Abstract
Bradyrhizobium sp. DOA9 isolated from the legume Aeschynomene americana exhibited a broad host range and divergent nodulation (nod) genes compared with other members of the Bradyrhizobiaceae. Genome analysis of DOA9 revealed that its genome comprised a single chromosome of 7.1 Mbp and a plasmid of 0.7 Mbp. The chromosome showed highest similarity with that of the nod gene-harboring soybean symbiont B. japonicum USDA110, whereas the plasmid showed highest similarity with pBBta01 of the nod gene-lacking photosynthetic strain BTAi1, which nodulates Aeschynomene species. Unlike in other bradyrhizobia, the plasmid of DOA9 encodes genes related to symbiotic functions including nodulation, nitrogen fixation, and type III/IV protein secretion systems. The plasmid has also a lower GC content (60.1%) than the chromosome (64.4%). These features suggest that the plasmid could be the origin of the symbiosis island that is found in the genome of other bradyrhizobia. The nod genes of DOA9 exhibited low similarity with those of other strains. The nif gene cluster of DOA9 showed greatest similarity to those of photosynthetic bradyrhizobia. The type III/IV protein secretion systems of DOA9 are similar to those of nod gene-harboring B. elkanii and photosynthetic BTAi1. The DOA9 genome exhibited intermediate characteristics between nod gene-harboring bradyrhizobia and nod gene-lacking photosynthetic bradyrhizobia, thus providing the evidence for the evolution of the Bradyrhizobiaceae during ecological adaptation. Bradyrhizobium sp. DOA9 isolated from the legume Aeschynomene americana exhibited a broad host range and divergent nodulation (nod) genes compared with other members of the Bradyrhizobiaceae. Genome analysis of DOA9 revealed that its genome comprised a single chromosome of 7.1 Mbp and a plasmid of 0.7 Mbp. The chromosome showed highest similarity with that of the nod gene-harboring soybean symbiont B. japonicum USDA110, whereas the plasmid showed highest similarity with pBBta01 of the nod gene-lacking photosynthetic strain BTAi1, which nodulates Aeschynomene species. Unlike in other bradyrhizobia, the plasmid of DOA9 encodes genes related to symbiotic functions including nodulation, nitrogen fixation, and type III/IV protein secretion systems. The plasmid has also a lower GC content (60.1%) than the chromosome (64.4%). These features suggest that the plasmid could be the origin of the symbiosis island that is found in the genome of other bradyrhizobia. The nod genes of DOA9 exhibited low similarity with those of other strains. The nif gene cluster of DOA9 showed greatest similarity to those of photosynthetic bradyrhizobia. The type III/IV protein secretion systems of DOA9 are similar to those of nod gene-harboring B. elkanii and photosynthetic BTAi1. The DOA9 genome exhibited intermediate characteristics between nod gene-harboring bradyrhizobia and nod gene-lacking photosynthetic bradyrhizobia, thus providing the evidence for the evolution of the Bradyrhizobiaceae during ecological adaptation.
Introduction
The interaction between leguminous plants and rhizobia results in the formation of root nodules. Within legume nodules rhizobia fix atmospheric nitrogen to ammonia, which can support the growth of host plants. Rhizobia possess a set of modulation (nod) genes that control the synthesis of lipochitooligosaccharides called nod factors (NFs) [1], which induce signal transduction cascades in host plants, ultimately leading to nodule formation [1]. The rhizobial nod genes were previously thought to be essential for nodule formation; however, the recent discovery of Bradyrhizobium spp. ORS278 and BTAi1, which lack nod genes, but are able to nodulate some species of the semi-aquatic legume genus Aeschynomene, contradicts this long-held dogma [2].
Aeschynomene species can be divided into two groups according to the mechanism of nodule initiation. One group, including A. afraspera and A. americana, initiates nodulation through NFs, whereas the other group, including A. indica and A. sensitiva, initiates nodulation without NFs. Although the genome of some Aeschynomene symbionts including ORS278 and BTAi1 were sequenced [2], the genetic basis for NF-independent nodulation and the evolutionary relationship between NF-independent and NF-dependent nodulation remains mostly unclear [2,3].
A recent interest in the origin and evolution of Bradyrhizobiaceae rhizobia has developed because the genomes of several strains have been sequenced. The genome analyses of B. japonicum USDA110 [4] and B. japonicum USDA6 T [5] revealed that genes for nodule symbiosis, such as nod, for nitrogen fixation, and for secretion systems are located on integrated genomic islands (symbiosis islands), suggesting that the symbiotic genes were acquired by lateral gene transfer from an unknown donor. This evolution scenario is supported by the detailed genome comparison between symbiotic (B. japonicum USDA110) and nonsymbiotic (Bradyrhizobium sp. S23321) bradyrhizobia [6]. However, the origin and transmission mechanism of symbiotic genes remains poorly understood.
We recently isolated several strains of bradyrhizobia from the root nodules of A. americana [7]. One isolate, Bradyrhizobium sp. DOA9, was found to nodulate a broad range of leguminous hosts, including dalbergioid, millettioid, and robinoid trives and was also found to be an endophyte of rice, although DOA9 did not form nodules on NF-independent groups of Aeschynomene [8]. DOA9 possesses highly divergent nod genes that could not be amplified by general primer sets designed on other bradyrhizobia [7]. Furthermore, Southern blot hybridization suggested that some of the nod genes and nitrogen fixation (nif) genes of DOA9 are localized on the plasmid unlike in other bradyrhizobia [8]. These results suggested that DOA9 possesses a novel type of genome with nod genes divergent from previously reported bradyrhizobia.
Here we analyzed the genome of DOA9 and compared it with the genomes of other bradyrhizobia. Several symbiosis-related genes, including those involved in nodulation, nitrogen fixation, and secretion systems, were investigated in order to highlight the genetic basis of the broad host range of DOA9 and the evolutionary relationships between nod gene-harboring soybean bradyrhizobia and nod gene-lacking Aeschynomene bradyrhizobia.
Materials and Methods
Bacterial strains and DNA preparation
Bradyrhizobium sp. DOA9 was cultured for 4 d at 28°C in arabinose–gluconate medium [9]. Genomic DNA was prepared as described by Wilson [10].
Sequencing and annotation
The genome sequence of DOA9 was determined by 454 pyrosequencing analysis using a GS FLX Titanium system (Roche Diagnostics Co., Indianapolis, IN, USA). Genomic DNA (5 μg) was sheared using nebulization to obtain fragments ranging from 300 to 800 bp. Template DNA was prepared according to the supplier’s protocol. The pyrosequencing data were assembled using the MIRA assembler ver. 3 [11], and were curated by comparison with the Newbler ver. 2.8 (Roche Diagnostics Co., Indianapolis, IN, USA) assembly or with some close reference strains. Gap closing and resequencing of low-quality regions of the assembled data were performed by PCR and Sanger sequencing. Regions encoding structural RNAs, rRNAs, tRNAs, tmRNAs, noncoding RNAs, and proteins were predicted using the Genaris Annotation System (Genaris, Inc., Kanagawa, Japan).
Genome comparisons and ortholog analysis
A circular genome map showing the GC skew and the GC content was created using the CGview server [12] with default parameters. Similarity was compared between DOA9 and other bacteria using GenomeMatcher [13]. Putative orthologous genes among Bradyrhizobium strains DOA9, USDA110, and ORS278 were identified using bidirectional BLASTN comparisons with an e-value cut-off of 10−20. Orthologous relationships were depicted in a Venn diagram. Phylogenetic analysis was performed by comparing the sequences aligned using the CLUSTALW program [14]. Neighbor-joining trees were constructed using MEGA version 5.02 [15] and 1,000 bootstrap replicates were used to generate a consensus tree.
