Abstract
Objective
Protracted, systemic inflammation has been associated with adverse effects on cognition and brain structure, and may accelerate neurodegenerative disease processes; however, it is less clear whether changes in inflammation are associated with brain structure.
Methods
We studied 276 black and white older adults (mean age=83 years at time of imaging) enrolled in a prospective study of aging. Inflammation (measured with CRP) was assessed repeatedly over 6 years (i.e. Year 2, 4, 6, & 8). Brain MRIs were obtained at years 10–11 with DTI; regions of interest included late-myelinating areas vulnerable to aging, including frontal-parietal [superior longitudinal fasciculus (SLF)-dorsal] and temporal (SLF-temporal; uncinate) white matter tracts.
Results
Mean CRP values significantly declined (t=−5.54, p<.0001) over 6 years, and subject-specific slopes (BLUPs) all showed a decline (mean=−.57, SD=.53) for our participant sample. More than 50% of study participants were still in the moderate to high cardiovascular risk range based on CRP values at Year 8. After controlling for demographics, vascular risk factors and MRI white matter hyperintensities, larger decreases in CRP values over time were significantly associated with higher fractional anisotropy in the SLF-dorsal [Beta=−0.0052, standard error (SE)=0.003; 95% confidence interval (CI)= −0.0103 to −0.0025, p=.04], SLF-temporal (Beta=−0.0109, SE=0.004; 95%CI=−0.0189 to −0.0029, p=.008), and uncinate (Beta=−0.0067, SE=0.003; 95%CI=−0.0132 to −0.0001, p=.05) fasciculi.
Conclusions
Results suggest that in a prospective cohort of older individuals, faster declines in inflammation over time are related to indicators of white matter health, even after accounting for vascular risk factors.
Keywords: CRP, pro-inflammatory, diffusion tensor imaging, longitudinal
1. Introduction
Sustained pro-inflammatory processes have damaging effects on neurological functioning that may hasten cognitive decline (Eikelenboom et al., 2012) and accelerate neurodegenerative disease course (Bermejo et al., 2008, Cunningham et al., 2009, Kravitz et al., 2009, McGeer and McGeer, 2001). While inflammation was initially conceptualized as a downstream or ‘bystander’ effect of neuronal death, it is now thought to be an early event that may precede the clinical manifestations of neurodegeneration (Bettcher and Kramer, 2013, Cagnin et al., 2001, Mancinella et al., 2009, Yaffe et al., 2004). Moreover, there is growing consensus that inflammatory processes play a contributory role in cognitive decline (Rosano et al., 2012, Tarkowski et al., 1999, Yaffe et al., 2003).
In particular, c-reactive protein (CRP) is an acute phase protein and marker of systemic, low-grade inflammation that has received considerable research focus, as it has clear clinical utility in predicting vascular events (e.g. stroke; myocardial infarction), is amenable to risk stratification (Ridker, 2003), and also correlates with age-related cognitive impairment (Noble et al., 2010). Higher levels of CRP have been associated with future development of Alzheimer’s Disease (AD) (Engelhart et al., 2004), and elevations in inflammatory mediators may be present years before a dementia diagnosis (Koyama et al., 2013).
Despite the strong association between higher baseline inflammation and later dementia (Tan et al., 2007), little is known about longitudinal trajectories of inflammation in aging (Metti et al, in press) or how they relate to changes in brain structure. Specifically, while previous studies have suggested that increases in inflammatory markers over time are related to cognitive impairment, cardiovascular disease and mortality (Danesh et al., 2008, Jenny et al., 2012), it is unclear how increases or decreases in inflammation may associate with neuroanatomy in older adults.
Recent evidence suggests that higher inflammation induces changes in vascular permeability (Cuff et al., 1996), endothelial function (Csiszar et al., 2004), and microvascular structure (Sprague and Khalil, 2009, Zhang et al., 2009), all of which may contribute to the pathogenesis of cerebrovascular disease and alterations in white matter microstructure. Consistent with these potential mechanisms, higher levels of inflammation have recently been associated with lower white matter microstructure in older adults using diffusion tensor imaging (Bettcher et al., 2013, Wersching et al., 2010). However, it is unclear whether the association between inflammation and white matter structure is driven by vascular risk factors, or if it is an independent contributor to myelin and axonal integrity. Furthermore, lower white matter microstructure has to date only been related to cross-sectional levels of inflammation, which may obscure the impact of longitudinal change in peripheral pro-inflammatory markers.
The goal of this study is to assess whether changes in the pro-inflammatory marker, CRP, are associated with white matter microstructure in a prospective cohort of community dwelling, black and white older adults. We hypothesized that change in CRP levels would predict white matter microstructure in late-myelinating temporal and frontal-parietal tracts, and this association would remain even after controlling for vascular risk and health history factors.
