Abstract
The landscape of alternative splicing is only beginning to unravel, and the functional consequences are often unclear. Two articles in Cell and Genome Research focus on a set of largely ignored yet highly conserved exons, microexons. These appear strongly regulated by RNA-binding proteins (RBPs) and functionally modulate protein–protein interactions with strong evidence for deregulation in autism spectrum disorder.
See also: M Irimia et al (December 2014) and YI Li et al (January 2015)
Whole genome sequencing revealed that gene number does not linearly scale with organism complexity as evidenced by humans and C. elegans carrying similar numbers of genes. Consequently, post-transcriptional regulation of gene expression is a major contributing factor to increase transcriptomic and proteomic diversity. Alternative splicing (AS) in particular expands the number of different transcripts enormously, and distinct splice variants often differ not only in their coding sequence but also in their localization and stability (Irimia & Blencowe, 2012). It is estimated that 95% of all human genes undergo AS with alternative exons often encoding for disordered regions important for protein–protein interactions, (Ellis et al, 2012).
Two recent reports (Irimia et al, 2014; Li et al, 2015) focus on a particular class of exons, called microexons, which can be as short as three nucleotides (nts) and have largely been missed in transcriptome profiling studies to date. Both articles offer a comprehensive analysis across species and various tissues. Remarkably, microexons are highly conserved and their lengths are usually multiples of three nts, which makes them less likely to disrupt the open reading frame compared to longer exons.
Consistent with previous reports on individual microexons (e.g., Black, 1991) and similar to reports on longer exons (Licatalosi & Darnell, 2010), both studies conclude that microexon regulation is most abundant in neurons. This supports the proposal that neurons have developed particular modes of RNA regulation to govern their highly specialized structures and functions (Darnell, 2013). Systematic profiling of microexons during the differentiation of embryonic stem cells to glutamatergic neurons revealed that the vast majority (70% of microexons) shows a regulated inclusion pattern, particularly during later differentiation stages. With most microexons increasing in their inclusion during differentiation, the authors argue that microexons could be important for terminal neurogenesis (Irimia et al, 2014).
Splicing is often regulated by RBPs, which recognize sequences close to 5′ and 3′ splice acceptor sites to control exon inclusion. Consistent with the particular importance of AS in the brain, many RBPs are predominantly or exclusively expressed in the nervous system, and their mutations have been linked to distinct neurological disorders (Darnell, 2013). The new studies describe clusters of highly conserved intronic flanks/regulatory sequences around AS microexons. Specific scanning of such regions for RBP-binding motifs surfaces an enrichment for RBFOX and PTBP1 binding motifs in 3′ and 5′ intronic flanks, respectively (Li et al, 2015). Recently published genome-wide RNA-binding maps for RBFOX proteins in the mouse brain suggested that 3′ intronic RBFOX binding increases exon inclusion (Weyn-Vanhentenryck et al, 2014). Consistently, RBFOX may facilitate the inclusion of microexons (Fig1). Conversely, upstream PTBP1 binding had been shown to prevent exon inclusion (Han et al, 2014), a notion corroborated here for microexons harboring upstream PTBP1 binding sites (Fig1; Li et al, 2015). A note of caution comes from the fact that PTBP1 does not seem to be expressed in neurons (Darnell, 2013). Therefore, PTBP1-mediated microexon regulation might be restricted to non-neuronal cells, and microexons in the brain might instead be regulated by the neuronal paralog PTBP2 (Licatalosi & Darnell, 2010).
Figure 1. Microexon inclusion regulates protein–protein interactions in neurons.
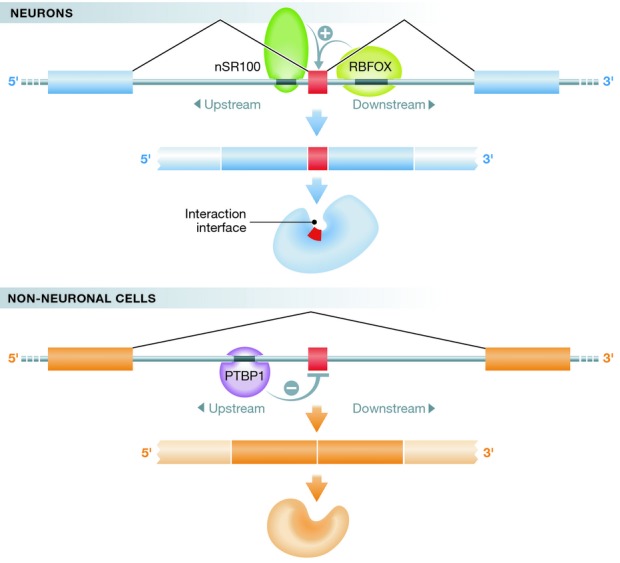
The regulation of microexons (shown in red) relies on RNA-binding proteins like nSR100, RBFOX, and PTBP1, which bind to conserved intronic flanks of microexons. nSR100 and RBFOX promote microexon inclusion in neurons, whereas PTBP1 seems to prevent microexon inclusion in non-neuronal cells. Microexons encode for domains important for protein–protein interactions, and their increased inclusion in neurons modulates protein interactions important for neuronal function.
Irimia et al (2014) undertook a complementary approach to investigate microexon regulation. Analyzing several previously published datasets with perturbed levels of splicing regulators, they found that nSR100 had the strongest effect on microexon regulation. Intriguingly, manipulating nSR100 levels had a much more dramatic effect on microexons when compared to longer exons. nSR100 is a highly expressed neuronal-specific splicing factor and has previously been shown to promote the inclusion of longer exons by binding to a UGC motif upstream of weak 3′ splice sites (Raj et al, 2014). Irimia et al (2014) observe a similar nSR100 binding pattern upstream of microexons that were affected by manipulating nSR100 protein levels, indicating that nSR100 promotes microexon inclusion (Fig1).
