Abstract
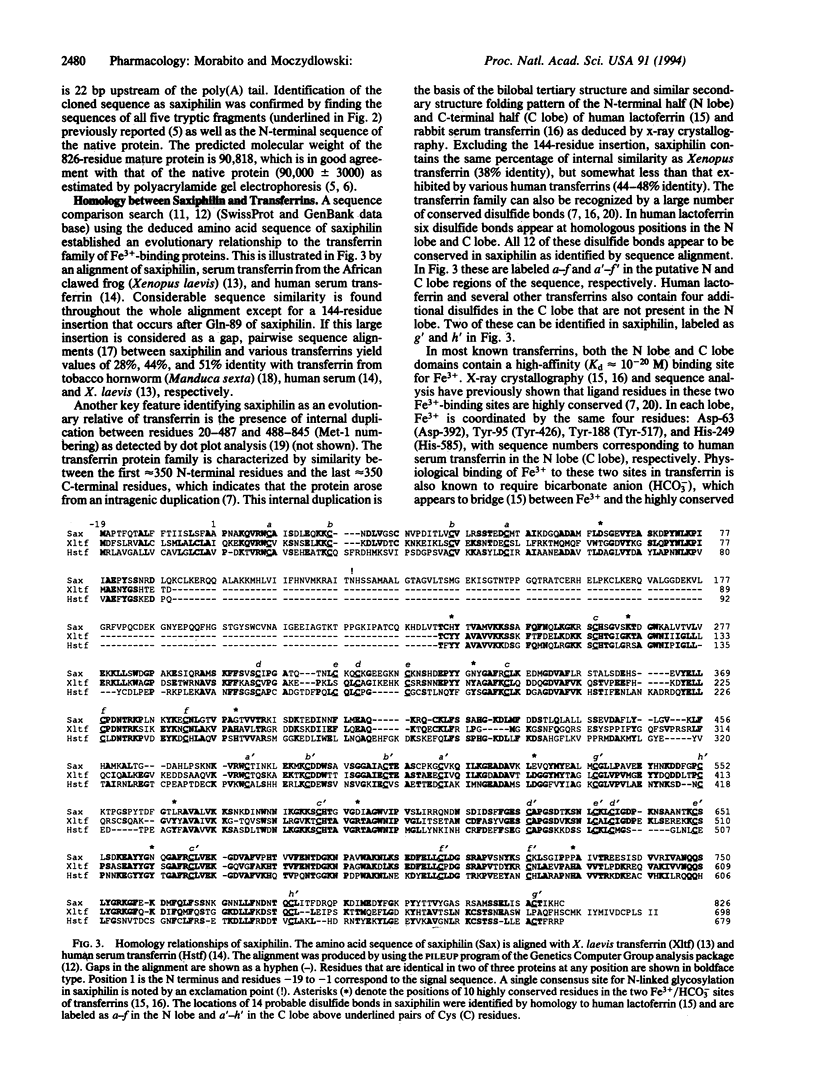
Plasma and tissue of certain vertebrates contain a protein called saxiphilin that specifically binds the neurotoxin saxitoxin with nanomolar affinity. We describe the isolation of a cDNA clone of saxiphilin from liver of the North American bullfrog (Rana catesbeiana). The cDNA sequence encodes a protein that is evolutionarily related to members of the transferrin family of Fe(3+)-binding proteins. Pairwise sequence alignment of saxiphilin with various transferrins reveals amino acid identity as high as 51% and predicts 14 disulfide bonds that are highly conserved. The larger size of saxiphilin (91 kDa) versus serum transferrin (approximately 78 kDa) is primarily due to a unique insertion of 144 residues. This insertion contains a 49-residue domain classified as a type 1 repetitive element of thyroglobulin, which is shared by a variety of membrane, secreted, and extracellular matrix proteins. Saxiphilin also differs from transferrins in 9 of 10 highly conserved amino acids in the two homologous Fe3+/HCO3-binding sites of transferrin. Identification of saxiphilin implies that transferrin-like proteins comprise a diverse superfamily with functions other than iron binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. F., Baker H. M., Norris G. E., Rice D. W., Baker E. N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989 Oct 20;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- Bailey S., Evans R. W., Garratt R. C., Gorinsky B., Hasnain S., Horsburgh C., Jhoti H., Lindley P. F., Mydin A., Sarra R. Molecular structure of serum transferrin at 3.3-A resolution. Biochemistry. 1988 Jul 26;27(15):5804–5812. doi: 10.1021/bi00415a061. [DOI] [PubMed] [Google Scholar]
- Baldwin G. S. Comparison of transferrin sequences from different species. Comp Biochem Physiol B. 1993 Sep;106(1):203–218. doi: 10.1016/0305-0491(93)90028-4. [DOI] [PubMed] [Google Scholar]
- Bartfeld N. S., Law J. H. Isolation and molecular cloning of transferrin from the tobacco hornworm, Manduca sexta. Sequence similarity to the vertebrate transferrins. J Biol Chem. 1990 Dec 15;265(35):21684–21691. [PubMed] [Google Scholar]
- Brinkman A., Groffen C., Kortleve D. J., Geurts van Kessel A., Drop S. L. Isolation and characterization of a cDNA encoding the low molecular weight insulin-like growth factor binding protein (IBP-1). EMBO J. 1988 Aug;7(8):2417–2423. doi: 10.1002/j.1460-2075.1988.tb03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann A. J. Stable and safe HIV provirus clones. Nucleic Acids Res. 1990 Oct 25;18(20):6153–6154. doi: 10.1093/nar/18.20.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D. D., Wong M., Tanaka J., Barr L. Saxitoxin binding sites in frog-myocardial cytosol. Science. 1982 Feb 26;215(4536):1117–1119. doi: 10.1126/science.6278588. [DOI] [PubMed] [Google Scholar]
- Idzerda R. L., Huebers H., Finch C. A., McKnight G. S. Rat transferrin gene expression: tissue-specific regulation by iron deficiency. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3723–3727. doi: 10.1073/pnas.83.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N., Lauer W., Habicht J., Dobberstein B. Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii). An alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO J. 1987 Jun;6(6):1677–1683. doi: 10.1002/j.1460-2075.1987.tb02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Llewellyn L., Moczydlowski E. Biochemical and immunochemical comparison of saxiphilin and transferrin, two structurally related plasma proteins from Rana catesbeiana. Mol Pharmacol. 1993 Oct;44(4):742–748. [PubMed] [Google Scholar]