Pulse-field gel electrophoresis
DNA plugs for pulse-field gel electrophoresis were prepared using CHEF Genomic DNA Plug Kits (Bio-Rad Laboratories Inc., Hercules, CA, USA) as follows. DOA9 was cultured for 6 d at 28°C in peptone salts yeast extract medium [16]. Bacterial cells were collected by centrifugation, washed twice with 0.85% NaCl, and resuspended with 0.85% NaCl to an OD600 of 5. The cell suspension (0.5 mL) was centrifuged at 8,000 × g for 5 min at 10°C, then resuspended with 0.5 mL of cell suspension buffer and mixed thoroughly with 0.5 mL of 2% CleanCut agarose (Bio-Rad Laboratories Inc., Hercules, CA, USA). The mixture was transferred to plug molds and solidified at 4°C for 30 min. The solidified agarose plugs were treated with lysozyme (1 mg/mL) at 37°C for 4 h and with proteinase K (30 U/mL) at 50°C overnight. Fragments of 225–6,000 kb and 225–2,200 kb were separated on 0.8% certified megabase agarose (Bio-Rad Laboratories Inc., Hercules, CA, USA) in TAE buffer or 1% certified megabase agarose in 0.5xTBE buffer, respectively. Contour-clamped homogeneous electric field (CHEF) electrophoresis was conducted at 14°C in a temperature-controlled cooling unit using the autoalgorithm mode in the CHEF Mapper gel electrophoresis system (Bio-Rad Laboratories Inc., Hercules, CA, USA). The gel was stained with 0.5 mg mL−1 ethidium bromide for 1 h and then destained in H2O for 1 h.
Results
Phylogeny of Bradyrhizobium sp. DOA9
To examine the relationships between Bradyrhizobium sp. DOA9 and other members of the Bradyrhizobiaceae, phylogenetic trees were constructed based on 16S rRNA and internal transcribed spacer sequences (Fig. 1 and S1 Fig.). Using both approaches, DOA9 sequences were clustered within a group of nonphotosyntetic nod gene-containing bradyrhizobia, including B. japonicum, B. liaoningense, and B. yuamingense and were separated from those of photosynthetic nod gene-lacking strains, such as Bradyrhizobium spp. ORS278 and BTAi1. These results suggest that the DOA9 genome resembles more closely those of nonphotosynthetic nod gene-containing soybean bradyrhizobia than those of photosynthetic nod gene-lacking bradyrhizobia.
Fig 1. Phylogenetic relationships of Bradyrhizobium sp. DOA9 and related bacteria based on 16S rRNA gene sequences.
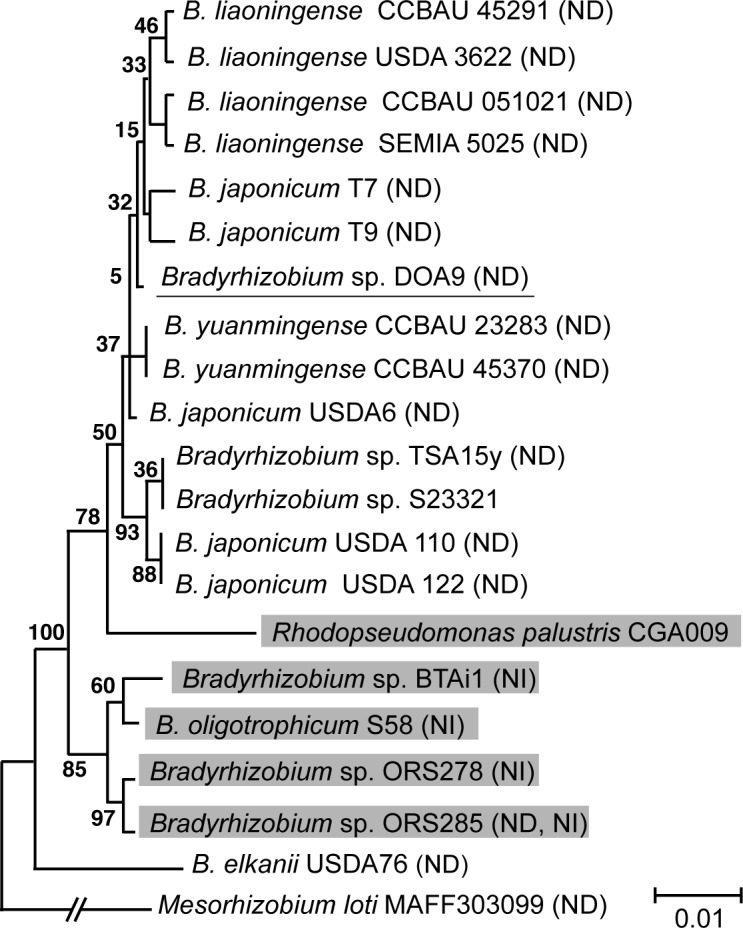
Bootstrap values are expressed as percentages of 1,000 replications. Evolutionary distances were computed using the Kimura two-parameter method. The bar represents one estimated substitution per 100-nucleotide positions. Strains capable of Nod factor-dependent and -independent nodulation are marked with (ND) and (NI), respectively. Photosynthetic strains are highlighted in gray.
Sequencing
The nucleotide sequence of the DOA9 genome was deduced by assembling 454 pyrosequencing data and Sanger sequencing data. In total, 1,853,745 reads from 454 pyrosequencing data were assembled using the MIRA assembler (version 4.0), and 591 contigs were generated. After curation of the MIRA software assembly by comparison with the Newbler software assembly or with the genomes of close reference strains, 48 contigs remained. Gap closing and resequencing of low-quality regions in the assembled data were performed by PCR and Sanger sequencing.
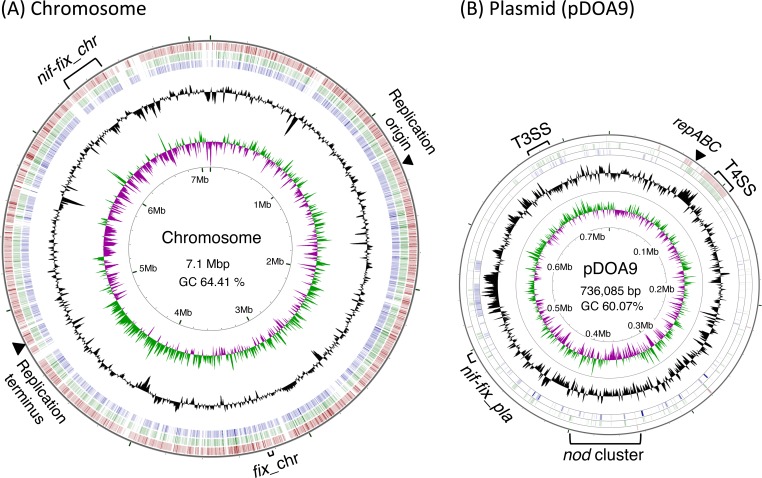
The final draft genome consisted of two circular scaffolds of about 7.1 Mbp and 0.7 Mbp (Fig. 2). The 7.1-Mb scaffold comprised five contigs and was considered to be the main chromosome of DOA9 (Fig. 2A). Due to the presence of small but highly repetitive elements, five gaps of 1–6 kb in the scaffold could not be closed. The smaller scaffold consisted of one contig of 736,085 bp (Fig. 2B) and was considered to be the plasmid.
Fig 2. The genome structure of Bradyrhizobium sp. DOA9.
(A) Circular representation of the chromosome of Bradyrhizobium sp. DOA9. The outermost, second, and third circles represent BLASTN comparisons with B. japonicum USDA110, Bradyrhizobium sp. ORS278, and Bradyrhizobium sp. ORS28, respectively (e-value < 10−10). The innermost and second-innermost circles show the GC skew and the GC content, respectively. The GC content circle shows the deviation from the average GC content of the entire sequence (higher than average GC content is represented in green, and lower than average content is represented in purple). The markings inside the innermost circle represent genome positions (in Mb). The positions of the putative replication origin, putative replication terminus, and nitrogen-fixation genes are shown outside of the outermost circle. (B) Circular representation of the plasmid (pDOA9) of DOA9. The outermost, second, and third circles represent BLASTN comparisons with the plasmid pBBta01 of Bradyrhizobium sp. BTAi1, the draft genome of Bradyrhizobium elkanii 587 (GenBank accession number AJJK00000000), and B. japonicum USDA110, respectively (e-value < 10−10). The innermost and second-innermost circles show the GC skew and the GC content, respectively. The GC content circle shows the deviation from the average GC content of the entire sequence (higher than average GC content in represented in green, and lower than average is represented in purple). The markings inside the innermost circle represent genome positions (in Mb). The positions of the repABC operon, T3/T4SS, nod genes, nif-fix gene clusters, and hup cluster are shown outside of the outermost circle.