Both articles make the observation that microexons often encode for protein domains that are surface accessible and suggest that microexons can regulate protein–protein interactions. In fact, neural-specific microexons were suggested to modulate protein interactions (Dergai et al, 2010; Ohnishi et al, 2014). While Li et al describe how conserved microexon-encoded amino acids of the beta-turn loop of tensins and APBB might modulate protein interactions, Irimia et al (2014) directly demonstrate that the inclusion of microexons promotes interactions between Apbb1 and Kat5, as well as AP1 subunits, proteins that have previously been linked to autism spectrum disorder (ASD).
Splicing misregulation due to mutations in RBPs has been linked to a number of diseases, including ASD. Because microexons seem to rely more heavily on RBPs for their regulation, microexons might be especially susceptible to dysregulation in disease. In fact, Irimia et al (2014) observed that, compared to 5% of longer exons, 30% of known microexons are differentially spliced in ASD patients and that in the majority of the cases, the inclusion of microexons decreased. The authors further show that nSR100 was downregulated in ASD patients and particularly nSR100-regulated microexons were affected. Misregulated microexons were enriched for GO terms related to ASD, suggesting that the disruption of microexon-mediated protein interactions underlies ASD (Irimia et al, 2014). Interestingly, RBFOX proteins and RBFOX-mediated splicing have recently been shown to be affected in autistic brain, suggesting that also RBFOX-mediated microexon regulation might be perturbed in ASD patients (Weyn-Vanhentenryck et al, 2014).
In conclusion, the new papers provide convincing evidence for the importance of microexon regulation in general and in neurons in particular. Compared to longer exons, microexons are strongly conserved, usually in-frame, follow tight modes of regulation, and may be relevant for the modulation of protein–protein interactions. Their dependence on RBPs makes them particularly vulnerable to dysregulation, which has been revealed already in ASD. All these make microexons an intriguing topic for future studies.
References
- Black DL. Does steric interference between splice sites block the splicing of a short c-src neuron-specific exon in non-neuronal cells? Genes Dev. 1991;5:389–402. doi: 10.1101/gad.5.3.389. [DOI] [PubMed] [Google Scholar]
- Darnell RB. RNA protein interaction in neurons. Annu Rev Neurosci. 2013;36:243–270. doi: 10.1146/annurev-neuro-062912-114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergai M, Tsyba L, Dergai O, Zlatskii I, Skrypkina I, Kovalenko V, Rynditch A. Microexon-based regulation of ITSN1 and Src SH3 domains specificity relies on introduction of charged amino acids into the interaction interface. Biochem Biophys Res Commun. 2010;399:307–312. doi: 10.1016/j.bbrc.2010.07.080. [DOI] [PubMed] [Google Scholar]
- Ellis JD, Barrios-Rodiles M, Colak R, Irimia M, Kim T, Calarco JA, Wang X, Pan Q, O'Hanlon D, Kim PM, Wrana JL, Blencowe BJ. Tissue-specific alternative splicing remodels protein-protein interaction networks. Mol Cell. 2012;46:884–892. doi: 10.1016/j.molcel.2012.05.037. [DOI] [PubMed] [Google Scholar]
- Han A, Stoilov P, Linares AJ, Zhou Y, Fu X-D, Black DL. De novo prediction of PTBP1 binding and splicing targets reveals unexpected features of its RNA recognition and function. PLoS Comput Biol. 2014;10:e1003442. doi: 10.1371/journal.pcbi.1003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia M, Blencowe BJ. Alternative splicing: decoding an expansive regulatory layer. Curr Opin Cell Biol. 2012;24:323–332. doi: 10.1016/j.ceb.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Irimia M, Weatheritt RJ, Ellis J, Parikshak NN, Gonatopoulos-Pournatzis T, Babor M, Quesnel-Vallières M, Tapial J, Raj B, O'Hanlon D, Barrios-Rodiles M, Sternberg MJE, Cordes SP, Roth FP, Wrana JL, Geschwind DH, Blencowe BJ. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell. 2014;159:1511–1523. doi: 10.1016/j.cell.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YI, Sanchez-Pulido L, Haerty W, Ponting CP. RBFOX and PTBP1 proteins regulate the alternative splicing of micro-exons in human brain transcripts. Genome Res. 2015 doi: 10.1101/gr.181990.114. doi: 10.1101/gr.181990.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Darnell RB. RNA processing and its regulation: global insights into biological networks. Nat Rev Genet. 2010;11:75–87. doi: 10.1038/nrg2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Shirane M, Hashimoto Y, Saita S, Nakayama KI. Identification and characterization of a neuron-specific isoform of protrudin. Genes Cells. 2014;19:97–111. doi: 10.1111/gtc.12109. [DOI] [PubMed] [Google Scholar]
- Raj B, Irimia M, Braunschweig U, Sterne-Weiler T, O'Hanlon D, Lin Z-Y, Chen GI, Easton LE, Ule J, Gingras A-C, Eyras E, Blencowe BJ. A global regulatory mechanism for activating an exon network required for neurogenesis. Mol Cell. 2014;56:90–103. doi: 10.1016/j.molcel.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyn-Vanhentenryck SM, Mele A, Yan Q, Sun S, Farny N, Zhang Z, Xue C, Herre M, Silver PA, Zhang MQ, Krainer AR, Darnell RB, Zhang C. HITS-CLIP and integrative modeling define the Rbfox splicing-regulatory network linked to brain development and autism. Cell Rep. 2014;6:1139–1152. doi: 10.1016/j.celrep.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


