Abstract
Transmembrane guanylyl cyclases (GCs), with activity regulated by peptide ligands and/or calcium-binding proteins, are essential for various physiological and sensory processes. The mode of activation of the GC subtype GC-G, which is expressed in neurons of the Grueneberg ganglion that respond to cool temperatures, has been elusive. In searching for appropriate stimuli to activate GC-G, we found that its enzymatic activity is directly stimulated by cool temperatures. In this context, it was observed that dimerization/oligomerization of GC-G, a process generally considered as critical for enzymatic activity of GCs, is strongly enhanced by coolness. Moreover, heterologous expression of GC-G in cultured cells rendered these cells responsive to coolness; thus, the protein might be a sensor for cool temperatures. This concept is supported by the observation of substantially reduced coolness-induced response of Grueneberg ganglion neurons and coolness-evoked ultrasonic vocalization in GC-G-deficient mouse pups. GC-G may be a novel thermosensory protein with functional implications for the Grueneberg ganglion, a sensory organ responding to cool temperatures.
Keywords: cyclic guanosine monophosphate, chemosensory, Grueneberg ganglion, transmembrane guanylyl cyclase GC-G, ultrasound vocalization
See also: M Kuhn (February 2015)
Introduction
Receptor guanylyl cyclases (GCs) are membrane-spanning enzymes that generate the second messenger cyclic guanosine monophosphate (cGMP) and play a crucial role in various important physiological and sensory processes, including blood pressure regulation, cell growth, intestinal electrolyte and water transport as well as vision and olfaction (Kuhn, 2009; Potter, 2011). Seven subtypes of transmembrane GC (GC-A through GC-G) have been identified in mice (Kuhn, 2009; Potter, 2011). The mode of activation of most subtypes has been identified: their activity is regulated by extracellular peptide ligands or intracellular calcium-binding proteins (Potter, 2011). However, the mode of activation of the subtype GC-G under physiological conditions is still elusive (Kuhn et al, 2004; Huang et al, 2006; Chao et al, 2010).
Recent studies have demonstrated that GC-G is expressed in neurons of the Grueneberg ganglion (GG) in the anterior region of the murine nose, whose cells are activated by distinct odorous compounds and cool temperature (Mamasuew et al, 2008, 2011a; Fleischer et al, 2009; Liu et al, 2009). Although the precise functional relevance of GC-G in GG neurons is still unclear, previous findings indicated that cGMP signaling might contribute to coolness-evoked responses in the GG (Mamasuew et al, 2010). This aspect is particularly relevant because these neuronal cells lack the ion channel TRPM8 (Fleischer et al, 2009), which is essential for somatosensory neurons responding to cool temperatures (Bautista et al, 2007; Colburn et al, 2007; Dhaka et al, 2007). In view of a possible involvement of GC-G in the responsiveness of GG neurons to coolness, it is interesting to note that the transmembrane GC subtype GCY-12 from the nematode Caenorhabditis elegans (C. elegans) showed maximum enzymatic activity at mild cool temperatures (20–25°C) (Yu et al, 1997). Moreover, in this context, previous studies demonstrated that the transmembrane GC subtype GCY-8 is a critical element for thermotransduction in sensory neurons from C. elegans (Inada et al, 2006; Wang et al, 2013). Yet, it is still unclear whether given transmembrane GCs serve indeed as thermoreceptors in C. elegans sensory cells.
In this study, we used a combination of biochemical and molecular biological methods as well as Ca2+ imaging to investigate a potential role of GC-G as a receptor protein for cool ambient temperatures, which would allow this enzyme to operate as a sensor for coolness in GG neurons.
Results
Stimulation of GC-G enzymatic activity by cool temperatures
As an initial step to scrutinize whether the transmembrane GC subtype GC-G is activated by coolness, we assessed its activity in vitro at warm and cool temperatures. After transient transfection of an empty vector or GC-G-encoding vector in HEK-293T cells, cells were harvested and incubated [in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX)] at 37 or 15°C. Exposure to the cool temperature indeed stimulated intracellular cGMP accumulation in HEK-293T cells expressing GC-G but not in the cells transfected with the empty vector (Fig1A). Similarly, with heterologous expression of GC-G, coolness increased the intracellular cGMP concentration in two other cell types tested: Chinese hamster ovary (CHO-K1) and mouse neuroblastoma (NG108) cells (Supplementary Fig S1).
Figure 1.
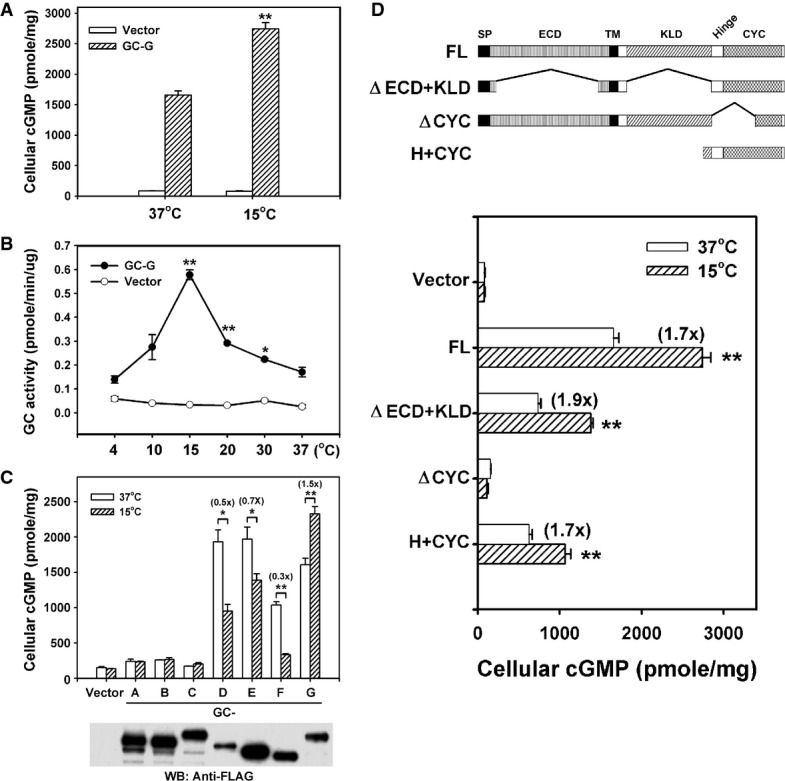
- Coolness stimulates intracellular cyclic guanosine monophosphate (cGMP) accumulation in HEK-293T cells expressing GC-G. Two days after transfection of empty vector or GC-G-encoding plasmid, cells were exposed to ambient temperatures (37 or 15°C) for 20 min and cellular cGMP concentration was measured.
- Temperature dependence of GC-G activity. Membrane preparations from HEK-293T cells transfected with empty vector or GC-G-encoding plasmid were used to determine cyclase activity at the indicated temperatures for 20 min (asterisks denote values significantly elevated compared to 37°C).
- GC-G is the only murine transmembrane GC that can be stimulated by cool temperatures. Expression plasmids encoding FLAG-tagged GC subtypes GC-A to GC-G were transfected into HEK-293T cells. Two days after transfection, membrane protein fractions were prepared and used for cyclase activity assays at 37 or 15°C. Protein expression of each receptor GC was confirmed by Western blot (WB) analysis with anti-FLAG antibody.
- The H+CYC domain is critical for stimulation by coolness. The domain structure of full-length (FL) GC-G and its mutant variants is shown in the upper panel. A FLAG tag was added at the N-terminus of each protein. The ΔECD+KLD mutant protein lacks amino acids 73–455 and 556–833; the ΔCYC variant lacks amino acids 834–1,003. The H+CYC mutant protein contains amino acids 806–1,100. ECD, extracellular domain; KLD, kinase-like domain; SP, signal peptide; TM, transmembrane region. HEK-293T cells expressing the indicated GC-G constructs were incubated at 37 or 15°C for 20 min, then intracellular cGMP concentration was measured (lower panel).
Data information: All data are mean ± SD from three experiments in triplicate. *P < 0.05; **P < 0.01.
To further confirm this coolness-induced increase in GC-G-mediated cGMP synthesis and analyze the temperature dependence of GC-G activity in more detail, membrane protein fractions from empty vector- or GC-G-expressing HEK-293T cells were used to monitor cyclase activity at a wide range of temperatures (Fig1B). Cyclase activity from membrane fractions of GC-G-expressing cells peaked at ∽15°C and returned to a basal level at ∽4°C and ∽37°C. By contrast, cyclase activity from membrane fractions of empty vector-transfected cells was low and unaltered by temperature changes. Furthermore, coolness (15°C) did not increase the cyclase activity in membrane preparations from HEK-293T cells expressing the other members of the transmembrane GC family (GC-A to GC-F; Fig1C). These results suggest that low ambient temperatures specifically stimulated the activity of GC-G, thereby increasing cellular cGMP concentration.