2. Methods
2.1. Study Participants
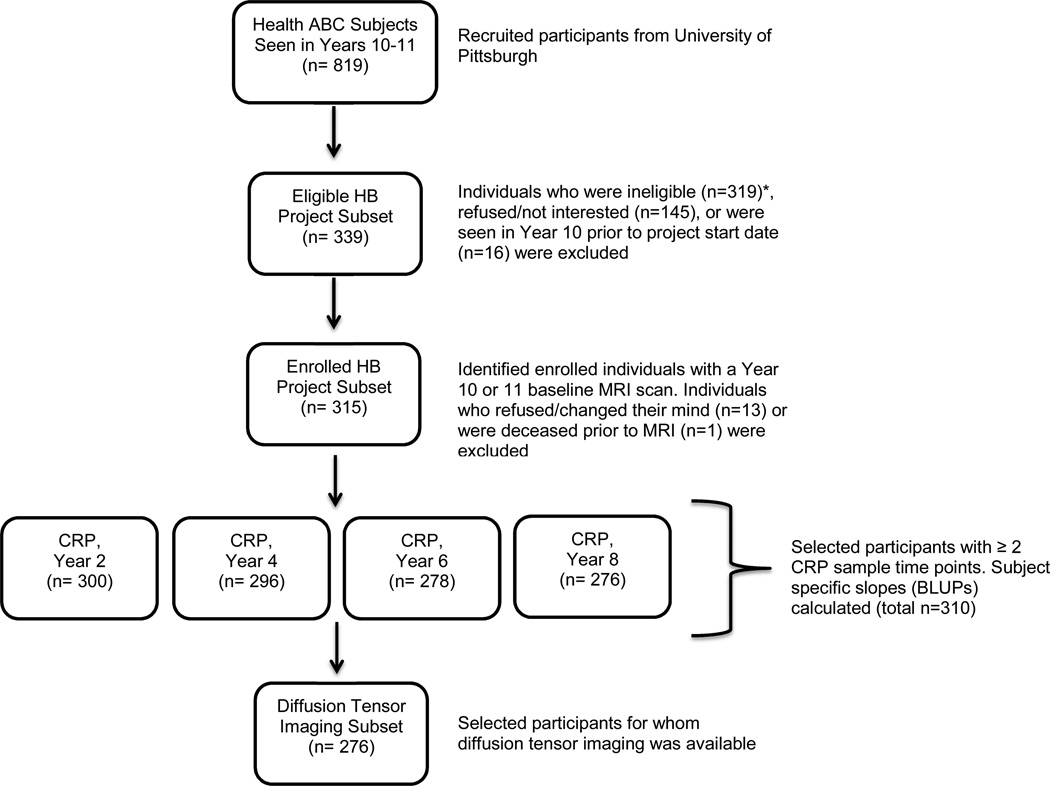
We studied a subset of participants enrolled in the Health, Aging, and Body Composition Study (Health ABC). The Health ABC began in 1997–1998 as a longitudinal, observational cohort study of 3075 well-functioning older white and black men and women, 70–79 years old, from Pittsburgh, PA and Memphis, TN (Simonsick et al., 2001) who reported no difficulty walking a quarter of a mile (400 m), climbing 10 steps, or performing activities of daily living. Participants were enrolled if they were free of life-threatening cancers with no active treatment within the prior 3 years, and had planned to remain within the study area for at least 3 years. Between 2006 and 2007 (i.e. study Years 10 and 11), 315 Health ABC participants from the Pittsburgh site who were interested and eligible for a brain 3T MRI (e.g. no metal implants, pacemaker, etc.) participated in the ancillary study called the Healthy Brain Project. Medical histories were reviewed to rule out endocrinal, neurological and psychological illnesses. Participants received a brain MRI in addition to Health ABC assessments. Among the 315 participants, 310 individuals had at least two measurements of blood between baseline and the time of MRI, and 276 had complete diffusion tensor imaging (DTI) data available (see Figure 1). A global cognitive function measure [Modified Mini Mental Status Exam (3MS)] and test of executive functions [Digit Symbol Substitution Test (DSST)] were also available for all participants.
Figure 1. Flow chart for selection of study participants.
Displays flow chart for selection of current study participants. HB= Healthy Brain. *Individuals deemed ineligible for Healthy Brain Project included those who displayed difficulty walking with assistance (n=74), could not complete a 6 meter walk (n=23), did not meet MRI criteria (n=106), and those who had “other reasons” based on chart review (n=29) or medical exclusions (n=87).
This study was approved by the institutional review boards of the clinical site (University of Pittsburgh) and the coordinating center (University of California, San Francisco).