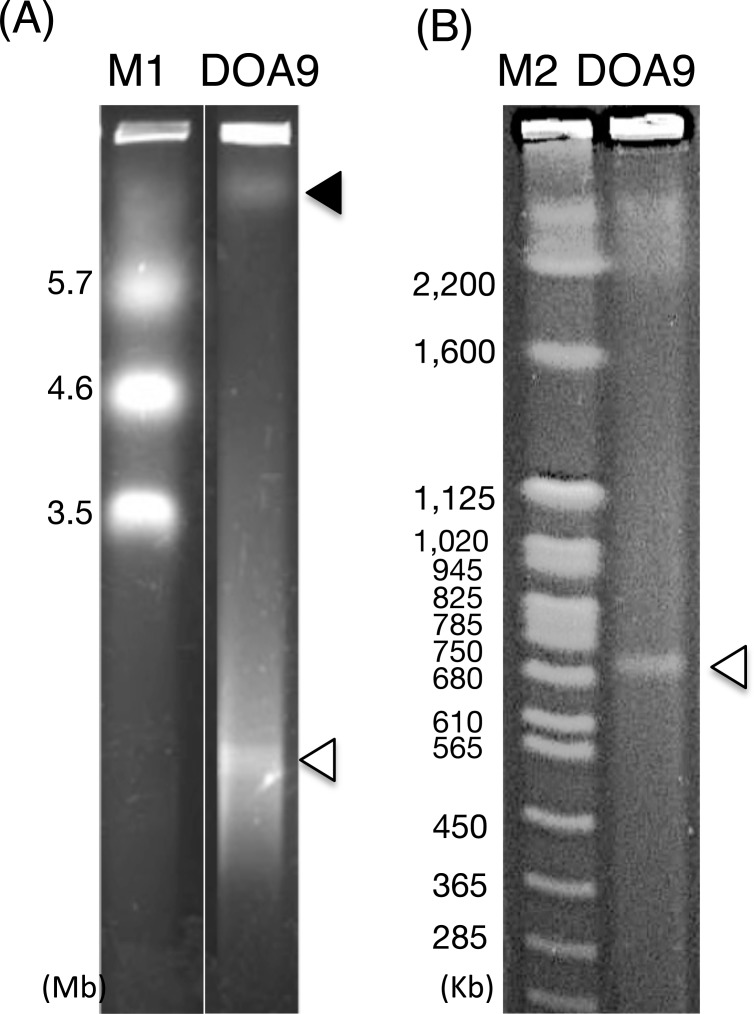
In order to confirm the replicon structure of DOA9, genomic DNA was analyzed by pulse-field gel electrophoresis. A larger fragment (more than 5 Mbp) and a smaller fragment (between 680 and 750 kb) were detected (Fig. 3), similar in size to the larger scaffold (7.1 Mb) and the smaller scaffold (736,085 bp) of the sequencing results, respectively. The canonical repABC operon, which is required for the replication of bacterial plasmids, was found in the smaller scaffold (BDOA9_0200660 to BDOA9_0200680, see below). Therefore, we concluded that the smaller scaffold was the plasmid of DOA9 and designated it as pDOA9. The sequences of the chromosome and plasmid pDOA9 are available in the DDBJ/GenBank/EMBL database (accession numbers DF820425 and DF820426, respectively).
Fig 3. Pulse-field gel electrophoresis of Bradyrhizobium sp. DOA9 genomic DNA.
DOA9 cells were digested in 1% pulse field grade (PFG) agarose plugs with proteinase K, as described in the Materials and Methods, and run 0.8% certified megabase agarose in TAE buffer to separate fragments of 225–6,000 kb (A), or 1% certified megabase agarose in 0.5 x TBE buffer (B) to separate fragments of 225–2,200 kb, respectively. Closed arrowheads and open arrowheads indicate the putative chromosome and the plasmid, respectively. Lane M1: PFGE marker, 3.5–5.7 Mb, Saccharomyces pombe chromosomal DNA. Lane M2: low-range (2.03–194 kb) PFG marker DNA ladder. DOA9: DOA9 genomic DNA.
General genome description
The average GC contents of the chromosome and the plasmid were 64.41% and 60.07%, respectively (Table 1). A GC skew analysis was performed to predict the locations of the putative replication origin and terminus of the chromosome. Two shifts of the GC skew were observed in the chromosome at coordinates 1.3 Mb and 4.86 Mb, respectively (Fig. 2A, innermost circle). The putative replication origin determined by comparison with the genome of B. japonicum USDA110 was found at 1.3 Mb. In B. japonicum USDA110, the putative replication origin was positioned between two conserved hypothetical proteins bll0636 and blr0637 [4]. The orthologs of these two genes, BDOA9_0112090 and BDOA9_0112080, were found at coordinates 1,313,579 to 1,313,070 and 1,312,684 to 1,311,839, respectively. The dif sequence, which is required to convert a dimer chromosome to monomers after aberrant DNA duplication, was found at coordinates 4,862,982 to 4,863,009 (Fig. 2A). The DNA replication may terminate in this region.
Table 1. General genome features of DOA9 and related bradyrhizobiaceae.
| B. japonicum | Bradyrhizobium sp. | |||||
|---|---|---|---|---|---|---|
| Strain | USDA110 | USDA6 | DOA9 a | ORS285 | ORS278 | BTAi1 |
| Genome size (bp) | 9,105,828 | 9,207,384 | 7,850,677 b (736,085) | 7,632,258 | 7,456,587 | 8,493,515 |
| G + C content (%) | 64.06 | 63.67 | 64.41 (60.07) | 65.23 | 65.51 | 64.80 |
| tRNA coding genes | 50 | 51 | 50 (1) | 51 | 51 | 51 |
| rRNA genes | 3 | 6 | 3 (0) | 4 | 6 | 6 |
| Genes | 8,374 | 8,882 | 7273 (676) | 6,848 | 6,748 | 7,723 |
| Photosynthetic | - | - | - | - | + | + |
| CI group | 1 | 1 | 1 | 2 | 3 | 3 |
| Nod-dependent (ND) or Nod-independent (NI) | ND | ND | ND | ND and NI | NI | ND |
| Original host plant | Soybean | Soybean | Aeschynomene | Aeschynomene | Aeschynomene | Aeschynomene |
a The values of plasmid is shown in parentheses.
b The estimated values with gaps.
DNA dot plot analysis was performed to compare the genome structures of Bradyrhizobiaceae. The genome of DOA9 showed extensive similarity with the chromosome of the nonphotosynthetic B. japonicum spp. USDA6 and USDA110 and the photosynthetic non-nodulating Bradyrhizobium sp. S23321 (S2 Fig.). Conversely, the chromosome of DOA9 showed low similarity with those of the photosynthetic strains, including Bradyrhizobium spp. BTAi1, ORS278, and S58 (S2 Fig.). These results are in agreement with the phylogenetic analysis based on 16S rRNA gene sequences (Fig. 1).
RNA- and protein-encoding genes
The DOA9 genome contains one copy of the rRNA gene cluster at coordinates 1,939,820 to 1,939,935 of the chromosome. In total, 50 tRNA genes, which correspond to all 20 of the standard amino acids, were scattered throughout the main chromosome. A total of 7,326 (chromosome: 6,650; plasmid: 676) opening reading frames were predicted and annotated.
A BiBlast comparison was conducted among bradyrhizobial strains DOA9, USDA110, and BTAi1 (Fig. 4). Overall, 3,896 genes (30.8%) are conserved among all three strains (Fig. 4) representing 53.6% (3,896/7,273) of the total number of DOA9 genes. The number of genes unique to DOA9 (1,337 genes; 10.6%) is lower than that of both B. japonicum sp. USDA110 (2,268 genes; 17.9%) and Bradyrhizobium sp. BTAi1 (2,453 genes; 19.4%). DOA9 and B. japonicum sp. USDA110 share 1,437 genes (11.3%) that were not found in Bradyrhizobium sp. BTAi1—a markedly higher number than the 556 genes (4.4%) shared by DOA9 and BTAi1. These results indicate that the DOA9 genome is similar to the genome of B. japonicum USDA110 in terms of gene content.
Fig 4. Comparative genomic analysis among Bradyrhizobium sp. DOA9, B. japonicum USDA110, and Bradyrhizobium sp. BTAi1.