Coolness directly activated the C-terminal domain of GC-G
Transmembrane GCs are single membrane-spanning proteins with an N-terminal extracellular domain (ECD) and a cytoplasmic region that can be further subdivided into a protein kinase-like domain (KLD), an amphipathic α-helical hinge (H) region and a C-terminal cyclase catalytic domain (CYC) (Kuhn, 2009; Potter, 2011). To investigate which domain is essential for activation by coolness, we compared the effect of cooling on full-length (FL) GC-G and three mutant GC-G proteins (Fig1D): the first (ΔECD+KLD) lacked both the extracellular and the KLD, the second (ΔCYC) lacked a functional cyclase catalytic domain, and the third (H+CYC) comprised only the hinge region and the cyclase domain. Western blot analysis verified that the FL GC-G and mutant proteins were expressed at a comparable level in HEK-293T cells transfected with expression plasmids encoding these GC-G variants (Supplementary Fig S2A). Exposure to a cool temperature of 15°C stimulated cellular cGMP production in the ΔECD+KLD-expressing HEK-293T cells to a similar degree as in cells expressing the FL GC-G protein (Fig1D), which suggests that the ECD and the KLD are not essential for GC-G activation by coolness. However, elimination of the CYC domain completely abolished the coolness-stimulated cGMP increase. By contrast, the H+CYC mutant protein containing only the H and the CYC domain retained substantial coolness-evoked GC activity, which suggests that these domains are critical for GC-G activation by cool temperatures.
To further scrutinize whether the H+CYC domain of GC-G serves as a thermosensor, we engineered a construct encoding a chimeric protein (designated GC-A/G) with the H+CYC domain of coolness-insensitive GC-A replaced by the H+CYC domain of GC-G (Fig2). Western blot and flow cytometry confirmed that the expression and cell-surface targeting of the chimeric GC-A/G protein were similar to that of GC-A and GC-G when expressed in HEK-293T cells (Supplementary Fig S2B and C). The chimeric protein GC-A/G was effectively activated by coolness, similar to GC-G (Fig2). Thus, replacing the H+CYC domain of GC-A with that of GC-G confers responsiveness to cool temperatures, so the molecular sensor for activation by cool temperatures resides within the H+CYC domain of GC-G. More detailed analyses with deletion constructs showed that residues at the N-terminal domain of the hinge region appeared to be critical for coolness-evoked activation of GC-G: on deletion of this portion of GC-G, the enzyme was no longer stimulated in a temperature-dependent manner (Fig3A–C).
Figure 2.
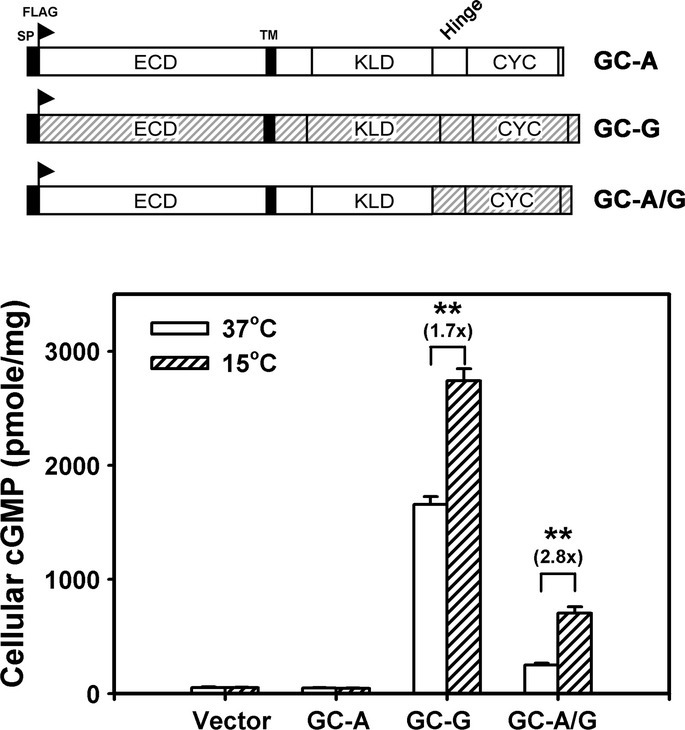
Chimeric GC-A/G receptor containing the H+CYC domain of GC-G acquires coolness-evoked activity
In the chimeric GC-A/G protein, the H+CYC domain of GC-A was replaced by that of GC-G (upper panel). HEK-293T cells expressing the indicated proteins were exposed to ambient temperature of 37 or 15°C for 20 min; then, intracellular cGMP concentration was measured. Data are mean ± SD from three experiments in triplicate. **P < 0.01.
Figure 3.
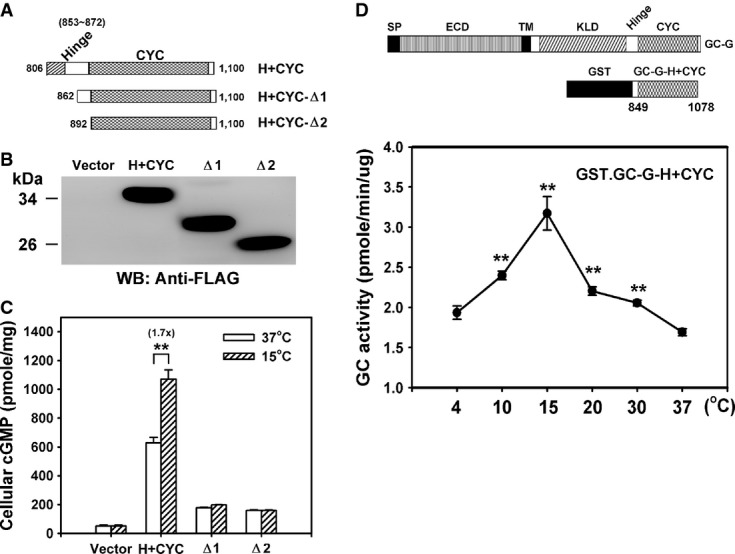
- A Representation of the domain structure from different C-terminal fragments of GC-G analyzed for coolness-induced activity (amino acids of GC-G encoded in each expression plasmid are indicated).
- B, C Protein expression and coolness-stimulated cGMP synthesis of the above shown divergent C-terminal fragments of GC-G with intact or ablated hinge (H) region. The expression plasmids encoding the H+CYC domain or a deletion construct (Δ1–Δ2) tagged with a FLAG epitope were transfected into HEK-293T cells. Two days after transfection, cells were exposed to the ambient temperatures (37 or 15°C) for 20 min and cellular cGMP concentration was measured (C). Protein expression of each construct was confirmed by Western blot (WB) analysis with anti-FLAG antibody (B).
- D A recombinant protein containing the H+CYC domain of GC-G acquires coolness-evoked activity. Diagram of recombinant GST.GC-G-CYC protein containing the H+CYC domain of GC-G (amino acids 849–1,078) compared to the domain structure of full-length GC-G (upper panel). The purified GST.GC-G-CYC protein was exposed to the indicated temperatures for 20 min, and GST.GC-G-CYC cyclase activity was measured (lower panel; asterisks denote values significantly elevated compared to 37°C).
Data information: All data are mean ± SD from three experiments in triplicate. **P < 0.01.
These experimental findings demonstrate that heterologously expressed GC-G (notably the H+CYC domain) enables coolness-evoked cGMP synthesis in HEK-293T cells and in membrane fractions prepared from these cells. However, whether GC-G alone is sufficient to mediate this coolness-induced cGMP generation remains unclear; alternatively, an additional cellular factor might be involved in the activation of GC-G by cool temperatures. To address this issue, we generated and purified a recombinant GST fusion protein (GST.GC-G-H+CYC) containing the H+CYC domain (residues 849–1,078) of GC-G (Fig3D and Supplementary Fig S2D). A cool temperature (15°C) significantly stimulated the cyclase activity of the purified GST.GC-G-H+CYC protein (Fig3D), analogous to the coolness-induced activation observed in GC-G-expressing cells (Fig1B). These data suggest that coolness directly activates the H+CYC domain of GC-G without the involvement of any additional cellular factor.
Dimerization/oligomerization of GC-G is enhanced by cool temperatures
It is so far unclear why GC-G—in contrast to other GCs—is activated by coolness (Fig1C). Similar to other GCs (Chinkers & Wilson, 1992; Lowe, 1992; Vaandrager et al, 1994; Goldberg & Molday, 1996; Yang & Garbers, 1997), GC-G forms heteromers or homomers (Kuhn et al, 2004). Interestingly, dimerization/oligomerization has been found to be essential for catalytic activity of other GCs (Thompson & Garbers, 1995; Wilson & Chinkers, 1995; reviewed by Lucas et al, 2000), suggesting that dimerization/oligomerization might be also relevant for GC-G enzymatic activity. Therefore, we tested whether the coolness-induced increase of GC-G enzymatic activity might be associated with an enhanced dimerization/oligomerization of this protein at cool temperatures. For this purpose, in HEK-293T cells, we heterologously co-expressed two different versions of GC-G, which were labeled by a FLAG (FLAG.GC-G) or a Myc tag (GC-G.Myc), respectively. Cell lysates were then subjected to immunoprecipitation with anti-Myc antibody at 15 or 25°C before Western blotting with anti-FLAG antibody was conducted. An immunoreactive band of the expected size was observed in the immunoprecipitates (Fig4A; upper left panel). Importantly, the intensity of this band was largely stronger when immunoprecipitation was conducted at 15°C compared to 25°C. When the same experiment was carried out with two variants of GC-A endowed with Myc and FLAG tag, respectively, the intensity of the immunoreactive band was very similar for immunoprecipitations conducted at 15 or 25°C (Fig4A; upper right panel). Statistical analyses revealed that in comparison with 25°C, the immunoreactive band for GC-G was more than fivefold stronger when the immunoprecipitation was carried out at 15°C (Fig4B), indicating that coolness enhances dimerization/oligomerization of GC-G. When both immunoprecipitation and Western blotting were performed with the anti-Myc antibody, intensity of the immunoreactive band was similar at both precipitation temperatures (Fig4A; middle panel). Similar results were obtained when immunoprecipitation and Western blotting were performed with the anti-FLAG antibody (Fig4A; lower panel). These findings demonstrate that changing temperatures did not affect the efficiency of immunoprecipitation.