2.2. Inflammatory Marker Assessment
Blood measures were obtained after an overnight fast, frozen at −70°C, and shipped to the Health ABC Core Laboratory at the University of Vermont. Serum and plasma levels of CRP were measured in duplicate by enzyme-linked immunosorbent essay based on purified protein and polyclonal anti-CRP antibodies (Calbiochem, San Diego, California). The CRP assay was standardized according to the World Health Organization’s First International Reference Standard, with a sensitivity of 0.08 µg/ml (for clinical risk comparisons, 1 µg/ml=1 mg/L) (Ridker, 2003). Blind duplicate analyses for CRP showed an average interassay coefficient of variation of 8.0%. CRP measures were available at study years: 2, 4, 6, and 8. Years 2, 4, and 6 utilized serum samples, whereas Year 8 employed citrated plasma samples. Year 8 CRP levels were recalibrated for longitudinal comparison with the three prior time points (Kalogeropoulos et al., 2010). Across all time points, 14 CRP measurements were considered outliers (i.e. > 30 µg/ml) and were removed from further analyses due to concerns that the individuals may have had an acute inflammatory event or an underlying chronic illness. According to standard guidelines for cardiovascular disease risk, CRP levels less than 1.0µg/ml are considered low, 1.0–2.9µg/ml intermediate, and greater than 3.0µg/ml high risk (Ridker, 2003).
2.3. Neuroimaging Evaluation
Magnetic resonance imaging acquisition
MRI scanning used a Siemens 12-channel head coil and was performed on a 3 T Siemens Tim TrioMR scanner at the MR Research Center of the University of Pittsburgh in Year 10 of the study. Magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted images were acquired in the axial plane: TR=2300ms; TE=3.43ms; TI=900 ms; flip angle=9; slice thickness=1 mm; FOV=256*224 mm; voxel size=1 mm*1mm; matrix size=256*224; and number of slices=176. Diffusion Weighted Images (DTI)were acquired using single-short spin-echo sequence with the following parameters: TR=5300 ms; TE=88 ms; TI=2500 ms; flip angle=90; FOV=256*256 mm; two diffusion values of b=0 and 1000 s/mm; 12 diffusion directions; four repeats; 40 slices; matrix size=128*128; voxel size=2 mm*2 mm; slice thickness=3 mm; and GRAPPA=2. A radiologist verified that the T1 MR images for this study did not have abnormalities with potential clinical relevance. No images were excluded because of unexpected findings.
Image processing and analysis
Full imaging protocol details are reported in prior publications (Rosano et al, 2012); in brief, white matter hyperintensity volumes were obtained from T2-weighted FLAIR images using an automated method for quantification and localization of WMH. The WMH quantification was done using a fuzzy connected algorithm with automated seed selection(Wu et al., 2006). Total WMH volume was estimated by summing all the voxels classified as WMH and normalized for brain volume. One parameter was obtained from the diffusion weighted images, fractional anisotropy (FA), which is a marker of white matter tract microstructure. The diffusion-weighted images were pre-processed using the FMRIB's Diffusion Toolbox (Smith et al., 2004) to remove unwanted distortions due to eddy current, the tensor were computed (Basser et al., 1994), and diagonalized to determine the eigenvalues from which the FA maps were computed (Pierpaoli and Basser, 1996). The FA maps were registered to the FMRIB58-FA template (Smith et al., 2004) using the FMRIB's Non-linear Image Registration Tool (FNIRT) (Andersson et al., 2007), similar to Tract-based Spatial Statistics (Smith et al., 2006).