- Li Y., Moczydlowski E. Purification and partial sequencing of saxiphilin, a saxitoxin-binding protein from the bullfrog, reveals homology to transferrin. J Biol Chem. 1991 Aug 15;266(23):15481–15487. [PubMed] [Google Scholar]
- Linnenbach A. J., Wojcierowski J., Wu S. A., Pyrc J. J., Ross A. H., Dietzschold B., Speicher D., Koprowski H. Sequence investigation of the major gastrointestinal tumor-associated antigen gene family, GA733. Proc Natl Acad Sci U S A. 1989 Jan;86(1):27–31. doi: 10.1073/pnas.86.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar J., Lukács G. L., Li Y., Hall S., Moczydlowski E. Pharmacological and biochemical properties of saxiphilin, a soluble saxitoxin-binding protein from the bullfrog (Rana catesbeiana). Toxicon. 1991;29(1):53–71. doi: 10.1016/0041-0101(91)90039-t. [DOI] [PubMed] [Google Scholar]
- Mahmood N. A., Carmichael W. W. Paralytic shellfish poisons produced by the freshwater cyanobacterium Aphanizomenon flos-aquae NH-5. Toxicon. 1986;24(2):175–186. doi: 10.1016/0041-0101(86)90120-0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthiéry Y., Lissitzky S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur J Biochem. 1987 Jun 15;165(3):491–498. doi: 10.1111/j.1432-1033.1987.tb11466.x. [DOI] [PubMed] [Google Scholar]
- Mann K., Deutzmann R., Aumailley M., Timpl R., Raimondi L., Yamada Y., Pan T. C., Conway D., Chu M. L. Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO J. 1989 Jan;8(1):65–72. doi: 10.1002/j.1460-2075.1989.tb03349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight A. J., Mason D. W., Barclay A. N. Sequence of a rat MHC class II-associated invariant chain cDNA clone containing a 64 amino acid thyroglobulin-like domain. Nucleic Acids Res. 1989 May 25;17(10):3983–3984. doi: 10.1093/nar/17.10.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Mahar J., Ravindran A. Multiple saxitoxin-binding sites in bullfrog muscle: tetrodotoxin-sensitive sodium channels and tetrodotoxin-insensitive sites of unknown function. Mol Pharmacol. 1988 Feb;33(2):202–211. [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikkarainen T., Eddy R., Fukushima Y., Byers M., Shows T., Pihlajaniemi T., Saraste M., Tryggvason K. Human laminin B1 chain. A multidomain protein with gene (LAMB1) locus in the q22 region of chromosome 7. J Biol Chem. 1987 Aug 5;262(22):10454–10462. [PubMed] [Google Scholar]
- Rose T. M., Plowman G. D., Teplow D. B., Dreyer W. J., Hellström K. E., Brown J. P. Primary structure of the human melanoma-associated antigen p97 (melanotransferrin) deduced from the mRNA sequence. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1261–1265. doi: 10.1073/pnas.83.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon B., Podolsky D. K., Moldenhauer G., Isselbacher K. J., Gattoni-Celli S., Brand S. J. Epithelial glycoprotein is a member of a family of epithelial cell surface antigens homologous to nidogen, a matrix adhesion protein. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2755–2759. doi: 10.1073/pnas.87.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Lum J. B., McGill J. R., Moore C. M., Naylor S. L., van Bragt P. H., Baldwin W. D., Bowman B. H. Human transferrin: cDNA characterization and chromosomal localization. Proc Natl Acad Sci U S A. 1984 May;81(9):2752–2756. doi: 10.1073/pnas.81.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]