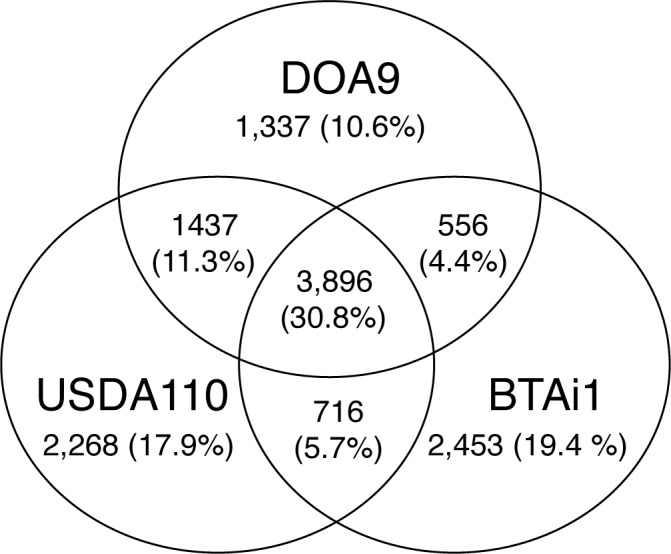
Each genome is represented by a circle, and the numbers of shared and unique genes are shown by the overlapping and nonoverlapping regions. The proportion of total genes represented by each area of the diagram is shown in parentheses. The total number of genes in each genome is shown in square brackets.
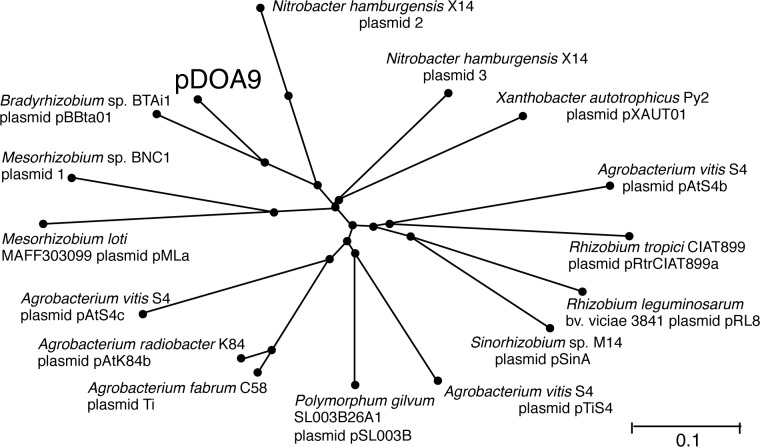
Properties of pDOA9
The BLASTN comparison indicated that pDOA9 showed similarity to the plasmids of Rhizobium and Agrobacterium. The dendrogram based on genomic dissimilarity revealed that pDOA9 is grouped with pBBTa01of Bradyrhizobium sp. BTAi1 (Fig. 5). There was no evidence of extensive synteny between pDOA9 and pBBTa01 or other symbiotic plasmids, except in gene regions with symbiosis-related functions such as nodulation, nitrogen fixation, type III/IV secretion systems, and hydrogenase (Fig. 2B). The GC content of pDOA9 (60.07%) was lower than that of the chromosome (64.95%); this characteristic is similar to Bradyrhizobium sp. BTAi1, where the GC content is 60.69% for pBBTa01 and 64.92% for the chromosome.
Fig 5. Phylogenetic relationships of pDOA9 and related plasmids.
Bootstrap values are expressed as percentages of 1,000 replications. Evolutionary distances were computed using the Kimura two-parameter method. The bar represents one estimated substitution per 100-nucleotide positions.
Nodulation genes
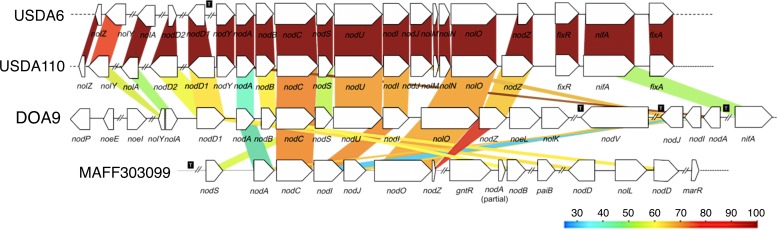
Nodulation genes encode proteins involved in the biosynthesis and transport of NFs, which induce nodule organogenesis in most rhizobia. A large nod gene cluster was found on pDOA9 (Fig. 2B). The organization and repertoire of nod genes are similar to those of B. japonicum, although losses, insertions, and inversions were found (Fig. 6 and Table 2). For example, B. japonicum USDA110 possesses nodYABCSUIJnolMNO arranged in a single operon, whereas DOA9 lacks nodY, nodJ, nolM, and nolN. In addition, B. japonicum has two nodD genes (nodD1 and nodD2) within the cluster, whereas DOA9 has only one (nodD1, locus tag: BDOA9_0203500) in the cluster and the other (nodD2, BDOA9_0205810) located 0.27 Mb away. Furthermore, nodQ, nodP, noeE, noeL, and nolK (BDOA9_0203370–0203390, 0203580–0203590) are incorporated into the nod gene cluster of DOA9 but are located far from the cluster in B. japonicum. Unlike B. japonicum USDA110, DOA9 possesses two copies of nodA (nodA1, BDOA9_0206740 and nodA2, BDOA9_0203720), one in the nodABCSUI operon and the other in the nodA2IJ operon. nodA1 shares only 32–36% identity with the copies in other Bradyrhizobium strains, whereas nodA2 shares 63–69% identity (Table 2). nodB and nodC share more than 60% identity with the corresponding genes in B. japonicum (Table 2). Phylogenetic analyses of the common nodulation genes nodA, nodB and nodC showed that those of DOA9 were placed on unclassified branches separated from the known nod gene-containing rhizobia (S3 Fig.). Although most of the nod genes involved in NF biosynthesis, such as nodABC, are located on pDOA9, some are also found on the chromosome (Table 2): nolG, nodV, nodW, nodQ, nodN, and nodT. Eight nod box consensus sequences [17], where NodD (the transcriptional activator that responds to flavonoids) binds to the DNA, were identified in pDOA9 (S4 Fig.). Most of the nod boxes preced genes involved in Nod factor biosynthesis and regulation, as in other rhizobia. No nod boxes were identified in the chromosome using our search criteria.
Fig 6. Comparison of nodulation gene clusters in Bradyrhizobium sp. DOA9 and related bacteria.
Double slash marks represent DNA regions that are not shown. Colored strips represent the conserved gene regions between the compared strains, and the color indicates the percentage similarity, as detailed in the key. T: region where the transposase genes were located.
Table 2. Nodulation genes detected in the genome of Bradyrhizobium sp. DOA9 and higher BLASTP similarity with other microorganisms.