ROI Selection
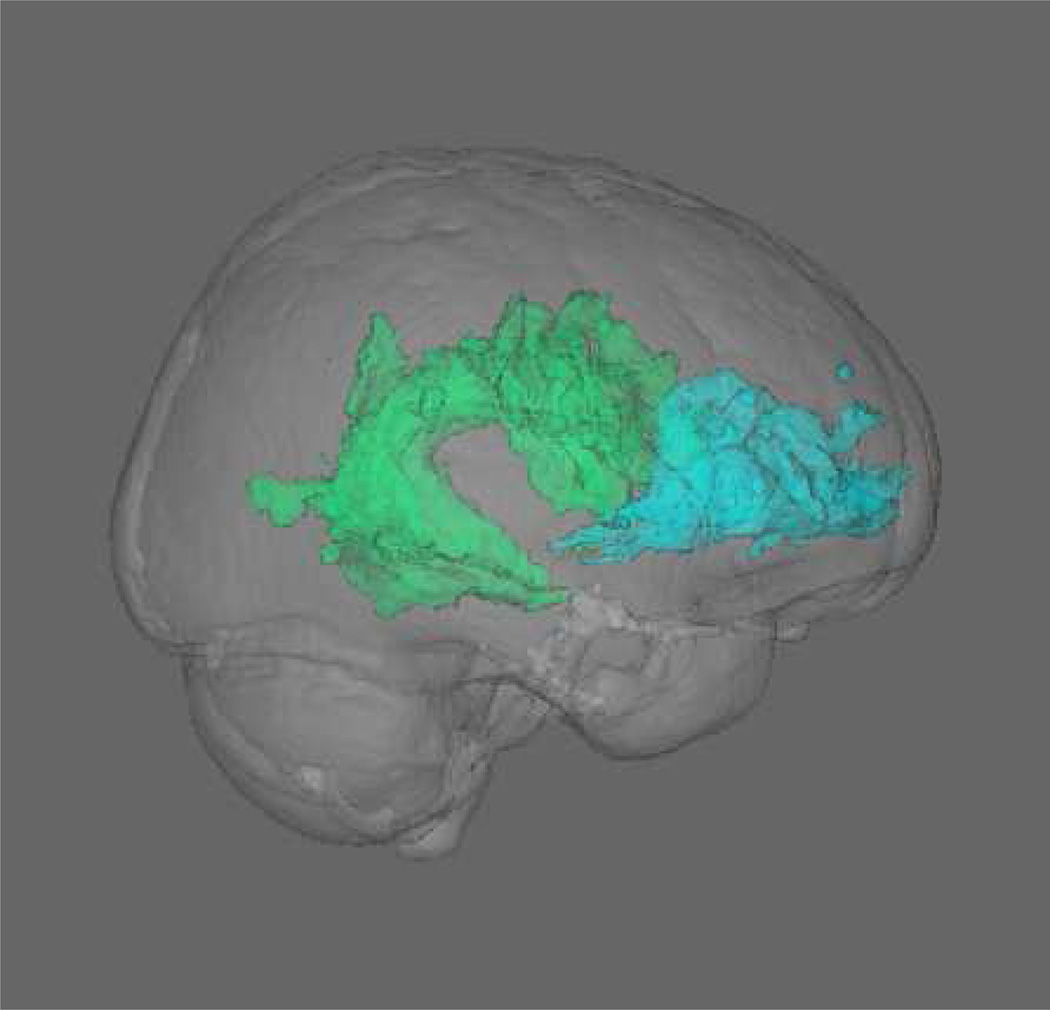
White matter tracts were masked using the Johns Hopkins University (JHU) White Matter Atlas (Smith et al, 2006), and FA values for both hemispheres were averaged to create single regions of interest (ROI). The Healthy Brain Project assessed late-myelinating fronto-temporal and fronto-parietal tracts (i.e. uncinate fasciculus, superior longitudinal fasciculus-temporal portion, and superior longitudinal fasciculus-dorsal portion; see Figure 2)(Lebel et al., 2012), which have been shown to be sensitive to aging (Brickman et al., 2012, Sullivan et al., 2010, Teipel et al., 2010).
Figure 2. Diffusion tensor imaging white matter ROI’s.
Displays a 3D representation of the uncinate fasciculus (blue) and superior longitudinal fasciculus-dorsal and temporal (green) ROI’s from the JHU atlas, shown within the MNI standard brain.
2.4. Covariates
Several covariates were incorporated into our statistical models. In terms of demographic variables, age, sex, education, and race were included in the model. In addition, pro-inflammatory markers are strongly associated with vascular risk and cardiovascular disease (Bettcher et al., 2013, Csiszar et al., 2004, Cuff et al., 1996, Sprague and Khalil, 2009), thus we controlled for additional health factors to isolate the independent contribution of longitudinal inflammation to brain structure. At the time of MRI, the following health information was collected and controlled for in subsequent analyses: prevalent hypertension, cardiovascular disease, stroke, diabetes, smoking status (never, former, or current), and body mass index. This information was based on prevalent disease algorithms that took into account self-report of physician diagnoses and recorded medications, along with measures from the clinical examination (e.g. fasting blood glucose for diabetes). Total white matter hyperintensity volume was also controlled for in analyses. Finally, depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies-Depression Scale (CES-D), with a score of ≥16 suggestive of possible depression (Radloff, 1977).
2.5. Statistical Analyses
We estimated subject specific change in CRP levels as the best linear unbiased predictors of slopes (BLUPs) from a linear mixed effect model. BLUPs display numerous advantages over ordinary least squares (OLS) slopes, as they borrow strength from other participant’s data and minimize the overall mean-squared error in creating a robust measure of change. We subsequently entered these CRP BLUPs in a series of general linear models, in which aforementioned covariates and CRP BLUPs served as the predictor variables, and white matter FA ROI’s served as separate outcome variables.
In follow-up exploratory analyses, we examined additional questions. First, considering the strong association between female gender and higher levels of inflammation (Ridker et al., 1998, Ridker et al., 2000), we examined whether there was an interaction between gender and change in CRP values over time in predicting white matter ROI’s. In addition, we examined the interaction between race and change in CRP values in predicting white matter ROI’s. Finally, we examined whether single measurements of CRP (i.e. Years 2, 4, 6, 8) were predictive of white matter ROI’s. The goal of this analysis was to determine if a strong and consistent relationship was seen between individual CRP values and white matter FA, apart from overall change in inflammation.
All analyses were conducted using SAS, version 9.4.
3. Results
3.1. Participant Characteristics and Longitudinal CRP Levels (See Table 1)
Table 1.