Figure 4.
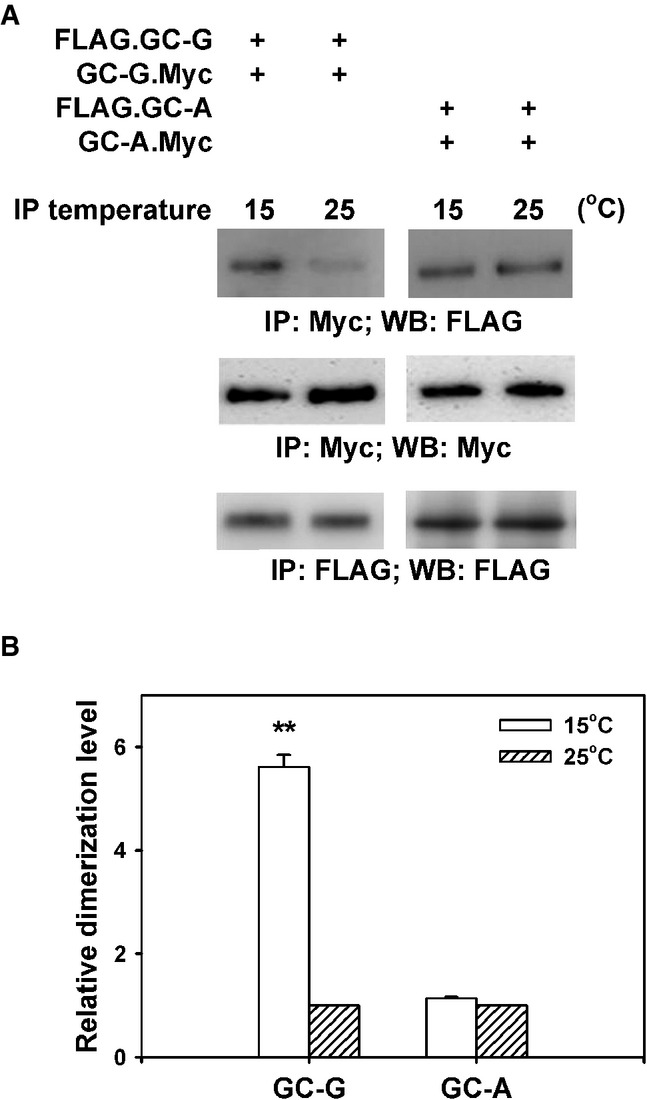
- HEK-293T cells were co-transfected with plasmids encoding two differential epitope-tagged (FLAG or Myc) versions of GC-G or GC-A, respectively. After 2 days, transfected cells were lysed with detergent and lysates were subjected to immunoprecipitation (IP) for 2 h with anti-Myc antibody at two different temperatures (15 or 25°C), followed by Western blotting (WB) using anti-FLAG antibody to determine the amount of dimerized/oligomerized GC proteins (upper panel). These approaches demonstrated that in contrast to GC-A, homomeric protein/protein association of GC-G seems to be enhanced by cool temperatures (upper panel). Cell lysates werealso precipitated and immunoblotted with the anti-Myc antibody, demonstrating that cooling does not interfere with immunoprecipitation of Myc-tagged GC-G via the anti-Myc antibody (middle panel). Likewise, precipitation and immunoblotting with the anti-FLAG antibody yielded bands of similar intensity for 15 and 25°C, respectively (lower panel).
- Relative quantification of homomeric protein/protein association of GC-G and GC-A was performed by densitometric scanning. Each intensity value from the upper panel was subsequently normalized to the total protein levels as determined in the middle and the lower panel of Fig4A (intensity of the immunoreactive bands obtained with the anti-Myc or anti-FLAG antibody, respectively). Relative dimerization level at 15°C was further calculated by normalizing to values obtained at 25°C; the latter were set as 1. Results are means ± SD of three experiments, **P < 0.01 (compared to 25°C).
Expression of GC-G renders HEK-293T cells responsive to cool temperatures
Thermosensitive GG neurons co-express GC-G and the cGMP-activated channel CNGA3, proteins proposed to mediate coolness-evoked GG responses (Fleischer et al, 2009; Mamasuew et al, 2010). To address the question of whether a signaling cascade comprising the coolness-activated enzyme GC-G and its effector CNGA3 is sufficient to render cells responsive to cool temperatures, we performed calcium imaging experiments with HEK-293T cells heterologously expressing CNGA3 and loaded with the Ca2+-sensitive dye Fura-2. In these cells, exposure to a cool temperature (15°C) did not increase intracellular Ca2+ concentration (Fig5; upper panel). By contrast, in HEK-293T cells co-expressing CNGA3 and (FL) GC-G, intracellular Ca2+ content increased with cooling. However, omitting Ca2+ from the extracellular buffer (−Ca2+) abolished this coolness-evoked Ca2+ signal (Fig5; middle panel), so the increase in intracellular Ca2+ content was due to Ca2+ influx. With expression of the H+CYC domain of GC-G, similar to FL GC-G, intracellular Ca2+ content increased with cooling (Fig5; lower panel). Therefore, a signal pathway comprising the thermosensory enzyme GC-G and the cGMP-activated and Ca2+-permeable channel CNGA3 was sufficient to allow coolness-induced responses in cells. In contrast to GC-G, co-expression of CNGA3 with GC-A did not enable coolness-evoked responses in HEK-293T cells (our unpublished observations).
Figure 5.
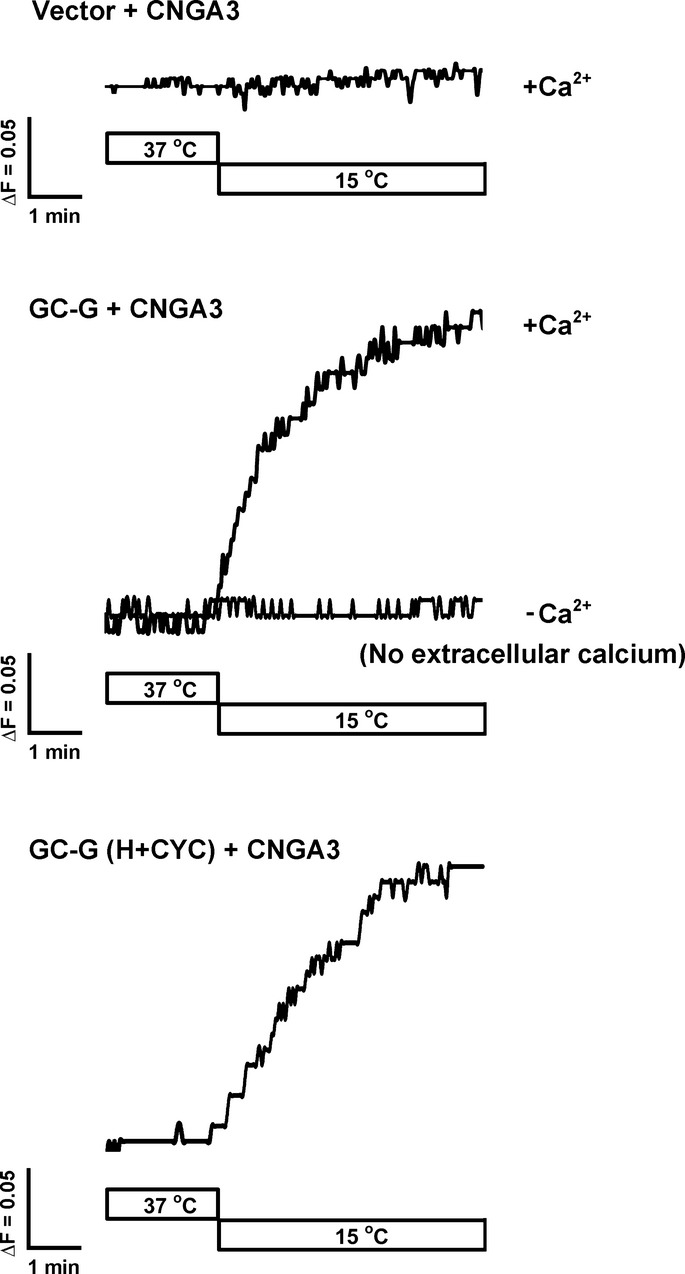
Increase in intracellular calcium [Ca2+]i concentration in HEK-293T cells co-expressing GC-G and CNGA3 in response to cool temperatures
HEK-293T cells expressing CNGA3 alone (upper panel) or together with GC-G (full-length in the middle panel and H+CYC domain in the lower panel) were loaded with the Ca2+ indicator Fura-2 to monitor intracellular [Ca2+]i concentration. Cells co-expressing GC-G and CNGA3 showed a rapid increase in [Ca2+]i when the temperature was lowered from 37 to 15°C (middle and lower panel); cells only expressing CNGA3 are not responsive to cool temperatures (top panel). Removal of extracellular Ca2+ (−Ca2+; middle panel) completely suppressed the response to cooling. ΔF represents changes in the ratio of the fluorescence intensity of Fura-2 at 340/380 nm excitation. The ratiometric Ca2+ responses are representative of 30 cells recorded from three experiments.