| Gene | Locus tag | Percentage of identity in BLASTP |
|---|---|---|
| Chromosome | ||
| nolG | BDOA9_0103430 | Bradyrhizobium sp. BTAi1 (91%), B. japonicum USDA110 (90%), Bradyrhizobium sp. ORS278 (76%), R. leguminosarum bv. viciae 3841 (74%) |
| nolG | BDOA9_0108870 | B. japonicum USDA110 (95%), Bradyrhizobium sp. ORS278 (83%), Bradyrhizobium sp. BTAi1 (83%), A. caulinodans ORS571 (56%) |
| nodV | BDOA9_0120640 | B. japonicum WSM2793 (40%), Methylobacterium sp. 10 (31%), Rhizobium sp. PDO1–076 (31%) |
| nodQ | BDOA9_0121760 | B. japonicum USDA110 (96%), Bradyrhizobium sp. ORS278 (80%), Bradyrhizobium sp. BTAi1 (80%), A. caulinodans ORS571 (60%) |
| nodW | BDOA9_0136710 | B. japonicum USDA110 (76%), S. meliloti 1021 (58%) |
| nodW | BDOA9_0136720 | B. japonicum USDA110 (66%), R. leguminosarum bv. viciae 3841 (40%) |
| nodV | BDOA9_0136850 | B. japonicum USDA110 (67%), S. meliloti 1021 (35%) |
| nodN | BDOA9_0136940 | B. japonicum USDA110 (92%), C. taiwanensis LMG 19424 (43%) |
| nodN | BDOA9_0139450 | B. japonicum USDA110 (96%), Bradyrhizobium sp. BTAi1 (83%), Bradyrhizobium sp. ORS278 (83%), M. loti MAFF303099 (46%) |
| nolG | BDOA9_0140510 | B. japonicum USDA110 (97%), Bradyrhizobium sp. BTAi1 (90%), Bradyrhizobium sp. ORS278 (90%), A. caulinodans ORS571 (76%) |
| nodV | BDOA9_0147240 | B. japonicum USDA110 (61%), R. leguminosarum bv. viciae 3841 (32%) |
| nodW | BDOA9_0147250 | B. japonicum USDA110 (78%), A. caulinodans ORS571 (62%) |
| nodW | BDOA9_0147260 | B. japonicum USDA110 (67%), A. caulinodans ORS571 (45%) |
| nodV | BDOA9_0151030 | B. japonicum USDA110 (88%), A. caulinodans ORS571 (34%) |
| nodW | BDOA9_0151040 | B. japonicum USDA110 (94%), A. caulinodans ORS571 (67%) |
| nodW | BDOA9_0151050 | B. japonicum USDA110 (89%), A. caulinodans ORS571 (44%) |
| nolG | BDOA9_0151210 | Bradyrhizobium sp. S23321 (98%), B. japonicum USDA110 (96%) |
| nodW | BDOA9_0151770 | B. japonicum USDA110 (89%), R. etli CFN42 (50%), C. taiwanensis LMG 19424 (47%) |
| nodV | BDOA9_0153480 | B. japonicum USDA110 (85%), Bradyrhizobium sp. BTAi1 (62%), Bradyrhizobium sp. ORS278 (61%) |
| nolG | BDOA9_0156320 | B. japonicum USDA110 (91%), Bradyrhizobium sp. BTAi1 (87%), Bradyrhizobium sp. ORS278 (86%) |
| nolO | BDOA9_0158070 | B. japonicum USDA6 (97%), R. etli CFN42 (35%), M. loti MAFF303099 (35%) |
| nodT | BDOA9_0162260 | Bradyrhizobium sp. ORS278 (56%), Bradyrhizobium sp. BTAi1 (55%), S. fredii NGR234 (54%) |
| nolG | BDOA9_0165280 | B. japonicum USDA110 (97%), Bradyrhizobium sp. BTAi1 (86%), Azospirillum sp. B510 (52%) |
| Plasmid (pDOA9) | ||
| nodW | BDOA9_0203300 | B. japonicum USDA110 (65%), Azoarcus sp. BH72 (42%) |
| nodW | BDOA9_0203310 | B. japonicum USDA110 (67%), Azoarcus sp. BH72 (57%) |
| nodQ | BDOA9_0203370 | Bradyrhizobium sp. ORS278 (53%), Mesorhizobium loti MAFF303099 (48%), Sinorhizobium fredii NGR234 (48%) |
| nodP | BDOA9_0203380 | Bradyrhizobium sp. ORS278 (73%), Azospirillum sp. B510 (71%), Bradyrhizobium sp. BTAi1 (70%) |
| noeE | BDOA9_0203390 | S. fredii NGR234 (55%) |
| nodW | BDOA9_0203420 | Mesorhizobium sp. WSM4349 (33%), S. terangae WSM1721 (31%) |
| noeI | BDOA9_0203430 | B. japonicum USDA110 (73%), S. fredii NGR234 (71%) |
| nolA | BDOA9_0203490 | Bradyrhizobium sp. NC92 (62%), Mesorhizobium sp. WSM4349 (60%), Bradyrhizobium sp. ORS 285 (47%) |
| nodD1 | BDOA9_0203500 | Rhizobium etli CFN42 (63%), B. japonicum USDA110 (62%), S. meliloti 1021 (61%), S. fredii NGR234 (61%) |
| nodA1 | BDOA9_0206740 | Bradyrhizobium sp. WSM1417 (36%), Rhizobium etli CFN42 (34%), B. japonicum USDA110 (32%) |
| nodB | BDOA9_0203510 | S. fredii NGR234 (65%), S. fredii HH103 (65%), B. japonicum USDA110 (62%), Azorhizobium caulinodans ORS571 (42%), Azospirillum sp. B510 (35%) |
| nodC | BDOA9_0203520 | B. japonicum USDA110 (69%), M. loti R7A (68%), R. etli CFN42 (66%) |
| nodS | BDOA9_0203530 | S. fredii NGR234 (59%), R. etli CFN42 (55%), M. loti MAFF303099 (55%), B. japonicum USDA6 (52%) |
| nodU | BDOA9_0203540 | B. japonicum USDA110 (69%), Cupriavidus taiwanensis LMG 19424 (64%), A. caulinodans ORS571 (54%) |
| nodI | BDOA9_0203550 | M. loti MAFF303099 (70%), S. meliloti 1021 (70%), R. etli CFN42 (68%) |
| nolO | BDOA9_0203560 | B. japonicum USDA110 (68%), M. loti MAFF303099 (67%), Rhizobium sp. NGR234 (67%), R. etli CFN42 (65%) |
| nodZ | BDOA9_0203570 | R. etli CFN42 (66%), M. loti MAFF303099 (66%), S. fredii NGR234 (64%) |
| noeL | BDOA9_0203580 | B. japonicum USDA110 (91%), Bradyrhizobium sp. BTAi1 (84%) |
| nolK | BDOA9_0203590 | B. japonicum USDA110 (89%), Bradyrhizobium sp. BTAi1 (80%), Bradyrhizobium sp. ORS278 (80%) |
| nodV | BDOA9_0203630 | B. japonicum USDA110 (50%) |
| nodJ | BDOA9_0203700 | R. etli CFN42 (66%), M. loti MAFF303099 (64%), S. fredii NGR234 (64%), R. leguminosarum bv. viciae 3841 (64%) |
| nodI | BDOA9_0203710 | B. japonicum USDA110 (47%), M. loti MAFF303099 (38%) |
| nodA2 | BDOA9_0203720 | B. japonicum USDA110 (71%), S. fredii NGR234 (70%), M. loti R7A (69%), C. taiwanensis LMG 19424 (69%) |
| nolY | BDOA9_0203790 | B. japonicum USDA110 (42%), S. fredii NGR234 (33%) |
| nodV | BDOA9_0205070 | B. japonicum USDA110 (85%), Bradyrhizobium sp. BTAi1 (79%), Bradyrhizobium sp. ORS278 (79%) |
| nodD2 | BDOA9_0205810 | B. japonicum USDA110 (69%), S. meliloti 1021 (66%), R. leguminosarum bv. viciae 3841 (63%) |
Nitrogen fixation genes
In DOA9 a single cluster of fix genes (fix_chr) was found on the chromosome and a nif-fix cluster was found on both the chromosome (nif-fix_chr) and the plasmid (nif-fix_pla) (Fig. 2 and S5 Fig.). The fix_chr cluster includ fixK-fixLJ, which encodes a factor involved in the regulation of symbiotic nitrogen fixation [18], fixNOQP, which encodes a microaerobically induced cytochrome oxidase complex [19], and fixGHIS, which encodes a membrane-bound complex including a cation pump involved in nitrogen fixation [19]. The comparative analysis showed that the organization of the fix_chr cluster is conserved among bradyrhizobial strains (S5 Fig.).
Comparative analysis showed that the organization of the nif-fix_chr cluster is almost identical to that in photosynthetic bradyrhizobial strains, including Aeschynomene symbionts (Bradyrhizobium spp. ORS278, BTAi1, S58, and ORS285) and the non-nodulating strain Bradyrhizobium sp. S23321. An exception was found for the nifH gene: photosynthetic bradyrhizobial strains possess two copies of nifH, which are located in the nifHQ and nifHDK operons; by contrast, DOA9 possesses only the nifHQ operon, and another copy of nifH might have been deleted from the nifHDK operon (S6 Fig.). Notably, a copy of nifV, which encodes the homocitrate synthase [20], was found in the cluster, similar to the photosynthetic strains Bradyrhizobium spp. ORS285, S58, BTAi1, ORS278, and S23321.
Another nif-fix cluster (nif-fix_pla) was also found in the plasmid of DOA9 (Fig. 2B and S6 Fig.). The nif-fix_pla is more divergent than the nif-fix_chr especially nifQ (BDOA9_0204570), which is involved in the incorporation of molybdenum into nitrogenase, and nifW (BDOA9_0204580), which is a nitrogenase stabilizing and protective protein. The nifH gene in the chromosome (BDOA9_0160870) and the plasmid (BDOA9_0204560) show 96% identity. The phylogenetic analysis showed that nifH of DOA9 is placed on a separate branch, distinct from those of nonphotosynthetic species, such as B. japonicum, B. elkanii, and B. yuanmingense (S7 Fig.). nifH on the chromosome of DOA9 shares higher similarity with the same gene of photosynthetic strains than that of nonphotosynthetic strains.