Participant characteristics and imaging results at time of MRI
| Mean (SD) or Proportion Total |
Range | ||
|---|---|---|---|
| Demographic Variables | |||
| Age (Years) | 82.96 (2.78) | 78 – 90 | |
| Gender (% Female) | 58 | ||
| Education (Years) | 13.94 (2.61) | 3 – 18 | |
| Race (% Black) | 40 | ||
| Health Variables | |||
| Current Smoker (%) | 2 | ||
| Former Smoker (%) | 46 | ||
| Stroke (%) | 8 | ||
| Diabetes (%) | 27 | ||
| Hypertension (%) | 82 | ||
| Cardiovascular Disease (%) | 29 | ||
| Body Mass Index (mean kg/m2) | 27.37 (4.34) | 16.89 – 39.11 | |
| CES-D Score | 6.86 (6.31) | 0 – 41 | |
| Neuroimaging Variables | |||
| Normalized WMH Volume | 0.006 (0.008) | 0.000 – 0.058 | |
| Uncinate FA | 0.398 (0.027) | 0.326 – 0.496 | |
| Superior Longitudinal Fasciculus FA | 0.376 (0.022) | 0.311 – 0.437 | |
| Superior Longitudinal Fasciculus-Temporal FA | 0.440 (0.033) | 0.347 – 0.523 | |
CES-D: Center for Epidemiological Studies Depression Scale; WMH: white matter hyperintensity; FA: fractional anisotropy
Study participants were an average of 83 years old at time of MRI, had some years of college (mean=14 years), and were comprised of primarily white (60%) and black (40%) individuals. Mean performance on measures of global cognitive functioning (3MS) and executive function (DSST) was within normal limits for age (3MS: M=92.50; SD=7.44; DSST: M=38.59; SD=12.57). Mean CRP values significantly declined (t=−5.54, p<.0001) across all four time points (Year 2: mean=4.50 µg/ml, SD=4.99; Year 4: mean=4.19 µg/ml, SD=5.51; Year 6: mean=3.77 µg/ml, SD=4.71; and Year 8: mean=2.91 µg/ml, SD=3.54), and subject-specific slopes (BLUPs) were all less than 0 (mean=−.5711, SD=.5289; min=−3.14; max=−.02) for our participant sample.1
Higher levels of CRP in the initial (Year 2) laboratory assessment were associated with faster declines in CRP over six years (Year 2, rs=−.82; p<.0001; Year 8, rs=−.62, p<.0001). Further investigation of the decline in CRP values using risk stratification (Ridker, 2003) (i.e. low risk: <1.0µg/ml; moderate risk: 1.0–2.9µg/ml; high-risk ≥3µg/ml) revealed that at the first time point (Year 2), approximately 45% of participants had CRP values in the high-risk range, whereas 30% displayed high-risk CRP levels at the last time point (Year 8).
In terms of associations between selected covariates and change in CRP values, after controlling for the effects of age, greater declines in CRP were related to prevalent hypertension (rs=−.23, p<.001), higher BMI (rs=−.31, p<.001), and black race (rs=−.17, p=.003). No other covariates were significantly related to longitudinal change in CRP.
3.2. Regression Analyses: Change in CRP and White Matter FA at Year 10 (Table 2)
Table 2.
Associations between subject-specific change in CRP values and white matter fractional anisotropy at year 10
| Model*: Demographics, White Matter Hyerintensities, Vascular Risk Factors Beta (Standard Error) [95% Confidence Interval], p-value |
||
|---|---|---|
| Superior Longitudinal Fasciculus- Dorsal FA | ||
| Δ in CRP | −0.0052 (0.003) [−0.0103 to −0.0025], p=.04 | |
| Superior Longitudinal Fasciculus- Temporal FA | ||
| Δ in CRP | −0.0109 (0.004) [−0.0189 to −0.0029], p=.008 | |
| Uncinate FA | ||
| Δ in CRP | −0.0067 (0.003) [−0.0132 to −0.0001], p=.05 | |
FA=fractional anisotropy. Δ in CRP represents the best linear unbiased predictor for subject-specific change in CRP values over 4 time points (i.e. BLUPS)
Adjusted for age, gender, education, race; smoking status; prevalent hypertension, cardiovascular disease, stroke, and diabetes; body mass index; white matter hyperintensity volume
All estimates and 95% CI’s for Model 2 are shown in Table 2. After controlling for demographic variables, white matter hyperintensity volumes, and vascular health history factors, faster decreases in CRP values over time were significantly associated with higher FA in the SLF-dorsal (t=−2.06; p=.04), SLF-temporal (t=−2.67, p=.008), and uncinate (=−2.00, p=.05) fasciculi. In addition, higher age (Uncinate: t=−2.22, p=.03), female gender (SLF-dorsal: t=3.45, p<.0001; Uncinate: t= 3.61, p<.0001), lower education (SLF-temp: t=3.34, p=.001; Uncinate: t=2.16, p=.03), white race (SLF-temp: t=−2.76, p=006), greater WMH (SLF-dorsal: t=−5.52, p<.001; SLF-temp: t=−3.30, p=.001), and higher BMI (Uncinate: t=−2.06, p=.04) were significantly associated with lower white matter FA for select ROI’s.