Deletion of GC-G reduced responsiveness to cool temperatures in the GG
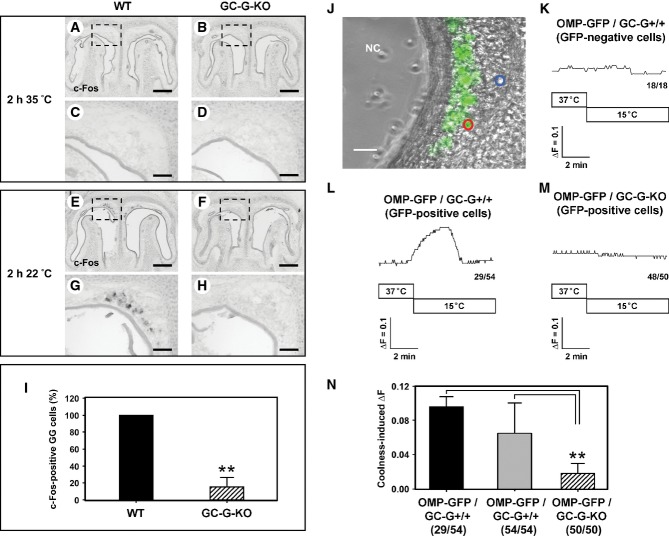
In GG neurons, cool temperatures induce responses that can be visualized by monitoring the expression of the activity-dependent gene c-Fos (Mamasuew et al, 2008, 2010). To assess whether GC-G is critical for GG responsiveness to coolness in vivo, we compared c-Fos expression at different temperatures in wild-type (WT) and GC-G-deficient (GC-G-KO) mice. Neonatal mouse pups [postnatal day 0–4 (P0–P4)] were exposed to 35 or 22°C for 2 h, and the GG was analyzed for c-Fos expression by in situ hybridization. On exposing pups to 35°C, c-Fos expression was absent from the GG of both WT and GC-G-KO pups (Fig6A–D). However, on exposing pups to 22°C, c-Fos expression was increased in a considerable number of GG neurons in WT animals (Fig6E and G). By contrast, in GC-G-KO animals, the expression of c-Fos was weak or even absent (Fig6F and H). In fact, the number of c-Fos-positive GG cells in GC-G-KO pups was about 85% lower than in WT counterparts (Fig6I).
Figure 6.
- A-H In situ hybridization experiments with an antisense probe for c-Fos on coronal sections through the GG of wild-type (WT; left panel) or GC-G-KO (right panel) mouse pups (P0–P4) exposed to warm (35°C; A–D) or cool (22°C; E–H) ambient temperature for 2 h. Panels (C, D, G, H) show higher magnifications of the boxed areas in (A, B, E, F). At 35°C (A–D), no c-Fos expression was detectable in the GG of both WT (A, C) and GC-G-KO (B, D) individuals. Upon exposure to 22°C, strong c-Fos expression was observed in the GG of WT pups (E, G), whereas c-Fos expression was barely detectable in the GG of GC-G-KO animals (F, H). The data shown are representative of 10 independent experiments. Pups originated from six different litters for each genotype. Scale bars: 200 μm in (A, B, E, F); 50 μm in (C, D, G, H).
- I Quantification of c-Fos-positive GG cells in WT and GC-G-KO pups on exposure to 22°C for 2 h. All stained cells on every section along the rostrocaudal extent of the GG were counted; the results shown are based on the above-mentioned 10 experiments. The number of c-Fos-positive cells in the GG of a GC-G-KO mouse was determined relative to that in a concomitantly processed WT pup; the latter number was set to 100%. Data are mean ± SD (n = 11 in each genotype), **P < 0.0001.
- J-N Calcium imaging of coronal sections through the GG of olfactory marker protein-green fluorescent protein (OMP-GFP)/GC-G+/+ or OMP-GFP/GC-G-KO pups (P1–P4). High magnification image (J) of a tissue slice through the GG of an OMP-GFP pup with GG neurons labeled by intrinsic GFP fluorescence (GFP fluorescence was merged with the transmitted-light channel). Cells analyzed are circled in blue (GFP-negative) or red (GFP-positive). NC, nasal cavity. Scale bar: 30 μm. (K–M) Representative ratiometric Ca2+ transients induced by cooling from 37 to 15°C in GFP-negative non-neuronal cells (K) and in GFP-positive GG neurons from OMP-GFP/GC-G+/+ (L) and OMP-GFP/GC-G-KO pups (M). The numbers in the bottom right hand corners are the number of cells with Ca2+ transient similar to what is shown in the respective graph (left) and total number of measured cells (right). (N) Quantification of coolness-induced ΔF in GG neurons from OMP-GFP/GC-G+/+ and OMP-GFP/GC-G-KO mice. Coolness-induced ΔF was calculated by subtracting the baseline fluorescence ratio (340/380 nm) at 37°C from the peak fluorescence ratio (340/380 nm) measured at 15°C. For OMP-GFP/GC-G+/+, the 29 coolness-responsive neurons (black bar) of all 54 analyzed neurons (gray bar) from five slices (obtained from different animals) were analyzed. For OMP-GFP/GC-G-KO, the 50 analyzed neurons (shaded bar) from five slices (obtained from different animals) were analyzed. Data are mean ± SD, **P < 0.01.
To further assess the relevance of GC-G for coolness-induced responses in the GG, we performed ratiometric Ca2+ imaging experiments on tissue slices through the GG from OMP-GFP mice (WT for GC-G) in which GG neurons fluoresce in green [due to expression of green fluorescent protein (GFP) in GG neurons positive for the olfactory marker protein (OMP); Fig6J]. Consistent with the findings of the c-Fos-based approaches, decreasing the temperature of the perfusion solution from 37 to 15°C elicited a substantial increase in intracellular Ca2+ content in 29 of 54 (∽54%) tested GFP-positive GG neurons (five slices originating from different animals) (Fig6L). By contrast, GFP-negative (non-neuronal) cells from these sections did not respond to cooling (Fig6K). In five tissue slices from different OMP-GFP/GC-G-KO mice, intracellular Ca2+ concentration was not or only weakly increased with cooling in 48 of 50 (96%) tested GFP-positive GG neurons (Fig6M). Consequently, the mean coolness-evoked Ca2+ signal was substantially lower in a GC-G-KO scenario than in GG neurons with GC-G (Fig6N). However, GG neurons form OMP-GFP/GC-G-KO mice clearly responded to potassium chloride (KCl) (Supplementary Fig S3), which demonstrated the viability and activation ability of these cells. Therefore, the results of both c-Fos experiments and Ca2+ imaging show that on elimination of GC-G, the GG is largely impaired in its responsiveness to cool temperatures, which suggests that GC-G significantly contributes to coolness-induced GG responses in vivo. Moreover, in additional experiments, the presence of the phosphodiesterase inhibitor IBMX increased the duration of Ca2+ elevation with cooling (our unpublished observations), which further supports that cGMP signaling is critical for coolness-evoked Ca2+ signals in GG neurons.
GC-G is absent from other thermosensory cell types
The above-mentioned observations strongly indicate that GC-G serves as a thermoreceptor in GG neurons, which raises the question of whether this molecule fulfills a similar function in other thermosensory neurons. To address this issue, potential GC-G expression was assessed by in situ hybridization in the two central nervous tissues harboring thermosensitive neurons: the trigeminal ganglion (TG) of the brain and the dorsal root ganglion (DRG) of the spinal cord of mouse. Coolness-responding neurons in the TG and DRG are endowed with the coolness-activated ion channel TRPM8 (Bautista et al, 2007; Colburn et al, 2007; Dhaka et al, 2007); however, they lack expression of GC-G (Supplementary Fig S4). Moreover, in situ hybridization revealed no expression of GC-G on sagittal sections through the mouse brain (Supplementary Figs S5 and S6), so in the mouse head, GC-G expression is confined to the GG.
