cGMP-mediated signal transduction cascade. Specifically, GG neurons co-express GC-G, the cGMP-stimulated regulatory proteins phosphodiesterase type 2A (PDE2) and protein kinase II (PKG II), as well as the cGMP-regulated calcium permeable channel CNGA3 (Hanke et al, 2013). In genetic mouse models with ablated GC-G or CNGA3, the responsiveness of the GG to certain odorants or to cold-ambient temperature was found to be significantly reduced, demonstrating that a signaling cascade involving GC-G and CNGA3 is important for both chemo- and thermosensation by the GG (Mamasuew et al, 2011; Hanke et al, 2013). But how coolness modulates the cGMP-forming activity of a transmembrane GC or the physiological relevance of GC-G in GG neurons remained to be determined. Intriguingly, the present paper by Chao and colleagues demonstrates that mild cool temperatures (20–25°C) directly enhance the enzymatic activity of GC-G by inducing the formation of GC-G homodimers. Coolness-induced stimulation of GC-G subsequently increases intracellular cGMP levels and thereby the probability of CNGA3 opening, ultimately elevating intracellular Ca2+ levels and the activity of the GG neurons. Finally, Chao et al show in behavioral studies in their genetic mouse models that independent of more classical sensors like the coolness-activated TRPM8 ion channel, GC-G is critical for coolness-evoked ultrasound-vocalization in abandoned mouse pups to elicit maternal-care behavior.
Of note, the temperature-sensing role of specific transmembrane GCs was already suggested by studies in the small nematode C. elegans. For instance, a biochemical study showed that a pGC in the worm (GCY-12) has maximal enzymatic cGMP-synthesizing activity at cool temperatures (Yu et al, 1997). In addition, genetic studies suggest that other pGCs (GCY-8, GCY-18, and GCY-23) function redundantly in thermosensory neurons within the head of the worm (Inada et al, 2006). Although these studies indicate that specific GCs and cGMP-mediated signal transduction have a role in thermosensation in C. elegans, the interdisciplinary approaches used by Chao et al provide new mechanistic insights into the molecular process of cold-induced guanylyl cyclase activation, and, importantly, for the first time demonstrate the thermosensitivity of a pGC in mammals, in the murine system, with a role in protective maternal behavior. Too bad that GC-G is a pseudogene in humans and good that we are smart enough to compensate for it!
Acknowledgments
Supported by the Deutsche Forschungsgemeinschaft (SFB 688).
References
- Brechbuhl J, Moine F, Klaey M, Nenniger-Tosato M, Hurni N, Sporkert F, Giroud C, Broillet MC. Mouse alarm pheromone shares structural similarity with predator scents. Proc Natl Acad Sci USA. 2013;110:4762–4767. doi: 10.1073/pnas.1214249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao YC, Chen CC, Lin YC, Breer H, Fleischer J, Yang RB. Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures. EMBO J. 2015;34:294–306. doi: 10.15252/embj.201489652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke W, Mamasuew K, Biel M, Yang RB, Fleischer J. Odorant-evoked electrical responses in Grueneberg ganglion neurons rely on cGMP-associated signaling proteins. Neurosci Lett. 2013;539:38–42. doi: 10.1016/j.neulet.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Huang YH, Wei CC, Su YH, Wu BT, Ciou YY, Tu CF, Cooper TG, Yeung CH, Chu ST, Tsai MT, Yang RB. Localization and characterization of an orphan receptor, guanylyl cyclase-G, in mouse testis and sperm. Endocrinology. 2006;147:4792–4800. doi: 10.1210/en.2005-1476. [DOI] [PubMed] [Google Scholar]
- Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–2252. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M. Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93:700–709. doi: 10.1161/01.RES.0000094745.28948.4D. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Cheng CF, Hou HH, Lian WS, Chao YC, Ciou YY, Djoko B, Tsai MT, Cheng CJ, Yang RB. Disruption of guanylyl cyclase-G protects against acute renal injury. J Am Soc Nephrol. 2008;19:339–348. doi: 10.1681/ASN.2007050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamasuew K, Breer H, Fleischer J. Grueneberg ganglion neurons respond to cool ambient temperatures. Eur J Neurosci. 2008;28:1775–1785. doi: 10.1111/j.1460-9568.2008.06465.x. [DOI] [PubMed] [Google Scholar]
- Mamasuew K, Hofmann N, Kretzschmann V, Biel M, Yang RB, Breer H, Fleischer J. Chemo- and thermosensory responsiveness of Grueneberg ganglion neurons relies on cyclic guanosine monophosphate signaling elements. Neurosignals. 2011;19:198–209. doi: 10.1159/000329333. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Qiu Y, Ogata CM, Misono KS. Crystal structure of hormone-bound atrial natriuretic peptide receptor extracellular domain: rotation mechanism for transmembrane signal transduction. J Biol Chem. 2004;279:28625–28631. doi: 10.1074/jbc.M313222200. [DOI] [PubMed] [Google Scholar]
- Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011;23:1921–1926. doi: 10.1016/j.cellsig.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, Garbers DL. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci USA. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]