3.3. Exploratory Analyses
We followed up our primary analyses with additional exploratory analyses. First, we examined whether there was an interaction between CRP slopes and gender in predicting white matter FA at year 10. Two trends emerged, indicating that for men, the faster their CRP values decline over time, the higher their SLF-dorsal and uncinate FA at Year 10 was (p=.08 for both) relative to women. We also examined whether there was an interaction between CRP slopes and race in predicting white matter FA at year 10; this interaction was not significantly associated with the SLF-dorsal (p=.91), SLF-temporal, (p=.25), or uncinate fasciculus (p=.17).
We further examined whether single time points of CRP values were also strong predictors of white matter. Regression analyses for SLF-dorsal FA indicated a positive association between Year 8 values only and white matter FA (t=2.57; beta=.0009, SE=.0004; 95%CI= .0002 to .00160; p=.01). For SLF-temporal FA and Uncinate FA, only Year 2 values were significant predictors (SLF-temporal: t=3.08, beta: .00136526, SE=.0004426; 95% CI=.0005 to .0022; p=.002), and positively related to white matter FA. No significant associations (all p’s >.05) were noted between white matter FA and Years 4 and 6 CRP values.
4. Discussion
Results from this prospective study indicate that in a cohort of older black and white individuals, faster declines in inflammation were related to higher white matter microstructure. Specifically, declines in CRP levels over six years predicted fractional anisotropy in temporal and fronto-parietal white matter tracts, and were not better accounted for by demographics, vascular risk factors, or white matter hyperintensities on MRI. This study builds on prior Health ABC work showing CRP levels (Yaffe et al., 2003) and trajectories (Metti et al., 2014) correlate with cognitive decline, as the current study examines a potentially more proximal indictor of overall brain health (white matter structure).To our knowledge, this is the first study to investigate the relation between longitudinal inflammation and brain structure in a cohort of community-dwelling, non-demented older adults, and highlights the important role of chronic immunological processes in healthy aging.
An important finding of the study is that faster declines in inflammation, particularly in men, predicted greater FA in the superior longitudinal fasciculus (SLF)-dorsal portion, SLF-temporal portion, and uncinate fasciculus. Although higher inflammation is linked with a wide range of cardiovascular factors (e.g. adiposity, hypertension) and vascular brain injury (e.g. stroke, white matter hyperintensities) (Fornage et al., 2008, Wilson et al., 2006, Zhang et al., 2009), the association between longitudinal change in CRP and white matter was not accounted for by vascular markers in our participants. Our results therefore provide preliminary support for the growing literature on inflammation and vascular risk as independent contributors to brain health (Bettcher et al., 2013, Ferretti and Cuello, 2011, Verstynen et al., 2013, Wyss-Coray and Mucke, 2002).
An additional consideration is whether single measurements of CRP are strongly and consistently associated with white matter microstructure, or if repeated measurements of inflammation are important to understanding brain health. In our exploratory analysis, CRP measurements from different study years were not consistently associated with white matter FA, and associations that were significant (specifically for Years 2 and 8) were counterintuitive (i.e. higher inflammation was associated with better white matter FA). Our findings suggest that cross-sectional studies may obscure the relationship between inflammatory trajectories and white matter FA, and further highlight the importance of accounting for repeated CRP measurements in elucidating the contribution of inflammation to white matter structure.
Notably, our cohort displayed an unexpected decrease in inflammation over six years, which is inconsistent with the commonly reportedly association between age and higher inflammation in cross-sectional studies (Ballou et al., 1996, Bruunsgaard et al., 1999, Cohen et al., 1997). Although our sample population was older in age (mean age=83 years at time of MRI), prior studies suggest than centenarians display higher peripheral levels of inflammation relative to eighty year olds, who in turn show higher inflammation than younger adults (Sandmand et al., 2002). One explanation for the overall decline in CRP values is a possible cohort effect; thus, individuals who live to a more advanced age with multiple comorbidities may exhibit different immunological profiles. In partial support of this hypothesis, it has been shown that centenarians with multiple health conditions display lower t-cell production of proinflammatory cytokines in response to stimulation than younger older adults, despite their higher levels of circulation markers (Sandmand et al., 2002, Sandmand et al., 2003). It is also important to highlight that despite declines in CRP values, more than 50% of study participants were still in the moderate to high-risk categories at Year 8. Thus, a decline in values did not necessarily indicate ‘low’ circulating levels of inflammation. Interestingly, a recent prospective study examining a different inflammatory analyte (interleukin-6) and cognition in the oldest old reported that higher levels of IL-6 were related to lower risk of dementia, further underscoring that inflammation may impart differential effects on younger and older individuals (Metti et al., In Press). Future studies examining longitudinal change in mRNA expression levels of cytokines (e.g. reverse transcription polymerase chain reaction, RT-PCR) may be particularly useful in clarifying the evolving immune phenotype in older adults, and may further isolate the source of immune alteration in cohorts with multiple vascular comorbidities.