GC-G is critical for ultrasound vocalization (USV) in pups
Previously, GG was proposed to influence USV (Mamasuew et al, 2008) generated by rodent pups to elicit maternal care on exposure to cool temperatures (Blumberg et al, 1992; Hashimoto et al, 2004; Szentgyorgyi et al, 2008). USVs are whistle-like sounds with a single component at frequencies between 30 and 100 kHz. Their sonographic pattern can be used to classify these calls ‘chevron’, downward, or complex calls (Scattoni et al, 2008). To further investigate the biological relevance of coolness-evoked cGMP signaling via GC-G in GG neurons, we compared coolness-induced USV between wild-type (WT) and GC-G-KO mouse pups. Littermates derived from a heterozygous intercross were taken from their nests and exposed to different temperatures (15, 23 or 35°C) for USV recording. Exposure of pups to 35°C, a temperature similar to the nest temperature in rodents (Cramer & Blass, 1982), or 23°C elicited a rather low rate of USV with no difference between WT and GC-G-KO neonatal pups (our unpublished observations). Only exposure to 15°C evoked substantial USV; therefore, we focused on this temperature for further USV recording experiments with pups at P2 to P6. Observation of pups with a thermal camera allowed us to determine the temperatures at the tip of the nose (corresponding to the region harboring the GG) and the periphery of the body (corresponding to the skin) (Supplementary Fig S7A). After exposure to 15°C for 5 min, in WT pups, the temperatures were reduced to 18.9°C (at the tip of the nose) or to 20.8°C (in the skin) (Supplementary Fig S7B). WT and GC-G-KO pups did not differ in temperatures at the tip of the nose and the skin (Supplementary Fig S7B). On analyzing USV in pups exposed to 15°C, similar USV call patterns were observed in WT mice and their GC-G-KO conspecifics (Supplementary Fig S8A). Moreover, the peak frequencies were similar between WT and GC-G-KO pups (Supplementary Fig S8B). However, the responsive rate to this cool temperature (i.e. the proportion of pups responsive to cooling) was markedly decreased in GC-G-KO pups at P6 as compared with their WT littermates (Fig7A). Despite no substantial differences in the responsive rate to coolness between genotypes at P2 (Fig7A), the number of ultrasound calls in GC-G-KO pups was significantly reduced (Fig7B) and the latency in emitting the first USV after exposure to 15°C was clearly elevated (Fig7C).
Figure 7.

- A Reduced responsive rate of coolness-stimulated USV in GC-G-KO pups. Pups were removed from their littermates and dams at postnatal (P) day 2, 4, and 6 and exposed to 15°C. USV was measured by use of the Anabat SD1 Bat detector. The number of responsive pups versus total number of pups tested for each WT and GC-G-KO group at different ages is indicated on the top of each bar. *P < 0.05.
- B, C Total number of USV calls (B) and response latency (C) in GC-G-KO and WT pups at P2. The horizontal bars indicate the mean. *P < 0.05; **P < 0.01.
Discussion
Transmembrane GCs comprise extracellular as well as intracellular domains, suggesting that these enzymes function as receptors for specific ligands. In fact, most of them are activated by peptide ligands. The GC subtype GC-G, however, is considered as an orphan receptor (Kuhn, 2009; Potter, 2011). Recently, it has been reported that the heterologously expressed intracellular cyclase domain of GC-G is activated by bicarbonate (Chao et al, 2010). This observation is supported by the finding that in GG neurons from transgenic mice lacking functional expression of GC-G, responsiveness to bicarbonate in calcium imaging experiments was dramatically reduced compared to GG neurons endowed with GC-G (our unpublished observations). Yet, it is uncertain whether GC-G indeed serves as a receptor for bicarbonate under physiological conditions. Toward the identification of stimuli activating GC-G, it is important to note that previous studies in C. elegans indicated that several transmembrane GC subtypes expressed in sensory cells function as receptor proteins in the chemosensory detection of distinct environmental stimuli, including alkaline pH, CO2 and salty substances (Ortiz et al, 2009; Hallem et al, 2011; Brandt et al, 2012; Murayama et al, 2013; Smith et al, 2013a,b). Moreover, given GC subtypes from C. elegans are considered to be involved in thermotransduction (Inada et al, 2006; Wang et al, 2013). In mice, chemo- and thermosensory neurons are localized to the GG. In the absence of the coolness-sensing channel TRPM8 in the GG, how such neurons can detect cool temperatures is unclear (Fleischer et al, 2009). Recently, we found that the temperature-gated potassium channel TREK-1 is expressed in GG neurons (Stebe et al, 2014). With a moderate reduction of GG activation by cool temperatures in the GG of TREK-1-KO mice, TREK-1 appears to be involved in coolness detection by GG neurons. Yet, TREK-1 cannot account for the substantial coolness-induced GG responses because even the GG of TREK-1-KO mice shows considerable responsiveness to cool temperatures. Thus, TREK-1 may not be indispensable for GG activation by coolness but rather enhances GG responses to cool temperatures (Stebe et al, 2014). In view of previous findings that coolness-responsive GG neurons express elements of a cGMP-mediated transduction cascade (notably the cGMP-synthesizing enzyme GC-G and the cGMP-activated ion channel CNGA3) and that elimination of CNGA3 resulted in strongly reduced responses to coolness (Fleischer et al, 2009; Mamasuew et al, 2010), cGMP signaling might contribute to GG responsiveness to cool temperatures. This concept was recently supported by the observation that the CNG channel inhibitor L-cis-diltiazem considerably decreased coolness-evoked Ca2+ signals in GG neurons (Brechbuhl et al, 2013). However, the actual thermosensor in GG cells remained elusive.
The present study is the first to provide biochemical evidence that GC-G serves as a molecular sensor for cool temperatures in mammals. Thus, transmembrane GCs are not only utilized as receptors for environmental stimuli in C. elegans but also in mammals. This is also in line with previous observations indicating that the GC-G-related transmembrane GC subtype GC-D serves as a chemosensory receptor for CO2, carbon disulfides, and given urinary peptides in the murine olfactory system (Hu et al, 2007; Leinders-Zufall et al, 2007; Duda & Sharma, 2010; Munger et al, 2010). Yet, at a first glance, a transmembrane GC seems to be an unusual candidate for a thermosensor, especially since mammals are supposed to detect physical stimuli (including temperature, pressure and sound) via ion channels in distinct thermo- and mechanosensory cell types. However, the transmembrane GC subtype GCY-12 from the nematode C. elegans is stimulated by mild cool temperatures (Yu et al, 1997). In addition, our findings indicate that the murine transmembrane GC subtype GC-G is activated by coolness because its enzymatic activity peaked at about 15°C (Fig1B). This trait is apparently not in line with the Q10 temperature coefficient for enzymatic reaction rates. How GC-G is activated by cool temperatures remains unclear. However, the finding that GC-G is activated by coolness in a heterologous expression system (Fig1) and as a purified protein points to an intramolecular mechanism. This view is further substantiated by the observation that even a truncated C-terminal fragment of GC-G comprising only the catalytic domain and the hinge region is stimulated by coolness (Fig3D). Thus, the enzyme's C-terminal region harboring the catalytic domain is directly activated by cool temperatures. This notion is substantially supported by the finding that transferring the C-terminal domain of GC-G to another murine GC subtype rendered this enzyme responsive to coolness (Fig2).
These results raise the question of why the C-terminal region of GC-G is so unique that it provides this enzyme with the unorthodox feature of high enzymatic activity at cool rather than warm temperatures. At a warm temperature (37°C), the (basal) activity of GC-G was similar to that of other GC subtypes (Fig1C). Therefore, lowering the temperature could elicit conformational changes in the C-terminal domain of GC-G to facilitate rate-limiting intermediate steps in the synthesis of cGMP such as the binding of guanosine triphosphate (GTP) to the catalytic domain of the enzyme. Alternatively, since GC-G forms dimers (Kuhn et al, 2004), an essential step for the catalytic activity of other GCs (Wilson & Chinkers, 1995; Yang & Garbers, 1997), cool temperatures may affect GC-G conformation to enhance dimerization/oligomerization. The latter notion is supported by our co-immunoprecipitation experiments indicating that dimerization/oligomerization of GC-G is markedly enhanced at cool temperatures, whereas dimerization/oligomerization of GC-A, which is not activated by coolness, appeared to be unaffected by temperature changes (Fig4). In this context, the hinge region might be of outstanding relevance since this domain is considered to be important for dimerization/oligomerization of GCs (Thompson & Garbers, 1995; Wilson & Chinkers, 1995; Lucas et al, 2000). Importance of the hinge region for GC-G enzymatic activity is also indicated by the observation that a deletion of some amino acid residues in the hinge region evoked an almost complete loss of GC-G coolness sensitivity; however, in these deletion variants, GC-G enzymatic activity was only decreased but not abolished (Fig3C). Consequently, temperature dependency of GC-G dimerization/oligomerization could be mediated by amino acids in the hinge region.
