Abstract
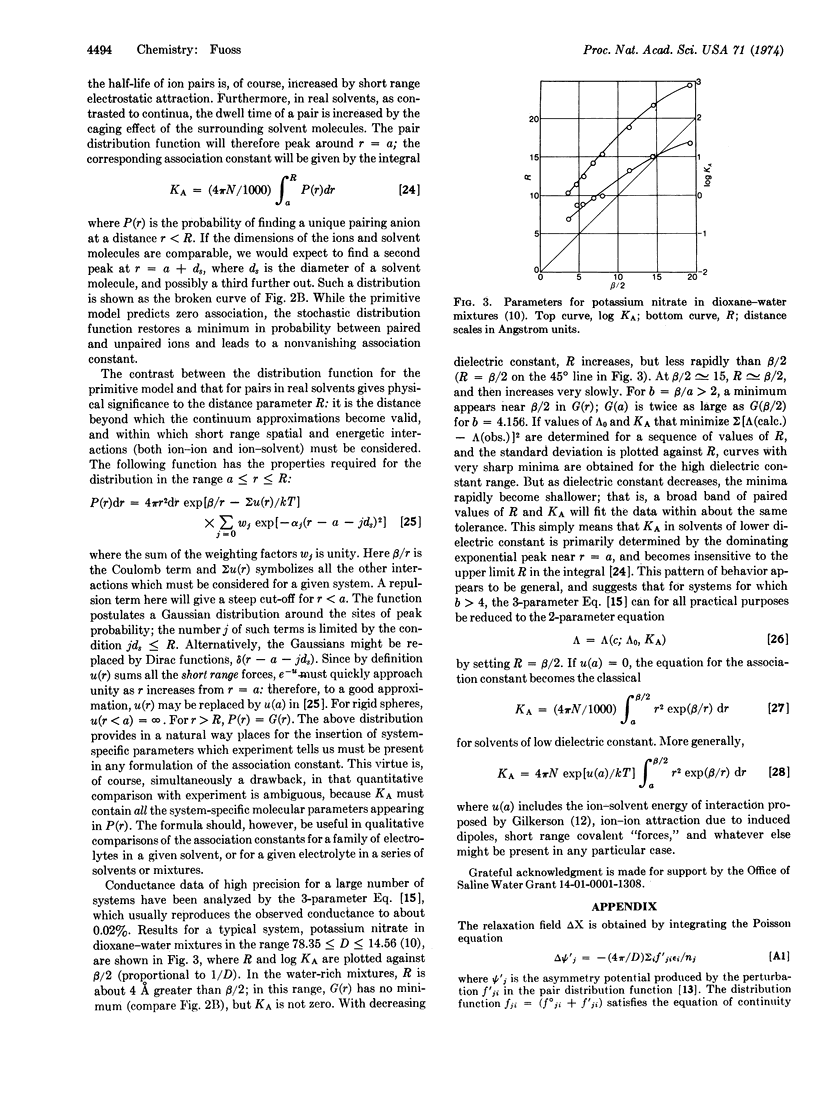
It is shown that three and only three parameters are sufficient to generate a function Λ (c; Λ0, KA, R) which reproduces observed equivalent conductance Λ as a function of concentration c within experimental error up to concentrations of about 2 × 10-7D3 eq/liter (D = dielectric constant). The three parameters are: Λ0, the limit of Λ(c) at zero concentration; KA, the association constant; and R, a distance. Realization that conductance data can provide only one distance parameter suggests a model for electrolytic solutions in which R is defined as the radius of the sphere inside of which a unique partner can be found for a paired ion, and outside of which continuum theory may be applied. All system-specific effects (ion-ion and ion-solvent interactions, and the inherent spatial discontinuity of real solutions) appear within the spheres of radius R centered at the cations of the ion pairs. The association constant therefore depends not only on electrostatic attraction but also on the multiplicity of molecular parameters that are needed to describe short range interactions.
Keywords: ion pairs, association constants, model electrolytes
Full text
PDF