This study displayed several strengths, including the prospective examination of longitudinal CRP levels in an older, well-characterized cohort, and the use of diffusion outcome metrics to measure subtle alterations in white matter microstructure. Considering the critical role of white matter microstructure in bolstering cognitive functioning (Kerchner et al., 2012, Sullivan et al., 2010), results from this study may have important clinical implications for older adults, as modulation of their inflammatory profile over time may improve overall brain health and functioning. Although the effects are not large, the association between change in inflammation and white matter FA is particularly notable given that we controlled for a range of vascular risk factors and comorbid health conditions.
A confine to interpretation of the study is that we cannot address whether increases in inflammation impart risk for lower white matter microstructure in the oldest old, given that our participant sample displayed overall decreases in CRP. Additional limitations to the study include the use of one peripheral pro-inflammatory marker, which may not adequately reflect inflammatory processes in the CNS. Although basic science and human research suggest a link between peripheral markers and CNS functioning (Lampa et al., 2012), direct comparisons to CNS levels cannot be made based on the current data. In addition, we focused our analyses on a clinical measure of non-specific inflammation (CRP), which may increase with underlying infections or illness. Given the exclusion of laboratory measurements reflecting high levels of CRP (CRP>30 µg/ml), we believe it is unlikely that the observed decline in values is attributable to acute infections in early years, but remains a consideration.
In terms of possible limitations regarding our MRI methodology and analysis, we elected to employ an ROI-based approach using a standard MNI template rather than a study-specific template. Notably, this approach depends on the accuracy of the ROI’s in the template as well as the reliability of the within-subject registration to the template. Although there are both advantages and disadvantages to using a standard template (Zhang and Arfanakis, 2013), we focused on the reliability of the standard atlas for extracting ROI’s, particularly given the need to account for white matter hyperintensity (WMH) extraction (masked from FLAIR T2-weighted images) in the study. Finally, one MRI time point was available, thereby limiting further analysis of longitudinal change in brain structure. Given that the temporal relationship between CRP declines and white matter changes remains unclear, caution should be applied when attempting to make a causal association between the two.
In summary, findings from the current study indicate that in a large, prospective cohort of community-dwelling non-demented older adults, faster declines in inflammation (CRP) over 6 years predicted better white matter integrity in SLF-dorsal, SLF-temporal and uncinate fasciculi, and were independent of vascular risk factors. These results highlight the importance of accounting for repeated measures of inflammatory factors to precisely estimate the contribution of inflammation to brain health, and suggest that inflammatory processes may play an independent role in white matter microstructure.
Bettcher et al. Highlights.
We examined whether change in CRP levels would predict white matter microstructure.
Mean CRP values significantly declined over 6 years in healthy older adults.
Larger CRP decreases associated with better microstructure in the SLF and uncinate.
Results suggest that declines in inflammation relate to indices of white matter health.
Acknowledgements
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, K23 AG042492-01 (Bettcher), RO1-AG028288 (Mei) and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging, as well as an Alzheimer’s Association New Investigator Grant (NIRP-12-259223; Bettcher).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Similar evaluations were conducted with the entire Health ABC sample, and overall declines in CRP were also noted.
The authors have no conflicts of interest or relevant disclosures.
References
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. 2007 [Google Scholar]
- Ballou SP, Lozanski FB, Hodder S, Rzewnicki DL, Mion LC, Sipe JD, Ford AB, Kushner I. Quantitative and qualitative alterations of acute-phase proteins in healthy elderly persons. Age Ageing. 1996;25:224–230. doi: 10.1093/ageing/25.3.224. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Ribera JM, Villar AM. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer's disease. 2008;117:198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Kramer JH. Inflammation and clinical presentation in neurodegenerative disease: a volatile relationship. 2013;19:182–200. doi: 10.1080/13554794.2011.654227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM, Walsh CM, Watson C, Miller JW, Green R, Patel N, Miller BL, Neuhaus J, Yaffe K, Kramer JH. Body mass and white matter integrity: the influence of vascular and inflammatory markers. PLoS One. 2013;8:e77741. doi: 10.1371/journal.pone.0077741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, Wasserman BT, Williams LM, Zimmerman ME. Testing the white matter retrogenesis hypothesis of cognitive aging. 2012;33:1699–1715. doi: 10.1016/j.neurobiolaging.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J. Gerontol. A Biol. Sci. Med. Sci. 1997;52:M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Cuff CA, Martiney JA, Berman JW, Brosnan CF. Differential effects of transforming growth factor-beta 1 on interleukin-1-induced cellular inflammation and vascular permeability in the rabbit retina. 1996;70:21–28. doi: 10.1016/s0165-5728(96)00103-8. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Hoozemans JJ, Veerhuis R, van Exel E, Rozemuller AJ, van Gool WA. Whether, when and how chronic inflammation increases the risk of developing late-onset Alzheimer's disease. 2012;4:15. doi: 10.1186/alzrt118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Ferretti MT, Cuello AC. Does a pro-inflammatory process precede Alzheimer's disease and mild cognitive impairment? 2011;8:164–174. doi: 10.2174/156720511795255982. [DOI] [PubMed] [Google Scholar]
