Abstract
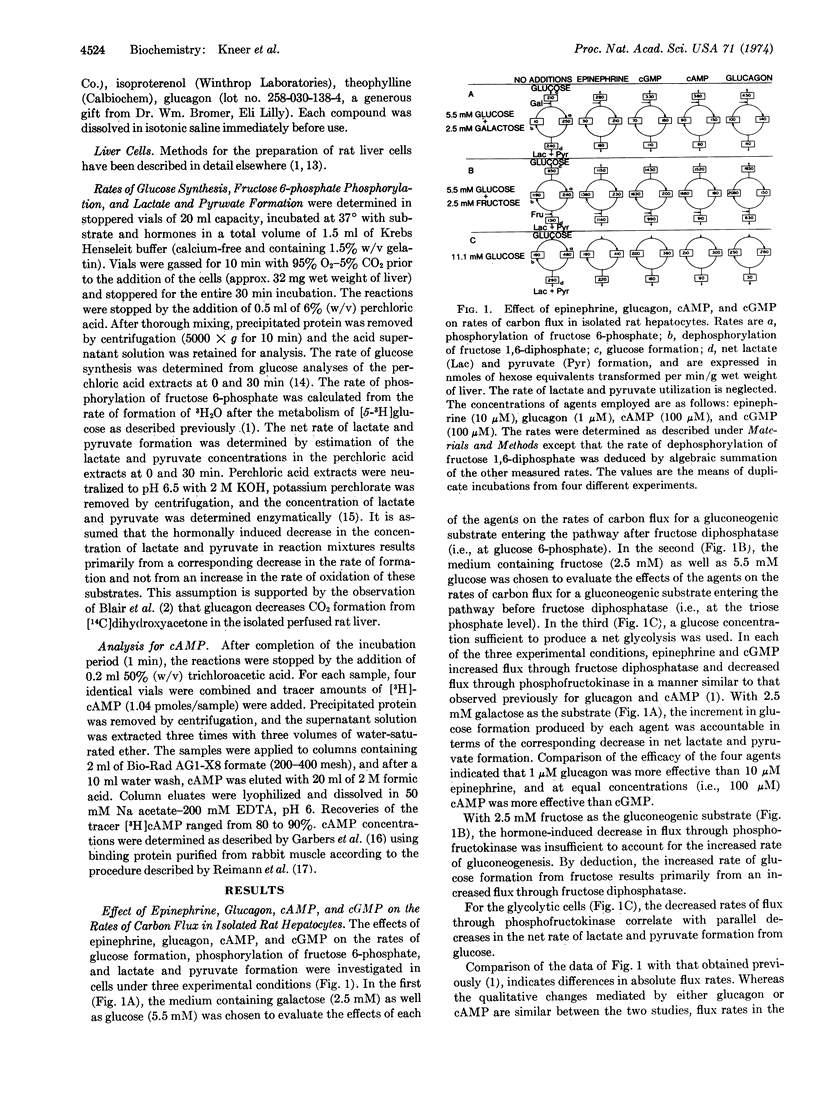
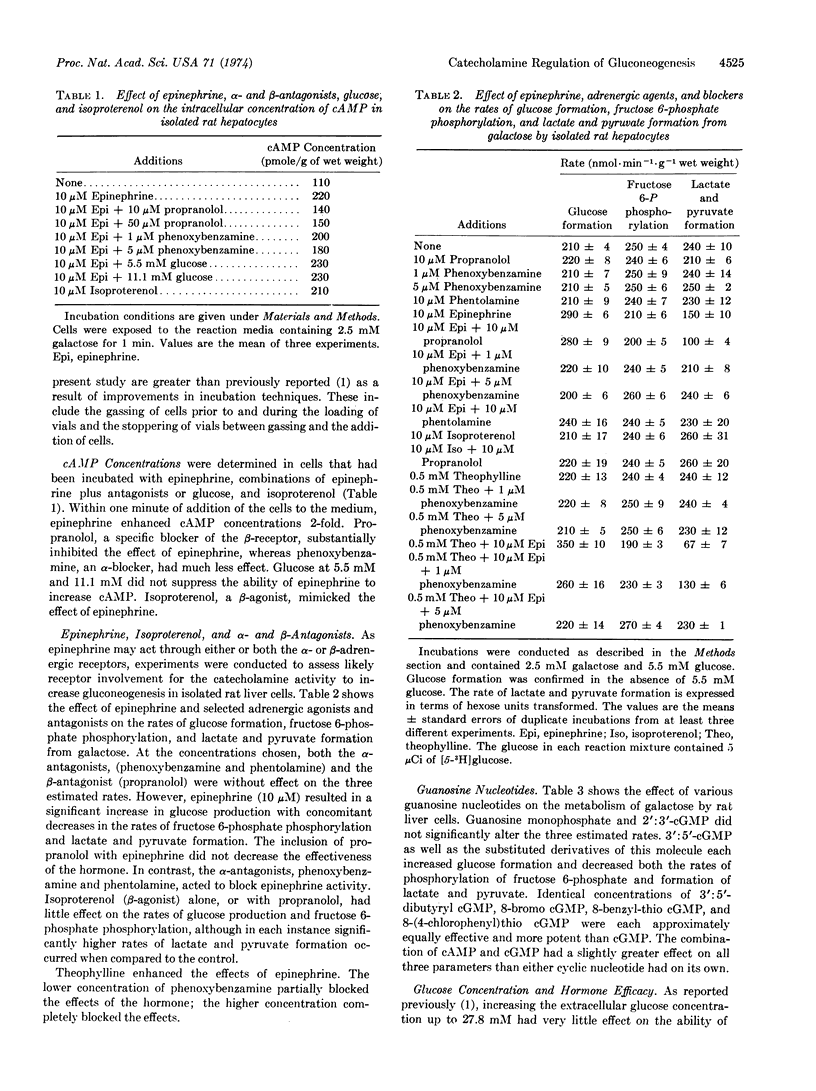
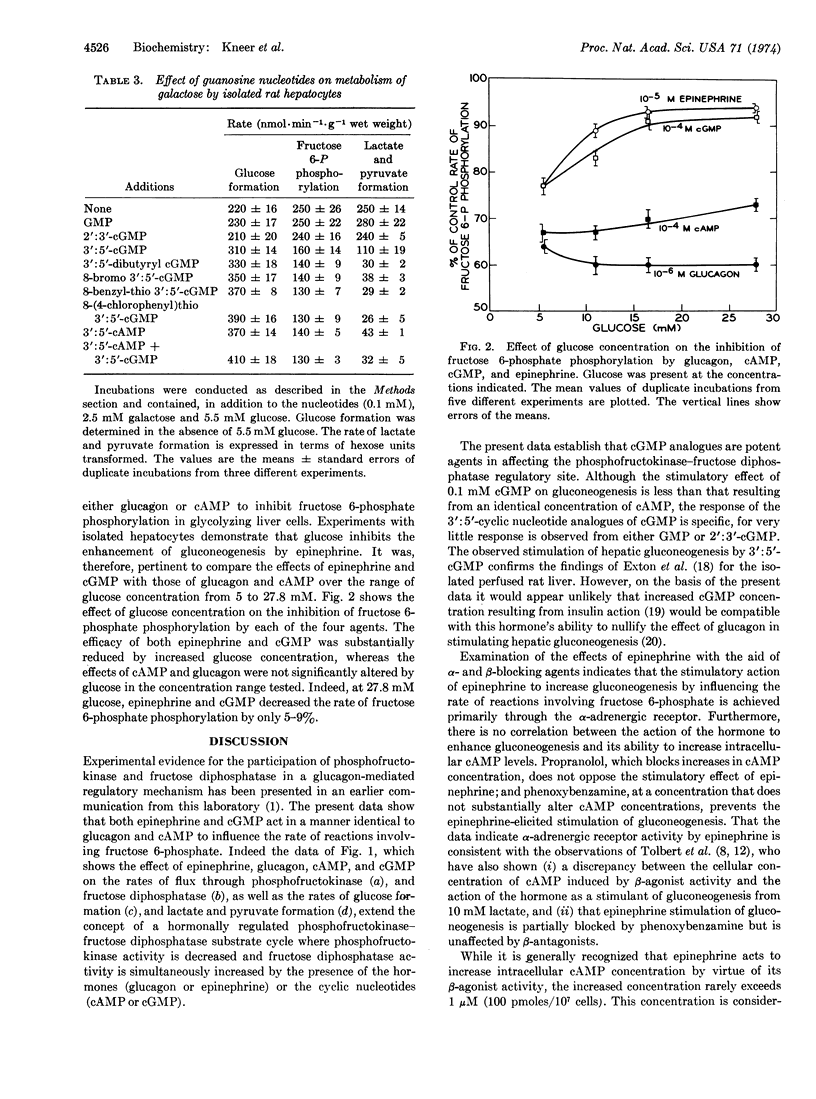
For isolated rat hepatocytes, glucagon, 3′:5′-cyclic AMP, 3′:5′-cyclic GMP, and epinephrine stimulate the rate of gluconeogenesis from substrates not involving pathways of mitochondrial metabolism. From estimation of the rates of glucose formation, fructose 6-phosphate phosphorylation, and lactate and pyruvate formation it is concluded that epinephrine and 3′:5′-cyclic GMP stimulate gluconeogenesis from either galactose or fructose by influencing the rate of reactions involving fructose 6-phosphate in a manner similar to that already reported for glucagon and 3′:5′-cyclic AMP. Each agent acts to inhibit flux through phosphofructokinase (EC 2.7.1.11) and enhance flux through fructose diphosphatase (EC 3.1.3.11), resulting in the re-direction of carbon from lactate and pyruvate formation to glucose synthesis. In addition to 3′:5′-cyclic GMP, dibutyryl 3′:5′-cyclic GMP, 8-bromo 3′:5′-cyclic GMP, 8-benzyl-thio 3′:5′-cyclic GMP and 8-(4-chlorophenyl)thio 3′:5′-cyclic GMP stimulate glucose formation and inhibit lactate and pyruvate formation from galactose. Guanosine monophosphate and 2′:3′-cyclic GMP are inactive. As the stimulatory effect of epinephrine is inhibited by phenoxybenzamine and not by propranolol, and is not simulated by isoproterenol, it is concluded that catecholamine activity is expressed through the α-receptor. Increased extracellular glucose concentration (>10 mM) decreases the stimulatory effect of epinephrine, 3′:5′-cyclic GMP, and partially that of 3′:5′-cyclic AMP but does not alter the efficacy of glucagon.