Our findings establish GC-G as a coolness-activated enzyme and provide evidence that this protein indeed serves as a thermosensor in cells: on heterologous expression of GC-G (together with its effector CNGA3), HEK-293T cells became responsive to coolness (Fig5). Moreover, coolness-induced GG responses were considerably diminished in GC-G-KO mice (Fig6). Yet, with GC-G deletion in c-Fos experiments, coolness-evoked responses persisted in about 15% of GG cells (Fig6I). In calcium imaging approaches, almost all tested GG neurons from GC-G-KO pups did not respond to coolness (Fig6M); however, two cells were weakly responsive to cool temperatures. This observation suggests an additional and GC-G-independent pathway contributing to coolness-induced GG responses—at least in a subset of GG neurons. In line with this concept, a recent report demonstrated that the temperature-sensitive potassium channel TREK-1 moderately contributes to GG activation by cool temperatures (Stebe et al, 2014). Consistent with this finding, Schmid and co-workers (Schmid et al, 2010) observed coolness responsiveness in individual GG neurons from CNGA3-deficient mice. Thus, cGMP signaling seems to be essential in most but not in all coolness-responsive GG neurons to mediate substantial reactivity to cool temperatures.
Because of its role as a thermosensor in GG neurons, GC-G is assumed to have a similar function in other thermosensory cells. However, our results demonstrate that GC-G is absent from two major types of thermosensory cells: neurons in the DRG and in the TG of mice (Supplementary Fig S4). Moreover, GC-G was not substantially expressed in the mouse brain (Supplementary Figs S5 and S6). These observations support the notion that a role of GC-G as a thermosensor may be restricted to the GG. In fact, expression of this protein has been observed in only a few other murine tissues, notably kidney, testis and spermatids (Kuhn et al, 2004; Huang et al, 2006; Lin et al, 2008); the function of GC-G in these tissues is currently unclear.
GG neurons use the enzyme GC-G for sensing coolness (this study), and other cold-responsive neurons use the TRPM8 channel (Bautista et al, 2007; Colburn et al, 2007; Dhaka et al, 2007), but why different cell types use distinct molecules for responding to cool temperatures remains unknown. For the relevant cells of the somatosensory system, the coolness-activated channel TRPM8 may be sufficient to reliably and rapidly respond to cool temperatures. However, in the dual sensory neurons of the GG, coolness-induced responses seem to cross-talk with signaling pathways activated by given odorants (Mamasuew et al, 2011b). To facilitate such cross-talks, GG neurons might rely on signaling elements that can function in both chemo- and thermotransduction. In fact, cGMP-associated signaling proteins are crucial for thermosensation in GG neurons (Mamasuew et al, 2010) (this study) and contribute to chemosensory responses in these cells (Mamasuew et al, 2011b; Hanke et al, 2013).
In mammals, sensing temperature is important for regulating the body temperature and for avoiding unpleasant or even noxious temperatures. Nocifensive behaviors allow mammals to escape from extreme temperatures that might elicit tissue damages. In the absence of their warmth-giving mother and being exposed to cool temperatures, rodent pups generate USV, thereby inducing pup-retrieval and maternal-care behaviors in dams. In fact, during the preweaning period, exposure to low ambient temperatures is considered the most important stimulus for eliciting USV (Allin & Banks, 1971; Okon, 1971; Oswalt & Meier, 1975; Blumberg et al, 1992; Szentgyorgyi et al, 2008). The sensory pathways underlying the generation of USV on exposure to coolness are unclear. Our findings demonstrate that GC-G is critical for eliciting coolness-evoked USV in mouse pups (Fig7) perhaps because of its function in the GG and/or in higher neuronal centers of the brain. Our in situ hybridization experiments contradict a substantial expression of GC-G in the mouse brain (Supplementary Figs S5 and S6); the reduced USV generation with cooling in GC-G-KO animals points to a relevance of the GG for ultrasound calling in pups. Our USV experiments also revealed some age-dependent aspects of coolness-evoked USV. While in wild-type pups exposure to a cool temperature evoked USV in about 40% of the pups at all ages tested (P2, P4, and P6), this proportion substantially varied in GC-G-deficient pups (Fig7A). In fact, in GC-G-knockout pups, the percentage of responding pups was very low at P6, whereas it was similar to wild-type conspecifics at P2. Although it has to be emphasized that even at P2, the number of ultrasound calls upon cooling was clearly reduced and their latency was increased in GC-G-deficient animals compared to wild-type pups (Fig7B and C), the reasons for these age-dependent differences remain unclear. In this regard, it has to be taken into consideration that GC-G-mediated detection of cool temperatures in the GG might not be the only signaling pathway contributing to coolness-evoked USV; others might exist as well inside or even outside GG neurons. For instance, the above-mentioned contribution of TREK channels to coolness-evoked activation of GG neurons (Stebe et al, 2014) could rescue responsiveness to coolness in the absence of GC-G; at least in a subset of GG neurons. It is conceivable that such alternative/additional mechanisms possibly contributing to coolness-induced USV might rescue responses to cool temperatures better at some ages than at others.
Materials and Methods
Cell culture, transfection, construction of expression plasmids, flow cytometry, preparation of GST fusion protein, membrane fraction isolation, immunoprecipitation and Western blot analyses. These experiments were performed as described previously (Kuhn et al, 2004; Chao et al, 2010).
GC activity assay
To investigate the temperature dependence of GC-G activity in HEK-293T cells (Figs1A and D, 2, 3C and Supplementary Fig S1), empty vector or GC-G-transfected HEK-293T cells were exposed to 37 or 15°C for 20 min (with 1 mM IBMX), then cGMP content was measured by use of the CatchPoint cGMP fluorescent assay kit (Molecular Devices, Sunnyvale, CA, USA). cGMP concentration was determined as picomole cGMP per milligram total cellular protein. For in vitro GC activity assay (Figs1B and C and 3D), membrane protein fractions (10 μg) from transfected cells or purified recombinant GST.GC-G-H+CYC protein (1 μg) were mixed with reaction buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM 3-isobutyl-1-methylxanthine, 2 mM ATP, 2 mM GTP, 0.5 mg/ml BSA, 5 mM MgCl2 and 10 mM sodium azide) to a final volume of 100 μl for 20 min at the indicated temperatures, then the reaction was stopped by adding 250 μl of ice-cold 100% ethanol. The supernatant was dried, and cGMP concentration was measured by use of the cGMP assay kit described above.
Mouse strains
This study involved wild-type (WT) mice (strain C57BL/6) and two GC-G-knockout mouse strains (designated GC-G-KO, backcrossed with C57BL/6 for at least 10 generations). Generation of the first knockout line was described recently (Lin et al, 2008), and generation of the second strain is detailed below and in Supplementary Fig S9. The c-Fos experiments involved the first GC-G-KO strain, and recording of USV and calcium imaging involved the second strain. In the OMP-GFP transgenic mouse line, green fluorescence protein (GFP) is expressed as a reporter under control of the olfactory marker protein (OMP) promoter (Potter et al, 2001). OMP-GFP/GC-G-KO mice (with GFP-labeled GG neurons lacking functional GC-G) were obtained by crossing GC-G-KO and OMP-GFP mice. Mice were kept on a standard 12-h light/dark cycle. All experiments complied with the current laws of Taiwan and Germany.
Calcium imaging
Before transfected HEK-293T cells were seeded, 30-mm cover slips were coated with poly-D-lysine (0.1 μg/ml, Millipore, dissolved in H2O) for 10 min at 37°C and air-dried for 2 h. HEK-293T cells were seeded and transfected with an expression plasmid encoding CNGA3 or together with an expression vector encoding GC-G fused to GFP for identification of transfected cells. Two days after transfection, cells were washed with Ca2+ recording buffer (20 mM HEPES pH 7.4, 125 mM NaCl, 2 mM MgCl2, 45 mM KCl, 10 mM glucose, 2 mM CaCl2), then incubated with 4 μM Fura-2AM (Sigma, dissolved in DMSO) for 30 min at room temperature and washed with Ca2+ recording buffer. Cover slips were placed in a POC open perfusion chamber (Pecon GmbH, Erbach, Germany) that was mounted on a Leica DMI 6000B microscope. The 340- and 380-nm excitation filters were used, and emission was measured at 510 nm. Light and the shutter were controlled by a LAMBDA DG-4 control device (Sutter Instrument, Novato, CA, USA). Images were collected by CCD-based imaging system running Metafluor software (Molecular Devices). The chamber was first filled with warm (37°C) Ca2+ recording buffer (supplemented with 1 mM of the phosphodiesterase inhibitor IBMX) for 2 min, then perfused with precooled (15°C) recording buffer (supplemented with 1 mM IBMX) for 5 min. Images were acquired every 2 s. Results are expressed as the ratio of 340-nm to 380-nm (R340/380) signals. Calcium imaging on coronal sections through the GG of mouse pups (P1–P4) was performed as described previously (Schmid et al, 2010).
Coolness-evoked GG responses (c-Fos) in mice pups
The experiments were conducted as described (Mamasuew et al, 2008, 2010). Female mice were kept together with their pups in a cage under a 12-h light/dark cycle (light on at 7:00 AM). Neonatal pups were transferred (without their mother) to a cage placed in an incubator (CERTOMAT BS-1; B. Braun Biotech International, Melsungen, Germany) for exposure to ambient temperature (22 or 35°C), then killed by decapitation immediately after exposure.