- Fornage M, Chiang YA, O'Meara ES, Psaty BM, Reiner AP, Siscovick DS, Tracy RPTLW., Jr Biomarkers of Inflammation and MRI-Defined Small Vessel Disease of the Brain: The Cardiovascular Health Study. 2008;39:1952–1959. doi: 10.1161/STROKEAHA.107.508135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, Ding J, Fried LP, Kritchevsky SB, Rifkin DE, Sarnak MJ, Newman AB. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. 2012;67:970–976. doi: 10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J Health ABC Study Investigators. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J. Am. Coll. Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Racine CA, Hale S, Wilheim R, Laluz V, Miller BL, Kramer JH. Cognitive processing speed in older adults: relationship with white matter integrity. 2012;7:e50425. doi: 10.1371/journal.pone.0050425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A, O'Brien J, Weuve J, Blacker D, Metti AL, Yaffe K. The role of peripheral inflammatory markers in dementia and Alzheimer's disease: a meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:433–440. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz BA, Corrada MM, Kawas CH. Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old. 2009;5:318–323. doi: 10.1016/j.jalz.2009.04.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampa J, Westman M, Kadetoff D, Agreus AN, Le Maitre E, Gillis-Haegerstrand C, Andersson M, Khademi M, Corr M, Christianson CA, Delaney A, Yaksh TL, Kosek E, Svensson CI. Peripheral inflammatory disease associated with centrally activated IL-1 system in humans and mice. Proc. Natl. Acad. Sci. U. S. A. 2012;109:12728–12733. doi: 10.1073/pnas.1118748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Mancinella A, Mancinella M, Carpinteri G, Bellomo A, Fossati C, Gianturco V, Iori A, Ettorre E, Troisi G, Marigliano V. Is there a relationship between high C-reactive protein (CRP) levels and dementia? 2009;49(Suppl 1):185–194. doi: 10.1016/j.archger.2009.09.028. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Innate immunity in Alzheimer's disease: a model for local inflammatory reactions. 2001;1:22–29. [PubMed] [Google Scholar]
- Metti AL, Yaffe K, Boudreau RM, Ganguli M, Lopez OL, Stone KL, Cauley JA. <br /> Change in Inflammatory Markers and Cognitive Status in the Oldest-Old Women from the Study of Osteoporotic Fractures. doi: 10.1111/jgs.12739. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metti AL, Yaffe K, Boudreau RM, Simonsick EM, Carnahan RM, Satterfield S, Harris TB, Ayonayon HN, Rosano C, Cauley JA Health ABC Study. Trajectories of inflammatory markers and cognitive decline over 10 years. Neurobiol. Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. 2010;67:87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. 1996;36:893–206. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. 1977:385–401. [Google Scholar]
- Ridker PM. Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. 2003;108:e81–e85. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Rosano C, Marsland AL, Gianaros PJ. Maintaining brain health by monitoring inflammatory processes: a mechanism to promote successful aging. 2012;3:16–33. [PMC free article] [PubMed] [Google Scholar]
- Sandmand M, Bruunsgaard H, Kemp K, Andersen-Ranberg K, Pedersen AN, Skinhoj P, Pedersen BK. Is ageing associated with a shift in the balance between Type 1 and Type 2 cytokines in humans? Clin. Exp. Immunol. 2002;127:107–114. doi: 10.1046/j.1365-2249.2002.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmand M, Bruunsgaard H, Kemp K, Andersen-Ranberg K, Schroll M, Jeune B. High circulating levels of tumor necrosis factor-alpha in centenarians are not associated with increased production in T lymphocytes. Gerontology. 2003;49:155–160. doi: 10.1159/000069174. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T Health ABC Study Group. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol. Aging. 2010;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Blennow K, Wallin A, Tarkowski A. Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. 1999;19:223–230. doi: 10.1023/a:1020568013953. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein KH, Filippi M, Ernemann U, Reiser MF, Hampel H. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J. Alzheimers Dis. 2010;22:507–522. doi: 10.3233/JAD-2010-100234. [DOI] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein A, Erickson KI, Sheu LK, Marsland AL, Gianaros PJ. Competing physiological pathways link individual differences in weight and abdominal adiposity to white matter microstructure. 2013;79C:129–137. doi: 10.1016/j.neuroimage.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, Conty M, Minnerup J, Ringelstein EB, Berger K, Deppe M, Knecht S. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. 2010;74:1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Ryan MC, Boyle AJ. The novel role of C-reactive protein in cardiovascular disease: risk marker or pathogen. 2006;106:291–297. doi: 10.1016/j.ijcard.2005.01.068. [DOI] [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, 3rd, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Arfanakis K. White matter segmentation based on a skeletonized atlas: Effects on diffusion tensor imaging studies of regions of interest. J. Magn. Reson. Imaging. 2013 doi: 10.1002/jmri.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]