Gene clusters of type III /IV secretion systems
A cluster of genes encoding the type III secretion system was found in pDAO9 spanning a 53-kb region. The organization of genes encoding the type III secretion apparatus (rhcC, rhcJ, rhcN, and rhcQRSTU) is well conserved among bradyrhizobial strains (S2 Table and S8 Fig.). The organization of the tts gene cluster of DOA9 is similar to that of B. elkanii USDA61, although losses, insertions, and inversions of some genes are present. Phylogenetic analysis using the conserved type III apparatus rhcTVRUC genes showed that the tts genes of DOA9 are grouped with those of other Bradyrhizobium strains (S8 Fig.). The DOA9 genome contains 10 tts boxes, which are conserved motifs located in the promoter region of genes encoding structural components and secrete proteins of the rhizobial tts cluster [21] (S9 Fig.). Most of the tts boxes precede genes that encode nodulation outer proteins (nopB and nopX) or structural components of the type III secretion machinery (y4yQ and rhcT). tts boxes were also identified in the upstream regions of the genes encoding ParA-like ATPase, heat shock protein, cupin, and hypothetical proteins. Homologues of these genes have not been reported so far as type III secreted proteins or related components.
The plasmid pDOA9 also contains genes encoding a VirB/D4 type IV secretion system (T4SS). The vir cluster includes virB1 to virB11, which encode the transmembrane complex and pili required for transfer proteins, and virD4, which encodes a protein that links the transferred substrates to the transfer apparatus (Ding et al. 2003). The vir genes of DOA9 are similar to that of BTAi1 (S10 Fig. and S3 Table). The phylogenetic analysis of two representative genes, virB2 and virB9, supports this finding (S10 Fig.).
Discussion
Bradyrhizobium sp. DOA9 is of particular biological interest because it possesses a broader host range and divergent nod genes compared with other bradyrhizobia [7]. The most prominent characteristic of the DOA9 genome is the presence of symbiosis-related genes on the plasmid rather than on the chromosome. A similar trait is seen in fast-growing rhizobia, whose symbiosis-related genes are often clustered on a large plasmid, such as pNGR234a (536,165 bp) of Sinorhizobium fredii NGR234 [22], p42d of Rhizobium etli CFN42 (371,254 bp) [23], and pRL10 of Rhizobium leguminosarum biovar 3841 (488,135 bp) [24]. Although plasmids were found in Bradyrhizobium sp. BTAi1 and some soybean bradyrhizobia, they did not contain any nod genes [25]. In nod gene-carrying bradyrhizobial strains such as B. japonicum USDA110, the symbiosis-related genes are clustered in regions of the chromosome with a low GC content, known as symbiosis islands [4,5]. For instance, in B. japonicum USDA110 the symbiosis island is a 680-kb region with a relatively low GC content (59.4%) compared with that of the entire genome (64.1%). Notably, in DOA9 the GC content of pDOA9 (60.07%) was lower than that of the chromosome (64.41%). Symbiosis islands have been found to integrate genes of the recipients into the tRNA. Sullivan et al. reported [26] that the symbiotic island of Mesorhizobium loti was transferred to nonsymbiotic strains present in the soil. It is possible that pDOA9 can be transferred to other nonsymbiotic bradyrhizobia by conjugal transfer and integrated into their chromosomes through a process mediated by an integrase, as reported for many other elements, including the pathogenicity islands of pathogenic bacteria [27]. However, the details of the molecular mechanisms and host range for plasmid transfer remain to be elucidated; the latter is particularly interesting because the plasmid might enhance the fitness of bacteria.
The nod genes of DOA9 are highly divergent compared with those of other rhizobia in terms of both gene organization and homology. DOA9 is phylogenetically close to B. japonicum (Fig. 1), and their chromosomes share a high degree of similarity (S2 Fig.). However, their host legume specificities are different and DOA9 is unable to nodulate soybean [7]. In B. japonicum, the common nodABC genes are cotranscribed with nodY [28–30]. The nod gene cluster in DOA9 consists of the nodABCSU genes but lacks nodY and nolMN (Fig. 6). nodY was reported to be Bradyrhizobium specific and to be induced by soybean seed extract and selected isoflavones, primarily genistein and daidzein, but not by flavones [31]. This might account for the nodulation deficiency of DOA9 on soybean. It is possible that soybean bradyrhizobia acquired nodY during coevolution with soybean from Aeschynomene bradyrhizobia.
Unlike B. japonicum, DOA9 harbors additional host-specific genes, nodQ, nodP, and noeE, in the nod gene cluster (Fig. 6 and Table 2). These genes exhibit no similarity with those of B. japonicum, suggesting that DOA9 acquired them independently from other rhizobia. The roles of these genes in nodulation and host specificity remain to be elucidated. Moreover, DOA9 harbors two copies of nodA in the nod gene cluster: nodA1, which is divergent, is incorporated into the nodA1BCSUI operon, whereas nodA2 is incorporated into the nodA2IJ operon. This structure is similar to the structure of Rhizobium tropici, which carries three copies of nodA on the symbiotic plasmid and whose nodA2 and nodA3 genes have no close homologues [32]. The NF acyl group attached by NodA might contribute to the determination of host range [33]. Additional and divergent nodA genes are likely to expand the diversity of NF acyl chains and might broaden the host range of the bacteria [33].
DOA9 harbors several copies of nodVW on both the chromosome and the plasmid: seven copies of nodV (five on the chromosome and two on the plasmid) and 10 copies of nodW (seven on the chromosome and three on the plasmid) (Table 2). NodVW is a member of the classical two-component regulatory family and is essential for the nodulation of cowpea, siratro, and mungbean [34]. In B. japonicum, NodVW is considered to recognize plant flavonoids and could therefore contribute to the nodulation of a broader range of host plants by increasing NF synthesis in combination with NodD [20,35]. Although most nodVW genes showed high similarity with those of B. japonicum, some—BDOA9_0120640, BDOA9_0203420, and BDOA9_0203630—exhibited low similarity (Table 2). These NodVW can recognize additional plant and environmental signals and hence activate symbiosis-related genes. Further study will be necessary to elucidate the involvement of nodVW genes in broad host specificity of DOA9.
The genome of DOA9 contains a full set of nitrogen-fixing genes (nif-fix) on the chromosome and an incomplete cluster of these genes on pDOA9. The nif-fix cluster on the chromosome (nif-fix_chr) is highly similar to that of photosynthetic bradyrhizobia as well as of the non-nodulating strain Bradyrhizobium sp. S23321. The nif-fix cluster on pDOA9 (nif-fix_pla) lacks most of the nif genes and those present are highly similar to those of photosynthetic bradyrhizobia. As in photosynthetic bradyrhizobia, nif-fix_chr cluster in DOA9 carries nifV, which encodes homocitrate synthase that is essential for nitrogenase activity in the free-living diazotrophs [20]. nifV is absent from most strains of rhizobia that perform nitrogen fixation only in symbiotic states, whereas it is presents in strains that also fix nitrogen in ex planta states such as Azorhizobium caulinodans [20], Bradyrhizobium spp. BTAi1 and ORS278 [36]. The presence of nifV in DOA9 suggests that DOA9 has retained the nitrogen-fixation system evolved from the ancestral photosynthetic bradyrhizobia and produces NifV to perform efficient nitrogen fixation during symbiosis.
Clusters of genes encoding elements of the type III secretion system (T3SS) were found in the genomes of various rhizobia including the nonphotosynthetic strains B. japonicum USDA6 and USDA110 and B. elkanii USDA61, and the photosynthetic strains Bradyrhizobium spp. ORS285; however, the cluster was not found in the genome of the most nod-independent bradyrhizobial strains BTAi1, ORS278, and S23321. The phylogenetic analysis using rhcTVRUC genes, which encode conserved proteins of the type III secretion apparatus, indicated that the genes encoding T3SS in DOA9 and other bradyrhizobial strains shared the same evolutionary origin (S8 Fig.). Among these, the components of the type III secretion apparatus of DOA9 showed high identities with those of the soybean symbionts B. elkanii USDA61 and B. japonicum USDA110 (S2 Table). The T3SS genes of these strains were expressed in the presence of the soybean flavonoid genistein, confirming that it functions in the infection of host roots. The induction of T3SS genes involves two regulators: NodD, which is activated by genistein; and TtsI, which is induced by the NodD protein. DOA9 possesses both of these regulators, and they show high identity with the soybean symbionts, despite the fact that DOA9 is unable to nodulate soybean. Expression analysis of T3SS genes and gene-deletion analysis could elucidate the regulation and symbiotic roles of T3SS in DOA9.