In situ hybridization
Heads (for the GG and TG) or necks (for the DRG) of mouse pups were embedded in Leica OCT Cryocompound ‘tissue freezing medium’ (Leica Microsystems, Bensheim, Germany) and quickly frozen on dry ice. Sections (12 μm) were cut by use of a CM3050S cryostat (Leica Microsystems) and adhered to Polysine slides (Menzel, Braunschweig, Germany). A digoxigenin-labeled antisense riboprobe was generated from a partial cDNA clone in a pGem-T plasmid encoding mouse c-Fos or GC-G by use of the T7/SP6 RNA transcription system (Roche Diagnostics, Mannheim, Germany) as recommended by the manufacturer. After fixation in 4% paraformaldehyde/0.1 M NaHCO3/pH 9.5 for 45 min at 4°C, slices were washed in 1× PBS (0.85% NaCl, 1.4 mM KH2PO4, 8 mM Na2HPO4, pH 7.4) for 1 min at room temperature, then incubated in 0.2 M HCl for 10 min, in 1% Triton X-100/1× PBS for 2 min and washed twice in 1× PBS for 30 s. Finally, sections were incubated in 50% formamide/5× SSC (0.75 M NaCl, 0.075 M sodium citrate, pH 7.0) for 10 min. Then, tissue was hybridized in hybridization buffer (50% formamide, 25% H2O, 25% Microarray Hybridization Solution v2.0; GE Healthcare, Freiburg, Germany) containing the probe and incubated in a humid box (50% formamide) at 65°C overnight.
Slides were washed twice in 0.1× SSC for 30 min at 65°C, then treated with 1% blocking reagent (Roche Diagnostics) in TBS (100 mM Tris, 150 mM NaCl, pH 7.5) with 0.3% Triton X-100 for 30 min at room temperature and incubated with an anti-digoxigenin alkaline phosphatase-conjugated antibody (Roche Diagnostics) diluted 1:750 in TBS/0.3% Triton X-100/1% blocking reagent at 37°C for 30 min. After two washes in TBS for 15 min each, slides were rinsed in DAP buffer (100 mM Tris, pH 9.5, 100 mM NaCl, 50 mM MgCl2). Hybridization signals were visualized with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate used as substrates. Sections were mounted in Vectamount mounting medium (Vector Laboratories, Burlingame, CA, USA) and photographed with use of an Axioskop 2 microscope (Carl Zeiss MicroImaging, Göttingen, Germany), an AxioCam MRc5 camera (Zeiss) and the Axiovision imaging system (Zeiss). For microscopy and photography of GC-G-specific in situ hybridization on sagittal sections of the head, a SteREO Discovery.V12 stereomicroscope (Zeiss), an AxioCam color camera (Zeiss) and the Axiovision imaging system (Zeiss) were used.
USV and thermal imaging in separated pups
Neonatal mouse littermates (11 litters) derived from a heterozygous GC-G+/Δ intercross were used. The day of birth was considered postnatal day 0 (P0). On the day of testing (P2, P4, and P6), each pup was separated from its littermates and dam and placed into a 600-ml glass beaker (diameter, 9 cm; height, 12.5 cm) located inside a low-temperature incubator (MCL-20A; BiOBiZ Corporation, Incheon, Korea) that was precooled to 15°C. A USV recorder (Anabat SD1 Bat Detector; Titley Electronics, Ballina, NSW, Australia) was placed on top of the beaker to record the USV of the pup for 5 min. USV was analyzed by use of AnaLook software (Titley Electronics). Crucial parameters of USV were defined as follows: (1) Positive response: any USV detected during the 5-min exposure to coolness (15°C). The cooling responsive rate was calculated as the proportion of pups responsive to cooling in each genotype at different ages. (2) Number of calls: the total number of USV calls detected during 5 min exposure to coolness. (3) Latency: time between the onset of coolness exposure and the first ultrasound call (measured to the first peak frequency).
For thermal imaging analyses, pups exposed to a cool ambient temperature (15°C) were filmed with a thermal camera (NEC Thermo Shot F30S) for 5 min. Temperatures at the tip of the nose (corresponding to the region harboring the GG) and the periphery of the body (corresponding to the skin) were calculated at 0.5, 3, and 5 min from thermal gradient scales.
Generation of a novel GC-G-KO mouse strain
For the GC-G-encoding gene (Gucy2g), we generated a novel conditional allele with a targeting vector that introduced two loxP sites flanking exons 16–18; the exons code for a critical part of the cyclase catalytic domain of GC-G (Supplementary Fig S9). After electroporation into ES cells (129/Sv R1 cell line), DNA samples from G418-resistant clones were digested with BamHI to examine correct targeting via Southern blotting analysis with an external probe. We then removed the FRT-flanked neomycin resistance gene cassette by transfecting the targeted ES cells with a FLP recombinase-encoding expression plasmid. The final targeted Flox allele contained a loxP site upstream of exon 16 and another FRT/loxP site downstream of exon 18 of the Gucy2g gene. ES cells were injected into C57BL/6 blastocysts to generate chimera. Germline transmission of the Flox allele was confirmed by PCR analysis. Heterozygous global Gucy2gΔ/+ mice were generated by crossing male Prm-Cre; Gucy2gFlox/+ mice to WT female mice. The Δ allele was backcrossed with C57BL/6 mice for at least seven generations. The GC-G-KO (GC-GΔ/Δ) littermates showed no apparent reproductive or morphological defects. Primers used for genotyping and RT-PCR analysis were as follows: WT allele: CU, 5′-TTT GCG GCC GCA TGG TTA ACA TAA CAA GCC TGTG-3′, and FD, 5′-TCA GTC GAC ATT TCT TGC CTT AGT TAG GGT GTG TGC TACT-3′; Δ allele: CU and JD, 5′-ATA GTC GAC AGG CCA TCA AGT CCA TAG AGG AAG-3′. F1, 5′-GTG AGC CCT ACT TGG TTG GTC TA-3′; R1, 5′-CAG CGC TTG GTA TGA TGG AT-3′; F2, 5′-GCT TCG GCC GTC CCT GTT GG-3′; R2, 5′-CCC TTG CCC TTG ACT GAA ATG GTG-3′.
Statistical analysis
For statistical analyses of c-Fos expression, from each animal investigated, all sections along the rostrocaudal extent of the GG were analyzed and all c-Fos-positive cells in sections were counted to determine the number of c-Fos-positive cells in the GG of a GC-G-KO mouse relative to that in a concomitantly processed WT pup; the latter number was set to 100%. In the bar chart of Fig6I, the standard deviation is indicated. The P-value was determined by two-tailed paired t-test (with 95% confidence interval) with GraphPad Prism 5.0. In Fig6N, coolness-induced ΔF (ratio of fluorescence intensity) was analyzed by two-tailed unpaired t-test. Cooling responsive rate between genotypes was analyzed by chi-square test. Number of calls and latency between genotypes were analyzed by two-tailed unpaired t-tests. P < 0.05 was considered statistically significant.
Acknowledgments
We thank Chien-Hsiun Chen for assistance in statistical analysis and Sin-Jhong Cheng, who is supported in part by the Neuroscience Program in Academia Sinica, for calcium imaging experiments. This study was supported by the National Science Council, Taiwan (NSC-97-2320-B-001-009-MY3 to R-BY) and the Deutsche Forschungsgemeinschaft (Br712/24-1 to HB and JF). JF was funded by the Bundesministerium für Bildung und Forschung (01PL11003). We thank the Taiwan Mouse Clinic (NSC 102-2325-B-001-042), which is funded by the National Research Program for Biopharmaceuticals (NRPB) at the National Science Council (NSC) of Taiwan, for technical support in thermal imaging experiments.