Keywords: fructose diphosphatase, phosphofructokinase, regulation, glucagon, cyclic purine nucleotides
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair J. B., Cook D. E., Lardy H. A. Influence of glucagon on the metabolism of xylitol and dihydroxyacetone in the isolated perfused rat liver. J Biol Chem. 1973 May 25;248(10):3601–3607. [PubMed] [Google Scholar]
- Clark M. G., Kneer N. M., Bosch A. L., Lardy H. A. The fructose 1,6-diphosphatase-phosphofructokinase substrate cycle. A site of regulation of hepatic gluconeogenesis by glucagon. J Biol Chem. 1974 Sep 25;249(18):5695–5703. [PubMed] [Google Scholar]
- Exton J. H., Hardman J. G., Williams T. F., Sutherland E. W., Park C. R. Effects of guanosine 3',5'-monophosphate on the perfused rat liver. J Biol Chem. 1971 Apr 25;246(8):2658–2664. [PubMed] [Google Scholar]
- Exton J. H., Robison G. A., Sutherland E. W., Park C. R. Studies on the role of adenosine 3',5'-monophosphate in the hepatic actions of glucagon and catecholamines. J Biol Chem. 1971 Oct 25;246(20):6166–6177. [PubMed] [Google Scholar]
- Garbers D., First N. L., Lardy H. A. The stimulation of bovine epididymal sperm metabolism by cyclic nucleotide phosphodiesterase inhibitors. Biol Reprod. 1973 Jun;8(5):589–598. doi: 10.1093/biolreprod/8.5.589. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Haynes R. C., Jr Hormonal control of glycogenolysis and gluconeogenesis in isolated rat liver cells. J Biol Chem. 1973 Aug 10;248(15):5333–5343. [PubMed] [Google Scholar]
- HAUGAARD E. S., HAUGAARD N. The effect of hyperglycemic-glycogenolytic factor on fat metabolism of liver. J Biol Chem. 1954 Feb;206(2):641–645. [PubMed] [Google Scholar]
- Illiano G., Tell G. P., Siegel M. E., Cuatrecasas P. Guanosine 3':5'-cyclic monophosphate and the action of insulin and acetylcholine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2443–2447. doi: 10.1073/pnas.70.8.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE R. A. EFFECT OF GLYCOGENOLYTIC AGENTS ON PHOSPHORYLASE ACTIVITY OF PERFUSED RAT LIVER. Am J Physiol. 1965 Feb;208:317–323. doi: 10.1152/ajplegacy.1965.208.2.317. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- SCHIMASSEK H., MITZKAT H. J. UBER EINE SPEZIFISCHE WIRKUNG DES GLUCAGON AUF DIE EMBDEN-MEYERHOF-KETTE IN DER LEBER. VERSUCHE AN DER ISOLIERT PERFUNDIERTEN RATTENLEBER. Biochem Z. 1963 Aug 14;337:510–518. [PubMed] [Google Scholar]
- Sudilovsky O., Pestana A., Hinderaker P. H., Pitot H. C. Cyclic adenosine 3',5'-monophosphate during glucose repression in the rat liver. Science. 1971 Oct 8;174(4005):142–144. doi: 10.1126/science.174.4005.142. [DOI] [PubMed] [Google Scholar]
- Tolbert M. E., Butcher F. R., Fain J. N. Lack of correlation between catecholamine effects on cyclic adenosine 3':5'-monophosphate and gluconeogenesis in isolated rat liver cells. J Biol Chem. 1973 Aug 25;248(16):5686–5692. [PubMed] [Google Scholar]
- Tolbert M. E., Fain J. N. Studies on the regulation of gluconeogenesis in isolated rat liver cells by epinephrine and glucagon. J Biol Chem. 1974 Feb 25;249(4):1162–1166. [PubMed] [Google Scholar]
- Veneziale C. M. Gluconeogenesis from D-glyceraldehyde and dihydroxyacetone in isolated rat liver. Stimulation by glucagon. Biochemistry. 1972 Aug 15;11(17):3286–3289. doi: 10.1021/bi00767a025. [DOI] [PubMed] [Google Scholar]
- Veneziale C. M. Gluconeogenesis from fructose in isolated rat liver. Stimulation by glucagon. Biochemistry. 1971 Aug 31;10(18):3443–3447. doi: 10.1021/bi00794a020. [DOI] [PubMed] [Google Scholar]
- Veneziale C. M., Lohmar P. H. Gluconeogenesis in isolated hepatic parenchymal cells. J Biol Chem. 1973 Nov 25;248(22):7786–7791. [PubMed] [Google Scholar]
- Zahlten R. N., Stratman F. W., Lardy H. A. Regulation of glucose synthesis in hormone-sensitive isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3213–3218. doi: 10.1073/pnas.70.11.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahlten R. N., Stratman F. W. The isolation of hormone-sensitive rat hepatocytes by a modified enzymatic technique. Arch Biochem Biophys. 1974 Aug;163(2):600–608. doi: 10.1016/0003-9861(74)90519-0. [DOI] [PubMed] [Google Scholar]