The VirB/D4 type IV secretion system (T4SS) has been identified in some rhizobia, including M. loti R7A[37] and R. etli CFN42[38] but is absent from bradyrhizobia with the exception of Bradyrhizobium sp. BTAi1[25]. M. loti R7A possesses a gene cluster (vir) encoding T4SS, similar to that of Agrobacterium tumefaciens. In M. loti R7A T4SS is transcriptionally regulated by a VirA/VirG two-component regulatory system [39]. In M. loti R7A the virA gene is preceded by a nod box, and T4SS is activated in a symbiosis-specific manner [39]. The DOA9 genome lacks homologues of M. loti virA and virG. Similarly, virA and virG homologues are absent from BTAi1, whereas R. etli CFN42 possess virA and virG that show 43% and 76% identities, respectively, with those of M. loti R7A. These findings suggest that the T4SS of Bradyrhizobium spp. DOA9 and BTAi1 share an origin that differs from that of M. loti R7A and other rhizobia.
In conclusion, the genome of DOA9 exhibits a mosaic structure with a unique repertoire of symbiotic genes with various origins. During adaptation to A. americana and other legumes, the ancestral strain of DOA9 acquired divergent nodulation genes and other symbiosis-related genes such as the type III and IV secretion systems. The present study also discovered the first symbiotic plasmid among Bradyrhizobiaceae. This plasmid will be a valuable tool for future studies on the genetics, physiology, and ecology of these species. For example, it will be interesting to test whether pDOA9 can transform non-symbiotic strains, such as S23321, into symbiotic strains or broaden the host range of BTAi1. Our efforts are now directed toward functional analysis of the divergent nod genes in pDOA9, to improve our understanding of the evolution of bradyrhizobia and of their host plants mediated by the symbiotic plasmid.
Supporting Information
Bootstrap values are expressed as percentages of 1,000 replications. Evolutionary distances were computed using the Kimura two-parameter method. The bar represents one estimated substitution per 100-nucleotide positions. Strains capable of Nod factor-dependent and -independent nodulation are marked with (ND) and (NI), respectively. Photosynthetic strains are highlighted in gray.
(TIF)
The positions on each chromosome of each bradyrhizobial strain are indicated on the x-axis and y-axis. The dot color indicates the percentage similarity, as indicated in the key.
(TIF)
Bootstrap values are expressed as percentages of 1,000 replications. The bar represents one estimated substitution per 100-nucleotide positions.
(TIF)
Nucleotides conserved in all cases are shown in bold uppercase letters. Numbers indicate the distance in base pairs between the nod box and the potential translational start site of the corresponding gene. In the consensus sequence capital letters are used for invariant nucleotides, and lowercase letters are used for nucleotides conserved in at least 50% of the sequences.
(TIF)
Double slash marks represent DNA regions that are not shown. Colored strips represent the conserved gene regions between the compared strains, and the color indicates the percentage similarity, as indicated by the key.
(TIF)
Double slash marks represent DNA regions that are not shown. Colored strips represent the conserved gene regions between the compared strains, and the color indicates the percentage similarity, as indicated by the key. T: region where the transposase genes were located. N: region of nodulation genes. G: region of groES-groEL regulatory genes.
(TIF)
Bootstrap values are expressed as percentages of 1,000 replications. The bar represents one estimated substitution per 100-nucleotide positions.
(TIF)
Asterisks represent the strains harboring the T3SS cluster in the plasmid and each plasmid name is shown in parentheses. Double slash marks represent DNA regions that are not shown. Colored stripes represent the conserved gene regions between the compared strains, and the color indicates the percentage similarity, as indicated by the key. T: region where the transposase genes were located.
(TIF)
Nucleotides conserved in all cases are shown in bold uppercase letters. Numbers indicate the distance in base pairs between the tts box and the potential translational start site of the corresponding gene. In the consensus sequence capital letters are used for invariant nucleotides, and lowercase letters are used for nucleotides conserved in at least 50% of the sequences.
(TIF)
(A) Comparative analysis of vir clusters of Bradyrhizobium sp. DOA9 and related bacteria. All the compared clusters were located on the symbiotic plasmid and each plasmid name is shown in parentheses. Double slash marks represent DNA regions that are not shown. Colored strips represent the conserved gene regions between the compared strains, and the color indicates the percentage similarity, as indicated by the key. (B) Phylogenetic trees based on a combination of the virB2 and virB9 sequences of DOA9 and other related rhizobia. Bootstrap values are expressed as percentages of 1,000 replications. The bar represents one estimated substitution per 100-nucleotide positions.
(TIF)
(XLSX)
(XLSX)
(XLSX)
Data Availability
The sequences of the DOA9 genome and plasmid have been deposited in the DNA Database of Japan (DDBJ)/GenBank/European Molecular Biology Laboratory (EMBL) databases with the accession numbers DF820425 and DF820426, respectively.
Funding Statement
This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI) in the Priority Area “Comprehensive Genomics” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (http://www.jsps.go.jp/english/e-grants/). The funding supported data collection and analysis of the present work.
References
- 1. Kouchi H, Imaizumi-Anraku H, Hayashi M, Hakoyama T, Nakagawa T, et al. (2010) How many peas in a pod? Legume genes responsible for mutualistic symbioses underground. Plant and Cell Physiology 51: 1381–1397. 10.1093/pcp/pcq107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giraud E, Moulin L, Vallenet D, Barbe V, Cytryn E, et al. (2007) Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316: 1307–1312. [DOI] [PubMed] [Google Scholar]
- 3. Chaintreuil C, Arrighi JF, Giraud E, Miché L, Moulin L, et al. (2013) Evolution of symbiosis in the legume genus Aeschynomene. New Phytologist 200: 1247–1259. 10.1111/nph.12424 [DOI] [PubMed] [Google Scholar]
- 4. Kaneko T, Nakamura Y, Sato S, Minamisawa K, Uchiumi T, et al. (2002) Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA research 9: 189–197. [DOI] [PubMed] [Google Scholar]
- 5. Kaneko T, Maita H, Hirakawa H, Uchiike N, Minamisawa K, et al. (2011) Complete genome sequence of the soybean symbiont Bradyrhizobium japonicum strain USDA6T. Genes 2: 763–787. 10.3390/genes2040763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okubo T, Tsukui T, Maita H, Okamoto S, Oshima K, et al. (2011) Complete genome sequence of Bradyrhizobium sp. S23321: insights into symbiosis evolution in soil oligotrophs. Microbes and environments/JSME 27: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noisangiam R, Teamtisong K, Tittabutr P, Boonkerd N, Toshiki U, et al. (2012) Genetic diversity, symbiotic evolution, and proposed infection process of Bradyrhizobium strains isolated from root nodules of Aeschynomene americana L. in Thailand. Applied and environmental microbiology 78: 6236–6250. 10.1128/AEM.00897-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teamtisong K, Songwattana P, Noisangiam R, Piromyou P, Boonkerd N, et al. (2014) Divergent Nod-Containing Bradyrhizobium sp. DOA9 with a Megaplasmid and its Host Range. Microbes and Environments. [DOI] [PMC free article] [PubMed]
- 9. Sadowsky MJ, Tully RE, Cregan PB, Keyser HH (1987) Genetic diversity in Bradyrhizobium japonicum serogroup 123 and its relation to genotype-specific nodulation of soybean. Applied and environmental microbiology 53: 2624–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson K (1987) Preparation of genomic DNA from bacteria. Current protocols in molecular biology: 2.4. 1–2.4. 5. [DOI] [PubMed]
- 11.Chevreux B, Wetter T, Suhai S (1999) Genome sequence assembly using trace signals and additional sequence information. pp. 45–56.