Author contributions
Y-CC, C-CC, JF and R-BY designed research. Y-CC, Y-CL and JF performed research. C-CC, HB, JF and R-BY analyzed data. JF, HB and R-BY wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary Figures
Review Process File
References
- Allin JT, Banks EM. Effects of temperature on ultrasound production by infant albino rats. Dev Psychobiol. 1971;4:149–156. doi: 10.1002/dev.420040206. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Efimova IV, Alberts JR. Ultrasonic vocalizations by rat pups: the primary importance of ambient temperature and the thermal significance of contact comfort. Dev Psychobiol. 1992;25:229–250. doi: 10.1002/dev.420250402. [DOI] [PubMed] [Google Scholar]
- Brandt JP, Aziz-Zaman S, Juozaityte V, Martinez-Velazquez LA, Petersen JG, Pocock R, Ringstad N. A single gene target of an ETS-family transcription factor determines neuronal CO2-chemosensitivity. PLoS One. 2012;7:e34014. doi: 10.1371/journal.pone.0034014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechbuhl J, Moine F, Broillet MC. Mouse Grueneberg ganglion neurons share molecular and functional features with C. elegans amphid neurons. Front Behav Neurosci. 2013;7:193. doi: 10.3389/fnbeh.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao YC, Cheng CJ, Hsieh HT, Lin CC, Chen CC, Yang RB. Guanylate cyclase-G, expressed in the Grueneberg ganglion olfactory subsystem, is activated by bicarbonate. Biochem J. 2010;432:267–273. doi: 10.1042/BJ20100617. [DOI] [PubMed] [Google Scholar]
- Chinkers M, Wilson EM. Ligand-independent oligomerization of natriuretic peptide receptors. Identification of heteromeric receptors and a dominant negative mutant. J Biol Chem. 1992;267:18589–18597. [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Cramer CP, Blass EM. The contribution of ambient temperature to suckling behavior in rats 3-20 days of age. Dev Psychobiol. 1982;15:339–348. doi: 10.1002/dev.420150406. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Duda T, Sharma RK. Distinct ONE-GC transduction modes and motifs of the odorants: uroguanylin and CO(2) Biochem Biophys Res Commun. 2010;391:1379–1384. doi: 10.1016/j.bbrc.2009.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer J, Mamasuew K, Breer H. Expression of cGMP signaling elements in the Grueneberg ganglion. Histochem Cell Biol. 2009;131:75–88. doi: 10.1007/s00418-008-0514-8. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Molday RS. Subunit composition of the peripherin/rds-rom-1 disk rim complex from rod photoreceptors: hydrodynamic evidence for a tetrameric quaternary structure. Biochemistry. 1996;35:6144–6149. doi: 10.1021/bi960259n. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Spencer WC, McWhirter RD, Zeller G, Henz SR, Ratsch G, Miller DM, 3rd, Horvitz HR, Sternberg PW, Ringstad N. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc Natl Acad Sci USA. 2011;108:254–259. doi: 10.1073/pnas.1017354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke W, Mamasuew K, Biel M, Yang RB, Fleischer J. Odorant-evoked electrical responses in Grueneberg ganglion neurons rely on cGMP-associated signaling proteins. Neurosci Lett. 2013;539:38–42. doi: 10.1016/j.neulet.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Moritani N, Aoki-Komori S, Tanaka M, Saito TR. Comparison of ultrasonic vocalizations emitted by rodent pups. Exp Anim. 2004;53:409–416. doi: 10.1538/expanim.53.409. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Huang YH, Wei CC, Su YH, Wu BT, Ciou YY, Tu CF, Cooper TG, Yeung CH, Chu ST, Tsai MT, Yang RB. Localization and characterization of an orphan receptor, guanylyl cyclase-G, in mouse testis and sperm. Endocrinology. 2006;147:4792–4800. doi: 10.1210/en.2005-1476. [DOI] [PubMed] [Google Scholar]
- Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–2252. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, Ng CK, Su YH, Kilic A, Mitko D, Bien-Ly N, Komuves LG, Yang RB. Identification of an orphan guanylate cyclase receptor selectively expressed in mouse testis. Biochem J. 2004;379:385–393. doi: 10.1042/BJ20031624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M. Function and dysfunction of mammalian membrane guanylyl cyclase receptors: lessons from genetic mouse models and implications for human diseases. Handb Exp Pharmacol. 2009;191:47–69. doi: 10.1007/978-3-540-68964-5_4. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Cheng CF, Hou HH, Lian WS, Chao YC, Ciou YY, Djoko B, Tsai MT, Cheng CJ, Yang RB. Disruption of guanylyl cyclase-G protects against acute renal injury. J Am Soc Nephrol. 2008;19:339–348. doi: 10.1681/ASN.2007050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Fraser SE, Koos DS. Grueneberg ganglion olfactory subsystem employs a cGMP signaling pathway. J Comp Neurol. 2009;516:36–48. doi: 10.1002/cne.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DG. Human natriuretic peptide receptor-A guanylyl cyclase is self-associated prior to hormone binding. Biochemistry. 1992;31:10421–10425. doi: 10.1021/bi00158a001. [DOI] [PubMed] [Google Scholar]
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- Mamasuew K, Breer H, Fleischer J. Grueneberg ganglion neurons respond to cool ambient temperatures. Eur J Neurosci. 2008;28:1775–1785. doi: 10.1111/j.1460-9568.2008.06465.x. [DOI] [PubMed] [Google Scholar]
- Mamasuew K, Michalakis S, Breer H, Biel M, Fleischer J. The cyclic nucleotide-gated ion channel CNGA3 contributes to coolness-induced responses of Grueneberg ganglion neurons. Cell Mol Life Sci. 2010;67:1859–1869. doi: 10.1007/s00018-010-0296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamasuew K, Hofmann N, Breer H, Fleischer J. Grueneberg ganglion neurons are activated by a defined set of odorants. Chem Senses. 2011a;36:271–282. doi: 10.1093/chemse/bjq124. [DOI] [PubMed] [Google Scholar]
- Mamasuew K, Hofmann N, Kretzschmann V, Biel M, Yang RB, Breer H, Fleischer J. Chemo- and thermosensory responsiveness of Grueneberg ganglion neurons relies on cyclic guanosine monophosphate signaling elements. Neurosignals. 2011b;19:198–209. doi: 10.1159/000329333. [DOI] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, McDougall LM, Cockerham RE, Schmid A, Wandernoth P, Wennemuth G, Biel M, Zufall F, Kelliher KR. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr Biol. 2010;20:1438–1444. doi: 10.1016/j.cub.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T, Takayama J, Fujiwara M, Maruyama IN. Environmental alkalinity sensing mediated by the transmembrane guanylyl cyclase GCY-14 in C. elegans. Curr Biol. 2013;23:1007–1012. doi: 10.1016/j.cub.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Okon E. The temperature relations of vocalization in infant Golden hamsters and Wistar rats. J Zool. 1971;164:227–237. [Google Scholar]
- Ortiz CO, Faumont S, Takayama J, Ahmed HK, Goldsmith AD, Pocock R, McCormick KE, Kunimoto H, Iino Y, Lockery S, Hobert O. Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr Biol. 2009;19:996–1004. doi: 10.1016/j.cub.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswalt GL, Meier GW. Olfactory, thermal, and tactual influences on infantile ultrasonic vocalization in rats. Dev Psychobiol. 1975;8:129–135. doi: 10.1002/dev.420080205. [DOI] [PubMed] [Google Scholar]
- Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011;23:1921–1926. doi: 10.1016/j.cellsig.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SM, Zheng C, Koos DS, Feinstein P, Fraser SE, Mombaerts P. Structure and emergence of specific olfactory glomeruli in the mouse. J Neurosci. 2001;21:9713–9723. doi: 10.1523/JNEUROSCI.21-24-09713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Pyrski M, Biel M, Leinders-Zufall T, Zufall F. Grueneberg ganglion neurons are finely tuned cold sensors. J Neurosci. 2010;30:7563–7568. doi: 10.1523/JNEUROSCI.0608-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Martinez-Velazquez L, Ringstad N. A chemoreceptor that detects molecular carbon dioxide. J Biol Chem. 2013a;288:37071–37081. doi: 10.1074/jbc.M113.517367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HK, Luo L, O'Halloran D, Guo D, Huang XY, Samuel AD, Hobert O. Defining specificity determinants of cGMP mediated gustatory sensory transduction in Caenorhabditis elegans. Genetics. 2013b;194:885–901. doi: 10.1534/genetics.113.152660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebe S, Schellig K, Lesage F, Breer H, Fleischer J. The thermosensitive potassium channel TREK-1 contributes to coolness-evoked responses of Grueneberg ganglion neurons. Cell Mol Neurobiol. 2014;34:113–122. doi: 10.1007/s10571-013-9992-x. [DOI] [PubMed] [Google Scholar]
- Szentgyorgyi H, Kapusta J, Marchlewska-Koj A. Ultrasonic calls of bank vole pups isolated and exposed to cold or to nest odor. Physiol Behav. 2008;93:296–303. doi: 10.1016/j.physbeh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Garbers DL. Dominant negative mutations of the guanylyl cyclase-A receptor. Extracellular domain deletion and catalytic domain point mutations. J Biol Chem. 1995;270:425–430. doi: 10.1074/jbc.270.1.425. [DOI] [PubMed] [Google Scholar]
- Vaandrager AB, van der Wiel E, Hom ML, Luthjens LH, De Jonge HR. Heat-stable enterotoxin receptor/guanylyl cyclase C is an oligomer consisting of functionally distinct subunits, which are non-covalently linked in the intestine. J Biol Chem. 1994;269:16409–16415. [PubMed] [Google Scholar]
- Wang D, O'Halloran D, Goodman MB. GCY-8, PDE-2, and NCS-1 are critical elements of the cGMP-dependent thermotransduction cascade in the AFD neurons responsible for C. elegans thermotaxis. J Gen Physiol. 2013;142:437–449. doi: 10.1085/jgp.201310959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EM, Chinkers M. Identification of sequences mediating guanylyl cyclase dimerization. Biochemistry. 1995;34:4696–4701. doi: 10.1021/bi00014a025. [DOI] [PubMed] [Google Scholar]
- Yang RB, Garbers DL. Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J Biol Chem. 1997;272:13738–13742. doi: 10.1074/jbc.272.21.13738. [DOI] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, Garbers DL. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci USA. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Review Process File