- 12. Grant JR, Stothard P (2008) The CGView Server: a comparative genomics tool for circular genomes. Nucleic acids research 36: W181–W184. 10.1093/nar/gkn179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohtsubo Y, Ikeda-Ohtsubo W, Nagata Y, Tsuda M (2008) GenomeMatcher: a graphical user interface for DNA sequence comparison. BMC bioinformatics 9: 376 10.1186/1471-2105-9-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic acids research 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution 28: 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regensburger B, Hennecke H (1983) RNA polymerase from Rhizobium japonicum. Archives of microbiology 135: 103–109. [DOI] [PubMed] [Google Scholar]
- 17. Spaink HP, Okker RJ, Wijffelman CA, Pees E, Lugtenberg BJ (1987) Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Molecular Biology 9: 27–39. 10.1007/BF00017984 [DOI] [PubMed] [Google Scholar]
- 18. Fischer H-M (1994) Genetic regulation of nitrogen fixation in rhizobia. Microbiological reviews 58: 352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dixon R, Kahn D (2004) Genetic regulation of biological nitrogen fixation. Nature Reviews Microbiology 2: 621–631. [DOI] [PubMed] [Google Scholar]
- 20. Zheng L, White RH, Dean DR (1997) Purification of the Azotobacter vinelandii nifV-encoded homocitrate synthase. Journal of bacteriology 179: 5963–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krause A, Doerfel A, Göttfert M (2002) Mutational and transcriptional analysis of the type III secretion system of Bradyrhizobium japonicum. Molecular plant-microbe interactions 15: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 22. Perret X, Viprey V, Freiberg C, Broughton W (1997) Structure and evolution of NGRRS-1, a complex, repeated element in the genome of Rhizobium sp. strain NGR234. Journal of bacteriology 179: 7488–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. González V, Santamaría RI, Bustos P, Hernández-González I, Medrano-Soto A, et al. (2006) The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proceedings of the National Academy of Sciences of the United states of America 103: 3834–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Young JPW, Crossman LC, Johnston AW, Thomson NR, Ghazoui ZF, et al. (2006) The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome biology 7: R34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cytryn EJ, Jitacksorn S, Giraud E, Sadowsky MJ (2008) Insights learned from pBTAi1, a 229-kb accessory plasmid from Bradyrhizobium sp. strain BTAi1 and prevalence of accessory plasmids in other Bradyrhizobium sp. strains. The ISME journal 2: 158–170. 10.1038/ismej.2007.105 [DOI] [PubMed] [Google Scholar]
- 26. Sullivan JT, Ronson CW (1998) Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. Proceedings of the National Academy of Sciences 95: 5145–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hacker J, Kaper JB (2000) Pathogenicity islands and the evolution of microbes. Annual Reviews in Microbiology 54: 641–679. [DOI] [PubMed] [Google Scholar]
- 28. Geelen D, Mergaert P, Geremia RA, Goormachtig S, Montagu M, et al. (1993) Identification of nodSUIJ genes in Nod locus 1 of Azorhizobium caulinodans: evidence that nodS encodes a methyltransferase involved in Nod factor modification. Molecular microbiology 9: 145–154. [DOI] [PubMed] [Google Scholar]
- 29. Göttfert M, Hitz S, Hennecke H (1990) Identification of nodS and nodU, two inducible genes inserted between the Bradyrhizobium japonicum nodYABC and nodIJ genes. Mol Plant-Microbe Interact 3: 308–316. [DOI] [PubMed] [Google Scholar]
- 30. Lewin A, Cervantes E, Wong C, Broughton WJ (1990) nodSU, two new nod genes of the broad host range Rhizobium strain NGR234 encode host-specific nodulation of the tropical tree Leucaena leucocephala. Mol Plant-Microbe Interact 3: 317–326. [DOI] [PubMed] [Google Scholar]
- 31. Banfalvi Z, Nieuwkoop A, Schell M, Besl L, Stacey G (1988) Regulation of nod gene expression in Bradyrhizobium japonicum. Molecular and General Genetics MGG 214: 420–424. [DOI] [PubMed] [Google Scholar]
- 32. Ormeño-Orrillo E, Menna P, Almeida LGP, Ollero FJ, Nicolás MF, et al. (2012) Genomic basis of broad host range and environmental adaptability of Rhizobium tropici CIAT 899 and Rhizobium sp. PRF 81 which are used in inoculants for common bean (Phaseolus vulgaris L.). BMC genomics 13: 735 10.1186/1471-2164-13-735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Debellé F, Plazanet C, Roche P, Pujol C, Savagnac A, et al. (1996) The NodA proteins of Rhizobium meliloti and Rhizobium tropici specify the N‐acylation of Nod factors by different fatty acids. Molecular microbiology 22: 303–314. [DOI] [PubMed] [Google Scholar]
- 34. Sanjuan J, Grob P, Göttfert M, Hennecke H, Stacey G (1994) NodW is essential for full expression of the common nodulation genes in Bradyrhizobium japonicum. MPMI-Molecular Plant Microbe Interactions 7: 364–369. 8075421 [Google Scholar]
- 35. Loh J, Stacey G (2003) Nodulation gene regulation in Bradyrhizobium japonicum: a unique integration of global regulatory circuits. Applied and environmental microbiology 69: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Terpolilli JJ, Hood GA, Poole PS (2012) What determines the efficiency of N2-fixing Rhizobium-legume symbioses? Advances in microbial physiology 60: 326. [DOI] [PubMed] [Google Scholar]
- 37. Hubber A, Vergunst AC, Sullivan JT, Hooykaas PJ, Ronson CW (2004) Symbiotic phenotypes and translocated effector proteins of the Mesorhizobium loti strain R7A VirB/D4 type IV secretion system. Molecular microbiology 54: 561–574. [DOI] [PubMed] [Google Scholar]
- 38. González V, Bustos P, Ramírez-Romero MA, Medrano-Soto A, Salgado H, et al. (2003) The mosaic structure of the symbiotic plasmid of Rhizobium etli CFN42 and its relation to other symbiotic genome compartments. Genome biology 4: R36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hubber AM, Sullivan JT, Ronson CW (2007) Symbiosis-induced cascade regulation of the Mesorhizobium loti R7A VirB/D4 type IV secretion system. Molecular plant-microbe interactions 20: 255–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bootstrap values are expressed as percentages of 1,000 replications. Evolutionary distances were computed using the Kimura two-parameter method. The bar represents one estimated substitution per 100-nucleotide positions. Strains capable of Nod factor-dependent and -independent nodulation are marked with (ND) and (NI), respectively. Photosynthetic strains are highlighted in gray.
(TIF)
The positions on each chromosome of each bradyrhizobial strain are indicated on the x-axis and y-axis. The dot color indicates the percentage similarity, as indicated in the key.
(TIF)
Bootstrap values are expressed as percentages of 1,000 replications. The bar represents one estimated substitution per 100-nucleotide positions.
(TIF)
Nucleotides conserved in all cases are shown in bold uppercase letters. Numbers indicate the distance in base pairs between the nod box and the potential translational start site of the corresponding gene. In the consensus sequence capital letters are used for invariant nucleotides, and lowercase letters are used for nucleotides conserved in at least 50% of the sequences.
(TIF)
Double slash marks represent DNA regions that are not shown. Colored strips represent the conserved gene regions between the compared strains, and the color indicates the percentage similarity, as indicated by the key.
(TIF)
Double slash marks represent DNA regions that are not shown. Colored strips represent the conserved gene regions between the compared strains, and the color indicates the percentage similarity, as indicated by the key. T: region where the transposase genes were located. N: region of nodulation genes. G: region of groES-groEL regulatory genes.
(TIF)
Bootstrap values are expressed as percentages of 1,000 replications. The bar represents one estimated substitution per 100-nucleotide positions.
(TIF)
Asterisks represent the strains harboring the T3SS cluster in the plasmid and each plasmid name is shown in parentheses. Double slash marks represent DNA regions that are not shown. Colored stripes represent the conserved gene regions between the compared strains, and the color indicates the percentage similarity, as indicated by the key. T: region where the transposase genes were located.
(TIF)
Nucleotides conserved in all cases are shown in bold uppercase letters. Numbers indicate the distance in base pairs between the tts box and the potential translational start site of the corresponding gene. In the consensus sequence capital letters are used for invariant nucleotides, and lowercase letters are used for nucleotides conserved in at least 50% of the sequences.
(TIF)
(A) Comparative analysis of vir clusters of Bradyrhizobium sp. DOA9 and related bacteria. All the compared clusters were located on the symbiotic plasmid and each plasmid name is shown in parentheses. Double slash marks represent DNA regions that are not shown. Colored strips represent the conserved gene regions between the compared strains, and the color indicates the percentage similarity, as indicated by the key. (B) Phylogenetic trees based on a combination of the virB2 and virB9 sequences of DOA9 and other related rhizobia. Bootstrap values are expressed as percentages of 1,000 replications. The bar represents one estimated substitution per 100-nucleotide positions.
(TIF)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The sequences of the DOA9 genome and plasmid have been deposited in the DNA Database of Japan (DDBJ)/GenBank/European Molecular Biology Laboratory (EMBL) databases with the accession numbers DF820425 and DF820426, respectively.